Cyclocurcumin as Promising Bioactive Natural Compound: An Overview
Abstract
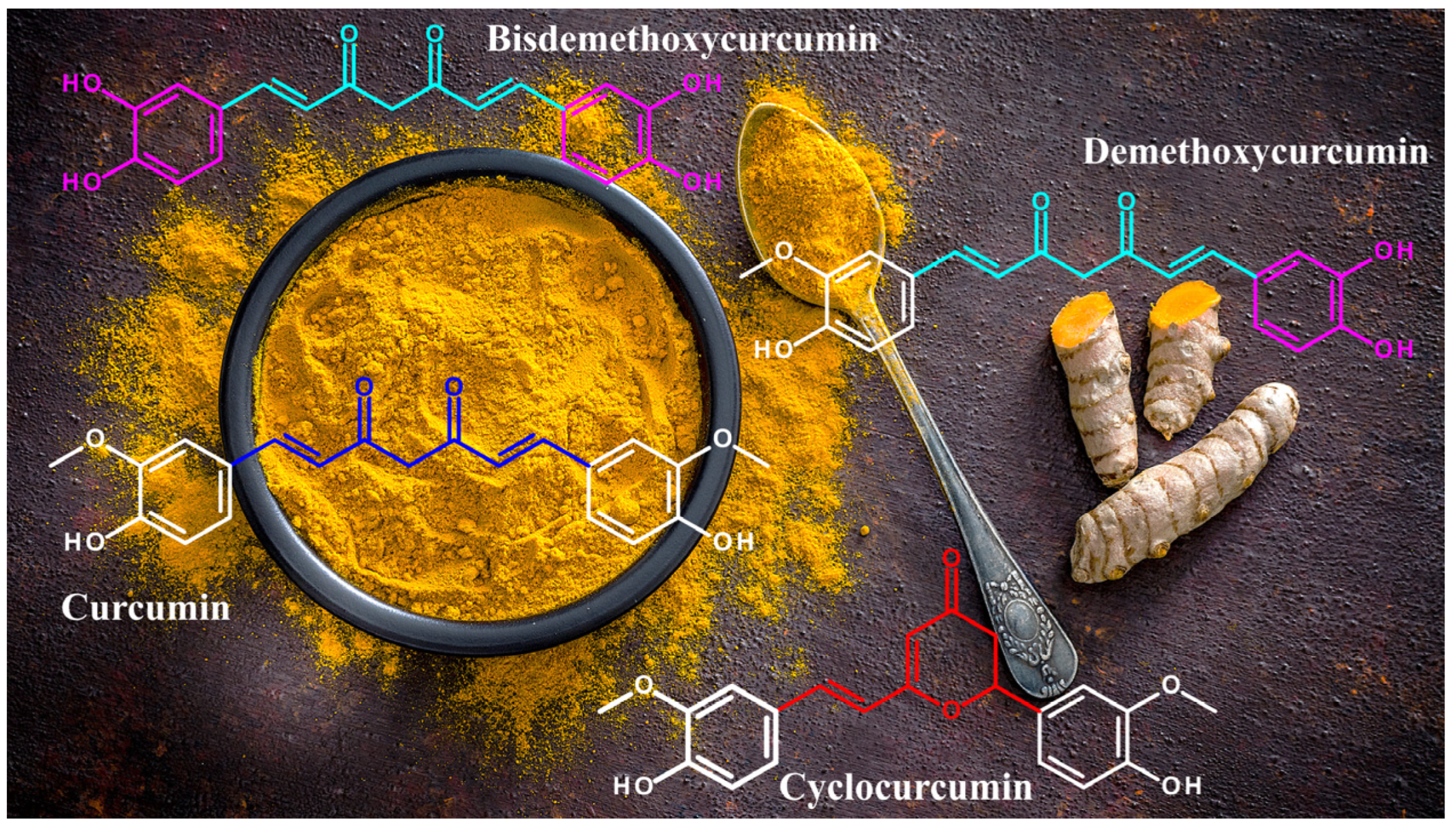
1. Introduction
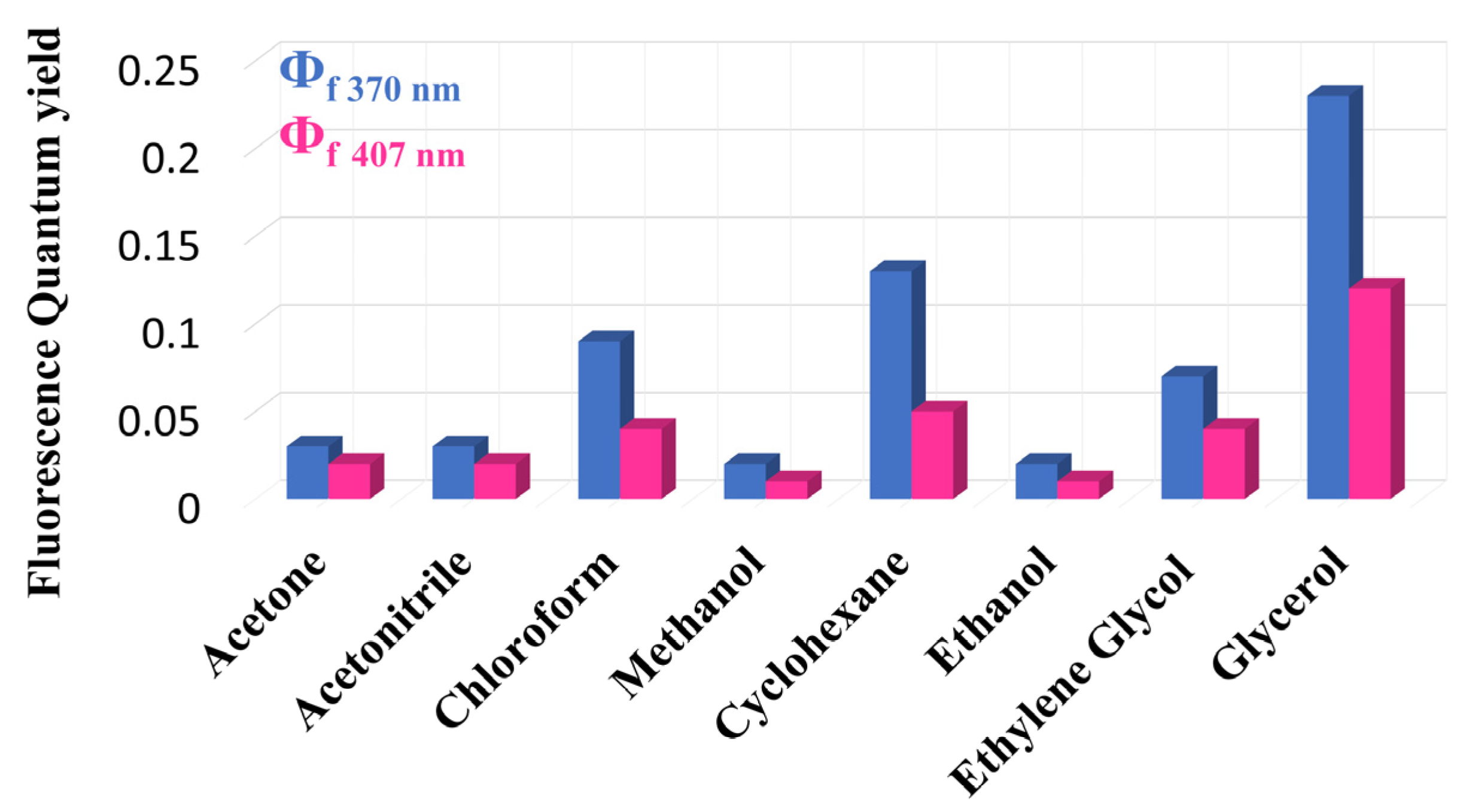
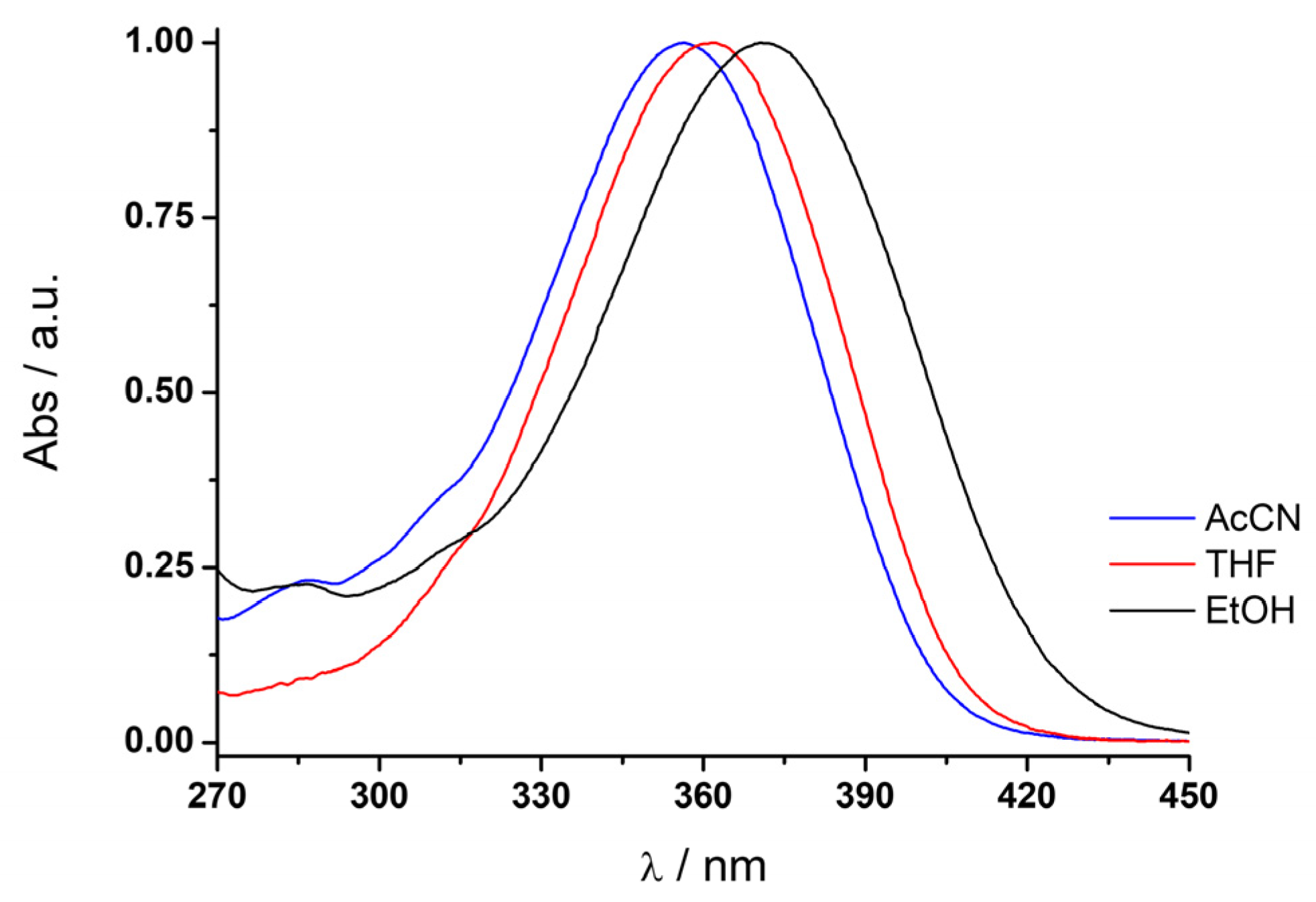
2. Structural and Chemical Properties
3. Computational Studies
4. Biological Activities
5. Future Prospectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lantz, R.C.; Chen, G.J.; Solyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine 2005, 12, 445–452. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Kurup, V.P.; Barrios, C.S. Immunomodulatory effects of curcumin in allergy. Mol. Nutr. Food Res. 2008, 52, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Surh, Y.J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem. Toxicol. 2002, 40, 1091–1097. [Google Scholar] [CrossRef]
- Al-Obaidi, L.F.H. Effect of adding different concentrations of turmeric powder on the chemical composition, oxidative stability and microbiology of the soft cheese. Plant Arch. 2019, 19, 317–321. [Google Scholar]
- de Carvalho, F.A.L.; Munekata, P.E.S.; de Oliveira, A.L.; Pateiro, M.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Turmeric (Curcuma longa L.) extract on oxidative stability, physicochemical and sensory properties of fresh lamb sausage with fat replacement by tiger nut (Cyperus esculentus L.) oil. Food Res. Int. 2020, 136, 109487. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural colorants from vegetable food waste: Recovery, regulatory aspects, and stability—A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529. [Google Scholar] [CrossRef]
- Masuda, T.; Hidaka, K.; Shinohara, A.; Maekawa, T.; Takeda, Y.; Yamaguchi, H. Chemical Studies on Antioxidant Mechanism of Curcuminoid: Analysis of Radical Reaction Products from Curcumin. J. Agric. Food Chem. 1999, 47, 71–77. [Google Scholar] [CrossRef]
- Dairam, A.; Limson, J.L.; Watkins, G.M.; Antunes, E.; Daya, S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J. Agric. Food Chem. 2007, 55, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.C.; Zhou, Y.Q.; Ren, D.; Wang, R.; Wang, C.; Lin, L.G.; Zhang, X.Q.; Ye, W.C.; Zhang, Q.W. Turmeric: A review of its chemical composition, quality control, bioactivity, and pharmaceutical application. In Natural and Artificial Flavoring Agents and Food Dyes. Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, UK, 2018; pp. 299–350. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Wright, J.S. Predicting the antioxidant activity of curcumin and curcuminoids. J. Mol. Struct. Theochem. 2002, 591, 207–217. [Google Scholar] [CrossRef]
