Aggregation-Induced Emission-Based Chemiluminescence Systems in Biochemical Analysis and Disease Theranostics
Abstract
1. Introduction
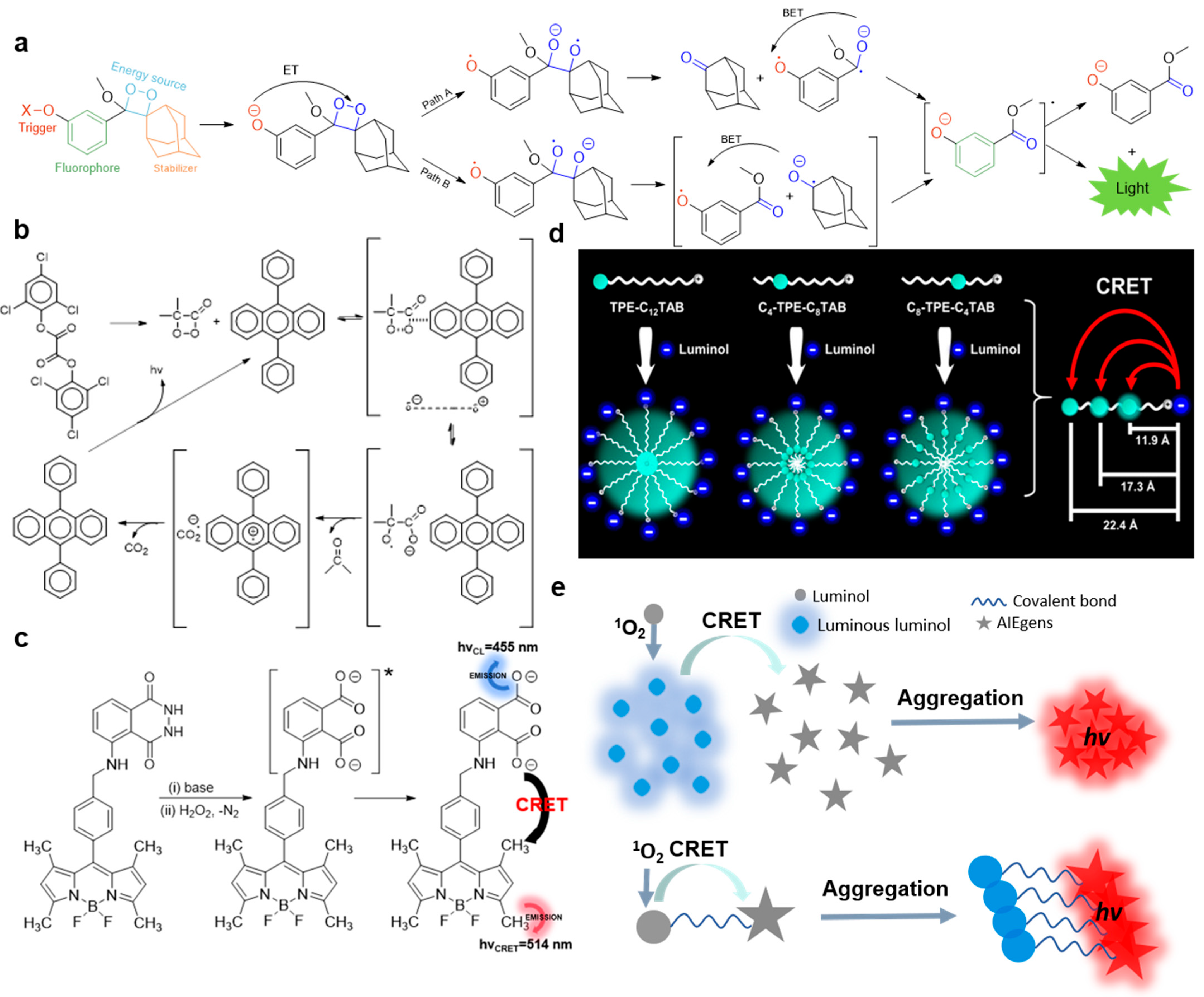
2. The Working Mechanism of CL Systems
3. Application in Biochemical Analysis In Vitro
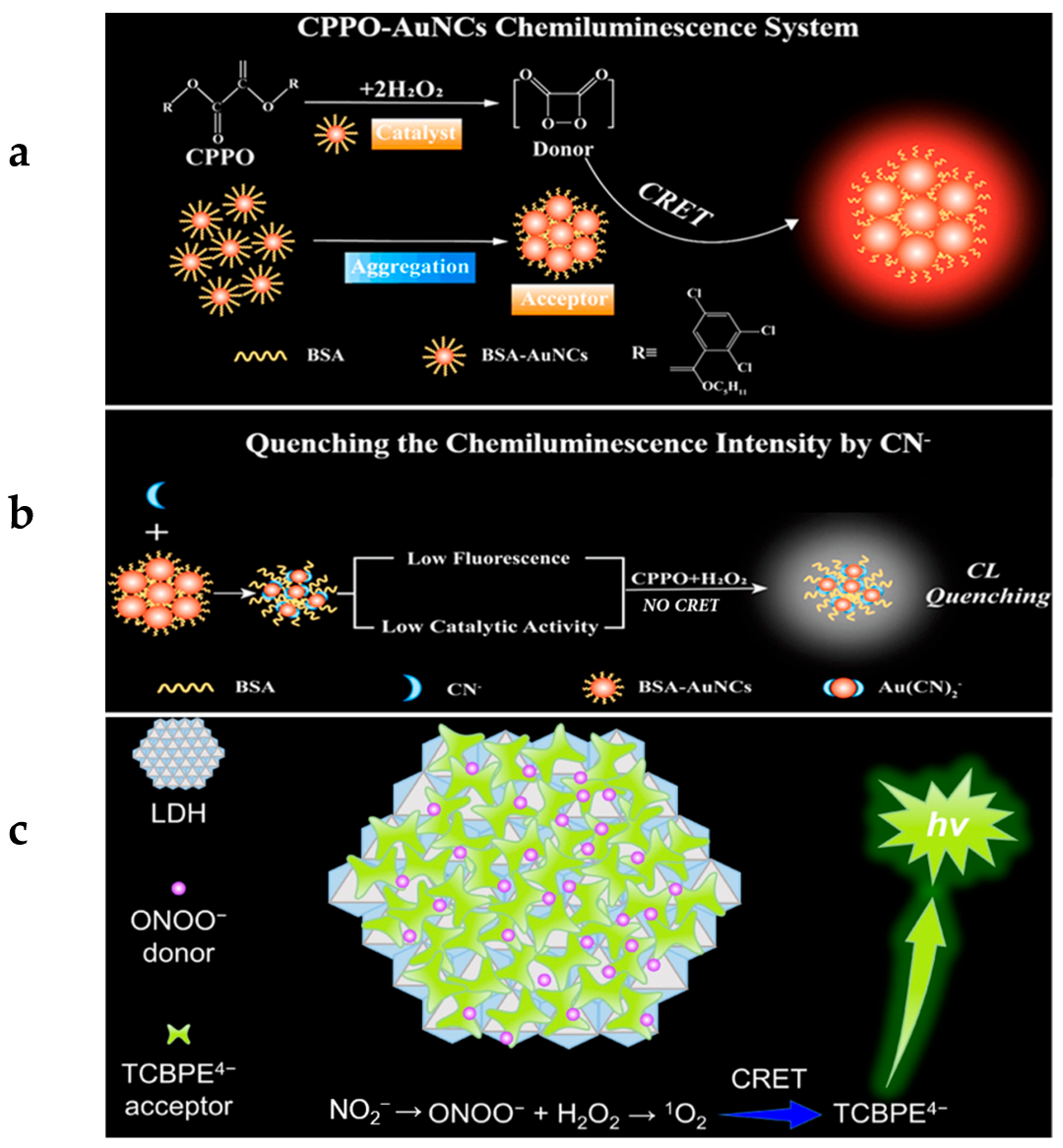
3.1. Ion Detection
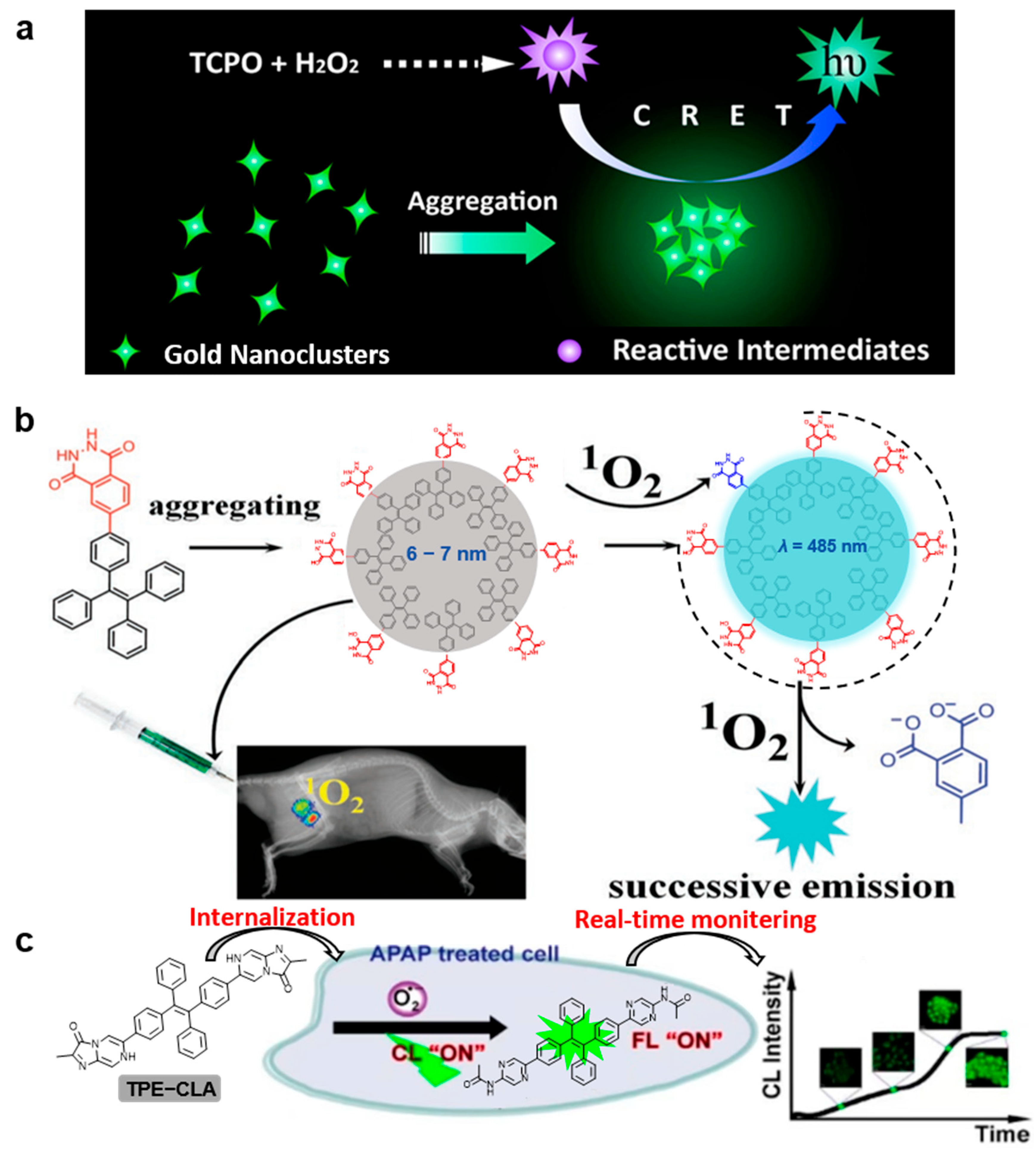
3.2. Reactive Oxygen Species (ROS) Detection
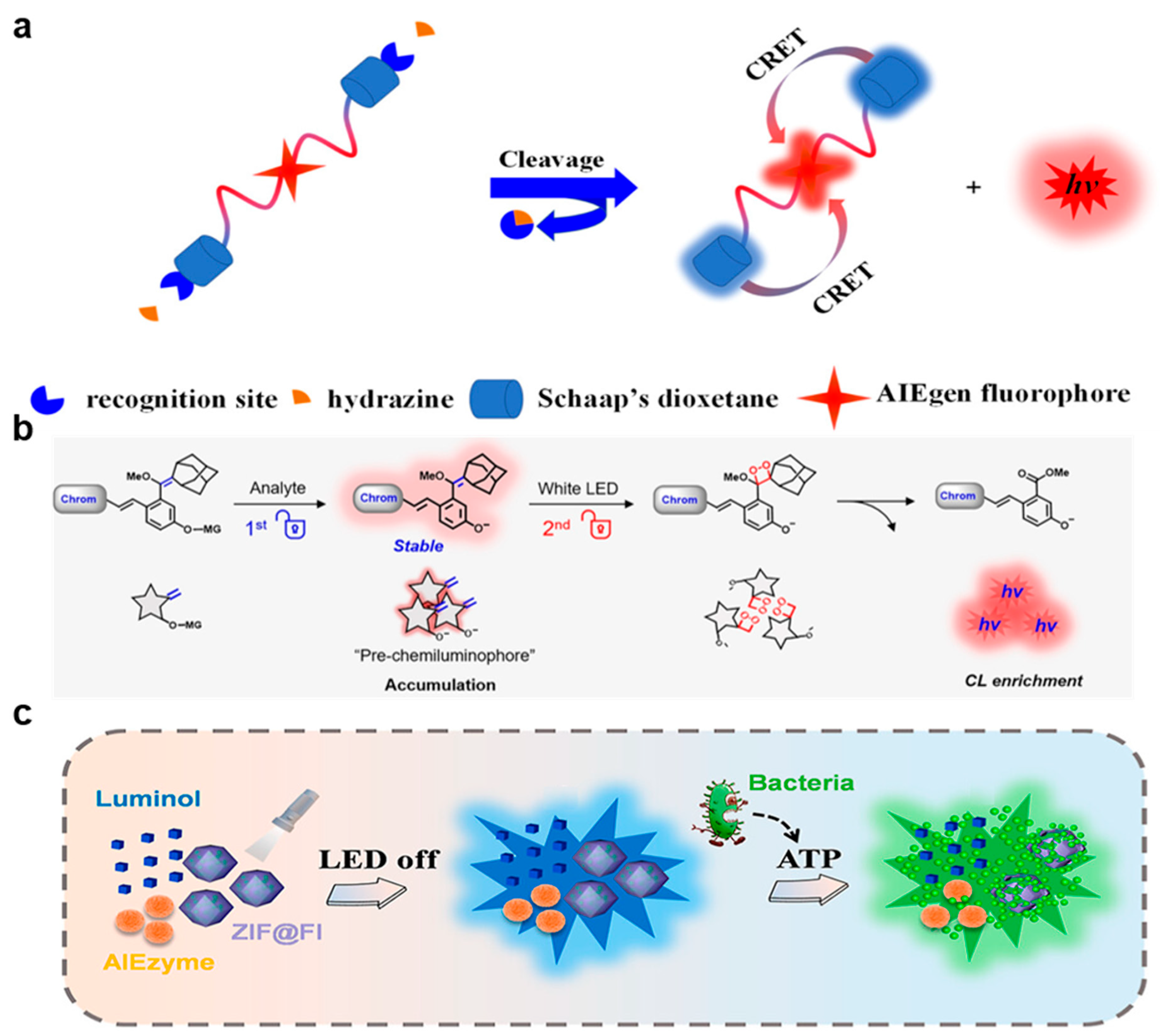
3.3. Molecular Sensing
3.4. Pathogen Assay
4. In Vivo Bioimaging and Image-Guided Therapy
4.1. External Light-Triggered CL Imaging and Disease Therapy
4.2. CL Imaging and Disease Therapy without External Light Triggering
5. Conclusions and Prospects
- (1)
- The range of substrates reported in AIE-CL systems is still limited to several common CL molecules, such as luminol, peroxalic acid, dioxetanes, etc., and the response of triggering CL is also limited to a very small amount of active substances, such as hydrogen peroxide and singlet oxygen. We believe that expanding the substrate range of AIE-CL systems and exploring more response modes is of great significance in the development of new CL systems with expanded applications.
- (2)
- It is well known that in order to facilitate deep tissue imaging, the development of NIR luminescence imaging dyes has always been pursued by researchers. However, the emission wavelengths of existing AIE-CL systems are generally located in the visible range. Although there are a few reports of CL with long wavelength emission, they still need to transfer the CL wavelength to the NIR range with the help of CRET/FRET, which is seriously limited by several critical parameters, including the number ratio of donors and acceptors, spatial distance, overlap coefficient of the emission spectra of donor and the absorption spectra of the acceptor, etc., and often requires complex and tedious molecular or nanostructural design. Therefore, the design of single molecular AIE-CL imaging substrates with NIR emissions is of particular importance and has application potential.
