Construction of a New Probe Based on Copper Chaperone Protein for Detecting Cu2+ in Cells
Abstract
1. Introduction
2. Results
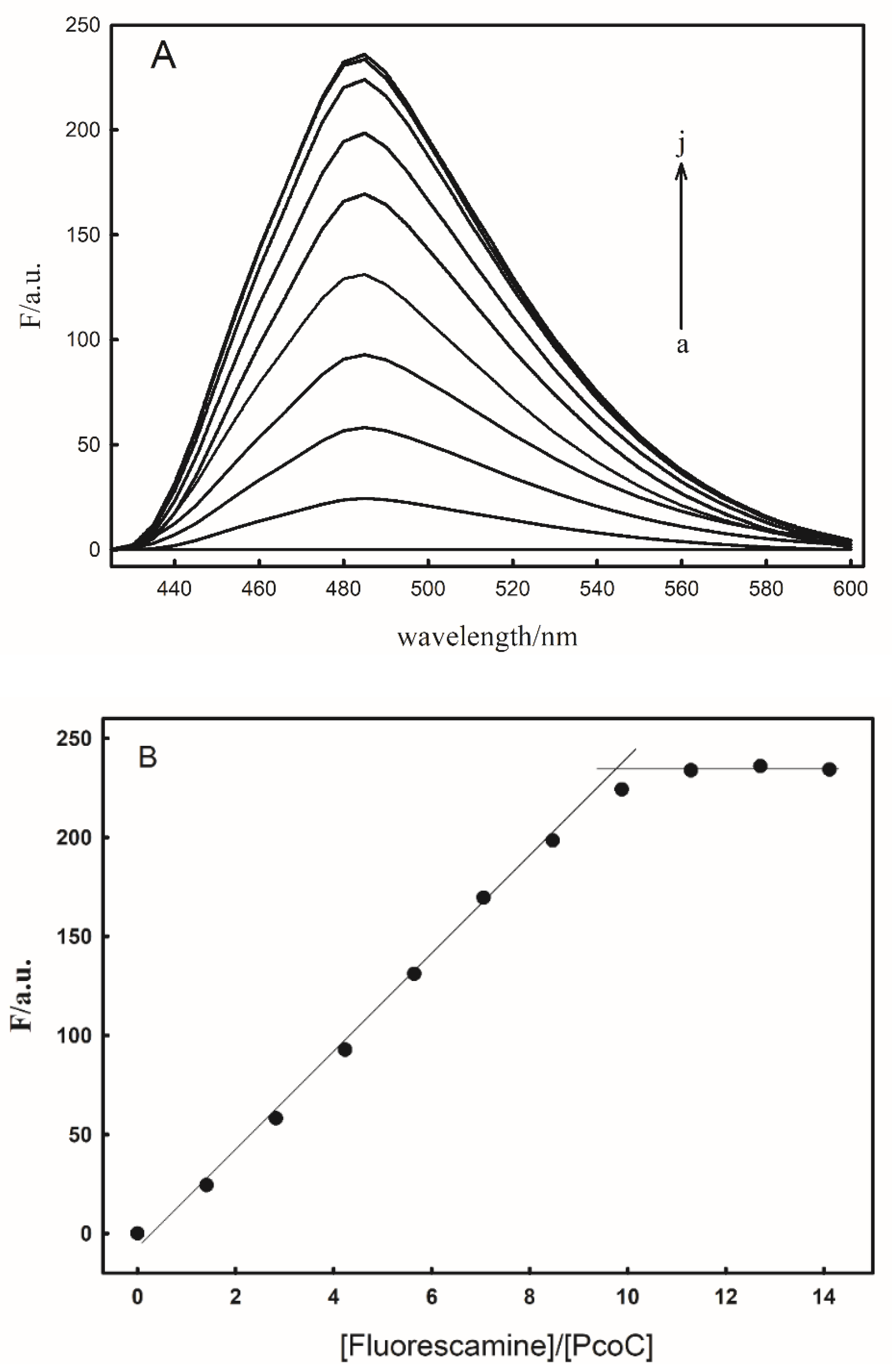
2.1. Construction of the Probe FP
2.2. Optimization of Reaction Conditions
2.2.1. The Best Reaction pH
2.2.2. The Best Reaction Time
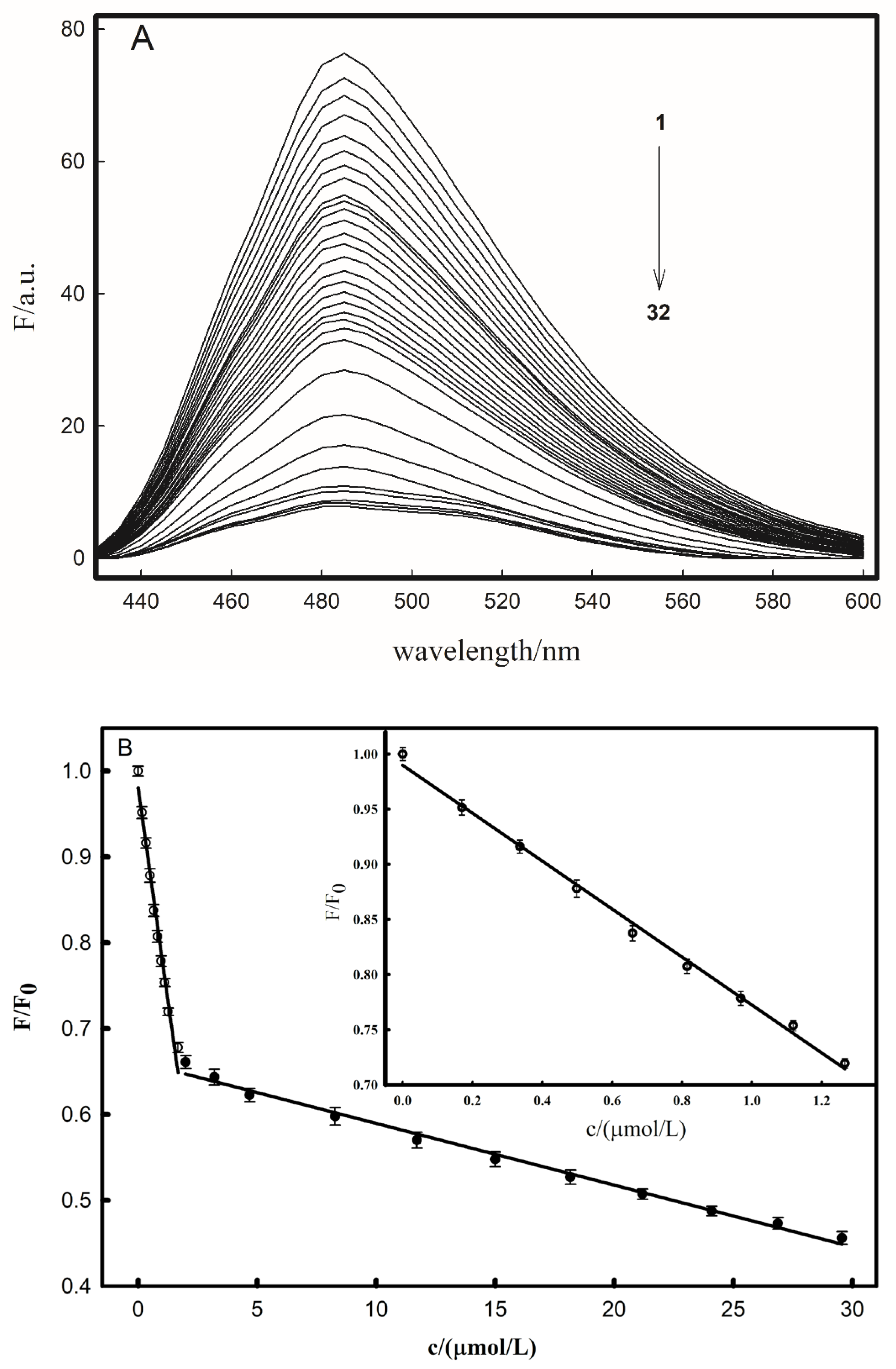
2.3. Interaction between Fluorescent Probe FP and Cu2+
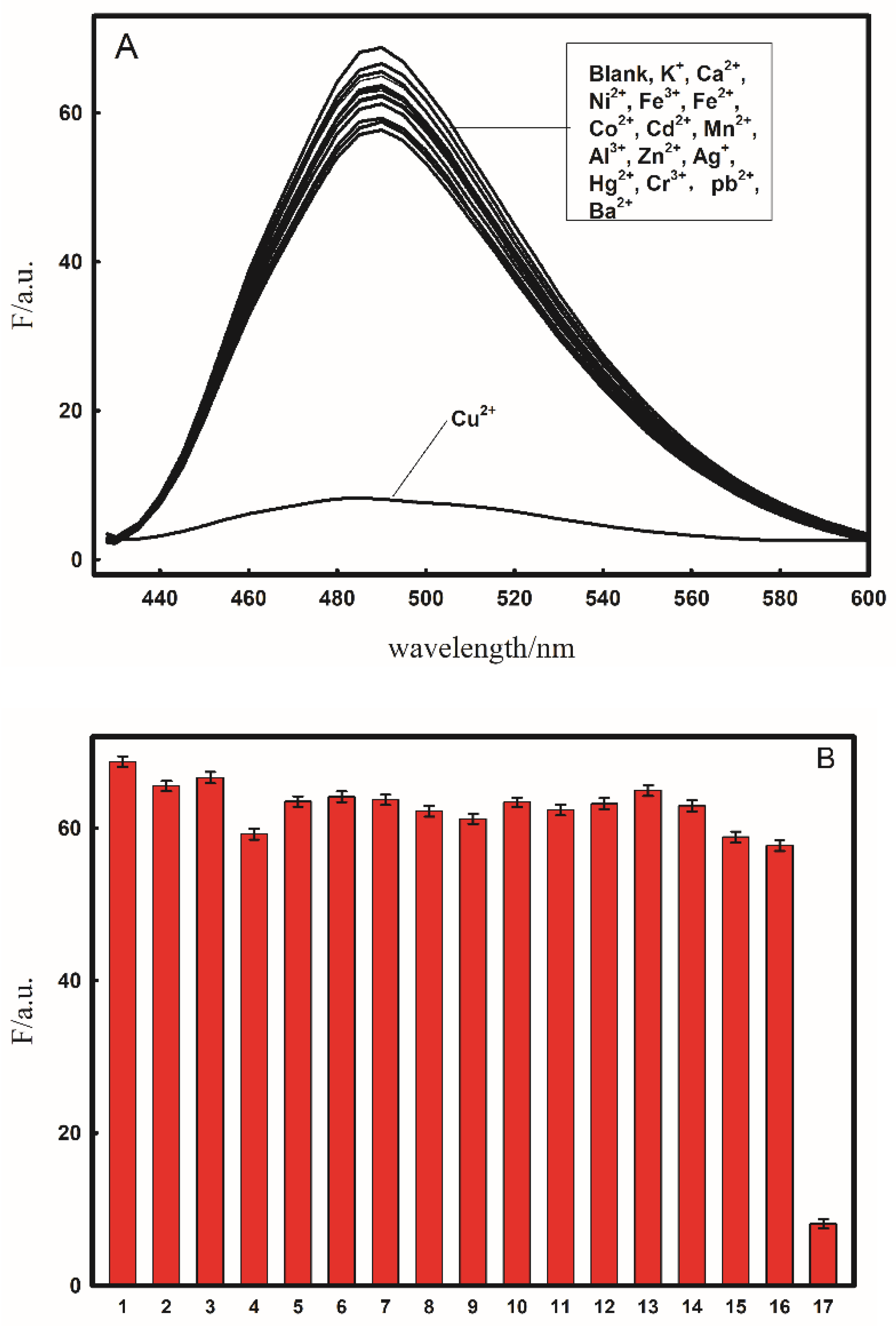
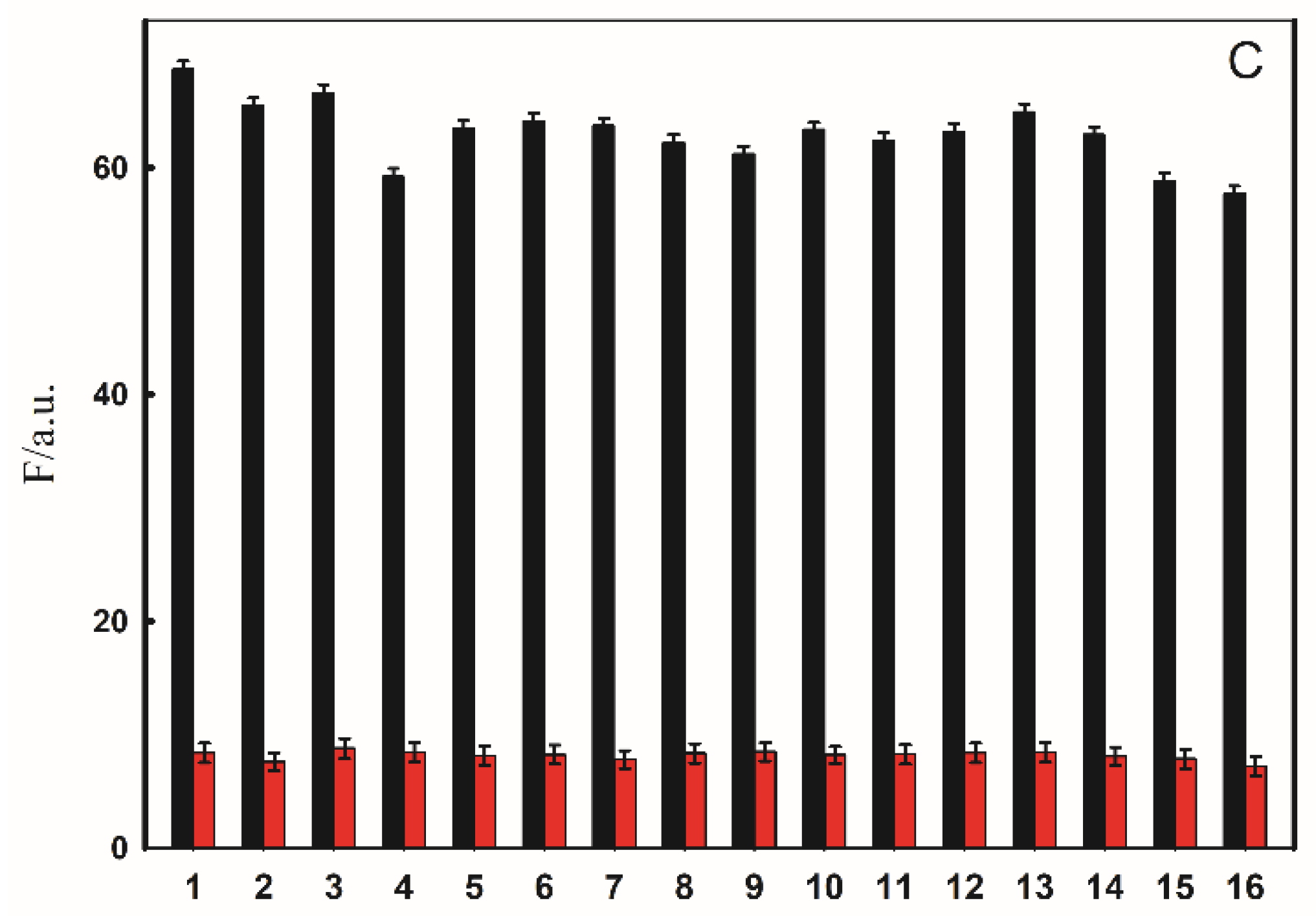
2.4. Selectivity of Fluorescent Probe for Cu2+
2.5. Cytotoxicity Study
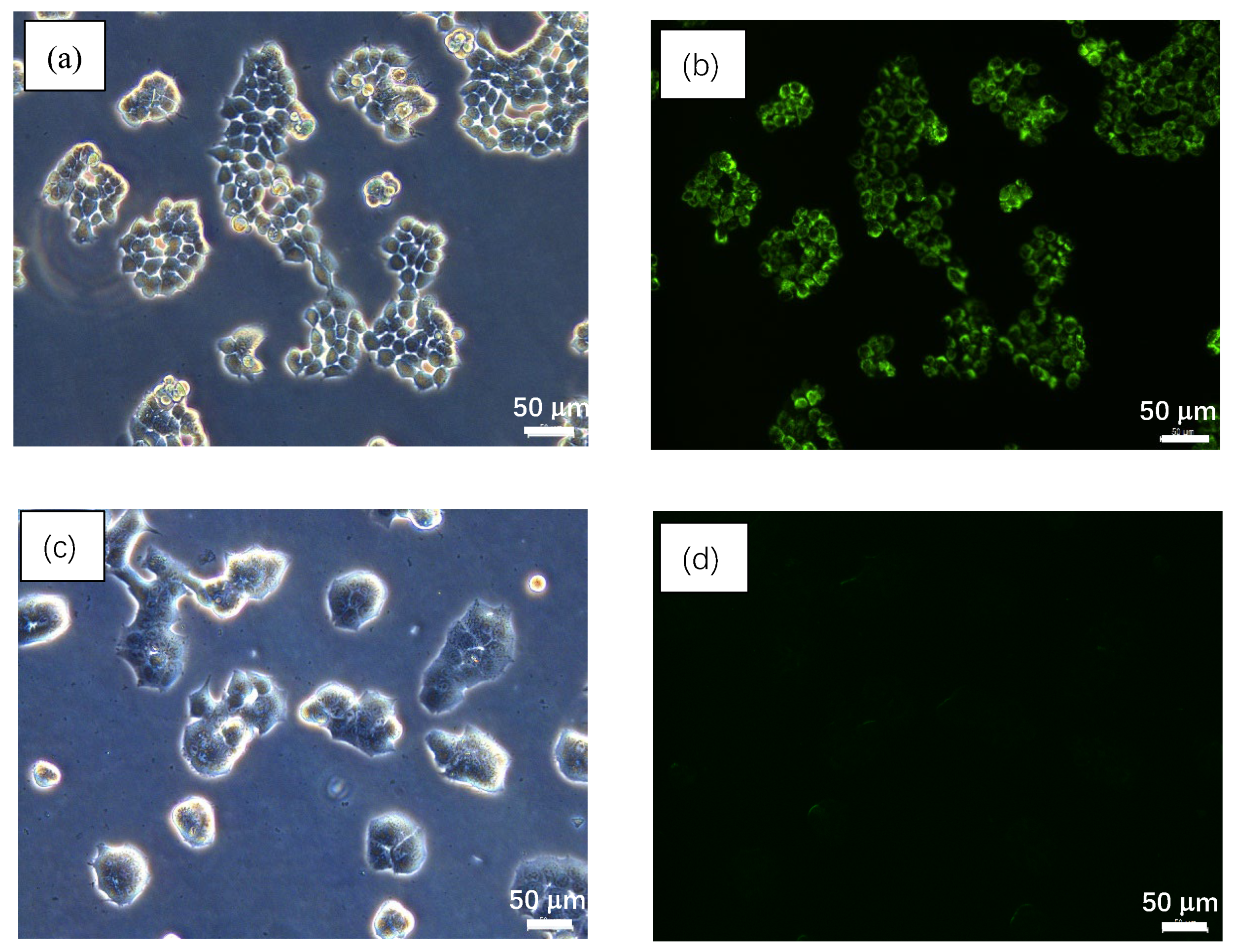
2.6. Cell Imaging Study
3. Discussion
4. Experimental Section
4.1. Materials and Instruments
4.2. Expression and Purification of PcoC
4.2.1. PcoC Protein Expression
4.2.2. Purification of the PcoC Protein
4.3. Preparation of Probe Fluorescamine-PcoC (FP)
4.4. Fluorescence Spectrum Analysis
4.5. Selection of Optimal pH
4.6. Selection of Reactive Time
4.7. Cytotoxicity
4.8. Cell Imaging
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abeywickrama, C.S. Large Stokes shift benzothiazolium cyanine dyes with improved intramolecular charge transfer (ICT) for cell imaging applications. Chem. Commun. 2022, 58, 9855–9869. [Google Scholar] [CrossRef]
- Ren, Y.W.; Cao, L.L.; Zhang, X.Y.; Jiao, R.; Ou, D.X.; Wang, Y.; Zhang, D.F.; Shen, Y.Z.; Ling, N.; Ye, Y.W. A novel fluorescence resonance energy transfer (FRET)-based paper sensor with smartphone for quantitative detection of Vibrio parahaemolyticus. Food Control 2023, 145, 109412. [Google Scholar] [CrossRef]
