Detection of Arsenic(V) by Fluorescence Sensing Based on Chlorin e6-Copper Ion
Abstract
1. Introduction
2. Results and Discussion
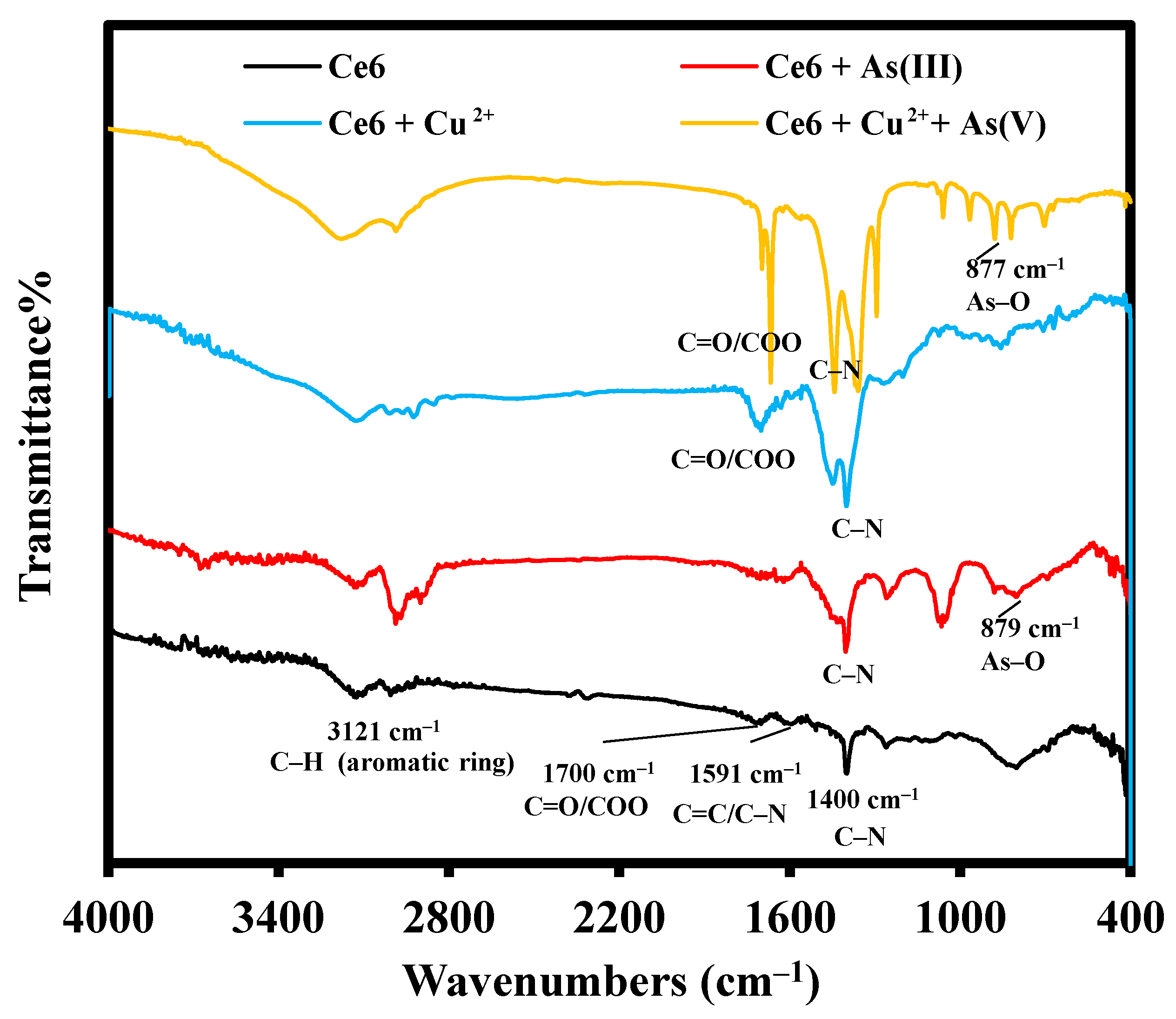
2.1. FT–IR
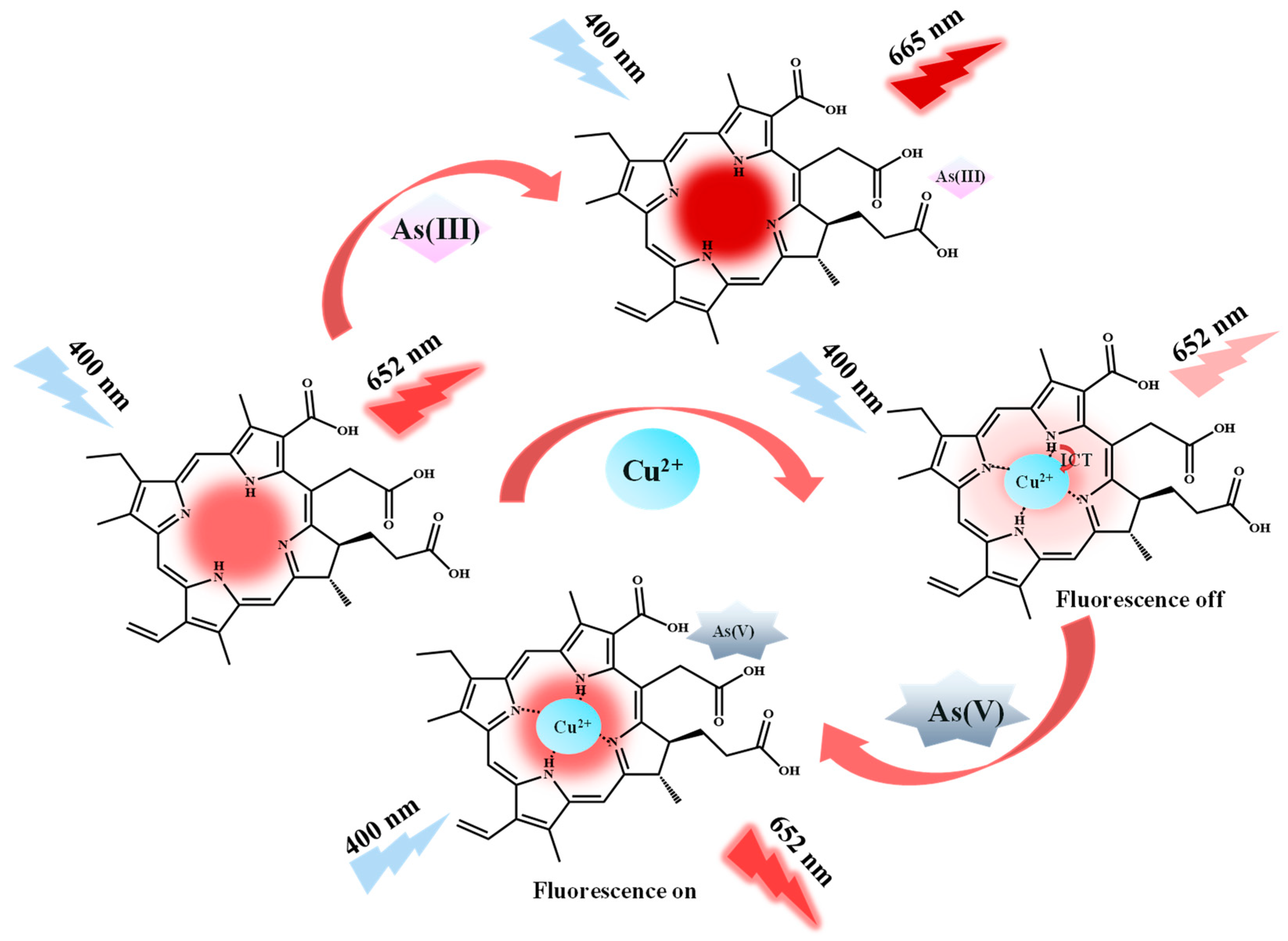
2.2. Feasibility of Fluorescence Detection of As(III), Cu2+, and As(V)
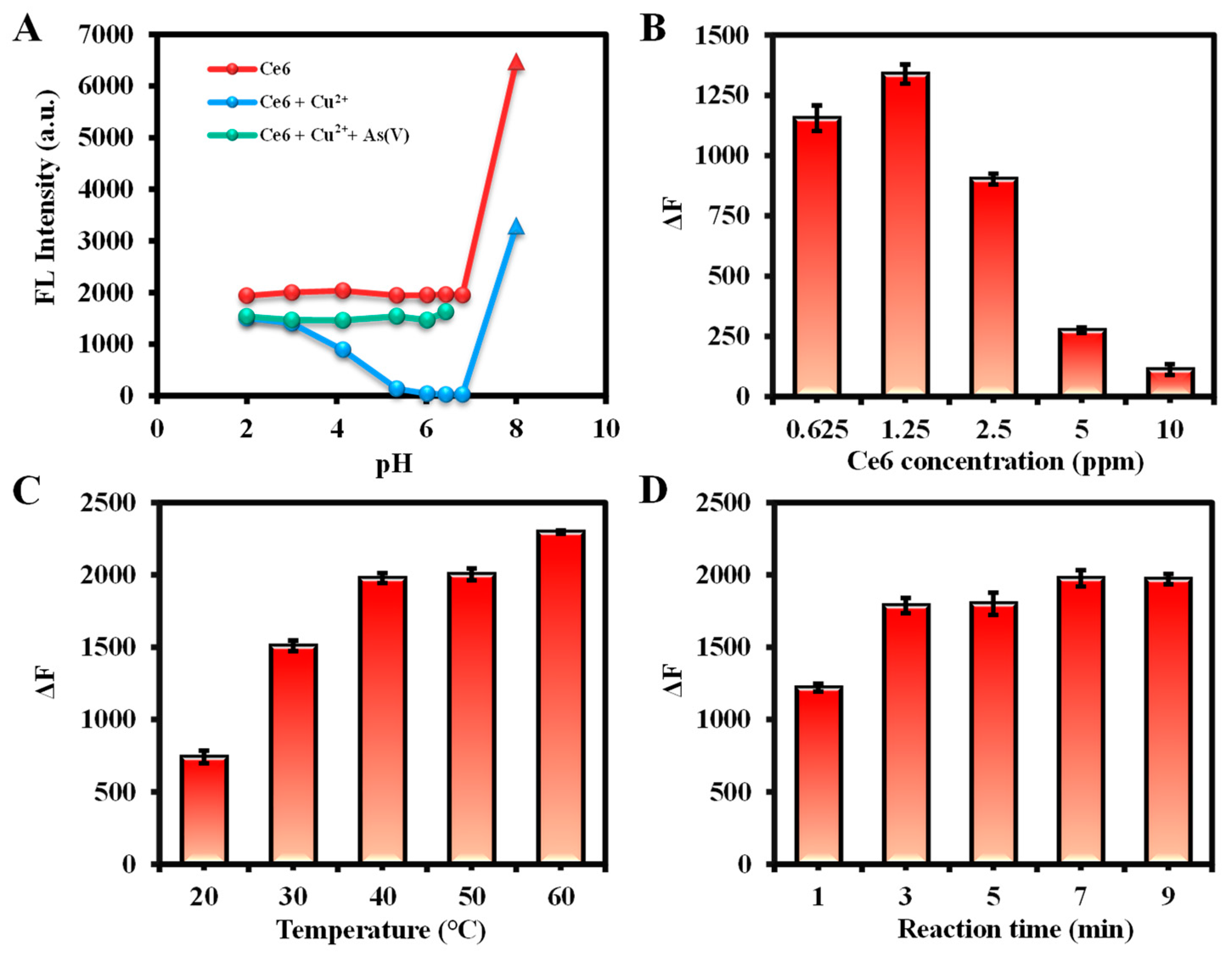
2.3. Optimization of Detection Condition
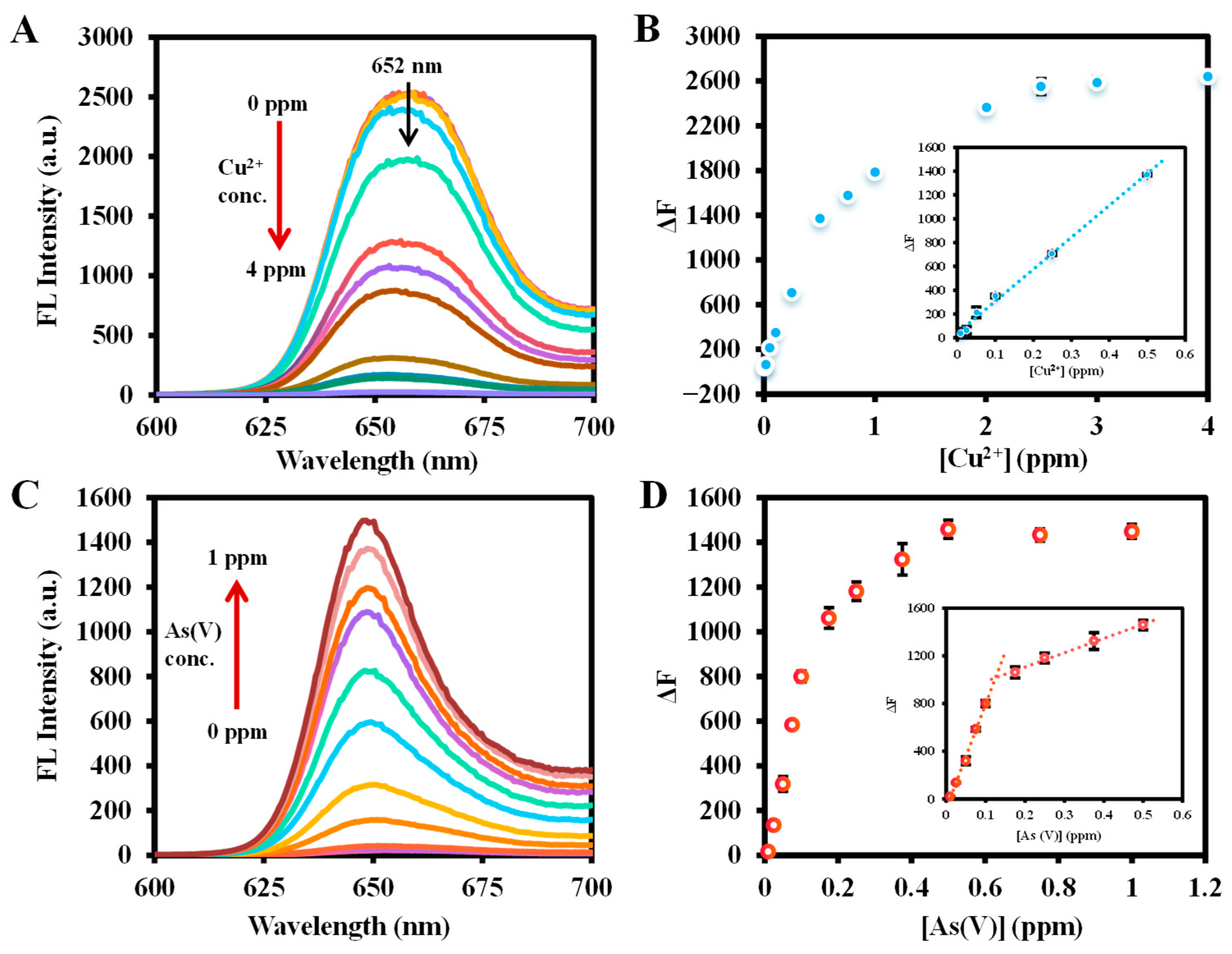
2.4. Sensitivity of Ce6 for As(III), Cu2+, and As(V) Detection
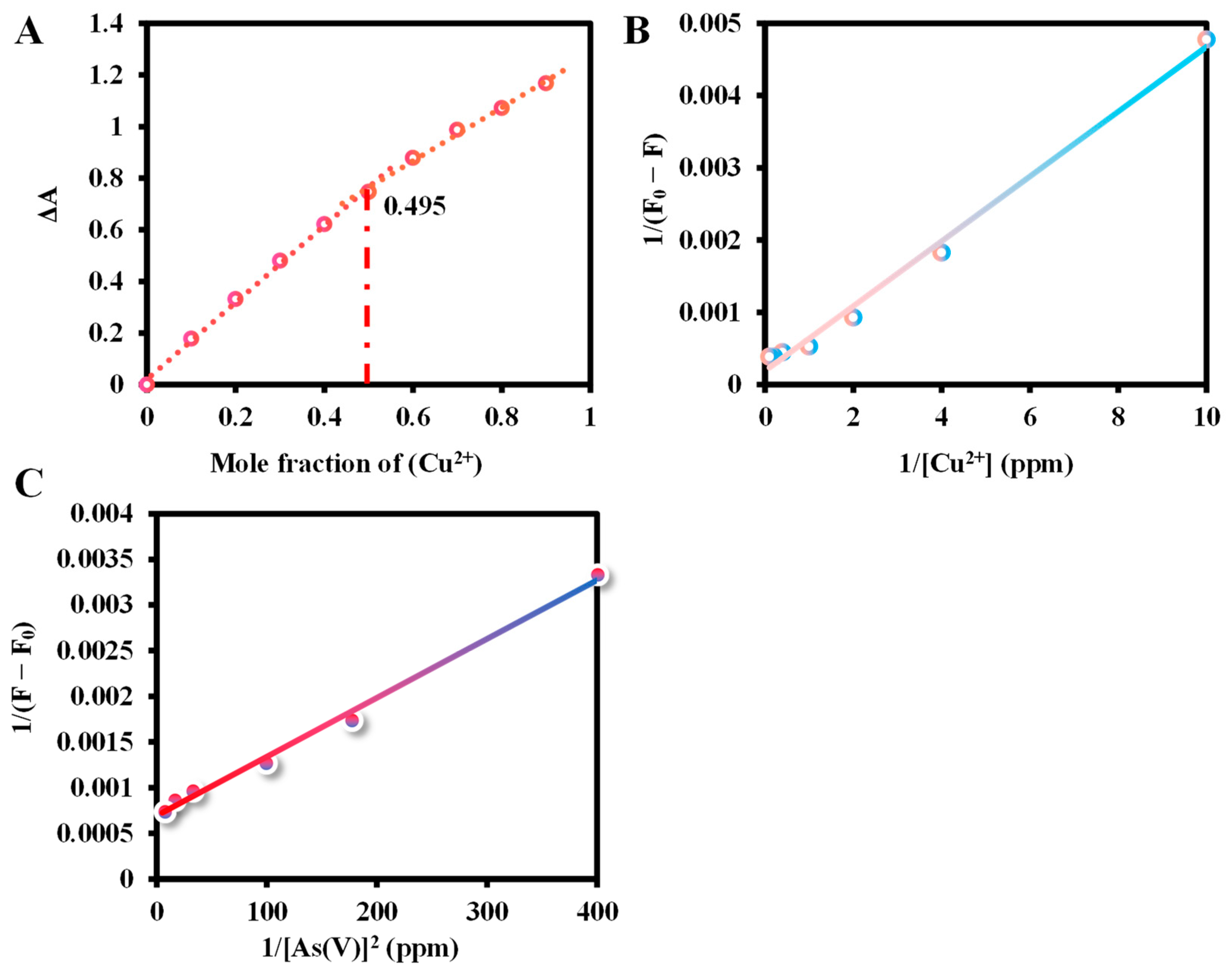
2.5. The Binding Study of Ce6 and Cu2+/As(V)
2.6. Detection Mechanisms
2.7. Interference and Selectivity Study
2.8. Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Fluorescence Detection of As(III) with Ce6
3.3. Fluorescence Detection of Cu2+ with Ce6
3.4. Fluorescence Detection of As(V) with Ce6-Cu2+
3.5. Interference Analysis
3.6. Detection of Cu2+, As(V), and As(III) in Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, N.; Singh, A.K. Dual anion colorimetric and fluorometric sensing of arsenite and cyanide ions. RSC Adv. 2016, 6, 100136–100144. [Google Scholar] [CrossRef]
- Nawarathne, M.; Weerasinghe, R.; Dharmarathne, C. Colorimetric and fluorometric detection of arsenic: Arsenate and arsenite. Anal. Methods Environ. Chem. J. 2023, 6, 29–57. [Google Scholar] [CrossRef]
- Saha, J.; Roy, A.D.; Dey, D.; Nath, J.; Bhattacharjee, D.; Hussain, S.A. Development of arsenic (V) sensor based on fluorescence resonance energy transfer. Sens. Actuator B Chem. 2017, 241, 1014–1023. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, S.; Li, L. Research progress in fluorescent probes for arsenic species. Molecules 2022, 27, 8497. [Google Scholar] [CrossRef]
- Lohar, S.; Sahana, A.; Banerjee, A.; Banik, A.; Mukhopadhyay, S.K.; Sanmartín Matalobos, J.; Das, D. Antipyrine based arsenate selective fluorescent probe for living cell imaging. Anal. Chem. 2013, 85, 1778–1783. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Y.; Liu, W.; Wang, Q. Paradoxical effects of arsenic in the lungs. Environ. Health Prev. 2021, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J. N-methyl-d-aspartate receptors mediate synaptic plasticity impairment of hippocampal neurons due to arsenic exposure. Neuroscience 2022, 498, 300–310. [Google Scholar] [CrossRef]
