Abstract
The global increase in antibiotic consumption is related to increased adverse effects, such as antibiotic-associated diarrhea (AAD). This study investigated the chemical properties of Zingiber officinale Rosc (ZO) extract and its ameliorative effects using a lincomycin-induced AAD mouse model. Intestinal tissues were evaluated for the expression of lysozyme, claudin-1, and α-defensin-1, which are associated with intestinal homeostasis. The cecum was analyzed to assess the concentration of short-chain fatty acids (SCFAs). The chemical properties analysis of ZO extracts revealed the levels of total neutral sugars, acidic sugars, proteins, and polyphenols to be 86.4%, 8.8%, 4.0%, and 0.8%, respectively. Furthermore, the monosaccharide composition of ZO was determined to include glucose (97.3%) and galactose (2.7%). ZO extract administration ameliorated the impact of AAD and associated weight loss, and water intake also returned to normal. Moreover, treatment with ZO extract restored the expression levels of lysozyme, α-defensin-1, and claudin-1 to normal levels. The decreased SCFA levels due to induced AAD showed a return to normal levels. The results indicate that ZO extract improved AAD, strengthened the intestinal barrier, and normalized SCFA levels, showing that ZO extract possesses intestinal-function strengthening effects.
1. Introduction
At the beginning of the 20th century, infectious diseases such as smallpox, measles, and pneumonia were widely prevalent and considered a leading cause of death worldwide [1]. In such serious situations, the introduction of antibiotics significantly decreased the incidence of mortality owing to infectious diseases. However, in addition to such changes, side effects due to the overuse of antibiotics and antibiotic resistance became problematic [2]. A study by Klein et al. analyzed the trends for antibiotic consumption between 2000 and 2015 in 76 countries and reported that the total antibiotic consumption rate increased by 39% [3]. Several studies have reported that the long-term use of antibiotics resulted in various side effects, such as hypersensitivity, direct toxic effects on tissues, and antibiotic-associated diarrhea, which is caused by antibiotics that can kill good bacteria and cause new infections [4,5]. Currently used antibiotics can be classified into different groups depending on their mechanism of action, chemical structure, and antimicrobial spectrum [6]. Among these antibiotics, lincomycin causes a change in the normal flora and induces diarrhea due to Clostridium difficile overgrowth [7]. C. difficile is a Gram-positive, spore-forming, anaerobic bacterium, which normally resides in small amounts in the intestinal tract. With the use of antibiotics, the levels of this bacterial flora increase, leading to diarrhea or infection caused by inflammation of the intestinal mucosa [8]. Major complications of AAD are associated with functional impairment of the intestinal wall. The intestinal wall consists of the mucosa, epithelium, and lamina propria, which form protein complexes called tight junctions (TJs), and play a role in protecting the intestine from harmful substances from the external environment and pathogens [9]. TJs connect intestinal epithelial cells and are composed of proteins such as claudin-1 and occluding, depending on the location of epithelium and permeability [10]. However, AAD-induced intestinal barrier impairment increases epithelial permeability, resulting in leakage of the gut and inflammation [11]. In the intestinal mucosal layer, bacterial flora interact with Paneth cells to secrete immunomodulatory substances, such as defensin, lysozyme, C-type lectins, and immunoglobulin A (IgA) [12]. However, the reduction in bacterial flora levels due to the use of antibiotics also reduces the secretion of antimicrobial substances, resulting in impaired intestinal immune function [13]. Furthermore, most carbohydrates are absorbed in the small intestine. However, some carbohydrates are fermented by normal flora and transformed into short-chain fatty acids (SCFAs), such as acetic acid, propionic acid, butyric acid, etc. [9]. The decreased levels of normal flora that result from the use of antibiotics cause an excessive amount of non-digestible carbohydrates to remain in the intestines, allowing them to move to the colon and absorb water via osmosis, ultimately leading to osmotic diarrhea [10].