- Niu, H.Y.; Liu, J.W.; O’Connor, H.M.; Gunnlaugsson, T.; James, T.D.; Zhang, H. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev. 2023, 52, 2322–2357. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.J.; Chen, J.; Liu, W.J.; Wang, C.; Qi, Q.K.; Qiao, Q.L.; Tan, T.M.; Xiong, K.M.; Liu, X.; Kang, K.; et al. A general descriptor ΔE enables the quantitative development of luminescent materials based on photoinduced electron transfer. J. Am. Chem. Soc. 2020, 142, 6777–6785. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef]
- Abo, M.; Urano, Y.; Hanaoka, K.; Terai, T.; Komatsu, T.; Nagano, T. Development of a Highly Sensitive Fluorescence Probe for Hydrogen Peroxide. J. Am. Chem. Soc. 2011, 133, 10629–10637. [Google Scholar] [CrossRef]
- Ungati, H.; Govindaraj, V.; Narayanan, M.; Mugesh, G. Probing the Formation of a Seleninic Acid in Living Cells by the Fluorescence Switching of a Glutathione Peroxidase Mimetic. Angew. Chem. Int. Ed. 2019, 58, 8156–8160. [Google Scholar] [CrossRef]
- Cai, Y.L.; Meng, X.M.; Wang, S.X.; Zhu, M.Z.; Pan, Z.W.; Guo, Q.X. A quinoline based fluorescent probe that can distinguish zinc (II) from cadmium (II) in water. Tetrahedron Lett. 2013, 54, 1125–1128. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.F.; Ming, Z.Z.; Hao, G.F.; Yang, W.C.; Yang, G.F. Coumarin-based novel fluorescent zinc ion probe in aqueous solution. Tetrahedron 2013, 69, 4743–4748. [Google Scholar] [CrossRef]