- Ezeh, V.C.; Harrop, T.C. A sensitive and selective fluorescence sensor for the detection of arsenic (III) in organic media. Inorg. Chem. 2012, 51, 1213–1215. [Google Scholar] [CrossRef]
- Niedzielski, P.; Siepak, M.; Novotny, K. Determination of inorganic arsenic species As (III) and As (V) by high performance liquid chromatography with hydride generation atomic absorption spectrometry detection. Open Chem. 2004, 2, 82–90. [Google Scholar] [CrossRef]
- Guo, X.; Peng, X.; Li, Q.; Mo, J.; Du, Y.; Wang, Z. Ultra-sensitive determination of inorganic arsenic valence by solution cathode glow discharge-atomic emission spectrometry coupled with hydride generation. J. Anal. At. Spectrom. 2017, 32, 2416–2422. [Google Scholar] [CrossRef]
- Corbini, G.; Dreassi, E.; Chiasserini, L.; Girolamo, M.M.; Mellace, P. Determination of copper by AAS in tear fluid of patients with keratoconus. Anal. Biochem. 2021, 623, 114174. [Google Scholar] [CrossRef] [PubMed]
- Alejkovec, Z.; van Elteren, J.T.; Aelih, V.S.; Aala, M.; Corns, W.T. Microanalysis of arsenic in solid samples by laser ablation-atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2017, 32, 299–304. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, M.; Wang, X.; Chen, Y.; Dong, K. Ecological risk assessment and source analysis of heavy metals in the soils of a lead-zinc mining watershed area. Water 2023, 15, 113. [Google Scholar] [CrossRef]
- Fleming, D.E.B.; Nader, M.N.; Foran, K.A.; Groskopf, C.; Reno, M.C.; Ware, C.S.; Tehrani, M.; Guimarães, D.; Parsons, P.J. Assessing arsenic and selenium in a single nail clipping using portable X-ray fluorescence. Appl. Radiat. Isot. 2017, 120, 1–6. [Google Scholar] [CrossRef]
- Li, F.; Lu, A.; Wang, J. Modeling of chromium, copper, zinc, arsenic and lead using portable X-ray fluorescence spectrometer based on discrete wavelet transform. Int. J. Environ. Res. Public Health 2017, 14, 1163. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, F.C.; Nunes, M.A.G.; Duarte, F.A.; Flores, É.M.D.M.; Hanzel, F.B.; Vaz, A.S.; Pozebon, D.; Dressler, V.L. Arsenic speciation analysis in rice milk using LC-ICP-MS. Food Chem. X 2019, 2, 100028. [Google Scholar] [CrossRef] [PubMed]
- Yamkate, P.; Funke, S.; Steiger, K.; Gold, R.M.; Lidbury, J.A.; Karst, U.; Steiner, J.M. Quantitative bioimaging of copper in frozen liver specimens from cats using laser ablation-inductively coupled plasma-mass spectrometry. J. Feline Med. Surg. 2023, 25, 1098612X–231186919X. [Google Scholar] [CrossRef]