Recently, studies have demonstrated that natural and plant-derived polysaccharides can help improve diarrhea and intestinal immunity [14,15,16,17]. Moreover, we have recently reported that the oral administration of ZO extract activates immunocytes in the Peyer’s patch, an intestinal lymphoid tissue, to increase the production of granulocyte–macrophage colony-stimulating factor and IgA in mice [18]. Ginger (Zingiber officinale Rosc.) is a perennial herbaceous plant native to tropical or subtropical regions and belongs to the Zingiberaceae family. It refers to the rhizome, or root stem, of the ginger plant [19]. The major components of ginger include carbohydrates (50–70%), lipids (3–8%), terpenes, and phenolic compounds such as gingerol, shogaol, and paradols. Additionally, it contains amino acids, fiber, proteins, phytosterols, vitamins (e.g., nicotinic acid and vitamin A), and minerals [20,21,22]. The terpenes in ginger, such as zingiberene and γ-cardinene, contribute to its distinctive smell [22]. The main components representing the pharmacological activity of ginger are known as 6-gingerol and 6-shogaol. These compounds exhibit antioxidant and anti-inflammatory properties, with 6-gingerol specifically showing antioxidant activity equivalent to 95% of ascorbic acid [23,24]. Furthermore, 6-shogaol has been proven to have various physiological effects, including antibacterial action [25], anti-inflammatory effects [26], anti-obesity properties [27], and activation of innate immunity [28,29]. However, the ameliorative effects of ZO extract on AAD and its mechanism of action have not yet been investigated. Therefore, this study evaluated the ameliorative effects of ZO extract on lincomycin-induced diarrhea and analyzed the levels of intestinal proteins and SCFAs to investigate its effects on intestinal health.
2. Results and Discussion
2.1. Chemical Properties of ZO Extract
In a previous study, six major index ZO compounds (6-Gingerol, 8-Gingerol, 6-Shogaol, 10-Gingerol, 8-Shogaol, and 10-Shogaol) were analyzed, with concentrations of 6.64, 0.22, 0.28, 0.16, and 0.01 mg/g, respectively, and the limit of quantitation was identified [18]. Furthermore, these six index compounds did not affect the proliferation of Peyer’s patch cells, GM-CSF, and IgA secretion.
Plant cell walls contain polysaccharides known as pectins, composed of homogalacturonan (HG) structures [30]. According to previous reports, polysaccharides can induce immune system activation, enhance intestinal immune function, and modulate the intestinal microbiome [15,31,32]. Therefore, we analyzed the chemical properties and composition of ZO extracts. As described in Table 1, the concentrations of total neutral sugar, acidic sugar, protein, and polyphenol in ZO extract were determined to be 86.4 ± 29.3, 8.8 ± 4.6, 4.0 ± 0.5, and 0.8 ± 1.1%, respectively. Furthermore, to analyze the monosaccharide composition of ZO, we derivatized the ZO extracts into 3-methyl-1-phenyl-2-pyrazolone (PMP) and analyzed them using high-performance liquid chromatography with ultraviolet detection (UV-HPLC) (Figure S1). The results indicated a glucose content of 97.3 ± 0.6% and galactose content of 2.7 ± 0.9 mole%. Thus, the ZO extract is primarily composed of neutral sugars, specifically glucose and galactose, with glucose being the main monosaccharide in ZO extracts.

Table 1.
Chemical properties of ZO extract.
2.2. Ameliorating Effect of ZO Extract on AAD in a Lincomycin-Induced-Diarrhea Mouse Model
To evaluate the ameliorative effects of ZO extract in an AAD mouse model, parameters including weight, diarrhea status score, and water intake were analyzed. During the treatment period using lincomycin, the rate of increase in weight was significantly lower in the group that received the antibiotic agent than in the normal group (Figure 1A). The group that received lincomycin showed an increase in water intake (Figure 1B). However, the rate of increase in weight was slightly higher in the ZO extract group than in the AAD group, confirming the likelihood of normalization. Furthermore, the increased water intake due to the use of lincomycin in the ZO extract group (100 and 300 mg/kg) was similar to the level of water intake of the normal group.
