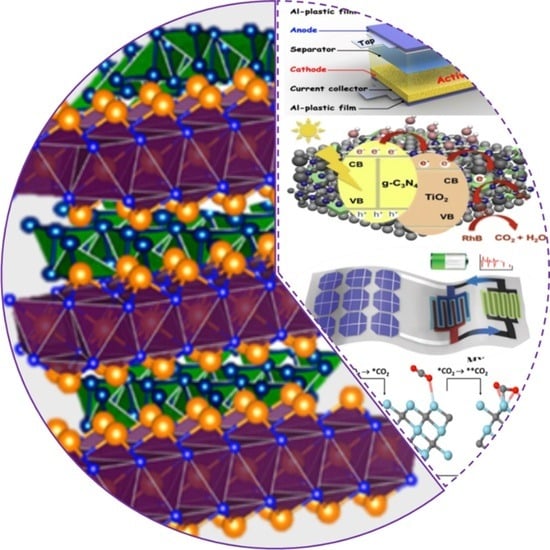
Structure, Synthesis, and Catalytic Performance of Emerging MXene-Based Catalysts
Abstract
1. Introduction
2. Structural Characteristic and Synthesis Method
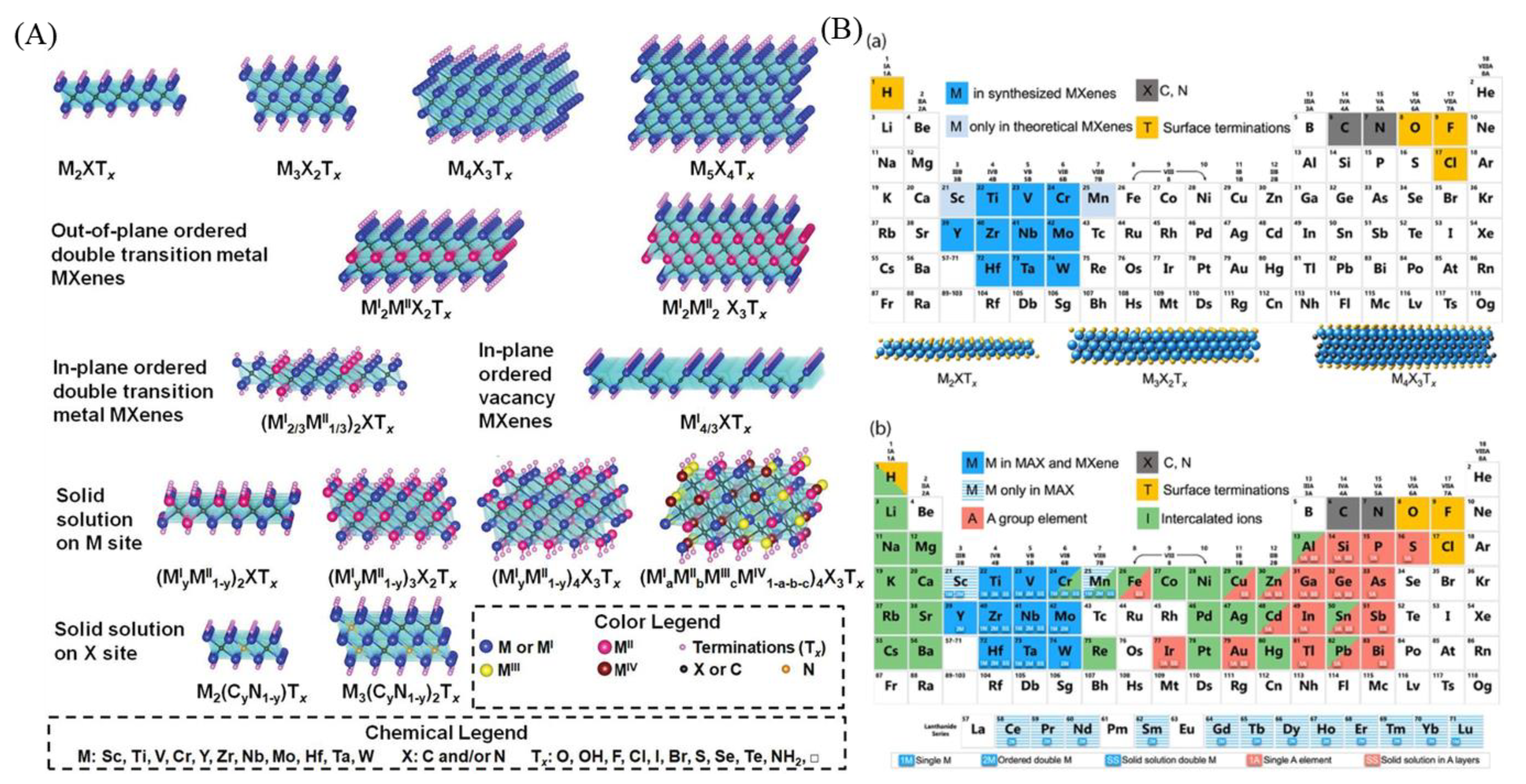
2.1. Structural Characteristics of MXenes
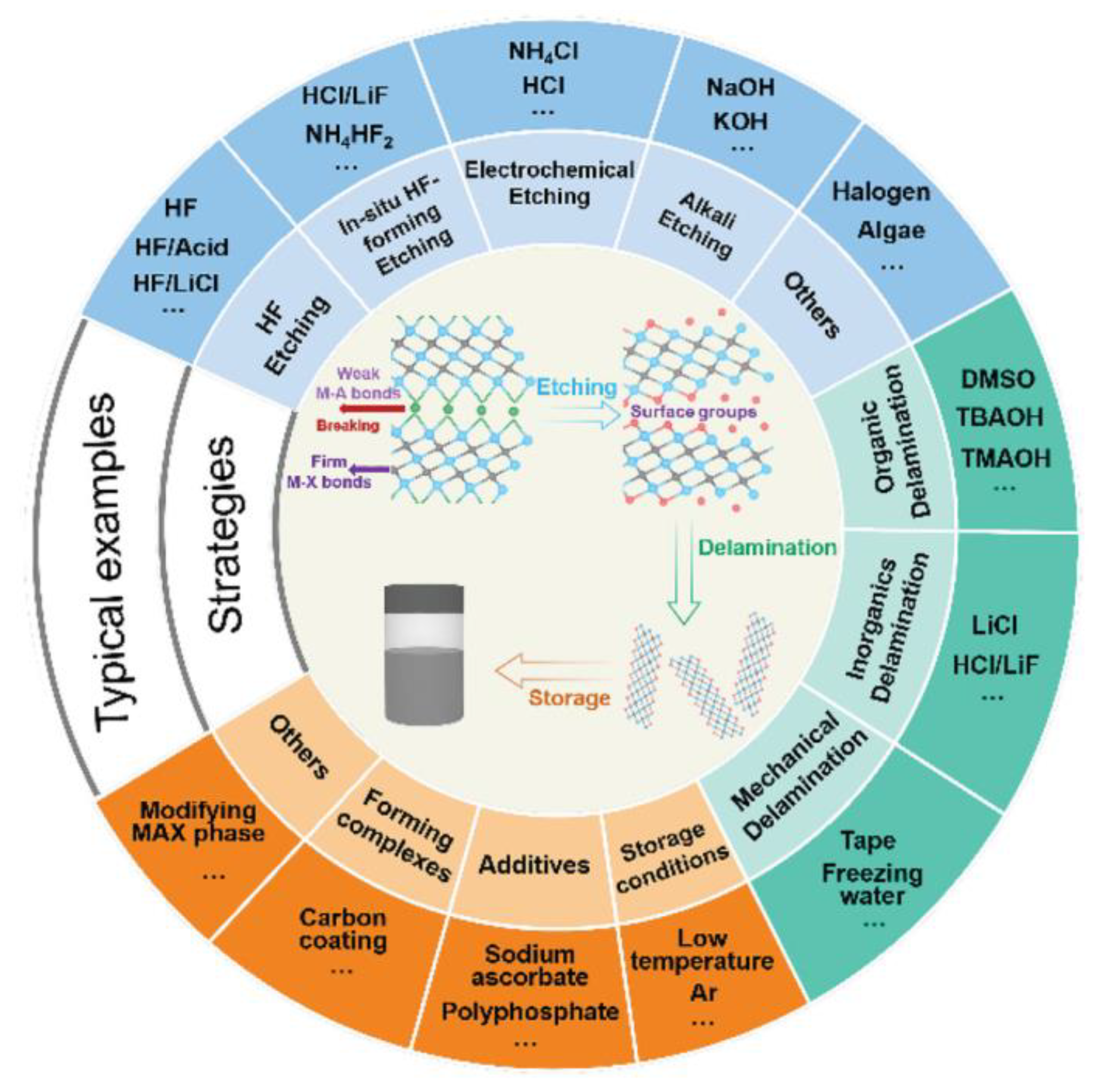
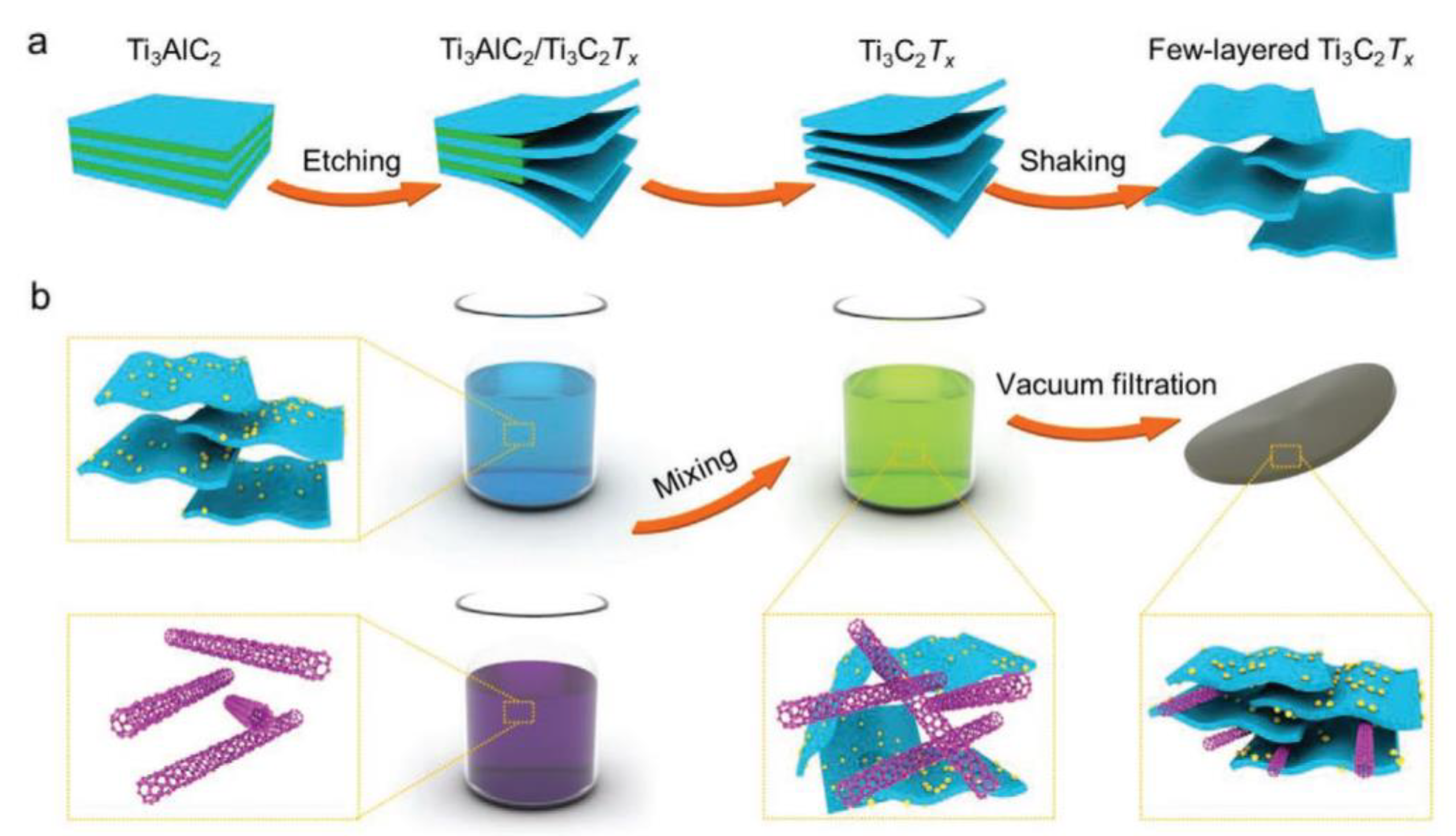
2.2. Methods for Synthesising MXenes
| Strategies | Advantages | Disadvantages | Influencing Factors | Ref. |
|---|---|---|---|---|
| Acid etching | Easy operation and low reaction temperature. | High corrosiveness, toxicity, and ecological and environmental risks. | Etchant concentration, etching temperature, and etching time. | [40] |
| Alkali etching | High etching efficiency and low impurity content. | Strong alkali and high temperatures in reaction conditions cause safety hazards. | The alkali concentration and reaction temperature. | [41] |
| Fluoride salt etching | Mild conditions and safe preparation. | Relatively high impurity content and low yield. | Acidity of organic anions and concentration of dissociated fluoride ions. | [30] |
| Electrochemical etching | Less acidic etchant content, mild conditions, and low energy consumption. | Low productivity and additional equipment costs. | Etching voltage window (etching potential) and etching time. | [42] |
| Molten salt etching | Mild reaction conditions and wide etching range. | The product structure has poor stability and the etching efficiency is unstable. | The type and concentration of molten salt. | [43] |
3. Catalytic Applications
3.1. Electrocatalysis
3.1.1. Hydrogen Evolution Reactions (HER)
3.1.2. Nitrogen Reduction Reaction (NRR)
3.2. Photocatalysis
| Photocatalysts | MXenes | Catalytic Activity | Ref. |
|---|---|---|---|
| C-TiO2/g-C3N4 | Ti3C2 | Photocatalytic hydrogen production activity was 1409 μmol/h/g. | [87] |
| TiO2/graphene/g-C3N4 | Ti3C2 | The degradation rate was TC (0.02442 min−1), CIP (0.01675 min−1), BPA (0.01935 min−1), and RhB (0.05586 min−1). | [88] |
| Ag/Nb2O5@Nb2CTx | Nb2CTx | HER activities were 682.2 and 824.2 μmol⋅g−1h−1, respectively. | [95] |
| MoS2/TiO2/Ti3C2 | Ti3C2 | The optimum H2 evolution rate of 6425.297 μmol/h/g was obtained on | [96] |
| CdLa2S4/Ti3C2 | Ti3C2 | The maximum hydrogen production rate was 11,182.4 μmol/h/g. | [97] |
| Ag3PO4/Ti3C2 | Ti3C2 | The photocatalytic performance toward tetracycline hydrochloride was 68.4%. | [98] |
| TiO2/Ti3C2 | Ti3C2 | The photocatalytic H2 production rate was 218.85 μmol g−1 h−1. | [99] |
| CdS/Ti3C | Ti3C2 | Visible light photocatalytic hydrogen production activity was 14,342 μmol h−1g−1. | [100] |
| MoxS@TiO2@Ti3C2 | Ti3C2 | The hydrogen production from photocatalytic water decomposition is 10,505.8 μmol g−1h−1. | [101] |
| Ti2C/3%TiO2/1%Ag | Ti2C | Salicylic acid (SA) photodegradation was 86.1–97.1% within 3 h; SA initial solution concentration was 100 μM. | [102] |
| Bi2WO6/Nb2CT | Nb2CTx | The degradation efficiencies of the photocatalysts were 99.8%, 92.7%, and 83.1% for RhB, MB, and TC-HCl, respectively. | [103] |
| MXene/ZnIn2S4 | Ti3C2Tx | Within 45 min under simulated visible light irradiation, the Cr(VI) reduction and MO degradation rates were as high as 93.4% and 96.9%, respectively. | [104] |
3.3. Renewable Energy and Energy Storage
| Catalysts | MXenes | Catalytic Activity | Ref. |
|---|---|---|---|
| Ti3C2Tx/C | Ti3C2Tx | The supercapacitor electrode exhibits a high specific capacity of 226 F g−1 at 1 A g−1 with 94% retention over 8000 cycles. | [114] |
| Graphite/ MXene | Ti3C2 | A high energy density of over 80 mW h/g and a rate capability of 75 mW h/g, with a capacity fading of 5% after 1500 cycles. | [115] |
| IL-MXene | Ti3C2 | The thermal conductivity is 0.82 W/m·K at 20 °C, and the specific capacity of pure IL aqueous solution is 2.374 J/g K. | [116] |
| V2CTx | V2CTx | With a gravimetric capacitance of 900 F g−1, the intercalated electrode exhibits excellent Coulombic efficiency (100%) for 10,000 GCD cycles at a current density of 2 A/g. | [117] |
| SSPCMs | Ti3C2Tx | The melting phase change enthalpy and relative enthalpy efficiency are 127.97 J/g and 76.96%, respectively, while the photothermal conversion efficiency (θ) is 90.45%. | [110] |
| PCM/MXene | Ti3C2 | Compared with pure paraffin, the absorbance of the composite increased by 39% and the maximum thermal conductivity increment was 16%. | [118] |
| MXene/EUPCM (1:99) | Ti3C2 | The addition of MXene resulted in a consistent reduction in heating and cooling times. | [119] |
| MoS2@MXene | Ti3C2 | The maximum electric displacement at room temperature is 10.96 μC/cm2, and the discharge energy density reaches 17.22 J/cm3. | [120] |
| MXene/MgCr2O4 | Ti3C2Tx | The maximum capacitance value observed in alkaline media is 542.6 F/g, while the minimum capacitance value is 454.1 F/g in acidic media. | [121] |
| MXene-C60 | Ti3SiC2 | The highest capacitance of MXene-C60 composite is 348 F g−1. | [122] |