- Wen, S.; Zhong, X.; Wu, Y.; Liang, R.; Zhang, L.; Qiu, J. Colorimetric assay conversion to highly sensitive electrochemical assay for bimodal detection of arsenate based on cobalt oxyhydroxide nanozyme via arsenate absorption. Anal. Chem. 2019, 91, 6487–6497. [Google Scholar] [CrossRef]
- Zhong, X.; Wen, S.; Wang, Y.; Luo, Y.; Li, Z.; Liang, R.; Zhang, L.; Qiu, J. Colorimetric and electrochemical arsenate assays by exploiting the peroxidase-like activity of FeOOH nanorods. Mikrochim. Acta 2019, 186, 732. [Google Scholar] [CrossRef]
- Chaiyo, S.; Apiluk, A.; Siangproh, W.; Chailapakul, O. High sensitivity and specificity simultaneous determination of lead, cadmium and copper using μPAD with dual electrochemical and colorimetric detection. Sens. Actuators B Chem. 2016, 233, 540–549. [Google Scholar] [CrossRef]
- Bradley, M.M.; Siperko, L.M.; Porter, M.D. Colorimetric-solid phase extraction method for trace level determination of arsenite in water. Talanta 2011, 86, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Boruah, B.S.; Biswas, R. Selective detection of arsenic (III) based on colorimetric approach in aqueous medium using functionalized gold nanoparticles unit. Mater. Res. Express 2018, 5, 15059. [Google Scholar] [CrossRef]
- Zhou, H.; Chai, T.; Peng, L.; Zhang, W.; Tian, T.; Zhang, H.; Yang, F. Bisubstrate multi-colorimetric assay based on the peroxidase-like activity of Cu2+-triethylamine complex for copper ion detection. Dye. Pigment. 2023, 210, 111028. [Google Scholar] [CrossRef]
- Zhou, H.; Peng, L.; Tian, T.; Zhang, W.; Chen, G.; Zhang, H.; Yang, F. Multicolor colorimetric assay for copper ion detection based on the etching of gold nanorods. Mikrochim. Acta 2022, 189, 420. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, Y.; Feng, T.; Wei, T.; Xue, C.; Li, Z.; Xu, J. Nitrogen-doped carbon dots/Fe3+-based fluorescent probe for the “off–on” sensing of As (V) in seafood. Anal. Methods 2023, 15, 1923–1931. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mohammadi, S.; Salimi, A.; Ahmadi, R. A chelation-enhanced fluorescence assay using thiourea capped carbonaceous fluorescent nanoparticles for As (III) detection in water samples. J. Fluoresc. 2022, 32, 145–153. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, G. A covalent organic framework containing bipyridine groups as a fluorescent chemical probe for the ultrasensitive detection of arsenic (III). J. Photochem. Photobiol. A Chem. 2021, 421, 113528. [Google Scholar] [CrossRef]