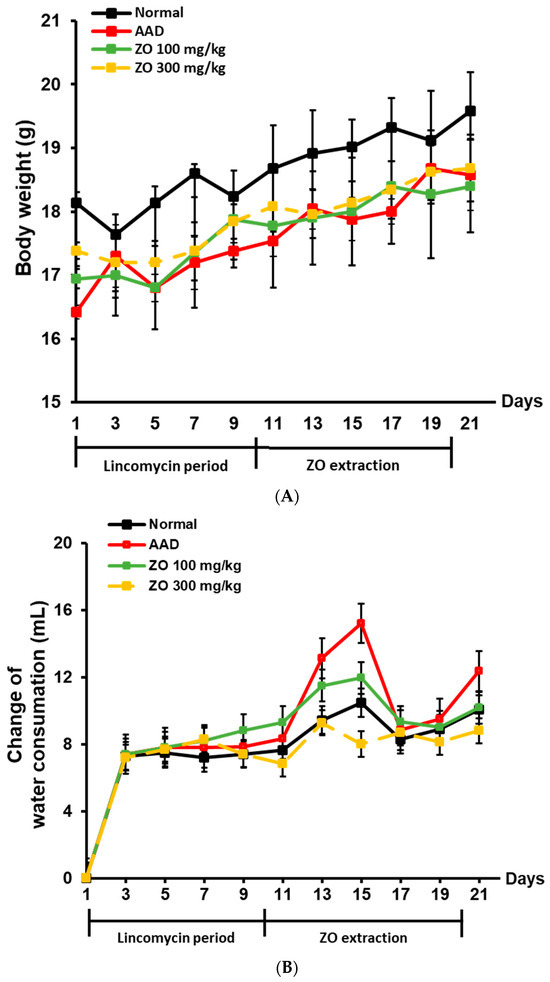
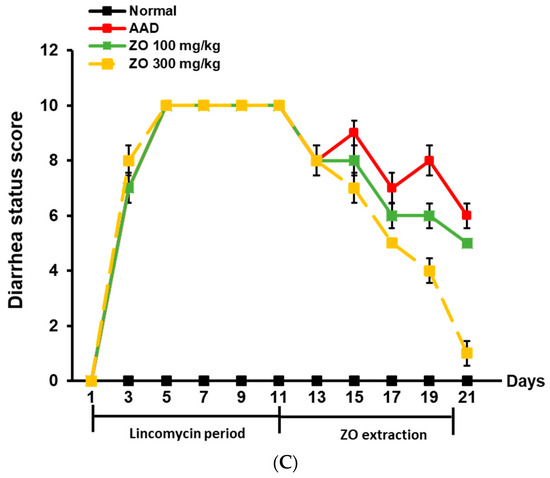
Figure 1.
Effect of ZO extraction on changes in body weight, diarrhea status score, and water intake in the AAD model using BALB/c mice. AAD models were established by treatment with or without lincomycin via oral administration for ten days in mice. After diarrhea induction was completed, ZO extracts were orally administered at concentrations of 100 mg/kg or 300 mg/kg for ten days. Mouse body weight was measured every two days (A). Changes in the water intake of mice were recorded every other day (B). Diarrhea status scores of mice were observed once every 2 days (C).
Figure 1C shows the total diarrhea status score evaluated according to the criteria listed in Table 2. The average diarrhea status score during the treatment period using lincomycin was 10 points, and the score dropped to 7 points when measured after treatment with lincomycin. Thereafter, the average diarrhea status scores in the ZO extract groups of 100 and 300 mg/kg were 6 and 1 point, respectively, indicating that ZO extract administration improved diarrhea. Based on these results, ZO extract ameliorated weight loss and diarrhea induced by lincomycin, and water intake.

Table 2.
Scoring criteria for lincomycin-induced antibiotic-associated diarrhea in BALB/c mice.
2.3. Analysis of the Effects of ZO Extract on Changes in the Expression of Intestinal Lysozyme and Claudin-1 in the Lincomycin-Induced-Diarrhea Mouse Model
The impairment of mucosal barrier function plays an important role in the occurrence of intestinal leakage and inflammation [33]. Tight junction (TJ) proteins in the intestinal epithelium play a crucial role in maintaining intestinal homeostasis [34]. Lysozyme exerts an antimicrobial activity by damaging the cell surface and inducing cytolysis [35]. The enzyme is secreted by Paneth cells, which are epithelial cells in the intestinal mucosa [23]. Lysozyme also plays a role in suppressing intestinal bacterial proliferation [27,28]. Claudin-1 enhances the adhesion between intestinal epithelial cells and regulates the passage of substances in the intestine [29,36].
We evaluated whether ZO extract improves the damaged intestinal barrier induced by lincomycin administration. Figure 2 shows that the expression of lysozyme was significantly reduced in the AAD group compared to that in the normal group. In the ZO extract group, the expression of lysozyme significantly increased compared to that in the AAD group. Moreover, the expression of claudin-1 significantly decreased in the AAD group compared to that in the normal group. The decreased expression of claudin-1 was significantly improved in the ZO extract group. However, a dose-dependent phenotype with the ZO extract was not observed, possibly because the ZO extract was prepared from the crude polysaccharide fraction; thus, further purification of the ZO extract is required.