- (3)
- Generally speaking, CL systems can produce light signals without external light excitation, but at the same time, CL signals often have poor capability to resist environmental interferences. Therefore, it is promising to design novel CL dyes with stabilized CL signals. The strategy of combining AIE systems with CL to make aggregated-state CL molecules has been proven to be feasible for enhancing the anti-interference ability of CL molecules to some extent. In addition, grafting CL molecules onto classical luminescent agents with verified stability (e.g., nanocluster, quantum dots) is likely to increase their photo- and structural stability.
- (1)
- In the previous reports, most of the developed AIE-CL systems were responsive to a single active substrate. Provided they can respond to more diverse targets or to multiple targets at the same time, they will become a more powerful tool in the field of biochemical analysis and bioimaging and detection;
- (2)
- The combination of multimodality signals can make use of the complementary effects of each individual imaging signal with different characteristics, resulting in significantly improved sensitivity, accuracy, and specificity in bioimaging. Therefore, the construction of a multimodality bioimaging platform, such as combining the highly sensitive CL signals of an AIE-CL system with magnetic resonance imaging (MRI) and computed tomography (CT) signals with the characteristics of high penetration depth and high spatial resolution can provide more effective candidates for accurate bioimaging. At the same time, the signal generated by a CL system without external light excitation is converted into a photothermal effect in situ, leading to the generation of an ultrasonic signal with the capability of deep-depth tissue penetration. Thus, it can realize combined CL and PA signals multimodal bioimaging;
- (3)
- Most of the developed AIE-CL systems only have an imaging function, but there are few reports of AIE-CL systems with therapeutic functions. The further incorporation of therapeutic functions into the existing AIE-CL imaging systems, such as PDT and RT, can not only have excellent imaging effects on disease tissues, but also achieve image-guided disease treatment, holding great potential to provide new strategies for applications in disease detection and treatment.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, Y.; Chen, Y.; Guo, X.; Liu, W.; Zhang, L.; Lv, C.; Xu, Y.; Jin, Y.; Li, B. Aggregation-induced chemiluminescence system for sensitive detection of mercury ions. Anal. Bioanal. Chem. 2021, 413, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, T.; Yang, J.; Zhang, X. A robust gold nanocluster-peroxyoxalate chemiluminescence system for highly sensitive detection of cyanide in environmental water. Sens. Actuators B Chem. 2022, 353, 131038. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, M.; Zhou, W.; Guan, W.; Lu, C. Disordered assembly of donors and acceptors on layered double hydroxides for high-efficiency chemiluminescence resonance energy transfer. Anal. Chem. 2021, 93, 7724–7731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, N.; Lu, C. Aggregation-induced emission: A simple strategy to improve chemiluminescence resonance energy transfer. Anal. Chem. 2015, 87, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, H.; Gu, M.; Zhao, N.; Cheng, M.; Lv, J. Real-time mapping of ultratrace singlet oxygen in rat during acute and chronic inflammations via a chemiluminescent nanosensor. Small 2019, 15, e1804662. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Fan, J.; Wang, X.; Xiao, Y.; Xie, X.; Jiao, X.; Sun, C.; Tang, B. Simultaneous fluorescence and chemiluminescence turned on by aggregation-induced emission for real-time monitoring of endogenous superoxide anion in live cells. Anal. Chem. 2017, 89, 7210–7215. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Li, Z.; Liu, W.; Deng, T.; Li, J. Photoactivatable red chemiluminescent AIEgen probe for in vitro/vivo imaging assay of hydrazine. Anal. Chem. 2021, 93, 10601–10610. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, C.; Guo, Z.; Liu, X.; Zhu, W.H. A sequential dual-lock strategy for photoactivatable chemiluminescent probes enabling bright duplex optical imaging. Angew. Chem. Int. Ed. 2020, 59, 9059–9066. [Google Scholar] [CrossRef]