- Luo, F.; Zhu, M.; Liu, Y.; Sun, J.; Gao, F. Ratiometric and visual determination of copper ions with fluorescent nanohybrids of semiconducting polymer nanoparticles and carbon dots. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2023, 295, 122574. [Google Scholar] [CrossRef]
- Ghorbanian, N.; Kajinehbaf, T.; Alizadeh, N. Picomolar detection of As (III) ions by using hydrothermal synthesis of functionalized polymer dots as a highly selective fluorescence sensor. Talanta 2023, 261, 124667. [Google Scholar] [CrossRef]
- Banerjee, M.; Ta, S.; Ghosh, M.; Ghosh, A.; Das, D. Sequential fluorescence recognition of molybdenum (VI), arsenite, and phosphate ions in a ratiometric manner: A facile approach for discrimination of AsO2−and H2PO4−. ACS Omega 2019, 4, 10877–10890. [Google Scholar] [CrossRef]
- Öksüz, N.; Saçmacı, S.; Saçmacı, M.; Ülgen, A. A new fluorescence reagent: Synthesis, characterization and application for speciation of arsenic (III)/(V) species in tea samples. Food Chem. 2019, 270, 579–584. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, R.; Kaur, V.; Talwar, D. Anthranilic acid schiff base as a fluorescent probe for the detection of arsenite and selenite: A detailed investigation of analytical parameters and mechanism for interaction. Anal. Sci. 2021, 37, 553–560. [Google Scholar] [CrossRef]
- Chauhan, K.; Singh, P.; Kumari, B.; Singhal, R.K. Synthesis of new benzothiazole schiff base as selective and sensitive colorimetric sensor for arsenic on-site detection at ppb level. Anal. Methods 2017, 9, 1779–1785. [Google Scholar] [CrossRef]
- Lohar, S.; Pal, S.; Sen, B.; Mukherjee, M.; Banerjee, S.; Chattopadhyay, P. Selective and sensitive turn-on chemosensor for arsenite ion at the ppb level in aqueous media applicable in cell staining. Anal. Chem. 2014, 86, 11357–11361. [Google Scholar] [CrossRef] [PubMed]