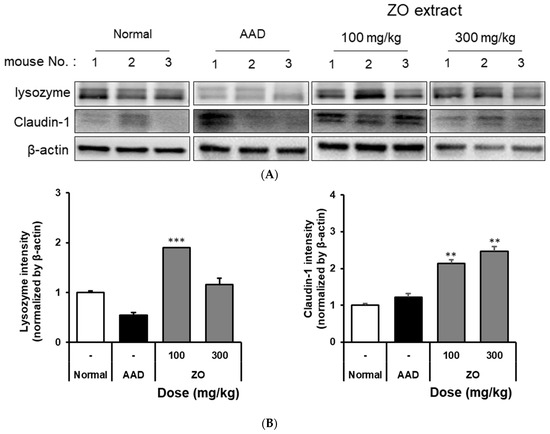
Figure 2.
The effect of oral administration of ZO extract on lysozyme and claudin-1 expression in the AAD model. AAD mice were orally administered the ZO extract (100 mg/kg or 300 mg/kg) daily for ten days. The intestinal tissue was extracted using a radioimmunoprecipitation assay buffer. Lysozyme and claudin-1 protein expression were determined by immunoblotting. β-actin was used as an internal loading control (A). Lysozyme and claudin-1 expression were analyzed using Image J software (B). Data are presented as the mean ± standard deviation (SD) of triplicate experiments. ***, p < 0.0001 and **, p < 0.001 vs. the AAD group.
2.4. Analysis of the Effects of ZO Extract on the mRNA Expression of Intestinal Claudin-1, α-Defensin-1, and Lysozyme in the Lincomycin-Induced-Diarrhea Mouse Model
Claudin-1 is a TJ protein in the intestinal mucosa and plays a role in regulating its permeability [37]. Claudin-1 normally resides between epithelial cells and controls intercellular gaps, absorption, and the secretion of substances inside the small intestine [38]. Furthermore, α-defensin-1 is an antimicrobial peptide and is produced in the epithelial cells of the intestinal mucosa. This peptide plays a role in defending the small intestine from infection by interrupting bacterial growth and controlling the number of bacteria in the small intestine [39]. Lysozyme is produced in the intestinal mucosa and destroys the bacterial cell wall in the extracellular matrix [40].
RT-qPCR was used to detect the mRNA expression of claudin-1, α-defensin-1, and lysozyme in the intestinal tissues of mice that received ZO extract in the lincomycin-induced-diarrhea model group. The results showed that the mRNA expression of claudin-1 in the AAD group was significantly reduced compared to that in the normal group. In contrast, in the group that received 300 mg/kg ZO extract, the mRNA expression of claudin-1 increased (Figure 3A). Further, the ZO extract group showed a dose-dependent increase in the expression of α-defensin-1 compared to that in the AAD group (Figure 3B). In the ZO extract group administered 100 mg/kg of ZO extract, the expression of lysozyme was significantly increased (Figure 3C). Based on these results, the use of ZO extract was shown to increase the mRNA expression of claudin-1, α-defensin-1, and lysozyme in the intestine of the lincomycin-induced-diarrhea model group.
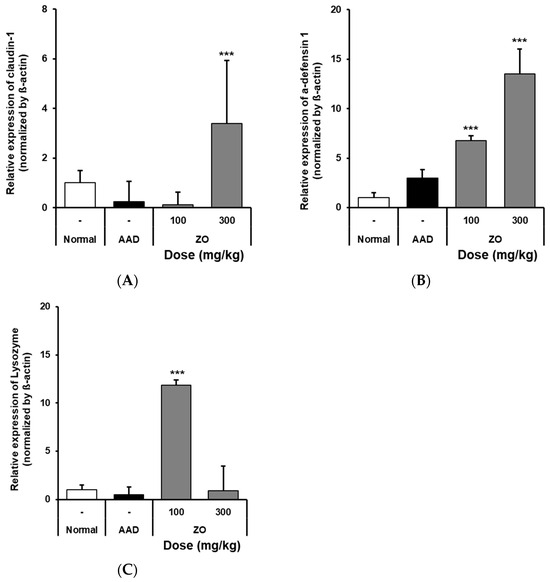
Figure 3.