| Paraffin/Ti3C2Tx@gelatin | Ti3C2Tx | The composite material has a high loading ratio (96.3–97.7%) and large melting enthalpy (184.7–199.9 J/g). | [123] |
| N-Ti3C2Tx-300 | Ti3C2Tx | Provides a maximum volumetric energy density of 21.0 Wh L−1 and an energy density of 10.2 Wh L−1 at a high power density of 18.3 kW L−1. | [124] |
| SMPCCs | Ti3C2 | The phase change material maintained up to 93 wt.% PEG loading without any leakage, with a relative thermal efficiency loss of only 1% after 100 heating-cooling cycles. | [125] |
3.4. Carbon Capture and Conversion
| Catalysts | MXenes | Catalytic Activity | Ref. |
|---|---|---|---|
| AC-MX-x | Ti2CTx | At 2.5 wt.% MXene, the adsorption capacity of AC increased from 46.46 cm3/g to 67.83 cm3/g. | [137] |
| AC/MXene sandwich | Ti3C2Tx | MXene containing ~4% has significant CO2 adsorption capacity (~8.9 mg/g). | [138] |
| Pebax/CMC@MXene MMMs | Ti3C2Tx | The CO2/N2 adsorption selectivity is 40.1, and after 60 h of testing, its separation performance has no significant change. | [139] |
| MXene-FO membrane | Ti3C2Tx | MXene sandwich TFC-FO membrane achieves higher water flux and lower specific solute flux. | [140] |
| Cr3C2 | Cr3C2 | The reaction energy value of Cr3C2 MXene is 1.05 eV, and the overpotential when the maximum Faradaic efficiency of CO2 to CO acts is 540 mV. | [131] |
| Pd-MXene | Pd-MXene | Displays a specific surface area of 97.5 m2g−1 and multiple pores and selectively electroreduces CO2 to CH3OH via multiple electron transfer. | [141] |
| Pd50-Ru50/MXene | Ti3C2Tx | The CO2 conversion efficiency of the Pd50-Ru50/MXene catalyst is as high as 78%, and the CH3OH yield is 76%. | [134] |
| MXene@CNF-3 membrane | Ti3C2Tx | The CO2 permeability is 156.7 Barrer, and the CO2/N2 and CO2/CH4 selectivities are 42.6 and 47.8, respectively. | [142] |
| MX-fluid-M2070 | Ti3C2Tx | Compared with pure epoxy resin, the flexural strength, flexural modulus, and impact strength increased by 15.32%, 6.42%, and 110.31%, respectively. | [143] |
| Pebax-Ti3C2TxTFC membranes | Ti3C2Tx | The composite membrane exhibits efficient CO2 permeability (1986.5 GPU) and CO2/N2 selectivity ≈ 42. | [144] |
| T-SDESM | Ti3C2Tx | The permeability of CO2 is approximately 26.35 GPU, and the selectivities for N2, CH4, and H2 are 319.15, 249.01, and 12.38, respectively. | [145] |
| MXene/PEG (600) | Ti3C2Tx | The mixed matrix membrane exhibits a CO2 permeability of 1626.99 GPU and CO2/N2 and CO2/CH4 selectivities of 32.18 and 27.84, respectively. | [146] |
4. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raffa, P. Where is research on fossil fuels going in times of climate change? A perspective on chemical enhanced oil recovery. MRS Commun. 2021, 11, 716–725. [Google Scholar] [CrossRef]
- Chandrasekar, K.; Sudhakar, S.; Rajappan, R.; Senthil, S.; Balu, P. Present developments and the reach of alternative fuel: A review. Mater. Today Proc. 2022, 51, 74–83. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Salim, O.; Mahmoud, K.; Pant, K.; Joshi, R. Introduction to MXenes: Synthesis and characteristics. Mater. Today Chem. 2019, 14, 100191. [Google Scholar] [CrossRef]
- Nan, J.; Guo, X.; Xiao, J.; Li, X.; Chen, W.; Wu, W.; Liu, H.; Wang, Y.; Wu, M.; Wang, G. Nanoengineering of 2D MXene-based materials for energy storage applications. Small 2021, 17, 1902085. [Google Scholar] [CrossRef] [PubMed]
- Shuai, T.-Y.; Qi-Ni, Z.; Xu, H.; Huang, C.-J.; Zhi-Jie, Z.; Li, G.-R. Recent Advances in the Synthesis and Electrocatalytic Applications of MXene Materials. Chem. Commun. 2023, 59, 3968–3999. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Li, Y.; Zhang, G. Two-dimensional MXene-based and MXene-derived photocatalysts: Recent developments and perspectives. Chem. Eng. J. 2021, 409, 128099. [Google Scholar] [CrossRef]
- Hui, X.; Ge, X.; Zhao, R.; Li, Z.; Yin, L. Interface chemistry on MXene-based materials for enhanced energy storage and conversion performance. Adv. Funct. Mater. 2020, 30, 2005190. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Rashad, M.; Hussain, I.; Abbas, A.; Aldosari, O.F.; Li, C. Green energy Harvesting from CO2 and NOx by MXene materials: Detailed Historical and Future Prospective. Appl. Catal. B Environ. 2023, 344, 123585. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.-M. Recent advances in structural engineering of MXene electrocatalysts. J. Mater. Chem. A 2020, 8, 10604–10624. [Google Scholar] [CrossRef]
- Kuang, P.; Low, J.; Cheng, B.; Yu, J.; Fan, J. MXene-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 18–44. [Google Scholar] [CrossRef]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V. 3D MXene architectures for efficient energy storage and conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Guo, S.; Mu, T.; Wei, L.; Lu, Y. CO2 capture and conversion to value-added products promoted by MXene-based materials. Green Energy Environ. 2022, 7, 394–410. [Google Scholar] [CrossRef]
- Devaraj, M.; Rajendran, S.; Hoang, T.K.; Soto-Moscoso, M. A review on MXene and its nanocomposites for the detection of toxic inorganic gases. Chemosphere 2022, 302, 134933. [Google Scholar] [CrossRef] [PubMed]
