Catalytic Transformation of Nitroarenes to Amines over Ba(1−x)SrxTiO3 (0 < x < 1) Perovskites in Water
Abstract
1. Introduction
2. Results and Discussion
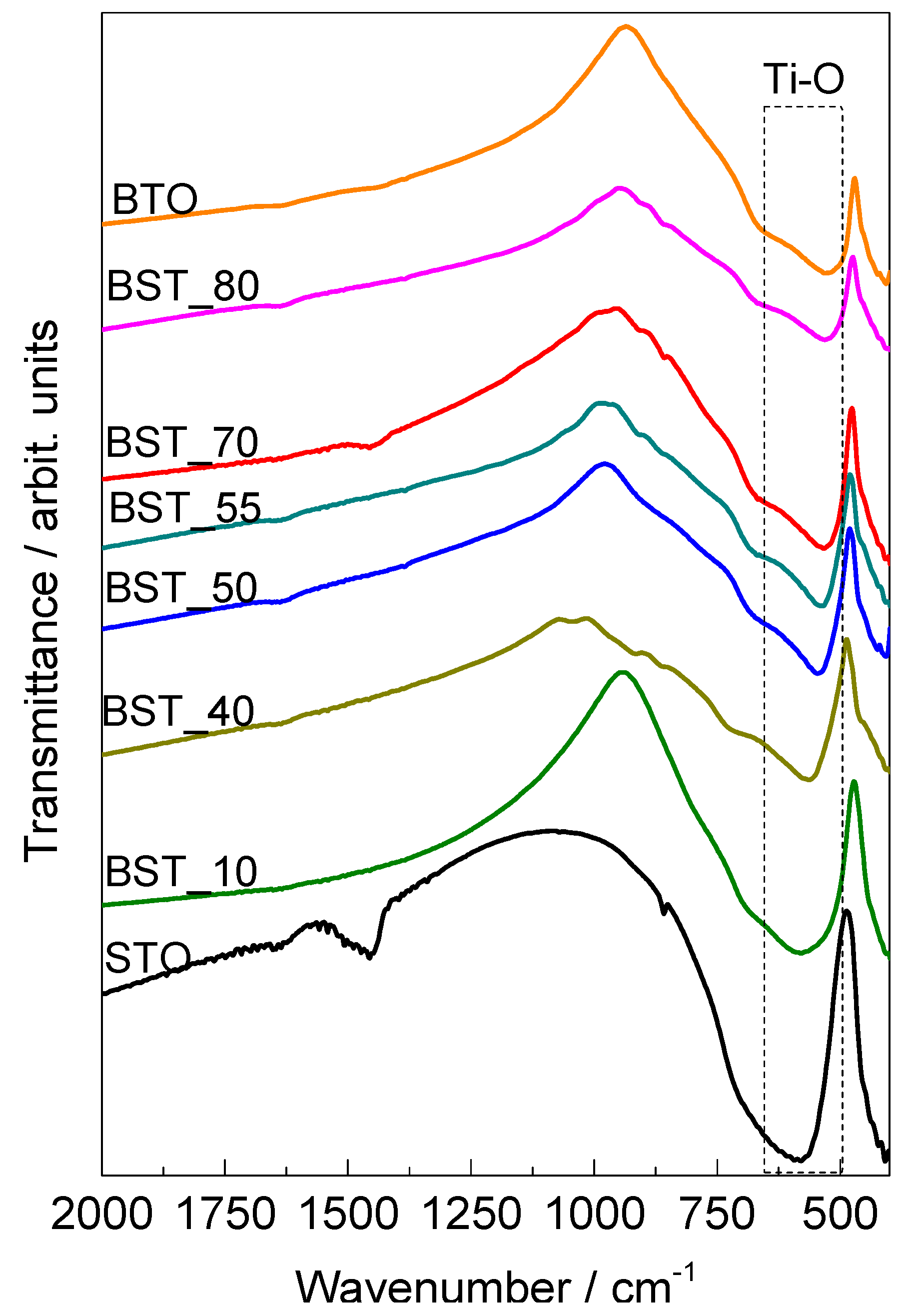
2.1. Characterization of the Ceramics
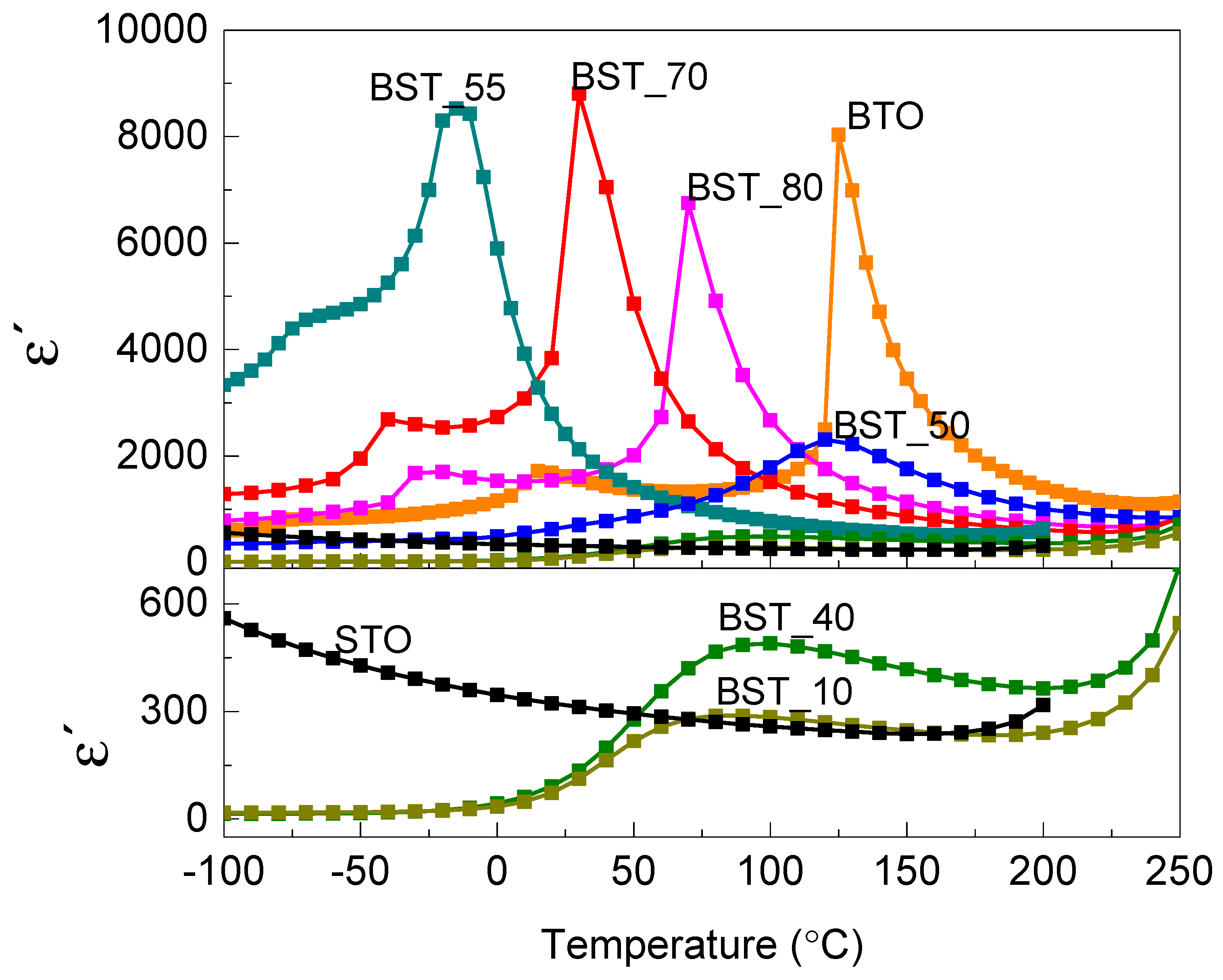
2.2. Dielectric Properties
2.3. Application in Catalysis
3. Materials and Methods
3.1. Materials, Reagents and Solvents
3.2. Preparation of the Ba(1−x)SrxTiO3 Ceramics
3.3. Chemical and Physicochemical Characterization
3.4. Catalytic Reduction of 4-NPh
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tchieno, F.M.M.; Tonle, I.K. p-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2018, 37, 20170019. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, H.; Zhang, W.; Lai, B.; Yao, G. Removal of nitrophenols and their derivatives by chemical redox: A review. Chem. Eng. J. 2019, 359, 13–31. [Google Scholar] [CrossRef]
- Sun, J.; Mu, Q.; Kimura, H.; Murugadoss, V.; He, M.; Du, W.; Hou, C. Oxidative degradation of phenols and substituted phenols in the water and atmosphere: A review. Adv. Compos. Hybrid Mater. 2022, 5, 627–640. [Google Scholar] [CrossRef]
- Matos, R.; Nunes, M.S.; Kuźniarska-Biernacka, I.; Rocha, M.; Guedes, A.; Estrada, A.C.; Lopes, J.L.; Trindade, T.; Freire, C. Graphene@Metal Sulfide/Oxide Nanocomposites as Novel Photo-Fenton-like Catalysts for 4-Nitrophenol Degradation. Eur. J. Inorg. Chem. 2021, 2021, 4915–4928. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic Assemblies for Catalytic Reduction of Nitrophenols: A Critical Review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef]
- Kuźniarska-Biernacka, I.; Santos, A.C.; Jarrais, B.; Valentim, B.; Guedes, A.; Freire, C.; Peixoto, A.F. Application of Fe-rich coal fly ashes to enhanced reduction of 4-nitrophenol. Clean. Chem. Eng. 2022, 2, 100019. [Google Scholar] [CrossRef]