Effects of oral administration of ZO extract on lysozyme and claudin-1 mRNA expression in the AAD model. AAD mice were orally administered ZO extracts (100 mg/kg or 300 mg/kg) daily for 10 days. Intestinal RNA was extracted and claudin-1 (A), α-defensin1 (B), and lysozyme (C) mRNA expressions were determined by RT-qPCR. Data are presented as the mean ± standard deviation (SD) of triplicate experiments. ***, p < 0.0001 vs. the AAD group.
2.5. Analysis of the Effects of ZO Extract on the Changes in the Levels of SCFAs in the Lincomycin-Induced-Diarrhea Model
Intestinal microorganisms use food ingested in the digestive tract to cause the fermentation of dietary fibers and the production of SCFAs, and they are affected by the physicochemical properties of dietary fibers [41]. SCFAs play a key role in maintaining the functions of epithelial cells and regulating the immune function [42]. Furthermore, SCFAs can alleviate diarrhea by absorption via colonic epithelial cells and stimulating the absorption of water and electrolytes [43]. Acetic, propionic, and butyric acids are well-known SCFAs. Acetic acid is a major SCFA that results due to lactic acid fermentation and is known to play an important role in inhibiting harmful bacteria in the intestine by reducing the intestinal pH, promoting immunocompetence, and regulating the intestinal environment [44] Butyric acid plays an important role in intestinal health by acting on colonocyte metabolism as a major SCFA, and is known to promote the proliferation and differentiation of intestinal epithelial cells and it helps prevent colon cancer [41,45]. Propionic acid is absorbed in the liver and inhibits cholesterol synthesis, and it strongly lowers blood cholesterol and lipid levels by regulating the expression of genes related to lipid synthesis enzymes [41].
We analyzed the effects of the ZO extract on SCFA levels in the appendix in the lincomycin-induced-diarrhea mouse model and found that the concentration of acetic acid in the AAD group decreased from 12.2 ± 4.8 to 3.3 ± 0.3 mM (Figure 4). In contrast, the concentrations of acetic acid in the groups that received 100 and 300 mg/kg of ZO extract were 5.3 ± 3.2 and 3.5 ± 0.7 mM, respectively. In the control group, the concentration of butyric acid was 2.6 ± 0.6 mM. In the AAD group, butyric acid was not detected. In the groups that received 100 and 300 mg/kg of ZO extract, the concentrations of butyric acid were 0.9 ± 0.1 and 0.8 ± 0.0 mM, respectively, indicating a recovery of butyric acid levels. The concentration of propionic acid in the AAD group decreased from 2.1 ± 0.6 to 1.6 ± 0.3 mM. No significant change in the concentration of propionic acid was found in the groups that received ZO extract compared to that in the AAD group (Figure 4). This result may be observed due to the effect of dietary fibers that contribute to the production of SCFAs and the composition of monosaccharides residing in crude polysaccharides, the major substance of ZO extract. Therefore, further studies on the composition of monosaccharides of ZO extract are required.
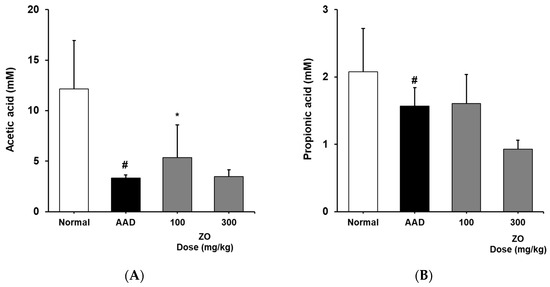
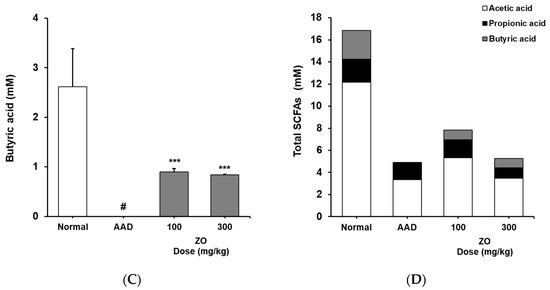
Figure 4.
Effects of ZO extract on short-chain fatty acids in the cecum of lincomycin-induced AAD mice. AAD mice were orally administered the ZO extract (100 mg/kg or 300 mg/kg) daily for ten days. Acetic acid (A), butyric acid (B), propionic acid (C), and total SCFA content (D) in the mouse cecum were determined using flame ionization detector–gas chromatography. Data are presented as the mean±standard deviation (SD) of triplicate experiments. #, p < 0.05 vs. the normal group; ***, p < 0.05 vs. the AAD group and *, p < 0.01 vs. the AAD group.