- Wu, H.; Fang, Y.; Tian, L.; Liu, X.; Zhou, X.; Chen, X.; Gao, H.; Qin, H.; Liu, Y. AIE nanozyme-based long persistent chemiluminescence and fluorescence for POCT of pathogenic bacteria. ACS Sens. 2023, 8, 3205–3214. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Liu, J.; Yue, Q.; Chen, S.; Lam, J.W.Y.; Luo, L.; Tang, B.Z. Near-infrared AIE dots with chemiluminescence for deep-tissue imaging. Adv. Mater. 2020, 32, e2004685. [Google Scholar] [CrossRef]
- Shen, H.; Sun, F.; Zhu, X.; Zhang, J.; Ou, X.; Zhang, J.; Xu, C.; Sung, H.H.Y.; Williams, I.D.; Chen, S.; et al. Rational design of NIR-II AIEgens with ultrahigh quantum yields for photo- and chemiluminescence imaging. J. Am. Chem. Soc. 2022, 144, 15391–15402. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, H.; Sun, S.; Qin, C.; Qiu, Q.; Feng, Y.; Liu, Y.; Li, Y.; Xu, L.; Ying, Y.; et al. Self-illuminating NIR-II chemiluminescence nanosensor for in vivo tracking H2O2 fluctuation. Adv. Sci. 2023, 10, e2207651. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Lu, L.; Zhang, Q.; Yu, P.; Fan, Y.; Zhang, F. NIR-II chemiluminescence molecular sensor for in vivo high-contrast inflammation imaging. Angew. Chem. Int. Ed. 2020, 59, 18380–18385. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, W.; Zheng, Y.; Ding, Y.; Xiang, Y.; Liu, B.; Tong, A. Organic nanoparticles with persistent luminescence for in vivo afterglow imaging-guided photodynamic therapy. Chem. Eur. J. 2021, 27, 6911–6916. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Zhang, X.; Duan, X.; Zheng, H.L.; Xue, X.S.; Ding, D. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 2019, 19, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.Q.; Zhang, Y.; Tang, Y.; Ding, D.; Li, W.; Liu, Q. Building highly light-harvesting near-infrared AIEgens using triazole-based luminescent core for improved intravital afterglow imaging. Adv. Funct. Mater. 2023, 33, 2212380. [Google Scholar] [CrossRef]
- Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hu, F.; Kong, D.; Ding, D.; Liu, B. Chemiluminescence-guided cancer therapy using a chemiexcited photosensitizer. Chem 2017, 3, 991–1007. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Yang, P.; Mao, D.; Liu, B. Near-infrared chemiluminescent nanoprobes for deep imaging and synergistic photothermal-nitric-oxide therapy of bacterial infection. Biomaterials 2022, 288, 121693. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Segawa, S.; He, X.; Tang, B.Z. Metal-free click and bioorthogonal reactions of aggregation-induced emission probes for lighting up living systems. Luminescence 2024, 39, e4619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Kwok, R.T.; Leung, C.W.; Lam, J.W.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Wang, D.; Tang, B.Z. Aggregation-induced emission luminogens for activity-based sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-induced emission: Recent advances in materials and biomedical applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, J.; Huang, J.; Yu, B.; Pu, K.; Xu, F.J. Chemiluminescence: From mechanism to applications in biological imaging and therapy. Aggregate 2021, 2, e140. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, W.; Guan, W.; Lu, C.; Tang, B.Z. Screening of photosensitizers by chemiluminescence monitoring of formation dynamics of singlet oxygen during photodynamic therapy. Anal. Chem. 2016, 88, 9707–9713. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Tseng, J.C.; Rochford, J. A bodipy-luminol chemiluminescent resonance energy-transfer (CRET) cassette for imaging of cellular superoxide. Org. Biomol. Chem. 2015, 13, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Tang, X.; Zhang, H.; Guan, W.; Lu, C. Chemiluminescence resonance energy transfer efficiency and donor-acceptor distance: From qualitative to quantitative. Angew. Chem. Int. Ed. 2021, 60, 13029–13034. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, G.; Wang, S.; Liu, K.; Hao, J. Mitigation options of atmospheric hg emissions in china. Environ. Sci. Technol. 2018, 52, 12368–12375. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Paul, S.; Roy, P.; Rayalu, S. Detection of cyanide ion by chemosensing and fluorosensing technology. Inorg. Chem. Commun. 2021, 128, 108562. [Google Scholar] [CrossRef]
- Peng, T.; Li, S.; Zhou, Y.; Liu, R.; Qu, J. Two cyanoethylene-based fluorescence probes for highly efficient cyanide detection and practical applications in drinking water and living cells. Talanta 2021, 234, 122615. [Google Scholar] [CrossRef]
- Mu, S.; Gao, H.; Li, C.; Li, S.; Wang, Y.; Zhang, Y.; Ma, C.; Zhang, H.; Liu, X. A dual-response fluorescent probe for detection and bioimaging of hydrazine and cyanide with different fluorescence signals. Talanta 2021, 221, 121606. [Google Scholar] [CrossRef]
- Guo, Z.; Niu, Q.; Yang, Q.; Li, T.; Chi, H. A highly selective and sensitive dual-mode sensor for colorimetric and turn-on fluorescent detection of cyanide in water, agro-products and living cells. Anal. Chim. Acta 2019, 1065, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Lan, L.; Li, T.; Guo, Z.; Jiang, T.; Zhao, Z.; Feng, Z.; Xi, J. A highly selective turn-on fluorescent and naked-eye colorimetric sensor for cyanide detection in food samples and its application in imaging of living cells. Sens. Actuators B Chem. 2018, 276, 13–22. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- He, X.; Lam, J.W.Y.; Kwok, R.T.K.; Tang, B.Z. Real-time visualization and monitoring of physiological dynamics by aggregation-induced emission luminogens (AIEgens). Annu. Rev. Anal. Chem. 2021, 14, 413–435. [Google Scholar] [CrossRef]
- Sun, J.; He, X. AIE-based drug/gene delivery system: Evolution from fluorescence monitoring alone to augmented therapeutics. Aggregate 2022, 3, e282. [Google Scholar] [CrossRef]
- de la Rica, R.; Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.-H.; Huang, S.; Huang, Y.; Yin, F.; Yang, F.; Zhang, Q.; Cheng, J.; Zhang, R.; He, X. Ultrasensitive visualization of virus via explosive catalysis of an enzyme muster triggering gold nano-aggregate disassembly. ACS Appl. Mater. Interfaces 2020, 12, 12525–12532. [Google Scholar] [CrossRef]
- Xiong, L.-H.; Cui, R.; Zhang, Z.-L.; Yu, X.; Xie, Z.; Shi, Y.-B.; Pang, D.-W. Uniform fluorescent nanobioprobes for pathogen detection. ACS Nano 2014, 8, 5116–5124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hu, M.; Liu, A.-A.; Lin, Y.; Liu, L.; Yu, B.; Zhou, X.; Pang, D.-W. Detection of sars-cov-2 by crispr/cas12a-enhanced colorimetry. ACS Sens. 2021, 6, 1086–1093. [Google Scholar] [CrossRef]
- He, X.; Zeng, T.; Li, Z.; Wang, G.; Ma, N. Catalytic molecular imaging of microRNA in living cells by DNA-programmed nanoparticle disassembly. Angew. Chem. Int. Ed. 2016, 55, 3073–3076. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Guo, Y.; Lu, S.; Du, Y.; Li, J.-J.; Zhang, X.; Leung, N.L.C.; Zhao, Z.; Niu, G.; et al. Phage-guided targeting, discriminative imaging, and synergistic killing of bacteria by AIE bioconjugates. J. Am. Chem. Soc. 2020, 142, 3959–3969. [Google Scholar] [CrossRef]