- Neal, R.D.; Hughes, R.A.; Sapkota, P.; Ptasinska, S.; Neretina, S. Effect of Nanoparticle Ligands on 4-Nitrophenol Reduction: Reaction Rate, Induction Time, and Ligand Desorption. ACS Catal. 2020, 10, 10040–10050. [Google Scholar] [CrossRef]
- Das, K.C.; Dhar, S.S. Fast catalytic reduction of p-nitrophenol by Cu/HAP/ZnFe2O4 nanocomposite. Mater. Sci. Eng. B 2021, 263, 114841. [Google Scholar] [CrossRef]
- Bai, X.-J.; Chen, D.; Li, Y.-N.; Yang, X.-M.; Zhang, M.-Y.; Wang, T.-Q.; Zhang, X.-M.; Zhang, L.-Y.; Fu, Y.; Qi, X.; et al. Two-dimensional MOF-derived nanoporous Cu/Cu2O networks as catalytic membrane reactor for the continuous reduction of p-nitrophenol. J. Membr. Sci. 2019, 582, 30–36. [Google Scholar] [CrossRef]
- Wei, Z.; Feng, D.; Li, J.; Lin, Y.; Zhang, H. Nanosheet array-like Cu@Cu2O-CuNiAl(O)/rGO composites for highly efficient reduction of nitrophenol: Electronic and structure promotion effect of nickel. Chem. Eng. J. 2022, 427, 131659. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Poplawski, K.; Lichtenberger, J.; Keil, F.J.; Schnitzlein, K.; Amiridis, M.D. Catalytic oxidation of 1,2-dichlorobenzene over ABO3-type perovskites. Catal. Today 2000, 62, 329–336. [Google Scholar] [CrossRef]
- Shen, Q.; Dong, S.; Li, S.; Yang, G.; Pan, X. A Review on the Catalytic Decomposition of NO by Perovskite-Type Oxides. Catalysts 2021, 11, 622. [Google Scholar] [CrossRef]
- Afzalinia, A.; Mirzaee, M. Fabrication of perovskite@MOF composites as an alternative for noble metal catalysts in hydrogenation of nitroarenes: An investigation of transition metals doping on catalytic performance and RSM modeling of reaction conditions. J. Mol. Struct. 2023, 1283, 135322. [Google Scholar] [CrossRef]
- Srilakshmi, C. Perovskite-Type Transition Metal Oxide Nanocatalysts. In Advanced Heterogeneous Catalysts Volume 1: Applications at the Nano-Scale; American Chemical Society: Washington, DC, USA, 2020; Volume 1359, pp. 319–351. [Google Scholar]