- Malaki, M.; Varma, R.S. Mechanotribological aspects of MXene-reinforced nanocomposites. Adv. Mater. 2020, 32, 2003154. [Google Scholar] [CrossRef] [PubMed]
- Biswal, L.; Mohanty, R.; Nayak, S.; Parida, K. Review on MXene/TiO2 nanohybrids for photocatalytic hydrogen production and pollutant degradations. J. Environ. Chem. Eng. 2022, 10, 107211. [Google Scholar] [CrossRef]
- Ding, B.; Ong, W.-J.; Jiang, J.; Chen, X.; Li, N. Uncovering the electrochemical mechanisms for hydrogen evolution reaction of heteroatom doped M2C MXene (M = Ti, Mo). Appl. Surf. Sci. 2020, 500, 143987. [Google Scholar] [CrossRef]
- Pu, L.; Zhang, J.; Jiresse, N.K.L.; Gao, Y.; Zhou, H.; Naik, N.; Gao, P.; Guo, Z. N-doped MXene derived from chitosan for the highly effective electrochemical properties as supercapacitor. Adv. Compos. Hybrid Mater. 2022, 5, 356–369. [Google Scholar] [CrossRef]
- Hong, X.; Lu, Z.; Zhao, Y.; Lyu, L.; Ding, L.; Wei, Y.; Wang, H. Fast fabrication of freestanding MXene-ZIF-8 dual-layered membranes for H2/CO2 separation. J. Membr. Sci. 2022, 642, 119982. [Google Scholar] [CrossRef]
- Fan, W.K.; Sherryna, A.; Tahir, M. Advances in Titanium Carbide (Ti3C2Tx) MXenes and Their Metal–Organic Framework (MOF)-Based Nanotextures for Solar Energy Applications: A Review. ACS Omega 2022, 7, 38158–38192. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Khan, M.A.; Gong, Y.; Javed, R.; Xu, Y.; Ye, D.; Zhao, H.; Zhang, J. Recent progress of MXenes and MXene-based nanomaterials for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 6089–6108. [Google Scholar] [CrossRef]
- Johnson, L.R.; Sridhar, S.; Zhang, L.; Fredrickson, K.D.; Raman, A.S.; Jang, J.; Leach, C.; Padmanabhan, A.; Price, C.C.; Frey, N.C. MXene materials for the electrochemical nitrogen reduction—Functionalized or not? ACS Catal. 2019, 10, 253–264. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Zubair, M.; Hassan, M.M.U.; Mehran, M.T.; Baig, M.M.; Hussain, S.; Shahzad, F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: A review. Int. J. Hydrogen Energy 2022, 47, 2794–2818. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Lei, D.; Liu, N.; Su, T.; Zhang, Q.; Wang, L.; Ren, Z.; Gao, Y. Roles of MXene in pressure sensing: Preparation, composite structure design, and mechanism. Adv. Mater. 2022, 34, 2110608. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten years of progress in the synthesis and development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes; ACS Publications: Washington, DC, USA, 2019; Volume 13, pp. 8491–8494. [Google Scholar]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.; Xu, B. Advances in the synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148. [Google Scholar] [CrossRef]
- Bharali, L.; Kalita, J.; Sankar Dhar, S. Several fundamental aspects of MXene: Synthesis and their applications. ChemistrySelect 2023, 8, e202301486. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A review on MXene synthesis, stability, and photocatalytic applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Anayee, M.; Kurra, N.; Alhabeb, M.; Seredych, M.; Hedhili, M.N.; Emwas, A.-H.; Alshareef, H.N.; Anasori, B.; Gogotsi, Y. Role of acid mixtures etching on the surface chemistry and sodium ion storage in Ti3C2Tx MXene. Chem. Commun. 2020, 56, 6090–6093. [Google Scholar] [CrossRef]
- Wang, C.; Shou, H.; Chen, S.; Wei, S.; Lin, Y.; Zhang, P.; Liu, Z.; Zhu, K.; Guo, X.; Wu, X. HCl-Based hydrothermal etching strategy toward fluoride-free MXenes. Adv. Mater. 2021, 33, 2101015. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Liu, L.; Zschiesche, H.; Antonietti, M.; Gibilaro, M.; Chamelot, P.; Massot, L.; Rozier, P.; Taberna, P.L.; Simon, P. In situ synthesis of MXene with tunable morphology by electrochemical etching of MAX phase prepared in molten salt. Adv. Energy Mater. 2023, 13, 2203805. [Google Scholar] [CrossRef]
- Yin, T.; Li, Y.; Wang, R.; Al-Hartomy, O.A.; Al-Ghamdi, A.; Wageh, S.; Luo, X.; Tang, X.; Zhang, H. Synthesis of Ti3C2Fx MXene with controllable fluorination by electrochemical etching for lithium-ion batteries applications. Ceram. Int. 2021, 47, 28642–28649. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Kumar, S. Fluorine-Free MXenes: Recent Advances, Synthesis Strategies, and Mechanisms. Small 2023, 6, 2308225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Zhang, N.; Hong, Y.; Yazdanparast, S.; Zaeem, M.A. Superior structural, elastic and electronic properties of 2D titanium nitride MXenes over carbide MXenes: A comprehensive first principles study. 2D Mater. 2018, 5, 045004. [Google Scholar] [CrossRef]
- Hassan, N.; Jalil, A.; Rajendran, S.; Khusnun, N.; Bahari, M.; Johari, A.; Kamaruddin, M.; Ismail, M. Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrogen Energy 2023, 52, 420–441. [Google Scholar] [CrossRef]