- Misra, A.; Shahid, M.; Srivastava, P. Optoelectronic behavior of bischromophoric dyads exhibiting Zn2+/F− ions induced “turn-On/Off” fluorescence. Sens. Actuators B 2012, 169, 327–340. [Google Scholar] [CrossRef]
- Ast, S.; Rutledge, P.J.; Todd, M.H. Reversing the Triazole Topology in a Cyclam-Triazole-Dye Ligand Gives a 10-Fold Brighter Signal Response to Zn2+ in Aqueous Solution. Eur. J. Inorg. Chem. 2012, 34, 5611–5615. [Google Scholar] [CrossRef]
- Domaille, D.W.; Que, E.L.; Chang, C.J. Synthetic fluorescent sensors for studying the cell biology of metals. Nat. Chem. Biol. 2008, 4, 168–175. [Google Scholar] [CrossRef]
- Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M. New fluorescent chemosensors for metal ions in solution. Coord. Chem. Rev. 2012, 256, 170–192. [Google Scholar] [CrossRef]
- Cankorur-Cetinkaya, A.; Eraslan, S.; Kirdar, B. Transcriptional remodelling in response to changing copper levels in the Wilson and Menkes disease model of Saccharomyces cerevisiae. Mol. Biosyst. 2013, 9, 2889–2908. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, D.J.; Bartnikas, T.B.; Gitlin, J.D. The role of copper in neurodegenerative disease. Neurobiol. Dis. 1999, 6, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, L.I.; Miller, T.M.; Cleveland, D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004, 27, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.B.; Nielsen, P.B.; Boe-Hansen, R.; Albrechtsen, H.J. Copper