- Angelini, G.; Pasc, A.; Gasbarri, C. Curcumin in silver nanoparticles aqueous solution: Kinetics of keto-enol tautomerism and effects on AgNPs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125235. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.H. Therapeutic Potential of Turmeric in Alzheimer’s Disease: Curcumin or Curcuminoids? Phytoter. Res. 2014, 28, 517–525. [Google Scholar] [CrossRef]
- Yovanovich, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar]
- Yanagisawa, D.; Shirai, N.; Amatsubo, T.; Taguchi, H.; Hirao, K.; Urushitani, M.; Morikawa, S.; Inubushi, T.; Kato, M.; Kato, F.; et al. Relationship between the tautomeric structures of curcumin derivatives and their Abeta-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 2010, 31, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.J.; Lin, H.Y.; Yang, Y.H.; Chou, C.J.; Chien, Y.C.; Wu, T.S.; Wu, C.H. Demethoxycurcumin, a major active curcuminoid from Curcuma longa, suppresses balloon injury induced vascular smooth muscle cell migration and neointima formation: An in vitro and in vivo study. Mol. Nutr. Food Res. 2013, 57, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Yang, Y.; Cui, L.; Yang, J.; Li, X.; Yang, Y.; Duan, H. Bisdemethoxycurcumin inhibits ovarian cancer via reducing oxidative stress mediated MMPs expressions. Sci. Rep. 2016, 6, 2877. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Rahbardar, M.G.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021, 35, 6459–6513. [Google Scholar] [CrossRef]
- Simon, A.; Allais, D.P.; Duroux, J.L.; Basly, J.P.; Durand-Fontanier, S.; Delage, C. Inhibition effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationship. Cancer Lett. 1998, 129, 111–116. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Adhikary, R.; Barnes, C.A.; Trampel, R.L.; Wallace, S.J.; Kee, T.W.; Petrich, J.W. Photoinduced trans-to-cis isomerization of cyclocurcumin. J. Phys. Chem. B 2011, 115, 10707–10714. [Google Scholar] [CrossRef]
- Kiuchi, F.; Goto, Y.; Sugimoto, N.; Akao, N.; Kondo, K.; Tsuda, Y. Nematocidal activity of turmeric: Synergistic action of curcuminoids. Chem. Pharm. Bull. 1993, 41, 1640–1643. [Google Scholar] [CrossRef]