- Mekjinda, N.; Phunnarungsi, S.; Ruangpornvisuti, V.; Ritchie, R.J.; Hamachi, I.; Ojida, A.; Wongkongkatep, J. Masking phosphate with rare-earth elements enables selective detection of arsenate by dipycolylamine-ZnII chemosensor. Sci. Rep. 2020, 10, 2656. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.S. Spectrofluorimetry-cloud point extraction determination of trace arsenic (III) with 2′,7′-dichlorofluorescein. J. Phys. Conf. Ser. 2019, 1294, 52060. [Google Scholar] [CrossRef]
- Nagaraj, K.; Nityananda Shetty, A.; Trivedi, D.R. Colorimetric chemosensors for the selective detection of arsenite over arsenate anions in aqueous medium: Application in environmental water samples and DFT studies. Anal. Chim. Acta 2023, 1265, 341355. [Google Scholar] [CrossRef]
- Xie, H.; Hu, Q.; Qin, X.; Zhang, Y.; Li, L.; Li, J. Naked-eye chemosensor with high absolute fluorescence quantum yield for selective detection of Cu(II) and cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 283, 121740. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, P.; Zhong, K.; Hou, S. Fluorescence relay enhancement sequential recognition of Cu2+ and CN− by a new quinazoline derivative. Sens. Actuators B Chem. 2013, 182, 439–445. [Google Scholar] [CrossRef]
- Heo, J.S.; Suh, B.; Kim, C. Selective detection of Cu2+ by benzothiazole-based colorimetric chemosensor: A DFT study. J. Chem. Sci. 2022, 134, 43. [Google Scholar] [CrossRef]
- Pan, J.; Yu, J.; Qiu, S.; Zhu, A.; Liu, Y.; Ban, X.; Li, W.; Yu, H.; Li, L. A novel dibenzimidazole-based fluorescent probe with high sensitivity and selectivity for copper ions. J. Photochem. Photobiol. A Chem. 2021, 406, 113018. [Google Scholar] [CrossRef]
- Sun, R.; Wang, L.; Jiang, C.; Du, Z.; Chen, S.; Wu, W. A highly efficient BODIPY based turn-off fluorescent probe for detecting Cu2+. J. Fluoresc. 2020, 30, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Okda, H.E.; El Sayed, S.; Otri, I.; Ferreira, R.C.M.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. A simple and easy-to-prepare imidazole-based probe for the selective chromo-fluorogenic recognition of biothiols and Cu(II) in aqueous environments. Dye. Pigment. 2019, 162, 303–308. [Google Scholar] [CrossRef]
- Bag, R.; Sikdar, Y.; Sahu, S.; Das Mukhopadhyay, C.; Drew, M.G.B.; Goswami, S. Benzimidazole based ESIPT active chemosensors enable nano-molar detection of Cu2+ in 90% aqueous solution, MCF-7 cells, and plants. J. Photochem. Photobiol. A Chem. 2022, 431, 114006. [Google Scholar] [CrossRef]