deficiency can limit nitrification in biological rapid sand filters for drinking water production. Water Res. 2016, 95, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo, J.A., Jr.; Aron, A.T.; Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar] [CrossRef]
- Ramos-Torres, K.M.; Kolemen, S.; Chang, C.J. Thioether coordination chemistry for molecular imaging of copper in biological systems. Isr. J. Chem. 2016, 56, 724–737. [Google Scholar] [CrossRef]
- Bou, R.; Guardiola, F.; Padró, A.; Pelfort, E.; Codony, R. Validation of mineralisation procedures for the determination of selenium, zinc, iron and copper in chicken meat and feed samples by ICP-AES and ICP-MS. J. Anal. At. Spectrom. 2004, 19, 1361–1369. [Google Scholar] [CrossRef]
- Wang, Z.P.; Wang, X.; Wang, Q.; Xiong, X.L.; Luo, H.; Huang, K. Recent developments in chemical vapor generation atomic spectrometry for zinc detection. Microchem. J. 2019, 149, 104052. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, F.; Zhong, Y.H.; Zhu, L.H.; Huang, Z.X.; Zhou, Z.; Zou, J.F.; Zeng, J.G.; Zhu, Z.; Yang, Z. A home-made sampling system coupled to hectowatt-MPT mass spectrometry in positive ion mode to confirm target ions of copper and zinc from Poyang Lake, China. Anal. Bioanal. Chem. 2022, 414, 6115–6126. [Google Scholar] [CrossRef]