- Randino, R.; Grimaldi, M.; Persico, M.; De Santis, A.; Cini, E.; Cabri, W.; Riva, A.; D’Errico, G.; Fattorusso, C.; D’Ursi, A.M.; et al. Investigating the neuroprotective effects of turmeric extract: Structural interactions of β-amyloid peptide with single curcuminoids. Sci. Rep. 2016, 6, 38846. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kar, S.K. Curcuminoids: The Novel Molecules of Nature. In Herbs and Spices-New Processing Technologies Edited by Rabia Shabir Ahmad; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulation. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Pundir, H.; Pathak, R.; Pant, T.; Pant, M.; Chandra, S.; Tamt, S. In Silico Screening of Phytochemicals Targeting SmdCD of Streptococcus mutans using Molecular Docking Approach. Trends Sci. 2023, 20, 6036. [Google Scholar] [CrossRef]
- Gasbarri, C.; Angelini, G. Combined calorimetric, spectroscopic, and microscopic investigation on the inclusion complex from cyclocurcumin and sulfobutylether-β-cyclodextrin in aqueous solution and Kinetics of thermal cis-trans isomerization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131149. [Google Scholar] [CrossRef]
- Catalán, J. Compounds with π(loc) → π*(deloc) electronic transitions and their solvatochromism. J. Phys. Org. Chem. 2015, 28, 497–503. [Google Scholar] [CrossRef]
- Schade, A.; Schreiter, K.; Rìffer, T.; Lang, H.; Spange, S. Interactions of enolizable barbiturate dyes. Chem. Eur. J. 2016, 22, 5734–5748. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Gansmüller, A.; Pecourneau, J.; Gasbarri, C. An insight into cyclocurcumin cis–trans isomerization: Kinetics in solution and in the presence of silver nanoparticle. J. Mol. Liquids 2021, 333, 116000. [Google Scholar] [CrossRef]
- Angelini, G.; Scotti, L.; Aceto, A.; Gasbarri, C. Silver nanoparticles as interactive media for the azobenzenes isomerization in aqueous solution: From linear to stretched kinetics. J. Mol. Liq. 2019, 284, 592–598. [Google Scholar] [CrossRef]
- Liu, X.M.; Jin, X.; Zhang, Z.X.; Wang, J.; Bai, F.Q. Theoretical study on the reaction mechanism of the thermal cis–trans isomerization of fluorine-substituted azobenzene derivatives. RSC Adv. 2018, 8, 11580–11588. [Google Scholar] [CrossRef]