- Burton, N.; Padilla, R.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021, 135, 110255. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Sun, X. Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting—A review. J. Energy Chem. 2019, 34, 111–160. [Google Scholar] [CrossRef]
- Verma, J.; Goel, S. Cost-effective electrocatalysts for hydrogen evolution reactions (HER): Challenges and prospects. Int. J. Hydrogen Energy 2022, 47, 38964–38982. [Google Scholar] [CrossRef]
- Cui, C.; Cheng, R.; Zhang, H.; Zhang, C.; Ma, Y.; Shi, C.; Fan, B.; Wang, H.; Wang, X. Ultrastable MXene@Pt/SWCNTs’ nanocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2020, 30, 2000693. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, M.; Liu, D.; Jian, K.; Zhang, R.; Dang, J. Synergetic effect of Ni2P and MXene enhances catalytic activity in the hydrogen evolution reaction. Inorg. Chem. 2021, 60, 1604–1611. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Sheng, M.; Yin, X.; Que, W. Synergistically Coupling phosphorus-doped molybdenum carbide with Mxene as a highly efficient and stable electrocatalyst for hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2020, 8, 12990–12998. [Google Scholar] [CrossRef]
- Gan, J.; Li, F.; Tang, Q. Vacancies-Engineered M2CO2 MXene as an efficient hydrogen evolution reaction electrocatalyst. J. Phys. Chem. Lett. 2021, 12, 4805–4813. [Google Scholar] [CrossRef]
- Arif, M.; Babar, M.; Azhar, U.; Sagir, M.; Tahir, M.B.; Mushtaq, M.A.; Yasin, G.; Mubashir, M.; Chong, J.W.R.; Khoo, K.S. Rational design and modulation strategies of Mo-based electrocatalysts and photo/electrocatalysts towards nitrogen reduction to ammonia (NH3). Chem. Eng. J. 2023, 451, 138320. [Google Scholar] [CrossRef]
- Islam, J.; Shareef, M.; Zabed, H.M.; Qi, X.; Chowdhury, F.I.; Das, J.; Uddin, J.; Kaneti, Y.V.; Khandaker, M.U.; Ullah, M.H. Electrochemical nitrogen fixation in metal-N2 batteries: A paradigm for simultaneous NH3 synthesis and energy generation. Energy Storage Mater. 2023, 54, 98–119. [Google Scholar] [CrossRef]
- Lv, X.W.; Weng, C.C.; Yuan, Z.Y. Ambient ammonia electrosynthesis: Current status, challenges, and perspectives. ChemSusChem 2020, 13, 3061–3078. [Google Scholar] [CrossRef] [PubMed]
- Estevez, R.; López-Tenllado, F.J.; Aguado-Deblas, L.; Bautista, F.M.; Romero, A.A.; Luna, D. Current research on green ammonia (nh3) as a potential vector energy for power storage and engine fuels: A review. Energies 2023, 16, 5451. [Google Scholar] [CrossRef]
- Sonker, M.; Tiwary, S.K.; Shreyash, N.; Bajpai, S.; Ray, M.; Kar, S.K.; Balathanigaimani, M. Ammonia as an alternative fuel for vehicular applications: Paving the way for adsorbed ammonia and direct ammonia fuel cells. J. Clean. Prod. 2022, 376, 133960. [Google Scholar] [CrossRef]
- Amrillah, T.; Hermawan, A.; Alviani, V.N.; Seh, Z.W.; Yin, S. MXenes and their derivatives as nitrogen reduction reaction catalysts: Recent progress and perspectives. Mater. Today Energy 2021, 22, 100864. [Google Scholar] [CrossRef]
- Kui, P.; Liming, H.; Jiada, H.; Guanhua, Z.; Leiming, T. Highly efficient electrochemical reduction of nitrate to ammonia on cobalt doped Ti3C2 MXene nanosheets. Inorg. Chem. Commun. 2024, 161, 112134. [Google Scholar]
- Johnson, D.; Hunter, B.; Christie, J.; King, C.; Kelley, E.; Djire, A. Ti2N nitride MXene evokes the Mars-van Krevelen mechanism to achieve high selectivity for nitrogen reduction reaction. Sci. Rep. 2022, 12, 657. [Google Scholar] [CrossRef]
- Peng, W.; Luo, M.; Xu, X.; Jiang, K.; Peng, M.; Chen, D.; Chan, T.S.; Tan, Y. Spontaneous atomic ruthenium doping in Mo2CTX MXene defects enhances electrocatalytic activity for the nitrogen reduction reaction. Adv. Energy Mater. 2020, 10, 2001364. [Google Scholar] [CrossRef]
- Fang, Q.; Gao, Y.; Zhang, W.; Sun, F.; Pan, J.; Zhuang, G.; Deng, S.; Yao, Z.; Wang, J. Oxygen groups enhancing the mechanism of nitrogen reduction reaction properties on Ru-or Fe-supported Nb2C MXene. J. Phys. Chem. C 2021, 125, 14636–14645. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Yang, Q.; Li, X.; Li, H.; Wang, Y.; Jiao, T.; Huang, Z.; Dong, B.; Zhang, W. Highly efficient electrochemical reduction of nitrogen to ammonia on surface termination modified Ti3C2Tx MXene nanosheets. ACS Nano 2020, 14, 9089–9097. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y. Vacancy and N dopants facilitated Ti3+ sites activity in 3D Ti3 − xC2Ty MXene for electrochemical nitrogen fixation. Appl. Catal. B Environ. 2021, 297, 120482. [Google Scholar] [CrossRef]
- Chu, K.; Li, X.; Li, Q.; Guo, Y.; Zhang, H. Synergistic enhancement of electrocatalytic nitrogen reduction over boron nitride quantum dots decorated Nb2CTx-MXene. Small 2021, 17, 2102363. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Liu, C.; Liu, Z.; Han, J.; Fang, Y.; Han, Y.; Niu, Y.; Wu, Y.; Sun, C.; Xu, Y. Rational design of hydroxyl-rich Ti3C2Tx MXene quantum dots for high-performance electrochemical N2 reduction. Adv. Energy Mater. 2020, 10, 2000797. [Google Scholar] [CrossRef]