- Fan, C.; Lv, X.; Liu, F.; Feng, L.; Liu, M.; Cai, Y.; Liu, H.; Wang, J.; Yang, Y.; Wang, H. Silver Nanoclusters Encapsulated into Metal-Organic Frameworks with Enhanced Fluorescence and Specific Ion Accumulation toward the Microdot Array-Based Fluorimetric Analysis of Copper in Blood. ACS Sens. 2018, 3, 441–450. [Google Scholar] [CrossRef]
- Wu, W.; Chen, A.; Tong, L.; Qing, Z.; Langone, K.P.; Bernier, W.E.; Jone, W.E., Jr. Facile Synthesis of Fluorescent Conjugated Polyelectrolytes Using Polydentate Sulfonate as Highly Selective and Sensitive Copper(II) Sensors. ACS Sens. 2017, 2, 1337–1344. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Colorimetric Cu2+ Detection with a Ligation DNAzyme and Nanoparticles. Chem. Commun. 2007, 46, 4872–4874. [Google Scholar] [CrossRef] [PubMed]
- Domaille, D.W.; Zeng, L.; Chang, C.J. Visualizing Ascorbate-Triggered Release of Labile Copper within Living Cells Using a Ratiometric Fluorescent Sensor. J. Am. Chem. Soc. 2010, 132, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Ai, K.; Zhang, G.; Li, H.; Lu, L. Dual-Emission Fluorescent Silica Nanoparticle-Based Probe for Ultrasensitive Detection of Cu2+. Anal. Chem. 2011, 83, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ardizzone, A.; Sui, B.; Anzola, M.; Ventosa, N.; Liu, T.; Veciana, J.; Belfield, K.D. Fluorenyl-Loaded Quatsome Nanostructured Fluorescent Probes. ACS Omega 2017, 2, 4112–4122. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Qing, T.; Mao, Z.; He, X.; Wang, K.; Zou, Z.; Shi, H.; He, D. dsDNA-specific Fluorescent Copper Nanoparticles as a “Green” Nano-Dye for Polymerization-Mediated Biochemical Analysis. Chem. Commun. 2014, 50, 12746–12748. [Google Scholar] [CrossRef]