- Yun, D.; Chae, J.B.; Kim, C. A novel benzophenone-based colorimetric chemosensor for detecting Cu2+ and F−. J. Chem. Sci. 2019, 131, 1. [Google Scholar] [CrossRef]
- Liao, S.; Cai, M.; Zhu, R.; Fu, T.; Du, Y.; Kong, J.; Zhang, Y.; Qu, C.; Dong, X.; Ni, J.; et al. Antitumor effect of photodynamic therapy/sonodynamic therapy/sono-photodynamic therapy of chlorin e6 and other applications. Mol. Pharm. 2023, 20, 875–885. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A promising photosensitizer in photo-based cancer nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Kiran Rompicharla, S.V.; Kumari, P.; Bhatt, H.; Ghosh, B.; Biswas, S. Lipid and poly (ethylene glycol)-conjugated bi-functionalized chlorin e6 micelles for NIR-light induced photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 29, 101633. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.; Serra, V.V.; Botequim, D.; Paulo, P.M.R.; Andrade, S.M.; Costa, S.M.B. Fluorescence spectroscopy of porphyrins and phthalocyanines: Some insights into supramolecular self-assembly, microencapsulation, and imaging microscopy. Molecules 2021, 26, 4264. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Tiwari, S.; Prasad, S.M. Toxicity assessment of arsenate and arsenite on growth, chlorophyll a fluorescence and antioxidant machinery in nostoc muscorum. Ecotoxicol. Environ. Saf. 2018, 157, 369–379. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, L.; Yang, Z.; Mubarak, H.; Tang, C. Chlorophyll fluorescence in leaves of Ficus tikoua under arsenic stress. Bull. Environ. Contam. Toxicol. 2016, 97, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Ma, Q.; Sun, B.; Wang, Q.; Ding, Z.; Zhang, H.; Chu, X.; Liu, M.; Wang, Z.; et al. Carrier-free, dual-functional nanorods via self-assembly of pure drug molecules for synergistic chemo-photodynamic therapy. Int. J. Nanomed. 2019, 14, 8665–8683. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Liu, F.; Lin, L.; Yan, N.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J. Control. Release 2020, 328, 631–639. [Google Scholar] [CrossRef]
- Ren, H.; Liu, C.; Yang, W.; Jiang, J. Sensitive and selective sensor based on porphyrin porous organic cage fluorescence to-wards copper ion. Dye. Pigment. 2022, 200, 110117. [Google Scholar] [CrossRef]
- Dongare, P.R.; Gore, A.H. Recent advances in colorimetric and fluorescent chemosensors for ionic species: Design, principle and optical signalling mechanism. ChemistrySelect 2021, 6, 5657–5669. [Google Scholar] [CrossRef]