- Lv, Z.; Ma, W.; Wang, M.; Dang, J.; Jian, K.; Liu, D.; Huang, D. Co-constructing interfaces of multiheterostructure on MXene (Ti3C2Tx)-modified 3D self-supporting electrode for ultraefficient electrocatalytic HER in alkaline media. Adv. Funct. Mater. 2021, 31, 2102576. [Google Scholar] [CrossRef]
- Li, L.-X.; Zhang, G.-C.; Sun, W.-J.; Zhang, H.-Y.; Wang, S.-X.; Wei, J.-L.; He, J.-H.; Song, K.; Lu, J.-M. Construction of ultra-small Pt nanoparticles@ Ti3C2Tx MXene electrocatalyst for efficient and stable electrochemical hydrodechlorination of chloramphenicol. Chem. Eng. J. 2022, 433, 134415. [Google Scholar] [CrossRef]
- Yue, Q.; Sun, J.; Chen, S.; Zhou, Y.; Li, H.; Chen, Y.; Zhang, R.; Wei, G.; Kang, Y. Hierarchical mesoporous MXene–NiCoP electrocatalyst for water-splitting. ACS Appl. Mater. Interfaces 2020, 12, 18570–18577. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Du, C.; Kuang, M.; Chen, M.; Huang, W.; Ren, H.; Xu, J.; Feldhoff, A.; Yan, Q. Boosting efficient ambient nitrogen oxidation by a well-dispersed Pd on MXene electrocatalyst. Chem. Commun. 2020, 56, 5779–5782. [Google Scholar] [CrossRef]
- Chen, J.; Long, Q.; Xiao, K.; Ouyang, T.; Li, N.; Ye, S.; Liu, Z.-Q. Vertically-interlaced NiFeP/MXene electrocatalyst with tunable electronic structure for high-efficiency oxygen evolution reaction. Sci. Bull. 2021, 66, 1063–1072. [Google Scholar] [CrossRef]
- Wang, Y.; Du, R.; Li, Z.; Song, H.; Chao, Z.; Zu, D.; Chong, D.; Gao, N.; Li, C. Rationally designed CdS/Ti3C2 MXene electrocatalysts for efficient CO2 reduction in aqueous electrolyte. Ceram. Int. 2021, 47, 28321–28327. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Y.; Zhuo, H.; Sun, X.; Gu, Y.; Zhuang, G.; Deng, S.; Zhong, X.; Wei, Z.; Li, X. Mo2TiC2 MXene: A promising catalyst for electrocatalytic ammonia synthesis. Catal. Today 2020, 339, 120–126. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Zou, G.; Fernandez, C.; Liu, B.; Zhang, Q.; Hu, J.; Peng, Q. Self-reduction synthesis of new MXene/Ag composites with unexpected electrocatalytic activity. ACS Sustain. Chem. Eng. 2016, 4, 6763–6771. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Handoko, A.D.; Johnson, L.R.; Meng, X.; Lin, M.; Subramanian, G.S.; Anasori, B.; Gogotsi, Y.; Vojvodic, A.; Seh, Z.W. 2h-Mos2 on Mo2ct X Mxene nanohybrid for efficient and durable electrocatalytic hydrogen evolution. ACS Nano 2020, 14, 16140–16155. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; He, M.; Zhu, B.; Yu, J.; Fan, K.; Jaroniec, M. 0D/2D NiS2/V-MXene composite for electrocatalytic H2 evolution. J. Catal. 2019, 375, 8–20. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, S.; Wang, Z.; Zhao, J.; Qiu, J. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual cocatalysts in TiO2 photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zboril, R.; Schmuki, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2018, 9, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhai, S.; Wang, Y.; Liu, A.; Chen, C. TiO2 photocatalysis for transfer hydrogenation. Molecules 2019, 24, 330. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pulgarin, C. Why five decades of massive research on heterogeneous photocatalysis, especially on TiO2, has not yet driven to water disinfection and detoxification applications? Critical review of drawbacks and challenges. Chem. Eng. J. 2023, 477, 146875. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, N. Positioning MXenes in the photocatalysis landscape: Competitiveness, challenges, and future perspectives. Adv. Funct. Mater. 2020, 30, 2002528. [Google Scholar] [CrossRef]
- Kim, H.; Anasori, B.; Gogotsi, Y.; Alshareef, H.N. Thermoelectric properties of two-dimensional molybdenum-based MXenes. Chem. Mater. 2017, 29, 6472–6479. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.; Fang, J.; Jiang, L.; Yang, H.; Xiao, Z. MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g–C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar] [CrossRef]
- Sergiienko, S.A.; Tobaldi, D.M.; Lajaunie, L.; Lopes, D.V.; Constantinescu, G.; Shaula, A.L.; Shcherban, N.D.; Shkepu, V.I.; Calvino, J.J.; Frade, J.R. Photocatalytic removal of benzene over Ti3C2Tx MXene and TiO2–MXene composite materials under solar and NIR irradiation. J. Mater. Chem. C 2022, 10, 626–639. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2020, 261, 127127. [Google Scholar] [CrossRef]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene loaded on g-C3N4 photocatalyst for the photocatalytic degradation of methylene blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Othman, Z.; Sinopoli, A.; Mackey, H.R.; Mahmoud, K.A. Efficient Photocatalytic Degradation of Organic Dyes by AgNPs/TiO2/Ti3C2Tx MXene Composites under UV and Solar Light. ACS Omega 2021, 6, 33325–33338. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W. Ultrathin ZnIn2S4 nanosheets anchored on Ti3C2TX MXene for photocatalytic H2 evolution. Angew. Chem. 2020, 132, 11383–11388. [Google Scholar] [CrossRef]