- Li, Y.; Toscano, M.; Mazzone, G.; Russo, N. Antioxidant properties and free radical scavenging mechanisms of cyclocurcumin. New J. Chem. 2018, 42, 12698. [Google Scholar] [CrossRef]
- Shakour, N.; Cabezas, R.; Gonzales Santos, J.; Barreto, G.E.; Jamialahmadi, T.; Hadizadeh, F.; Sahebkar, A. Curcumin can bind and interact with CRP: An in silico study. Adv. Exp. Med. Biol. 2021, 1308, 91–100. [Google Scholar]
- Wongrattanakamon, P.; Ampasavate, C.; Sirithunyalug, B.; Jiranusornkul, S. An integrated molecular modeling approach for the tryptase monomer-curcuminoid recognition analysis: Conformational and bioenergetic features. J. Bioenerg. Biomemb. 2018, 50, 447–459. [Google Scholar] [CrossRef]
- Kumar, A.; Bora, U. Molecular docking studies of curcumin natural derivatives with DNA Topoisomerasi I and II-DNA complexes. Interdiscip. Sci. Life Sci. 2014, 6, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Misra, K. In silico approach for designing potent inhibitors against Polymerase PB2 (Influenza a virus: H1N1). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2012, 82, 365–373. [Google Scholar] [CrossRef]
- Subbarao, E.K.; London, W.; Murphy, B.R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993, 67, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Bussey, K.A.; Bousse, T.L.; Desmet, E.A.; Kim, B.; Takimoto, T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza a virus in mammalian host cells. J. Virol. 2010, 84, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, R.N.; Nafisa, B.B.; Arifin, M.S.; Santosaningsih, D.; Muti’ah, R. Antiviral Activitiy of Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin and Cyclocurcumin compounds of Curcuma longa against NSP3 on SARS-CoV-2. Indones. J. Cancer Chemoprevent. 2022, 13, 166–174. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Rathinavel, T.; Periyannan, V.; Ammashi, S.; Marimuthu, S.; Iqbal, M.N. Molecular insight of phytocompounds from Indian spices and its hyaluronic acid conjugates to block SARS-Cov-2 viral entry. J. Biom. Struct. Dyn. 2023, 41, 7386–7405. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Saeed, A.; Alam, M.J.; Alreshidi, M. Computational hunting of natural active compounds as an alternative for Remdesivir to target RNA-dependent polymerase. Cell Mol. Biol. 2021, 67, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Fut. J. Pharm. Sci. 2020, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.J.; Walencewicz-Wasserman, A.J.; Kosmoski, J.; Cribbs, D.H.; Glabe, C.G.; Cotman, C.W. Structure-Activity Analyses of Beta-Amyloid Peptides—Contributions of the Beta-25–35 Region to Aggregation and Neurotoxicity. J. Neurochem. 