- Kelele, K.G.; Tadesse, A.; Desalegn, T.; Ghotekar, S.; Balachandran, R.; Murthy, H.C.A. Synthesis and characterizations of metal ions doped barium strontium titanate (BST) nanomaterials for photocatalytic and electrical applications: A mini review. Int. J. Mater. Res. 2021, 112, 665–677. [Google Scholar] [CrossRef]
- Rout, S.K.; Panigrahi, S. Mechanism of phase formation of BaTiO3–SrTiO3 solid solution through solid-oxide reaction. Indian J. Pure Appl. Phys. 2006, 44, 606–611. [Google Scholar]
- Maity, S.; Sasmal, A.; Sen, S. Comprehensive characterization of Ba1−xSrxTiO3: Correlation between structural and multifunctional properties. J. Alloys Compd. 2021, 884, 161072. [Google Scholar] [CrossRef]
- Seo, S.S.A.; Lee, H.N.; Noh, T.W. Infrared spectroscopy of CaTiO3, SrTiO3, BaTiO3, Ba0.5Sr0.5TiO3 thin films, and (BaTiO3)5/(SrTiO3)5 superlattice grown on SrRuO3/SrTiO3(001) substrates. Thin Solid Film. 2005, 486, 94–97. [Google Scholar] [CrossRef]
- Smolensky, G. Ferroelectrics with diffuse phase transition. Ferroelectrics 1984, 53, 129–135. [Google Scholar] [CrossRef]
- Bokov, A.A.; Ye, Z.G. Recent progress in relaxor ferroelectrics with perovskite structure. J. Mater. Sci. 2006, 41, 31–52. [Google Scholar] [CrossRef]
- Tkach, A.; Amaral, L.; Vilarinho, P.M.; Senos, A.M.R. Oxygen vacancies as a link between the grain growth and grain boundary conductivity anomalies in titanium-rich strontium titanate. J. Eur. Ceram. Soc. 2018, 38, 2547–2552. [Google Scholar] [CrossRef]
- Buessem, W.R.; Cross, L.E.; Goswami, A.K. Phenomenological Theory of High Permittivity in Fine-Grained Barium Titanate. J. Am. Ceram. Soc. 1966, 49, 33–36. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Iben Ayad, A.; Luart, D.; Ould Dris, A.; Guénin, E. Kinetic Analysis of 4-Nitrophenol Reduction by “Water-Soluble” Palladium Nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef]