- Jin, S.; Jing, H.; Wang, L.; Hu, Q.; Zhou, A. Construction and performance of CdS/MoO2@Mo2C-MXene photocatalyst for H2 production. J. Adv. Ceram. 2022, 11, 1431–1444. [Google Scholar] [CrossRef]
- Peng, C.; Xie, X.; Xu, W.; Zhou, T.; Wei, P.; Jia, J.; Zhang, K.; Cao, Y.; Wang, H.; Peng, F. Engineering highly active Ag/Nb2O5@Nb2CTx (MXene) photocatalysts via steering charge kinetics strategy. Chem. Eng. J. 2021, 421, 128766. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Ji, G.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, X.; Cui, H. 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B Environ. 2019, 246, 12–20. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Q.; Li, J.; Liu, H. Boosting the photocatalytic activity of CdLa2S4 for hydrogen production using Ti3C2 MXene as a co-catalyst. Appl. Catal. B Environ. 2020, 267, 118379. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Gao, T.; Li, H.; Ren, Y.; Zhou, G. Ti3C2 MXene co-catalyst assembled with mesoporous TiO2 for boosting photocatalytic activity of methyl orange degradation and hydrogen production. Chin. J. Catal. 2022, 43, 461–471. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Liang, Z.; Xue, Y.; Cui, H.; Tian, J. Synergetic effect of defects rich MoS2 and Ti3C2 MXene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2. Chem. Eng. J. 2020, 383, 123178. [Google Scholar] [CrossRef]
- Wojciechowski, T.; Rozmysłowska-Wojciechowska, A.; Matyszczak, G.; Wrzecionek, M.; Olszyna, A.; Peter, A.; Mihaly-Cozmuta, A.; Nicula, C.; Mihaly-Cozmuta, L.; Podsiadło, S. Ti2C MXene modified with ceramic oxide and noble metal nanoparticles: Synthesis, morphostructural properties, and high photocatalytic activity. Inorg. Chem. 2019, 58, 7602–7614. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Guo, R.; Xiao, H.; Ren, E.; Song, Q.; Xiang, C.; Lai, X.; Lan, J.; Jiang, S. Bi2WO6/Nb2CTx MXene hybrid nanosheets with enhanced visible-light-driven photocatalytic activity for organic pollutants degradation. Appl. Surf. Sci. 2020, 505, 144595. [Google Scholar] [CrossRef]
- Guo, S.; Luo, H.; Bao, Y.; Li, Y.; Guan, H.; Zhu, Y. Construction of hierarchical Ti3C2Tx MXene/ZnIn2S4 heterostructures for efficiently photocatalytic reduction of Cr (VI) under visible light. Appl. Surf. Sci. 2022, 575, 151753. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Wu, Z.-S. MXene for energy storage: Present status and future perspectives. J. Phys. Energy 2020, 2, 032004. [Google Scholar] [CrossRef]
- Wei, C.; Xi, B.; Wang, P.; Wang, Z.; An, X.; Tian, K.; Feng, J.; Xiong, S. In Situ Growth Engineering on 2D MXenes for Next-Generation Rechargeable Batteries. Adv. Energy Sustain. Res. 2023, 4, 2300103. [Google Scholar] [CrossRef]
- Liao, L.; Jiang, D.; Zheng, K.; Zhang, M.; Liu, J. Industry-scale and environmentally stable Ti3C2Tx MXene based film for flexible energy storage devices. Adv. Funct. Mater. 2021, 31, 2103960. [Google Scholar] [CrossRef]
- Lin, P.; Xie, J.; He, Y.; Lu, X.; Li, W.; Fang, J.; Yan, S.; Zhang, L.; Sheng, X.; Chen, Y. MXene aerogel-based phase change materials toward solar energy conversion. Sol. Energy Mater. Sol. Cells 2020, 206, 110229. [Google Scholar] [CrossRef]
- Gong, S.; Ding, Y.; Li, X.; Liu, S.; Wu, H.; Lu, X.; Qu, J. Novel flexible polyurethane/MXene composites with sensitive solar thermal energy storage behavior. Compos. Part A 2021, 149, 106505. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Jiang, H.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D.; Wu, H.; Mei, Y. Flame-retardant and form-stable phase change composites based on MXene with high thermostability and thermal conductivity for thermal energy storage. Chem. Eng. J. 2021, 420, 130466. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, H.; Das, P.; Zhang, Y.; Cao, Y.; Ma, J.; Liu, S.; Wu, Z.S. Multitasking MXene inks enable high-performance printable microelectrochemical energy storage devices for all-flexible self-powered integrated systems. Adv. Mater. 2021, 33, 2005449. [Google Scholar] [CrossRef] [PubMed]
- Qian, A.; Pang, Y.; Wang, G.; Hao, Y.; Liu, Y.; Shi, H.; Chung, C.-H.; Du, Z.; Cheng, F. Pseudocapacitive charge storage in MXene–V2O5 for asymmetric flexible energy storage devices. ACS Appl. Mater. Interfaces 2020, 12, 54791–54797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, X.; Zhao, R.; Liao, K.; Chan, V. Dual-functional Ti3C2Tx MXene for wastewater treatment and electrochemical energy storage. Sustain. Energy Fuels 2020, 4, 3566–3573. [Google Scholar] [CrossRef]
- Shpigel, N.; Malchik, F.; Levi, M.D.; Gavriel, B.; Bergman, G.; Tirosh, S.; Leifer, N.; Goobes, G.; Cohen, R.; Weitman, M. New aqueous energy storage devices comprising graphite cathodes, MXene anodes and concentrated sulfuric acid solutions. Energy Storage Mater. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Said, Z.; Sharma, P.; Aslfattahi, N.; Ghodbane, M. Experimental analysis of novel ionic liquid-MXene hybrid nanofluid’s energy storage properties: Model-prediction using modern ensemble machine learning methods. J. Energy Storage 2022, 52, 104858. [Google Scholar] [CrossRef]