1995, 64, 253–265. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. Medicine—The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Pike, C.J.; Burdick, D.; Walencewicz, A.J.; Glabe, C.G.; Cotman, C.W. Neurodegeneration Induced by Beta-Amyloid Peptides Invitro—The Role of Peptide Assembly State. J. Neurosci. 1993, 13, 1676–1687. [Google Scholar] [CrossRef]
- Terzi, E.; Holzemann, G.; Seelig, J. Alzheimer Beta-Amyloid Peptide-25–35—Electrostatic Interactions with Phospholipid Membranes. Biochemistry 1994, 33, 7434–7441. [Google Scholar] [CrossRef]
- D’Ursi, A.M.; Armenante, M.R.; Guerrini, R.; Salvadori, S.; Sorrentino, G.; Picone, D. Solution structure of amyloid beta-peptide (25–35) in different media. J. Med. Chem. 2004, 47, 4231–4238. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Pierre, Y. Gregory Gregoriadis: Introducing liposomes to drug delivery. J. Drug Target 2008, 16, 518–519. [Google Scholar] [CrossRef]
- Angelini, G.; Campestre, C.; Boncompagni, S.; Gasbarri, C. Liposomes entrapping β-cyclodextrin/ibuprofen inclusion complex: Role of the host and the guest on the bilayer integrity and microviscosity. Chem. Phys. Lipids 2017, 209, 61–65. [Google Scholar] [CrossRef]
- Chi, E.Y.; Ege, C.; Winans, A.; Majewski, J.; Wu, G.; Kjaer, K.; Lee, K.Y.C. Lipid membrane templates the ordering and induces the fibrillogenesis of Alzheimer’s disease amyloid-beta peptide. Proteins 2008, 72, 1–24. [Google Scholar] [CrossRef]
- Vagt, T.; Zschörnig, O.; Huster, D.; Koksch, B. Membrane Binding and Structure of De Novo Designed α-Helical Cationic Coiled- Coil-Forming Peptides. Chem. Phys. Chem. 2006, 7, 1361–1371. [Google Scholar] [CrossRef]
- Rhee, S.K.; Quist, A.P.; Lal, R. Amyloid β protein-(1–42) forms calcium-permeable, Zn2+-sensitive channel. J. Biol. Chem. 1998, 273, 13379–13382. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bhatia, R.; Lal, R. Amyloid β protein forms ion channels: Implications for Alzheimer’s disease pathophysiology. FASEB J. 2001, 15, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