- Wang, F.; Song, J.; Wang, T.; Du, C.; Su, Y. Photosynergy of Ag In Situ Anchored on AgNb1–xTaxO3 Solid Solutions as an Efficient and Durable Catalyst toward Nitrobenzene Reduction: Uncovering the Relevance of the Electronic Structure, Active Sites, and Catalytic Activity. J. Phys. Chem. C 2021, 125, 385–395. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, C.; Cao, J.; Hao, Y.; Lei, M.; Bi, K.; Sun, Q.; Wang, Z.L. Enhanced photocatalytic H2 evolution by plasmonic and piezotronic effects based on periodic Al/BaTiO3 heterostructures. Nano Energy 2019, 62, 513–520. [Google Scholar] [CrossRef]
- Corma, A.; Concepción, P.; Serna, P. A Different Reaction Pathway for the Reduction of Aromatic Nitro Compounds on Gold Catalysts. Angew. Chem. Int. Ed. 2007, 46, 7266–7269. [Google Scholar] [CrossRef]
- Grzeschik, R.; Schäfer, D.; Holtum, T.; Küpper, S.; Hoffmann, A.; Schlücker, S. On the Overlooked Critical Role of the pH Value on the Kinetics of the 4-Nitrophenol NaBH4-Reduction Catalyzed by Noble-Metal Nanoparticles (Pt, Pd, and Au). J. Phys. Chem. C 2020, 124, 2939–2944. [Google Scholar] [CrossRef]
- Datta, K.J.; Rathi, A.K.; Kumar, P.; Kaslik, J.; Medrik, I.; Ranc, V.; Varma, R.S.; Zboril, R.; Gawande, M.B. Synthesis of flower-like magnetite nanoassembly: Application in the efficient reduction of nitroarenes. Sci. Rep. 2017, 7, 11585. [Google Scholar] [CrossRef]
- Bae, S.; Gim, S.; Kim, H.; Hanna, K. Effect of NaBH4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol. Appl. Catal. B Environ. 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and their evaluation as non-noble metal catalysts for efficient reduction of 4-nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar]
- Wu, Z.; Zhang, Y.; Wang, X.; Zou, Z. Ag@SrTiO3 nanocomposite for super photocatalytic degradation of organic dye and catalytic reduction of 4-nitrophenol. N. J. Chem. 2017, 41, 5678–5687. [Google Scholar] [CrossRef]