- Zahra, S.A.; Murshed, M.M.; Naeem, U.; Gesing, T.M.; Rizwan, S. Cation-assisted self-assembled pillared V2CTx MXene electrodes for efficient energy storage. Chem. Eng. J. 2023, 474, 145526. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Gowthami, D.; Sharma, R.; Khalid, M. 2D MXene based nanocomposites for solar driven renewable energy storage utilizing binary eutectic phase change material. J. Mol. Liq. 2023, 391, 123246. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Wang, J.-W.; Wang, X.-Z.; Gao, X.-H.; Zhuang, G.-C.; Yang, J.-B.; Ren, H. Dielectric properties and energy storage performance of PVDF-based composites with MoS2@MXene nanofiller. Chem. Eng. J. 2022, 437, 135431. [Google Scholar] [CrossRef]
- Shafique, R.; Rani, M.; Batool, K.; Shah, A.A.; Bahajjaj, A.A.A.; Sillanpää, M.; Alsalmah, H.A.; Janjua, N.K.; Arshad, M. Nanoengineering of novel MXene (Ti3C2Tx) based MgCr2O4 nanocomposite with detailed synthesis, morphology and characterization for enhanced energy storage application. Mater. Sci. Eng. B 2024, 299, 117036. [Google Scholar] [CrossRef]
- Bukhari, H.; Iqbal, A.M.; Awan, S.U.; Hussain, D.; Shah, S.A.; Rizwan, S. Intercalation of C60 into MXene Multilayers: A Promising Approach for Enhancing the Electrochemical Properties of Electrode Materials for High-Performance Energy Storage Applications. ACS Omega 2023, 9, 227–238. [Google Scholar] [CrossRef]
- Liu, X.; Lin, F.; Zhang, X.; Liu, M.; Sun, Z.; Zhang, L.; Min, X.; Mi, R.; Huang, Z. Paraffin/Ti3C2Tx Mxene@ Gelatin aerogels composite Phase-Change materials with high Solar-Thermal conversion efficiency and enhanced thermal conductivity for thermal energy storage. Energy Fuels 2021, 35, 2805–2814. [Google Scholar] [CrossRef]
- Tian, Y.; Que, W.; Luo, Y.; Yang, C.; Yin, X.; Kong, L.B. Surface nitrogen-modified 2D titanium carbide (MXene) with high energy density for aqueous supercapacitor applications. J. Mater. Chem. A 2019, 7, 5416–5425. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Huang, D.; Zhang, J.; Lin, P.; Zhang, L.; Sheng, X.; Chen, Y.; Lu, X. One-step construction of novel phase change composites supported by a biomass/MXene gel network for efficient thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 241, 111729. [Google Scholar] [CrossRef]
- Jones, W.D. Carbon Capture and Conversion; ACS Publications: Washington, DC, USA, 2020; Volume 142, pp. 4955–4957. [Google Scholar]
- Sabri, M.A.; Al Jitan, S.; Bahamon, D.; Vega, L.F.; Palmisano, G. Current and future perspectives on catalytic-based integrated carbon capture and utilization. Sci. Total Environ. 2021, 790, 148081. [Google Scholar] [CrossRef] [PubMed]
- Morales-Salvador, R.; Gouveia, J.D.; Morales-Garcia, A.; Vines, F.; Gomes, J.R.; Illas, F. Carbon capture and usage by MXenes. ACS Catal. 2021, 11, 11248–11255. [Google Scholar] [CrossRef]
- Persson, I.; Halim, J.; Lind, H.; Hansen, T.W.; Wagner, J.B.; Näslund, L.Å.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O. 2D transition metal carbides (MXenes) for carbon capture. Adv. Mater. 2019, 31, 1805472. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Y.; Sa, B.; Fang, Y.; Lin, J.; Huang, Y.; Tang, C.; Zhou, J.; Miao, N.; Sun, Z. M2C-type MXenes: Promising catalysts for CO2 capture and reduction. Appl. Surf. Sci. 2020, 521, 146436. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.; Ong, W.-J.; MacFarlane, D.R.; Zhao, X.; Cheetham, A.K.; Sun, C. Understanding of electrochemical mechanisms for CO2 capture and conversion into hydrocarbon fuels in transition-metal carbides (MXenes). ACS Nano 2017, 11, 10825–10833. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cao, Z.; Zhang, Y.; Chen, H.; Gu, J.; Du, Z.; Shi, Y.; Li, B.; Yang, S. Single-Atom Sites on MXenes for Energy Conversion and Storage. Small Sci. 2021, 1, 2100017. [Google Scholar] [CrossRef]
- Liu, F.-Q.; Liu, X.; Sun, L.; Li, R.; Yin, C.-X.; Wu, B. MXene-supported stable adsorbents for superior CO2 capture. J. Mater. Chem. A 2021, 9, 12763–12771. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Hai, A.; Othman, I.; Ponpandian, N.; Banat, F.; Show, P.L. Hybrid Pd50-Ru50/MXene (Ti3C2Tx) nanocatalyst for effective hydrogenation of CO2 to methanol toward climate change control. Chem. Eng. J. 2021, 414, 128869. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, Y.; Yu, D.; Liu, Q.; Zhou, L.; Zhang, K.; Li, P.; Hu, F.; Li, L.; Chou, S. Hierarchical Ti3C2Tx MXene/Carbon Nanotubes for Low Overpotential and Long-Life Li-CO2 Batteries. ACS Nano 2021, 15, 8407–8417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, C.; Hu, R.; Du, Z.; Gu, J.; Cui, Y.; Chen, X.; Xu, W.; Cheng, Z.; Li, S. Selective etching quaternary MAX phase toward single atom copper immobilized MXene (Ti3C2Clx) for efficient CO2 electroreduction to methanol. ACS Nano 2021, 15, 4927–4936. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Yusuf, B.O.; Abdullah, A.; Bakare, A.I.; Mustapha, U.; Hakeem, A.S.; Ganiyu, S.A. Ti2C-MXene/activated carbon nanocomposite for efficient CO2 capture: Insights into thermodynamics properties. Sep. Purif. Technol. 2024, 340, 126737. [Google Scholar] [CrossRef]