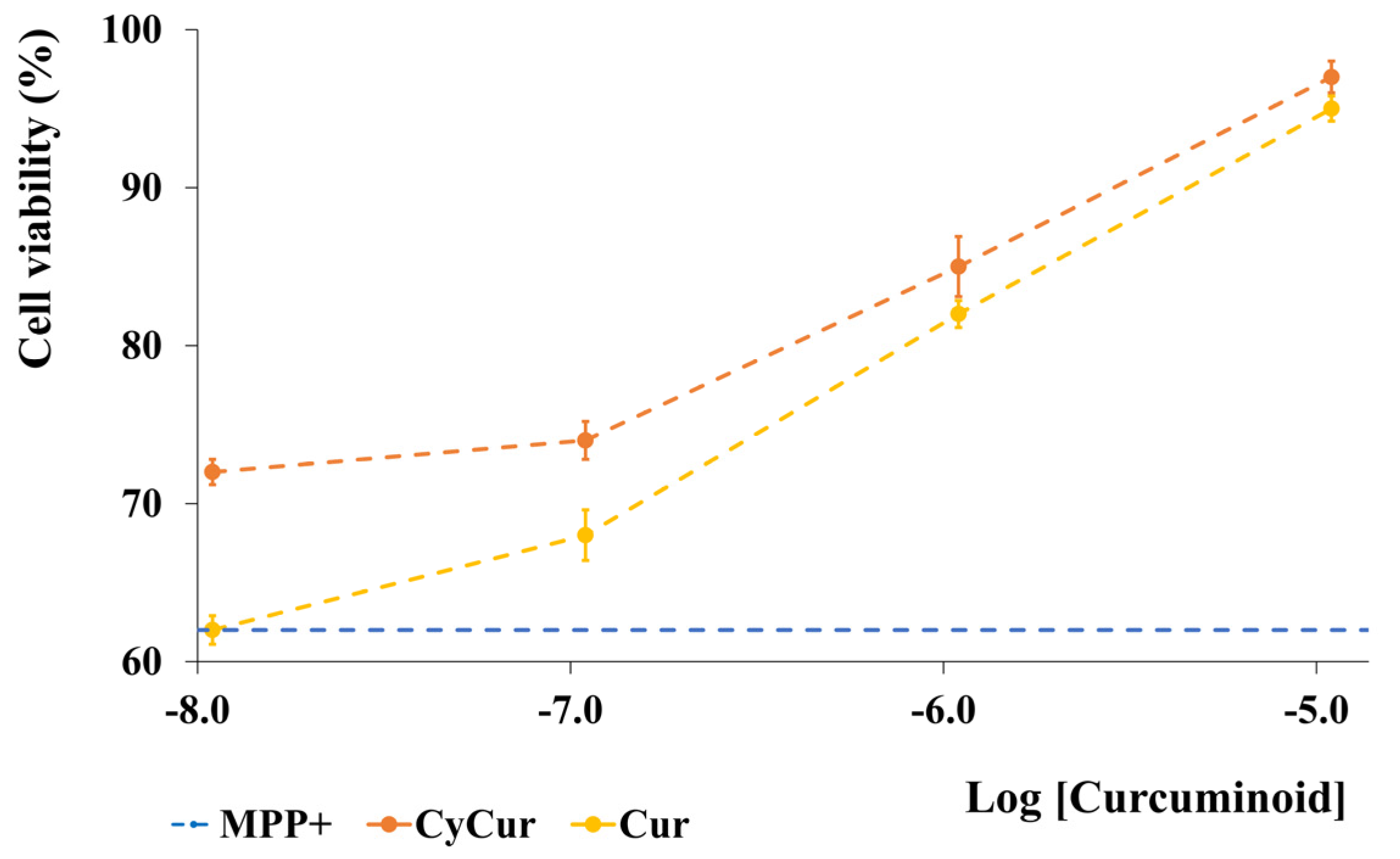
- Chakraborty, S.; Karmenyan, A.; Tsai, J.W.; Chiou, A. Inhibitory effects of curcumin and cyclocurcumin in 1-methyl-4-phenylpyridinium (MPP+) induced neurotoxicity in differentiated PC12 cells. Sci. Rep. 2017, 7, 16977. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Vila, M. MPTP: A review of its mechanisms and neurotoxicity. Clin. Neurosci. Res. 2001, 1, 407–418. [Google Scholar] [CrossRef]
- Westerink, R.H.S.; Erwing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.J.; Forouzanfar, M.; Ghaedi, K.; Kiani-Esfahani, A.; Peymani, M.; Nejati, S.; Izadi, T.; Karbalaie, K.; Noorbakhshnia, M.; Rahgozar, S.; et al. Nurr1 and PPARc protect PC12 cells against MPP+ toxicity: Involvement of selective genes, anti-inflammatory, ROS generation, and antimitochondrial impairment. Mol. Chem. Biochem. 2016, 420, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Nowaczyk, H.K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Nian, F.S.; Tsai, J.W.; Karmenyan, A.; Chiou, A. Quantification of the metabolic state in cell-model of Parkinson’s disease by fluorescence lifetime imaging microscopy. Sci. Rep. 2016, 6, 19145. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Chen, L.; Zhang, L.; Yu, X.; Yang, Q. Cyclocurcumin, a curcumin derivate, exhibits immune-modulating ability and is a potential compound for the treatment of rheumatoid arthritis as predicted by the MM-PBSA method. Int. J. Mol. Med. 2017, 39, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Van der Helm-van Mil, A.H.; Huizinga, T.W. Advances in the genetics of rheumatoid arthritis point to subclassification into distinct disease subsets. Arthritis Res. Ther. 2008, 10, 205. [Google Scholar] [CrossRef]
- Choy, E.H.; Isenberg, D.A.; Garrood, T.; Farrow, S.; Ioannou, Y.; Bird, H.; Cheung, N.; Williams, B.; Hazleman, B.; Price, R.; et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: A randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002, 46, 3143–3150. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.J.; Jung, Y.; Noh, J.Y.; Syed, A.S.; Kim, C.Y.; Lee, M.Y.; Lim, K.M.; Bae, O.N.; Chung, J.H. Cyclocurcumin, an Antivasoconstrictive Constituent of Curcuma longa (Turmeric). J. Nat. Prod. 2017, 80, 196–200. [Google Scholar] [CrossRef]