- Lam, K.W.K.; Chau, J.H.C.; Yu, E.Y.; Sun, F.; Lam, J.W.Y.; Ding, D.; Kwok, R.T.K.; Sun, J.; He, X.; Tang, B.Z. An alkaline phosphatase-responsive aggregation-induced emission photosensitizer for selective imaging and photodynamic therapy of cancer cells. ACS Nano 2023, 17, 7145–7156. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, G.; Shen, Y.; Guo, N.; Ma, N. DNA-templated magnetic nanoparticle-quantum dot polymers for ultrasensitive capture and detection of circulating tumor cells. Adv. Funct. Mater. 2018, 28, 1707152. [Google Scholar] [CrossRef]
- Yue, R.; Chen, M.; Ma, N. Dual microRNA-triggered drug release system for combined chemotherapy and gene therapy with logic operation. ACS Appl. Mater. Interfaces 2020, 12, 32493–32502. [Google Scholar] [CrossRef]
- Yue, R.; Li, Z.; Wang, G.; Li, J.; Ma, N. Logic sensing of microRNA in living cells using DNA-programmed nanoparticle network with high signal gain. ACS Sens. 2019, 4, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, Z.; Wang, G.; Ma, N. Photocaged nanoparticle sensor for sensitive microRNA imaging in living cancer cells with temporal control. ACS Sens. 2018, 3, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.-H.; Tu, J.-W.; Zhang, Y.-N.; Yang, L.-L.; Cui, R.; Zhang, Z.-L.; Pang, D.-W. Designer cell-self-implemented labeling of microvesicles in situ with the intracellular-synthesized quantum dots. Sci. China Chem. 2020, 63, 448–453. [Google Scholar] [CrossRef]
- Zhang, L.; Jean, S.R.; Li, X.; Sack, T.; Wang, Z.; Ahmed, S.; Chan, G.; Das, J.; Zaragoza, A.; Sargent, E.H.; et al. Programmable metal/semiconductor nanostructures for mRNA-modulated molecular delivery. Nano Lett. 2018, 18, 6222–6228. [Google Scholar] [CrossRef]
- Li, D.; He, W.; Lin, X.; Cui, X.; Nagl, S.; Wu, A.R.; Kwok, R.T.K.; Wu, R.; Tang, B.Z. Enzyme-free photothermally amplified fluorescent immunosorbent assay (PAFISA) for sensitive cytokine quantification. Aggregate 2023, 4, e384. [Google Scholar] [CrossRef]
- Wang, D.; Lee, M.M.S.; Xu, W.; Shan, G.; Zheng, X.; Kwok, R.T.K.; Lam, J.W.Y.; Hu, X.; Tang, B.Z. Boosting non-radiative decay to do useful work: Development of a multi-modality theranostic system from an AIEgen. Angew. Chem. Int. Ed. 2019, 58, 5628–5632. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, L.; Ji, J.; Li, Y.; Liu, T.; Lei, J. Cleancap-regulated aggregation-induced emission strategy for highly specific analysis of enzyme. Anal. Chem. 2020, 92, 4726–4730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, B.; Huang, Y.; Gu, Y.; Yan, J.; Chen, J.; Li, C.; Zhang, Y.; Wong, S.H.D.; Yang, M. A dual “turn-on” biosensor based on AIE effect and FRET for in situ detection of miR-125b biomarker in early Alzheimer’s disease. Biosens. Bioelectron. 2023, 230, 115270. [Google Scholar] [CrossRef] [PubMed]
- Garrod, S.; Bollard, M.E.; Nicholls, A.W.; Connor, S.C.; Connelly, J.; Nicholson, J.K.; Holmes, E. Integrated metabonomic analysis of the multiorgan effects of hydrazine toxicity in the rat. Chem. Res. Toxicol. 2004, 18, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Frazier, J.M. Involvement of apoptosis in hydrazine induced toxicity in rat primary hepatocytes. Toxicol. Vitr. 2003, 17, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, M.; Li, X.; Jia, X.; Wang, X.; Zhang, P.; Wei, C.; Li, X. A hepatocyte-targeting fluorescent probe for imaging isoniazid-induced hydrazine in HEPG2 cells and zebrafish. Chem. Commun. 2020, 56, 14183–14186. [Google Scholar] [CrossRef]
- Bai, H.; He, W.; Chau, J.H.C.; Zheng, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIEgens for microbial detection and antimicrobial therapy. Biomaterials 2021, 268, 120598. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, F. NIR luminescent nanomaterials for biomedical imaging. J. Mater. Chem. B 2014, 2, 2422–2443. [Google Scholar] [CrossRef] [PubMed]