- Arifutzzaman, A.; Musa, I.N.; Aroua, M.K.; Saidur, R. MXene based activated carbon novel nano-sandwich for efficient CO2 adsorption in fixed-bed column. J. CO2 Util. 2023, 68, 102353. [Google Scholar] [CrossRef]
- Luo, W.; Niu, Z.; Mu, P.; Li, J. Pebax and CMC@MXene-Based Mixed Matrix Membrane with High Mechanical Strength for the Highly Efficient Capture of CO2. Macromolecules 2022, 55, 9851–9859. [Google Scholar] [CrossRef]
- Wu, X.; Fernandes, D.; Feron, P.; Ding, M.; Xu, H.; Xie, Z. Production of cooling water by Ti3C2Tx MXene interlayered forward osmosis membranes for post-combustion CO2 capture system. J. Membr. Sci. 2022, 641, 119877. [Google Scholar] [CrossRef]
- Govindan, B.; Madhu, R.; Abu Haija, M.; Kusmartsev, F.V.; Banat, F. Pd-Decorated 2D MXene (2D Ti3C2Tix) as a High-Performance Electrocatalyst for Reduction of Carbon Dioxide into Fuels toward Climate Change Mitigation. Catalysts 2022, 12, 1180. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, Y.; Zhang, X.-F.; Xu, C.; Yao, J. Integrating two-dimensional MXene fillers into nanocellulose for the fabrication of CO2 separation membranes. Sep. Purif. Technol. 2023, 326, 124704. [Google Scholar] [CrossRef]
- Wang, D.; Xin, Y.; Wang, Y.; Li, X.; Wu, H.; Zhang, W.; Yao, D.; Wang, H.; Zheng, Y.; He, Z. A general way to transform Ti3C2Tx MXene into solvent-free fluids for filler phase applications. Chem. Eng. J. 2021, 409, 128082. [Google Scholar] [CrossRef]
- Shamsabadi, A.A.; Isfahani, A.P.; Salestan, S.K.; Rahimpour, A.; Ghalei, B.; Sivaniah, E.; Soroush, M. Pushing rubbery polymer membranes to be economic for CO2 separation: Embedment with Ti3C2Tx MXene nanosheets. ACS Appl. Mater. Interfaces 2019, 12, 3984–3992. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Gong, K.; Hykys, P.; Chen, D.; Ying, W.; Sofer, Z.; Yan, Y.; Li, Z.; Peng, X. Nanoconfined deep eutectic solvent in laminated MXene for efficient CO2 separation. Chem. Eng. J. 2021, 405, 126961. [Google Scholar] [CrossRef]
- Luo, W.; Niu, Z.; Mu, P.; Li, J. MXene/poly (ethylene glycol) mixed matrix membranes with excellent permeance for highly efficient separation of CO2/N2 and CO2/CH4. Colloids Surf. A 2022, 640, 128481. [Google Scholar] [CrossRef]

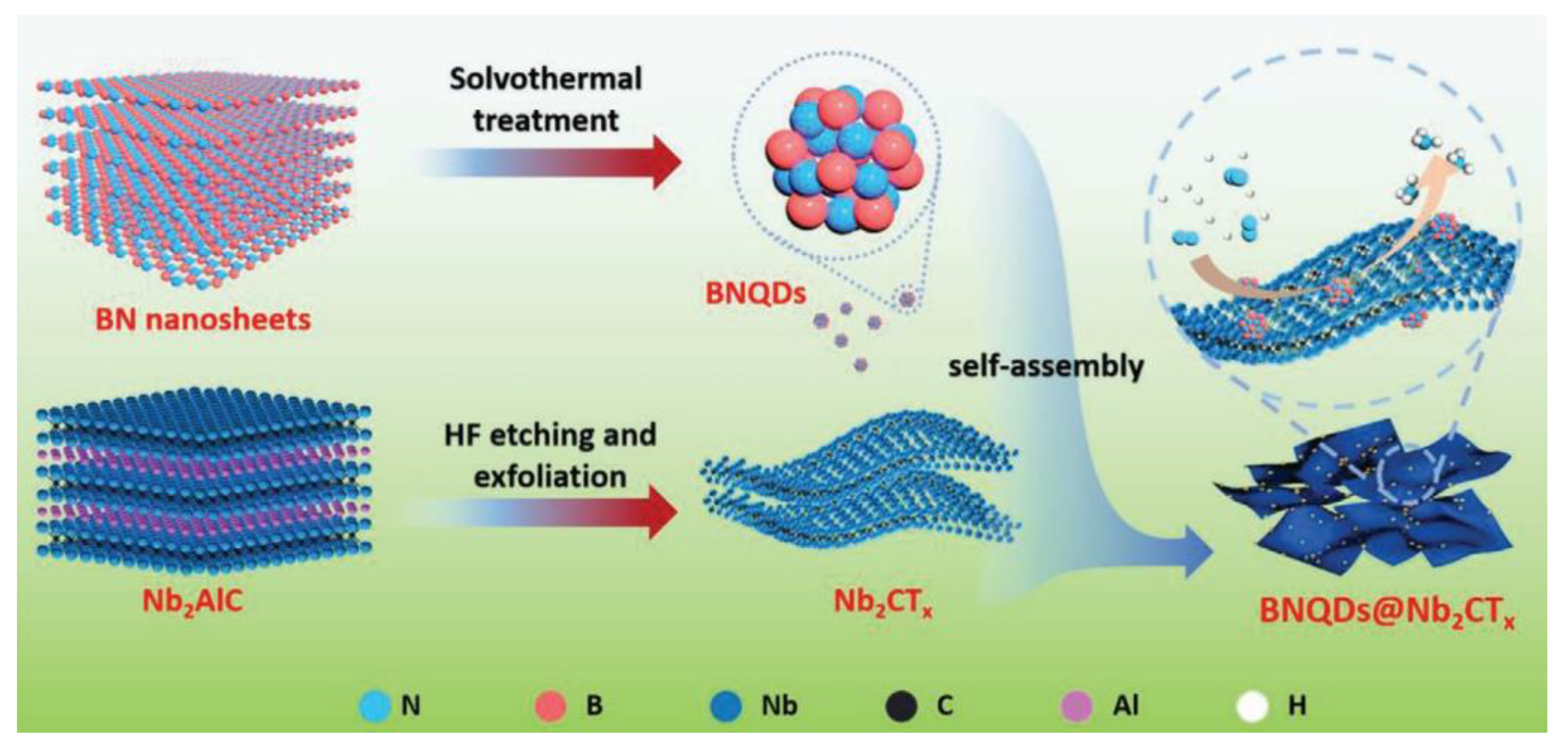
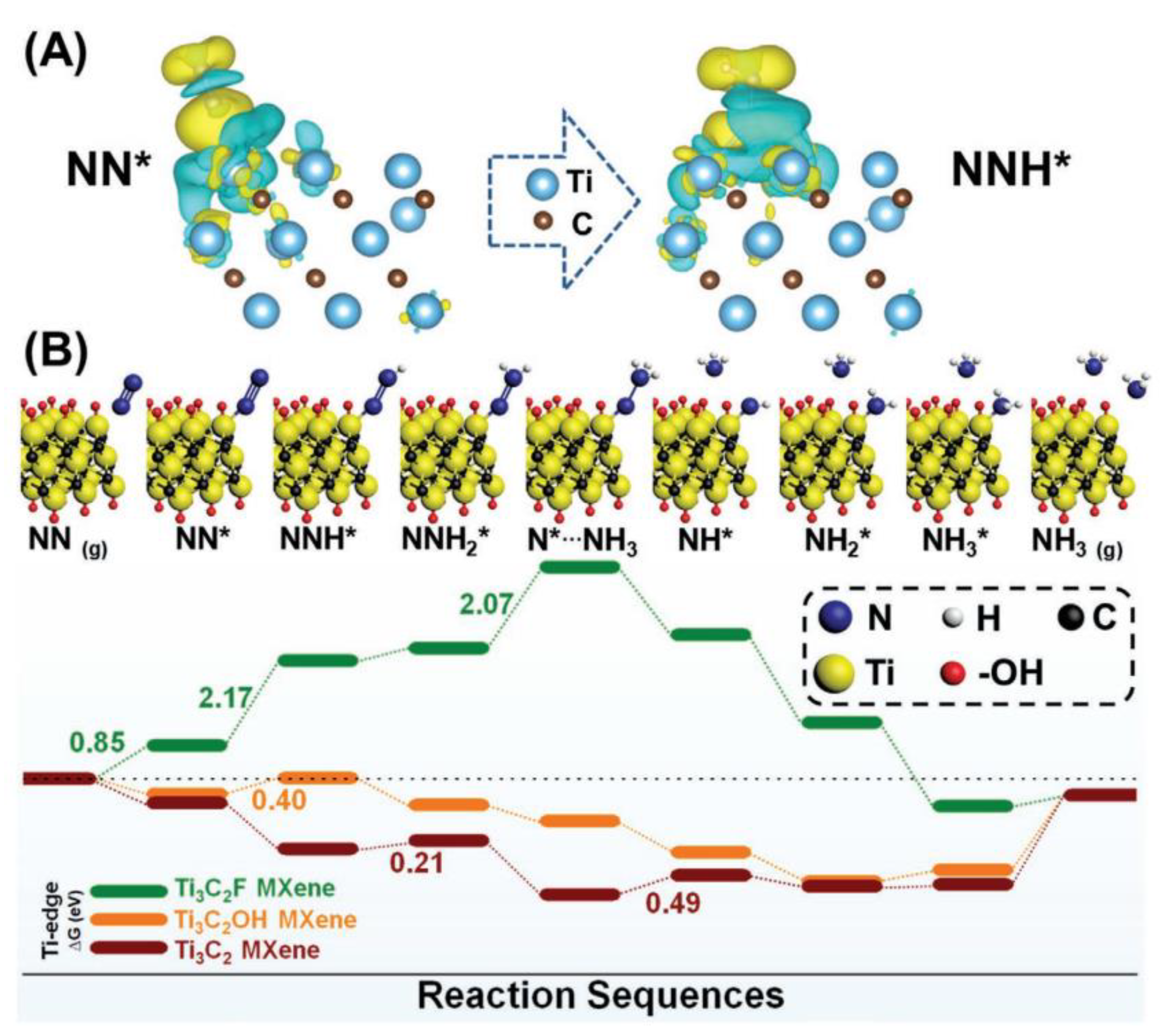
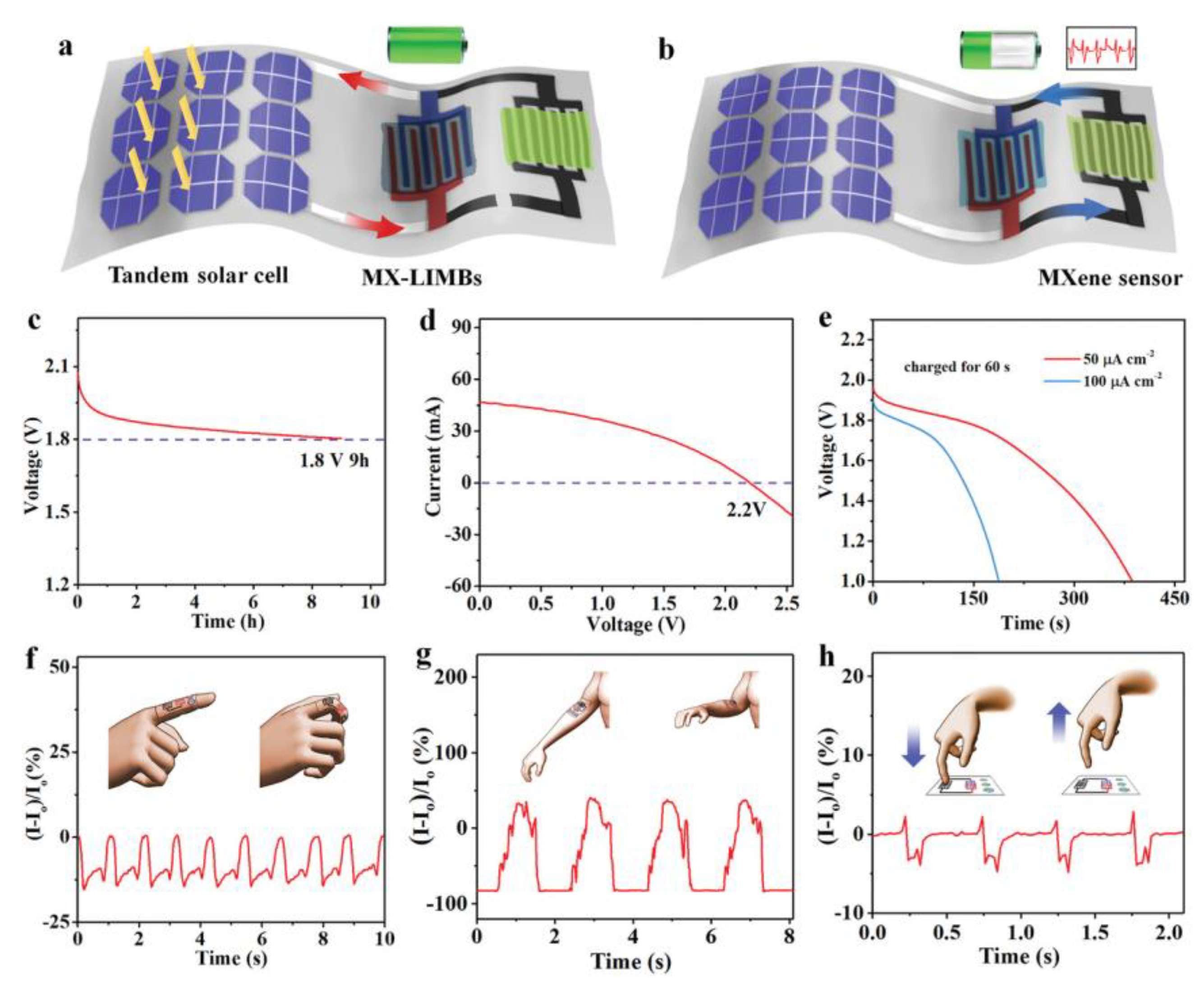
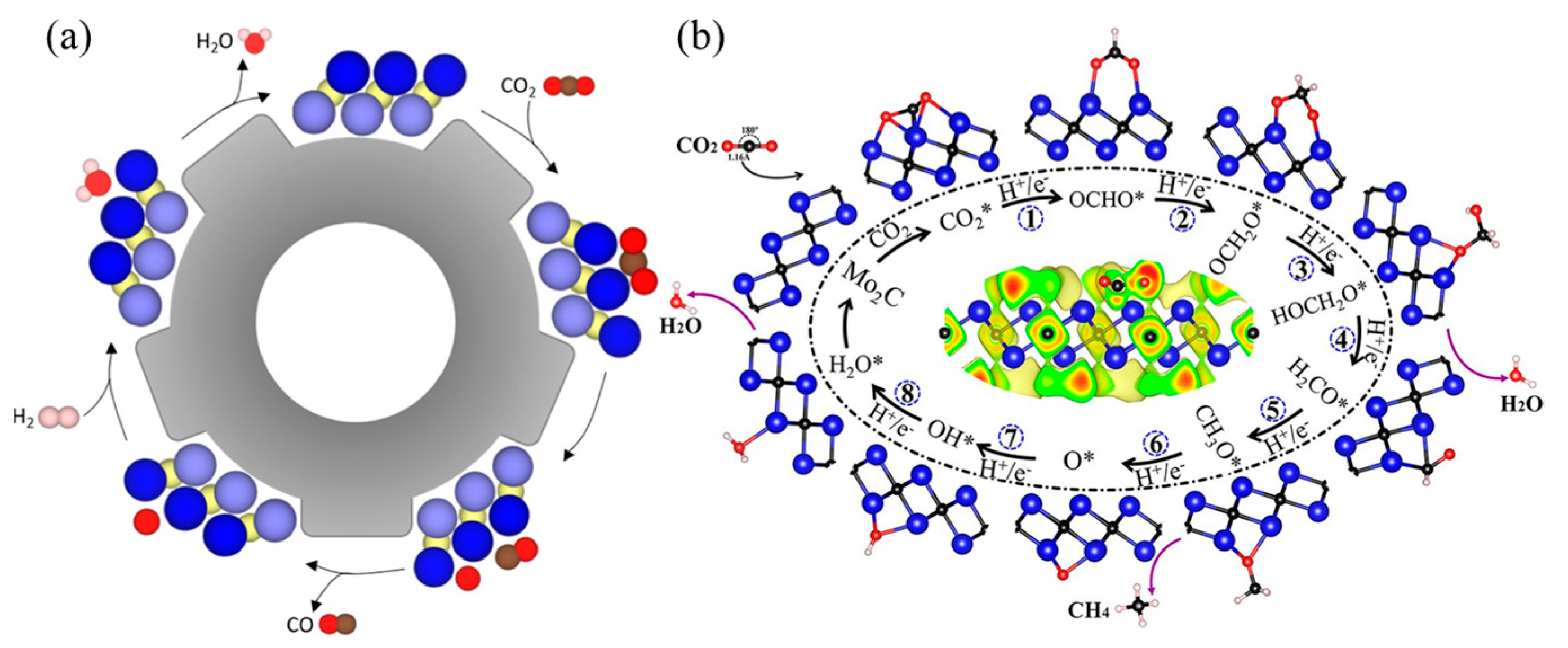
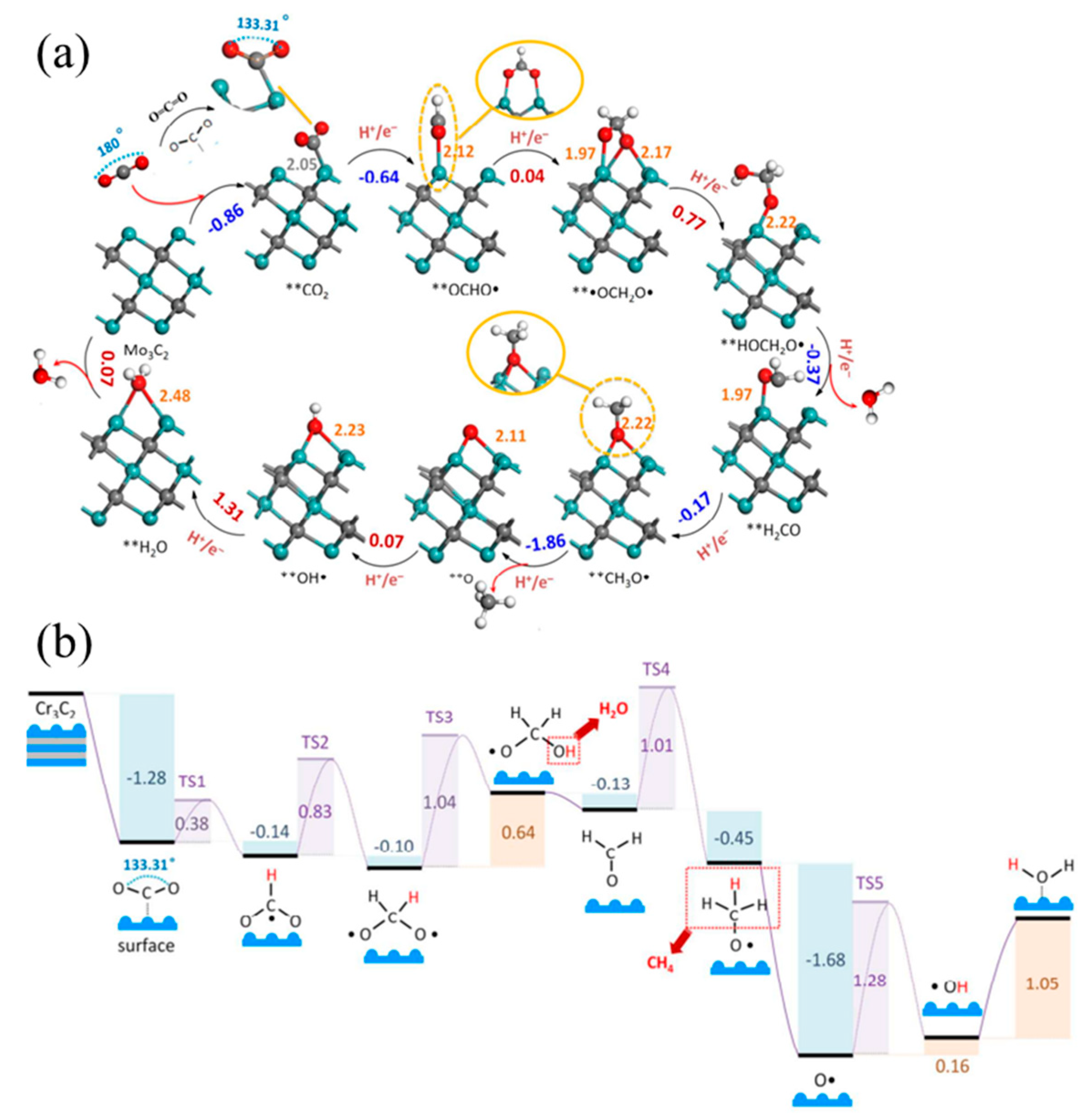
| Electrocatalysts | MXene | Catalytic Activity | Ref. |
|---|---|---|---|
| Co2P/N@Ti3C2Tx@NF | Ti3C2Tx | Ultra-low overpotential of 15 mV, achieving a current density of 10 mA∙cm−2. | [67] |
| Pt@Ti3C2Tx | Ti3C2Tx | 1% Pt@MXene can completely reduce CAP by 98.7% within 90 min and maintain 86.5% after 25 cycles. | [68] |
| Ti3C2@mNiCoP | Ti3C2 | Water splitting performance remains unchanged after 12 h of operation. | [69] |
| Pd-MXene | Ti3C2Tx | Excellent nitrate yield (2.80 µg h−1 mgcat −1) and Faradaic efficiency (11.34%). | [70] |
| NiFeP/MXene | Ti3C2 | Exhibiting a low overpotential of 286 mV at 10 mA∙cm−2 and a current density of 10 mA∙cm−2 at a cell voltage of 1.61 V. | [71] |
| CdS/Ti3C2 | Ti3C2 | Faradaic efficiency is as high as 94% at −1.0 V. | [72] |
| Mo2TiC2 | Mo2TiC2 | The overpotential is 0.26 V with high NRR activity, which meets the balance of N2 activation and overpotential reduction. | [73] |
| MXene/NW-Ag0.9Ti0.1 | Ti3C2 | It exhibits onset potential (EORR) and half-wave potential (E1/2) at 1600 rpm, which are 0.921 V (RHE) and 0.782 V (RHE), respectively. | [74] |
| Mo2CTx/2H-MoS2 | Mo2CTx | Maintains current densities in excess of −450 mA∙cm−2geom with less than 30 mV overpotential decay after 100,000 consecutive cyclic voltammetry cycles. | [75] |
| NiS2/V-MXene | Ti3C2Tx | The overpotential is 179 mV to achieve a catalytic current density of −10 mA∙cm2. | [76] |
| FeNi-LDH/Ti3C2-MXene | Ti3C2 | A current density of 50 mA∙cm−2 is achieved at η = 370 mV. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wang, R.; Matulis, V.E.; Vladimir, K. Structure, Synthesis, and Catalytic Performance of Emerging MXene-Based Catalysts. Molecules 2024, 29, 1286. https://doi.org/10.3390/molecules29061286
Sun Z, Wang R, Matulis VE, Vladimir K. Structure, Synthesis, and Catalytic Performance of Emerging MXene-Based Catalysts. Molecules. 2024; 29(6):1286. https://doi.org/10.3390/molecules29061286
Chicago/Turabian StyleSun, Zhengxiang, Rui Wang, Vitaly Edwardovich Matulis, and Korchak Vladimir. 2024. "Structure, Synthesis, and Catalytic Performance of Emerging MXene-Based Catalysts" Molecules 29, no. 6: 1286. https://doi.org/10.3390/molecules29061286
APA StyleSun, Z., Wang, R., Matulis, V. E., & Vladimir, K. (2024). Structure, Synthesis, and Catalytic Performance of Emerging MXene-Based Catalysts. Molecules, 29(6), 1286. https://doi.org/10.3390/molecules29061286