- Ngo, T.; Kim, K.; Bian, Y.; An, G.J.; Bae, O.N.; Lim, K.M.; Chung, J.H. Cyclocurcumin from Curcuma longa selectively inhibits shear stress-induced platelet aggregation. J. Funct. Foods 2019, 61, 103462. [Google Scholar] [CrossRef]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharm. 1999, 58, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Misra, A.; Surin, W.R.; Jain, M.; Bhatta, R.S.; Pal, R.; Kanwal, R.; Barthwal, M.K.; Dikshit, M. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb. Res. 2011, 127, 111–118. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Schaff, M.; Peter, K. Current and future antiplatelet therapies: Emphasis on preserving haemostasis. Nat. Rev. Card. 2018, 15, 181–191. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Hedge, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Racz, L.Z.; Racz, C.P.; Pop, L.C.; Tomoaia, G.; Mocanu, A.; Barbu, I.; Sárközi, M.; Roman, I.; Avram, A.; Tomoaia-Cotisel, M.; et al. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules 2022, 27, 6854. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Sukamtoh, E.; Xiao, H.; McClements, D.J.; Zhang, G. Curcumin: Recent Advances in the Development of Strategies to Improve Oral Bioavailability. Ann. Rev. Sci Technol. 2019, 10, 597–617. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef]
- Jager, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, C.; Meregalli, M.; Belicchi, M.; Farini, A.; Arosio, M.; Bestetti, D.; Villa, C.; Valenti, L.; Brambilla, P.; Torrente, Y. Preliminary evidences of safety and efficacy of flavonoids- and omega 3-based compound for muscular dystrophies treatment: A randomized double-blind placebo controlled pilot clinical trial. Front. Neurol. 2019, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Jacob, J.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.A.; George, R.; Sreeraj, T.R.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative oral absorption of curcumin in a natural turmeric matrix with two other curcumin formulations: An open-label parallel arm study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Jude, S.; Varma, K.; Jacob, J.; Gopi, S.; Luwafemi, O.S.; Thomas, S. Preparation of a novel bioavailable curcuminoid formulation (Cureit™) using Polar-Nonpolar-Sandwich (PNS) technology and its characterization and applications. Mater. Sci. Eng. C 2017, 75, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; George, R.; Sriraam, V.T. Cell culture study on the effects of “cureit” hyaluronidase inhibition—Antiaging effects. Int. J. Curr. Res. 2014, 6, 8473–8474. [Google Scholar]