- Tang, L.; Zheng, Z.; Huang, Z.; Zhong, K.; Bian, Y.; Nandhakumar, R. Multi-analyte, ratiometric and relay recognition of a 2,5-diphenyl-1,3,4-oxadiazole-based fluorescent sensor through modulating esipt. RSC Adv. 2015, 5, 10505–10511. [Google Scholar] [CrossRef]
- Hao, F.; Jing, M.; Zhao, X.; Liu, R. Spectroscopy, calorimetry and molecular simulation studies on the interaction of catalase with copper ion. J. Photochem. Photobiol. B 2015, 143, 100–106. [Google Scholar] [CrossRef]
- Rahimi, F.; Anbia, M.; Farahi, M. Aqueous synthesis of L-methionine capped PbS quantum dots for sensitive detection and quantification of arsenic (III). J. Photochem. Photobiol. A Chem. 2021, 417, 113361. [Google Scholar] [CrossRef]
- van de Weert, M.; Stella, L. Fluorescence quenching and ligand binding: A critical discussion of a popular methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- Sakuma, T.; Ohta, T.; Yagyu, T.; Takagi, H.D.; Inamo, M. Copper (II)-assisted charge transfer quenching of the excited state of a zinc (II) porphyrin complex bearing a peripheral bipyridine moiety. Inorg. Chem. Commun. 2013, 38, 108–111. [Google Scholar] [CrossRef]
- Song, R.; Ma, Y.; Bi, A.; Feng, B.; Huang, L.; Huang, S.; Huang, X.; Yin, D.; Chen, F.; Zeng, W. Highly selective and sensitive detection of arsenite ions (III) using a novel tetraphenylimidazole-based probe. Anal. Methods 2021, 13, 5011–5016. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.; Chen, G.; Chen, L.; Wang, J.; Zhang, C.; Yang, F. Adenine phosphate-Cu nanozyme with multienzyme mimicking activity for efficient degrading phenolic compounds and detection of hydrogen peroxide, epinephrine and glutathione. Anal. Chim. Acta 2023, 1279, 341771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Q.; Tang, C.; Zhang, M.; Huang, Z.; Cai, Z. Fluorescent folic acid-capped copper nanoclusters for the determination of rifampicin based on inner filter effect. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 121944. [Google Scholar] [CrossRef]
- Leng, X.; Wang, D.; Mi, Z.; Zhang, Y.; Yang, B.; Chen, F. Novel fluorescence probe toward Cu2+ based on fluorescein derivatives and its bioimaging in cells. Biosensors 2022, 12, 732. [Google Scholar] [CrossRef]