- Ren, L.; Yi, X.; Tong, L.; Zhou, W.; Wang, D.; Liu, L.; Ye, J. Nitrogen-doped ultrathin graphene encapsulated Cu nanoparticles decorated on SrTiO3 as an efficient water oxidation photocatalyst with activity comparable to BiVO4 under visible-light irradiation. Appl. Catal. B Environ. 2020, 279, 119352. [Google Scholar] [CrossRef]
- Farhadi, S.; Siadatnasab, F.; Kazem, M. Microwave-Assisted Rapid and efficient Reduction of Aromatic Nitro Compounds to Amines with Propan-2-ol over Nanosized Perovskite-type SmFeO3 powder as a New Recyclable Heterogeneous Catalyst. J. Chem. Res. 2011, 35, 104–108. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Zhao, X.; Li, R.; Wang, X. Enhanced photocatalytic activity of surface disorder-engineered CaTiO3. Mater. Res. Bull. 2018, 105, 286–290. [Google Scholar] [CrossRef]
- Ma, C.; Shen, D.; Qing, J.; Ng, T.-W.; Lo, M.-F.; Lee, C.-S. Heat Treatment for Regenerating Degraded Low-Dimensional Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 4860–4865. [Google Scholar] [CrossRef]
- Śmiga, W.; Garbarz-Glos, B.; Kalvane, A.; Antonova, M.; Suchanicz, W.; Sternberg, A. Strontium concentration dependence of selected structural and mechanical properties of polycrystalline Ba1−XSrXTiO3. Integr. Ferroelectr. 2009, 108, 77–88. [Google Scholar] [CrossRef]



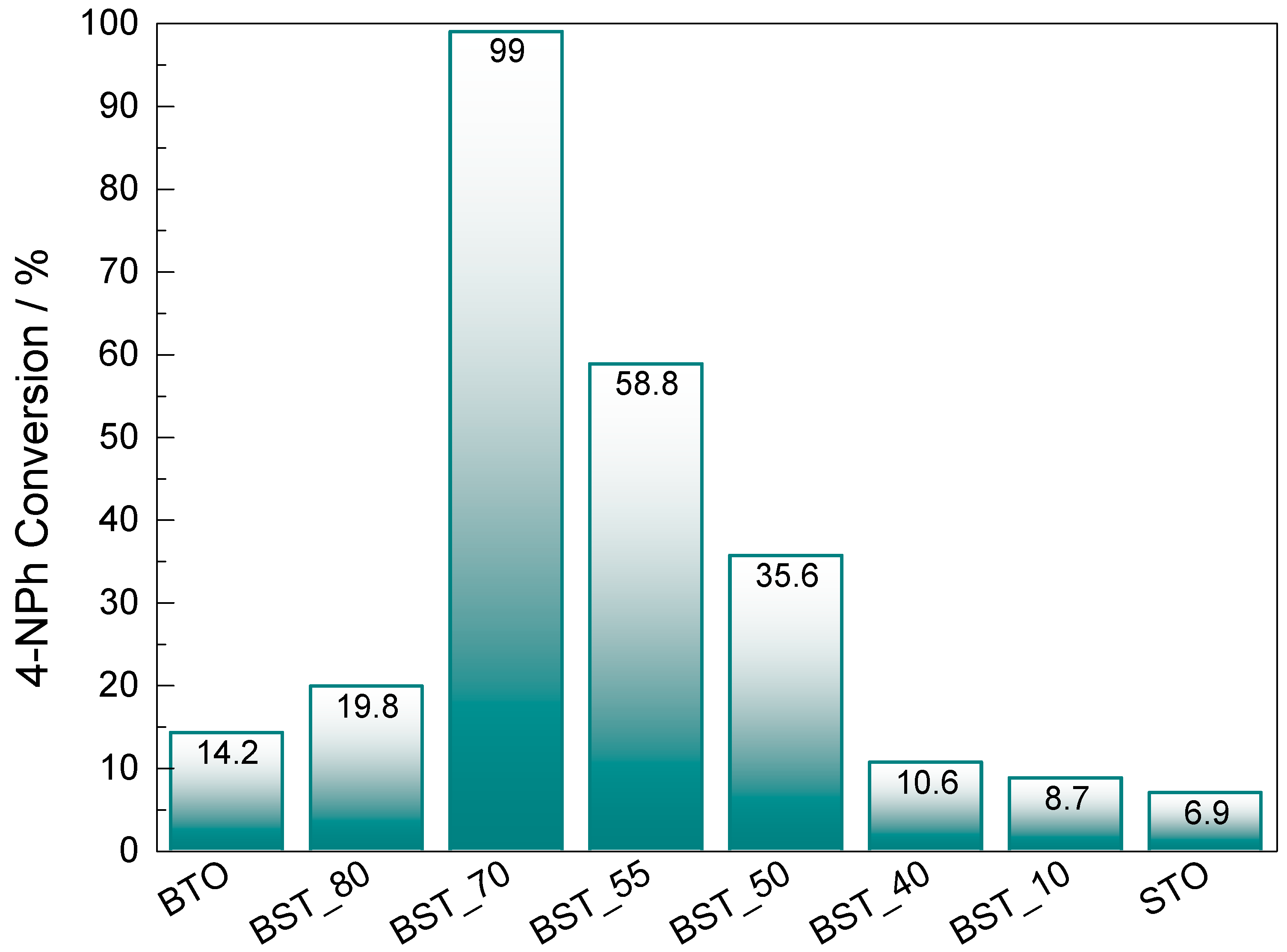


| Material | Found (Calculated) | ||
|---|---|---|---|
| Ba [%] | Sr [%] | Ti [%] | |
| BTO | 58.71 ± 0.17 (58.89) | ND 2 | 19.46 ± 1.15 (20.53) |
| BST_80 | 50.02 ± 0.52 (49.21) | 7.90 ± 0.12 (7.85) | 21.87 ± 0.52 (21.44) |
| BST_70 | 44.09 ± 0.53 (44.04) | 12.45 ± 0.58 (12.04) | 21.76 ± 0.17 (21.93) |
| BST_55 | 36.86 ± 0.58 (35.83) | 19.11 ± 0.76 (18.70) | 22.74 ± 0.12 (22.71) |
| BST_50 | 34.01 ± 1.24 (32.96) | 21.42 ± 0.24 (21.03) | 23.10 ± 0.13 (22.98) |