- Deng, Y.; Pang, Y.; Guo, Y.; Ren, Y.; Fen, W.; Liao, X.; Yang, B. Host-guest inclusion systems of daidzein with 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) and sulfobutyl ether-β-cyclodextrin (SBE-β-CD): Preparation, binding behaviors, and water solubility. J. Mol. Struct. 2016, 1118, 307–315. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef]
- Gasbarri, C.; Angelini, G. An overview on the role of cyclodextrins in the synthesis of silver nanoparticles by chemical reduction. Arkivoc 2022, 2022, 112–132. [Google Scholar] [CrossRef]
- Angelini, G.; Gasbarri, C. Green synthesis and properties of silver nanoparticles in sulfobutylether-β-cyclodextrin aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127924. [Google Scholar] [CrossRef]
- Wong, S.N.; Hu, S.; Ng, W.W.; Xu, X.; Lai, K.L.; Lee, W.Y.T.; Chow, A.H.L.; Sun, C.C.; Chow, S.F. Cocrystallization of curcumin with benzenediols and benzenetriols via rapid solvent removal. Cryst. Growth Des. 2018, 18, 5534–5546. [Google Scholar] [CrossRef]


 | trans δ (ppm) | cis δ (ppm) |
|---|---|---|
| 2 | 2.97 d, 3.02 d | 2.94, 2.99 |
| 4 | 5.56 s | 5.54 s |
| 6 | 6.76 (d, J = 15.9 Hz) | 5.99 (1H, d, J = 12.8 Hz) |
| 7 | 7.21 (d, J = 15.9 Hz) | 6.73 (1H, d, J = 12.8 Hz) |
| 2′ | 7.13 d | 7.05 d |
| 5′ | 6.83 d | 6.69 d |
| 2″ | 7.24 d | 6.89 d |
| 5″ | 6.77 d | 6.75 d |
| 6″ | 7.07 dd | 6.79 dd |
| OMe | 3.81 s | 3.57 s, 2.68 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasbarri, C.; Angelini, G. Cyclocurcumin as Promising Bioactive Natural Compound: An Overview. Molecules 2024, 29, 1451. https://doi.org/10.3390/molecules29071451
Gasbarri C, Angelini G. Cyclocurcumin as Promising Bioactive Natural Compound: An Overview. Molecules. 2024; 29(7):1451. https://doi.org/10.3390/molecules29071451
Chicago/Turabian StyleGasbarri, Carla, and Guido Angelini. 2024. "Cyclocurcumin as Promising Bioactive Natural Compound: An Overview" Molecules 29, no. 7: 1451. https://doi.org/10.3390/molecules29071451
APA StyleGasbarri, C., & Angelini, G. (2024). Cyclocurcumin as Promising Bioactive Natural Compound: An Overview. Molecules, 29(7), 1451. https://doi.org/10.3390/molecules29071451