- Cheng, Z.; Jin, X.; Liu, Y.; Zheng, L.; He, H. An ESIPT-based fluorescent probe for aqueous cu+ detection through strip, nanofiber and living cells. Molecules 2023, 28, 3725. [Google Scholar] [CrossRef]




| Receptors | Detection Ion | Liner Range (ppm) | LOD (ppb) | Ref. |
|---|---|---|---|---|
| Acf and RhB | As(V) | 0.04–0.09 | 10 | [3] |
| APSAL | As(V) | 4.65–37.2 | 557.73 | [5] |
| AF1 | As(III) | – a | 0.24 | [9] |
| SB | As(III) | 0.1–14 | 3.12 | [33] |
| DMBD | As(III) | 0–500 | 0.22 | [32] |
| HL | As(III) | 0–015 | 4.1 | [35] |
| 2′,7′-dichlorofluorescein | As(III) | 0.005–0.05 | 0.102 | [37] |
| Ce6 Ce6-Cu2+ | As(III) As(V) | 0.01–2.5 0.01–0.25 | 89 1.375 | This work |
| Samples | Added (ppm) | Founded (ppm) | Recovery a (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Yun Lake water | 0.00 | – b | ||
| 0.01 | 0.0091 | 91.3 | 4.1 | |
| 0.05 | 0.0513 | 102.6 | 7.6 | |
| 0.10 | 0.0961 | 96.1 | 4.9 | |
| Jin Lake water | 0.00 | – b | ||
| 0.01 | 0.0119 | 118.9 | 2.7 | |
| 0.05 | 0.0400 | 80.1 | 5.6 | |
| 0.10 | 0.0815 | 81.5 | 1.8 | |
| Yun Lake Soil | 0.00 | – b | ||
| 0.01 | 0.0106 | 106.3 | 2.7 | |
| 0.05 | 0.0454 | 90.9 | 3.2 | |
| 0.10 | 0.0897 | 89.7 | 2.1 | |
| Jin Lake Soil | 0.00 | – b | ||
| 0.01 | 0.0111 | 111.4 | 5.7 | |
| 0.05 | 0.0420 | 84.1 | 6.6 | |
| 0.10 | 0.1049 | 104.9 | 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.-L.; Chen, G.-Y.; Wang, J.-L.; Chai, T.-Q.; Qian, Z.-M.; Li, W.-J.; Yang, F.-Q. Detection of Arsenic(V) by Fluorescence Sensing Based on Chlorin e6-Copper Ion. Molecules 2024, 29, 1015. https://doi.org/10.3390/molecules29051015
Luo M-L, Chen G-Y, Wang J-L, Chai T-Q, Qian Z-M, Li W-J, Yang F-Q. Detection of Arsenic(V) by Fluorescence Sensing Based on Chlorin e6-Copper Ion. Molecules. 2024; 29(5):1015. https://doi.org/10.3390/molecules29051015
Chicago/Turabian StyleLuo, Mao-Ling, Guo-Ying Chen, Jia-Li Wang, Tong-Qing Chai, Zheng-Ming Qian, Wen-Jia Li, and Feng-Qing Yang. 2024. "Detection of Arsenic(V) by Fluorescence Sensing Based on Chlorin e6-Copper Ion" Molecules 29, no. 5: 1015. https://doi.org/10.3390/molecules29051015
APA StyleLuo, M.-L., Chen, G.-Y., Wang, J.-L., Chai, T.-Q., Qian, Z.-M., Li, W.-J., & Yang, F.-Q. (2024). Detection of Arsenic(V) by Fluorescence Sensing Based on Chlorin e6-Copper Ion. Molecules, 29(5), 1015. https://doi.org/10.3390/molecules29051015