- Pei, H.; Lu, N.; Wen, Y.; Song, S.; Liu, Y.; Yan, H.; Fan, C. A DNA Nanostructure-based Biomolecular Probe Carrier Platform for Electrochemical Biosensing. Adv. Mater. 2010, 22, 4754–4758. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.Y.; Yin, S.H.; Zhang, X.Q.; Hu, J.W.; Zhang, T.; Zhao, G.H. 16-Mer ferritin-like protein templated gold nanoclusters for bioimaging detection of methylmercury in the brain of living mice. Anal. Chim. Acta 2020, 1127, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Chen, H.; Zang, J.C.; Zhang, X.Q.; Zhao, G.H. Re-designing ferritin nanocages for mercuric ion detection. Analyst 2019, 144, 5890–5897. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.Q.; Dai, R.; Hu, P.Y.; Li, Q.Q.; Xiong, X.L.; Huang, K.; Huo, F. A traffic light-type sensitive visual detection of mercury by golden nanoclusters mixed with fluorescein. Microchem. J. 2018, 141, 163–169. [Google Scholar] [CrossRef]
- Singh, R.; Majhi, S.; Sharma, K.; Ali, M.; Sharma, S.; Choudhary, D.; Tripathi, C.S.P.; Guin, D. BSA stabilized copper nanoclusters as a highly sensitive and selective probe for fluorescence sensing of Fe3+ ions, Chem. Phys. Lett. 2021, 787, 139226. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Xiao, Z.G.; Wedd, A.G. Copper resistance in E. coli: The multicopper oxidase PcoA catalyzes oxidation of copper(I) in CuICuII-PcoC. ChemBioChem 2008, 9, 1579–1582. [Google Scholar] [CrossRef]