| BST_40 | 26.73 ± 1.15 (27.01) | 26.59 ± 0.68 (25.85) | 23.73 ± 0.16 (23.54) |
| STO | ND 2 | 47.83 ± 0.22 (47.75) | 25.23 ± 0.35 (26.09) |
| Non-Linear Fitting of First-Order Kinetic Reaction | |||
|---|---|---|---|
| Sample | k b (min−1) | R2 | k’ c (min−1g−1) |
| BTO | 2.71 × 10−3 | 0.95 | 5.42 × 10−2 |
| BST_80 | 3.21 × 10−3 | 0.85 | 6.42 × 10−2 |
| BST_70 | 1.19 × 10−1 | 0.99 | 2.38 × 100 |
| BST_55 | 1.04 × 10−2 | 0.99 | 2.08 × 10−1 |
| BST_50 | 6.16 × 10−3 | 0.95 | 1.23 × 10−1 |
| BST_40 | 1.11 × 10−3 | 0.96 | 2.22 × 10−2 |
| BST_10 | 1.10 × 10−3 | 0.94 | 2.20 × 10−2 |
| STO | 1.09 × 10−3 | 0.98 | 2.18 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźniarska-Biernacka, I.; Garbarz-Glos, B.; Skiba, E.; Maniukiewicz, W.; Monteiro, M.; Bąk, W.; Szydłowski, D.; Freire, C. Catalytic Transformation of Nitroarenes to Amines over Ba(1−x)SrxTiO3 (0 < x < 1) Perovskites in Water. Molecules 2024, 29, 1416. https://doi.org/10.3390/molecules29071416
Kuźniarska-Biernacka I, Garbarz-Glos B, Skiba E, Maniukiewicz W, Monteiro M, Bąk W, Szydłowski D, Freire C. Catalytic Transformation of Nitroarenes to Amines over Ba(1−x)SrxTiO3 (0 < x < 1) Perovskites in Water. Molecules. 2024; 29(7):1416. https://doi.org/10.3390/molecules29071416
Chicago/Turabian StyleKuźniarska-Biernacka, Iwona, Barbara Garbarz-Glos, Elżbieta Skiba, Waldemar Maniukiewicz, Marta Monteiro, Wojciech Bąk, Dariusz Szydłowski, and Cristina Freire. 2024. "Catalytic Transformation of Nitroarenes to Amines over Ba(1−x)SrxTiO3 (0 < x < 1) Perovskites in Water" Molecules 29, no. 7: 1416. https://doi.org/10.3390/molecules29071416
APA StyleKuźniarska-Biernacka, I., Garbarz-Glos, B., Skiba, E., Maniukiewicz, W., Monteiro, M., Bąk, W., Szydłowski, D., & Freire, C. (2024). Catalytic Transformation of Nitroarenes to Amines over Ba(1−x)SrxTiO3 (0 < x < 1) Perovskites in Water. Molecules, 29(7), 1416. https://doi.org/10.3390/molecules29071416