- Epps, S.V.; Harvey, R.B.; Hume, M.E.; Phillips, T.D.; Anderson, R.C.; Nisbet, D.J. Foodborne campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health 2013, 10, 6292–6304. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Gammon, S.T.; Moss, B.L.; Rauch, D.; Harding, J.; Heinecke, J.W.; Ratner, L.; Piwnica-Worms, D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. [Google Scholar] [CrossRef] [PubMed]

| Advantages | Reference | |
|---|---|---|
| 1 | Fast, sensitive, and selective detection of Hg2+; a linear detection range of 0.005–10 μg mL−1 and limit of detection (LOD) of 3 ng mL−1 | [30] |
| 2 | Detection range of 2.5–125 μg/L CN− with a LOD down to 0.55 μg/L; high sensitivity, high selectivity, and anti-interference capability | [31] |
| 3 | Detection range of NaNO2 from 1.0 to 100 μM with a LOD as low as 0.5 μM; recovery was as high as 98−106% and accuracy was good | [36] |
| 4 | Remarkably enhanced CL signals and faster reaction rate | [4] |
| 5 | Low cytotoxicity and good animal compatibility; high energy transfer efficiency; high CL amplification; LOD was as low as 4.6 × 10−9 M | [5] |
| 6 | Highly sensitive to O2•− with LOD of 0.21 nM for FL and 0.38 nM for CL | [6] |
| 7 | Simplicity, good specificity, and sensitivity for the detection of hydrazine; good stability and photoactivity; LOD down to 0.18 µM (5.72 ppb) | [7] |
| 8 | Stimuli-controlled, bright, and enriched CL signals with advantages in stability, brightness, and imaging flexibility | [8] |
| 9 | Long persistent luminescence; strong CL intensity; excellent capability of ROS generation; good anti-interference capability; outstanding stability; free of H2O2 and external light sources; high detection accuracy | [9] |
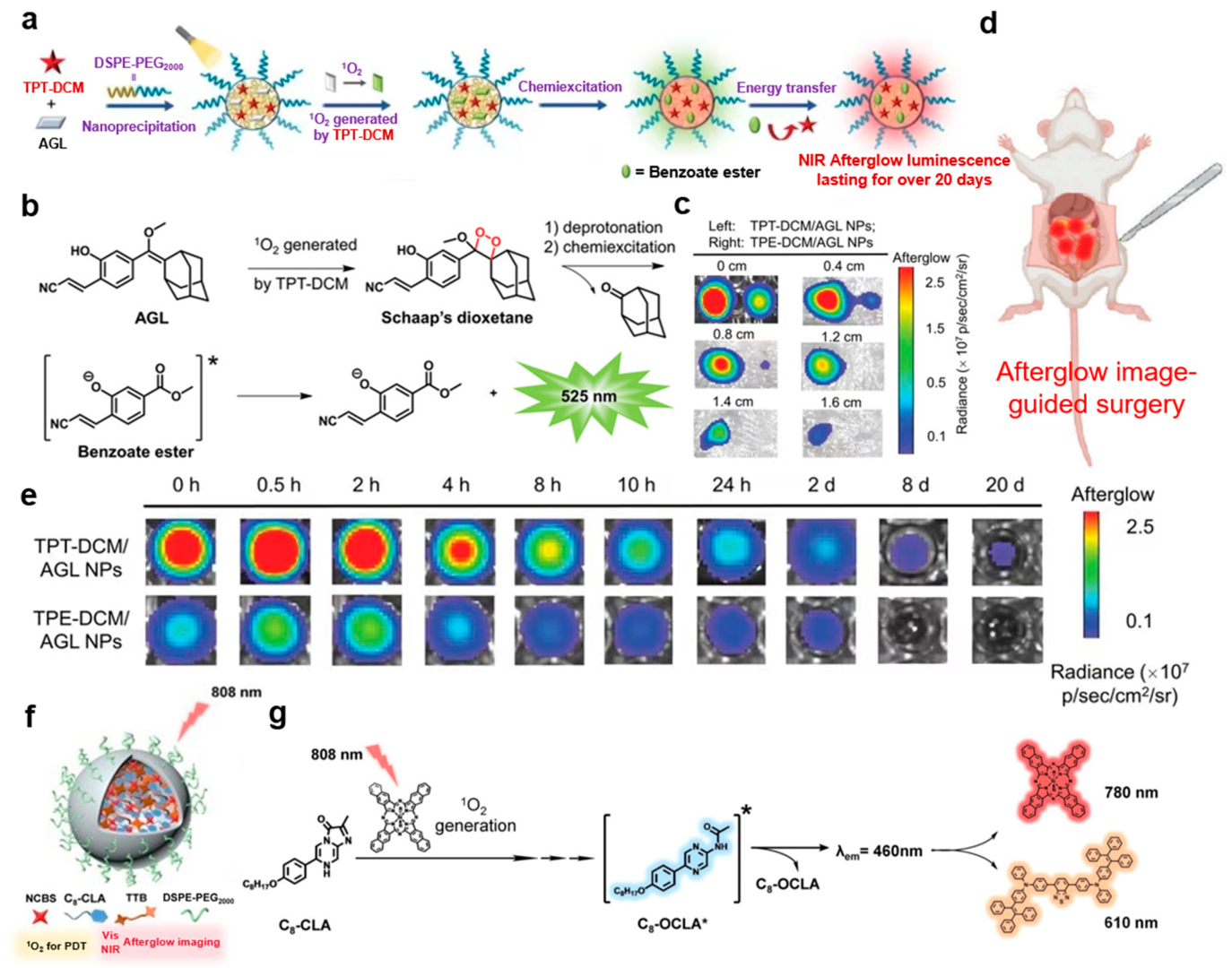
| 10 | High NIR afterglow luminescence persisting over 10 days; deeper tissue penetration; ultrahigh tumor-to-liver signal ratio; low afterglow background noise | [15] |
| 11 | High molar extinction coefficient; good brightness; excellent reactive oxygen species generation rate; ultralong NIR afterglow luminescence (up to 20 days); ultrahigh tumor-to-liver signal ratio (up to 187-fold) | [16] |
| 12 | Persistent luminescence; good biocompatibility; high SBR; good tissue penetration; abundant singlet oxygen generation | [14] |
| 13 | High NIR CL emission; tissue penetration depth of over 3 cm | [10] |
| 14 | Ultrahigh relative QYs; high signal-to-background ratio; high energy transfer efficiency; excellent continuous imaging | [11] |
| 15 | Excellent selectivity; high sensitivity to hydrogen peroxide; long-lasting luminescence performance; high signal-to-background ratio | [12] |
| 16 | Deep penetration depth; high signal-to-background ratio; large Stokes shift (>100 nm) and extremely high FRET efficiency (94.12%) | [13] |
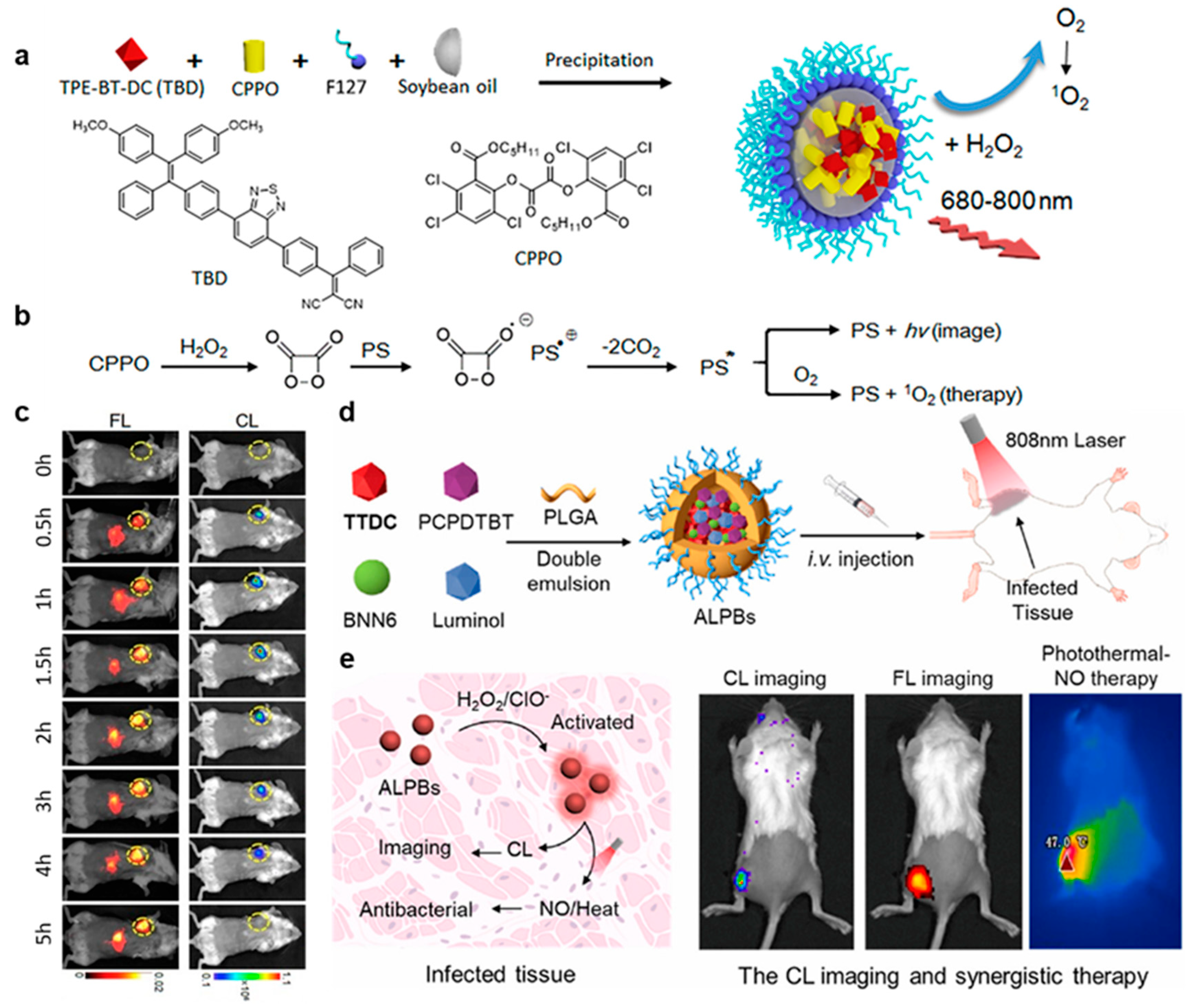
| 17 | Bright FR/NIR self-luminescence and significant 1O2 production in the presence of H2O2 | [17] |
| 18 | Excellent photothermal conversion efficiency; simultaneous CL and photothermal–NO therapy for deep tissue infection | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; He, X. Aggregation-Induced Emission-Based Chemiluminescence Systems in Biochemical Analysis and Disease Theranostics. Molecules 2024, 29, 983. https://doi.org/10.3390/molecules29050983
Shi Y, He X. Aggregation-Induced Emission-Based Chemiluminescence Systems in Biochemical Analysis and Disease Theranostics. Molecules. 2024; 29(5):983. https://doi.org/10.3390/molecules29050983
Chicago/Turabian StyleShi, Yixin, and Xuewen He. 2024. "Aggregation-Induced Emission-Based Chemiluminescence Systems in Biochemical Analysis and Disease Theranostics" Molecules 29, no. 5: 983. https://doi.org/10.3390/molecules29050983
APA StyleShi, Y., & He, X. (2024). Aggregation-Induced Emission-Based Chemiluminescence Systems in Biochemical Analysis and Disease Theranostics. Molecules, 29(5), 983. https://doi.org/10.3390/molecules29050983