- Peariso, K.; Huffman, D.L.; Penner-Hahn, J.E.; O’Halloran, T.V. The PcoC copper resistance protein coordinates Cu(I) via novel S-methionine interactions. J. Am. Chem. Soc. 2003, 125, 342–343. [Google Scholar] [CrossRef]
- Perry, R.D.; Bobrov, A.G.; Fetherston, J.D. The role of transition metal transporters for iron, zinc, manganese, and copper in the pathogenesis of Yersinia pestis. Metallomics 2015, 7, 965–978. [Google Scholar] [CrossRef]
- Wernimont, A.K.; Huffman, D.L.; Finney, L.A.; Demeler, B.; O’Halloran, T.V.; Rosenzweig, A.C. Crystal structure and dimerization equilibria of PcoC, a methionine-rich copper resistance protein from Escherichia coli. J. Biol. Inorg. Chem. 2003, 8, 185–194. [Google Scholar] [CrossRef]
- Derayea, S.M.; Samir, E. A review on the use of fluorescamine as versatile and convenient analytical probe. Microchem. J. 2020, 156, 104835. [Google Scholar] [CrossRef]
- Omar, M.A.; Nagy, D.M.; Halim, M.E. Fluorescamine-based fluorophore for spectrofluorimetric determination of heptaminol in human plasma; application to spiked human plasma. Spectrochim. Acta A 2020, 227, 117711. [Google Scholar] [CrossRef]
- Duan, Y.K.; Liu, Y.; Shen, W.; Zhong, W.W. Fluorescamine Labeling for Assessment of Protein Conformational Change and Binding Affinity in Protein-Nanoparticle Interaction. Anal. Chem. 2017, 89, 12160–12167. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Wang, Z.; Xiao, Z.; Fang, C.J. A fluorescent probe for the detection of Cu (II) in water and tumor cells. Inorg. Chem. Commun. 2021, 126, 108471. [Google Scholar] [CrossRef]
- Zhu, A.; Qu, Q.; Shao, X.; Kong, B.; Tian, Y. Carbon-Dot-Based Dual-Emission Nanohybrid Produces a Ratiometric Fluorescent Sensor for In Vivo Imaging of Cellular Copper Ions. Angew. Chem. Int. Ed. 2012, 51, 7185–7189. [Google Scholar] [CrossRef] [PubMed]
- Durgadas, C.V.; Sharma, C.P.; Sreenivasan, K. Fluorescent gold clusters as nanosensors for copper ions in live cells. Analyst 2011, 136, 933–940. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Li, L.; Han, H.; Chen, Y.; Qin, Z.; Song, Z. Construction of a New Probe Based on Copper Chaperone Protein for Detecting Cu2+ in Cells. Molecules 2024, 29, 1020. https://doi.org/10.3390/molecules29051020
Ren J, Li L, Han H, Chen Y, Qin Z, Song Z. Construction of a New Probe Based on Copper Chaperone Protein for Detecting Cu2+ in Cells. Molecules. 2024; 29(5):1020. https://doi.org/10.3390/molecules29051020
Chicago/Turabian StyleRen, Jing, Lin Li, Hongfei Han, Yi Chen, Ziying Qin, and Zhen Song. 2024. "Construction of a New Probe Based on Copper Chaperone Protein for Detecting Cu2+ in Cells" Molecules 29, no. 5: 1020. https://doi.org/10.3390/molecules29051020
APA StyleRen, J., Li, L., Han, H., Chen, Y., Qin, Z., & Song, Z. (2024). Construction of a New Probe Based on Copper Chaperone Protein for Detecting Cu2+ in Cells. Molecules, 29(5), 1020. https://doi.org/10.3390/molecules29051020