Based on these results, we infer that the use of ZO extract helps in recovering the reduced concentration of SCFAs in the lincomycin-induced-diarrhea mouse model and may contribute to the maintenance of intestinal homeostasis.
3. Materials and Methods
3.1. Preparation of ZO Extract
Zingiber officinale Roscoe (ZO) (30 g) was extracted with 300 mL of distilled water at 100 °C for 60 min. The extract was filtered through a non-woven fabric. Finally, 240 mL of supernatant was lyophilized using a freeze dryer (EYELA, Tokyo, Japan), and the yield was calculated by measuring the dried amount (Table 3).

Table 3.
Zingiber officinale Roscoe freeze-dried yield.
3.2. Chemicals and Antibodies
To analyze the components of the extract, galactose (≥99%, Sigma-Aldrich, St. Louis, MO, USA) was used as a standard, and the neutral sugar content was measured using phenol–sulfuric acid [46]. Additionally, galacturonic acid (≥99%, Sigma-Aldrich, St. Louis, MO, USA) was used as a standard to measure acidic sugar content using m-hydroxybiphenyl [47]. The protein content was measured using the Bradford method with BSA (≥99%, Bio-Rad, Hercules, CA, USA) as the standard, and the phenolic compound content was quantitatively analyzed using gallic acid (≥99%, Sigma-Aldrich, St. Louis, MO, USA) as the standard via the Folin–Ciocalteu method [48,49]. The antibodies used included anti-lysozyme (Abcam, ab108508, Cambridge, UK), anti-claudin-1 (Abcam, ab180158), and anti-GAPDH (Cell Signaling Technology, #4967), and they were purchased from Cell Signaling Technology (Danvers, MA, USA). GAPDH levels were used as a loading control.
3.3. Monosaccharide Composition Analysis of ZO Extract
For sugar analysis, 2 M trifluoroacetic acid (TFA) from Sigma was added to ZO extract, and the reaction was incubated at 120 °C for 1 h, followed by hydrolysis and drying for 30 min. Next, 100 μL of 0.3 M NaOH and 120 μL of 0.5 M 3-methyl-1-phenyl-2-pyrazolone (PMP) in methanol were added, and the reaction was incubated at 70 °C for 1 h. The mixture was neutralized with 100 μL of 0.3 M HCl, thoroughly dried, and separated using a chloroform/H2O two-phase solvent system. The water layer was recovered, filtered, and analyzed by HPLC. The HPLC conditions are described in Table 4. The mole% of each constituent sugar was calculated by comparing the peak area and molecular weight of the sample relative to the internal standard.

Table 4.
Analytical conditions for high-performance liquid chromatography (HPLC) for evaluating monosaccharide composition.
3.4. Animal and Experimental Design
Animal experiments were conducted as per the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Gachon University (Approval No: GU1-2022-IA0050-00). BALB/C mice (seven weeks, female) were purchased from OrientBio (Seoungnam, Korea). The experimental animals were exposed to a 12 h light–dark cycle and provided ad libitum access to food and water in an environment with a temperature of 22 ± 2 °C and humidity of 50–55%. After a 7-day adaptation period, the animals were assigned to the following groups: control (normal), antibiotic-induced-diarrhea (AAD), low-dose treatment (AAD + ZO 100 mg/kg), and high-dose treatment groups (AAD + ZO 300 mg/kg). Subsequently, the control group received oral administration of physiological saline, and the AAD group received oral administration of lincomycin (Dongkwang Pharm, Seoul, Korea) at a concentration of 3 g/kg once daily for ten days. After the completion of lincomycin administration, both the control and AAD groups received oral administration of sterilized 0.5 w/v % methyl cellulose 400 solution (CMC) (Wako, Tokyo, Japan). The ZO group was orally administered lincomycin for ten days and subsequently orally administered concentrations of 100 or 300 mg/kg for ten days (Figure 5).
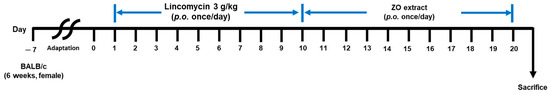
Figure 5.
Oral administration schedule for ZO extraction in the lincomycin-induced AAD model. AAD models were induced by the administration of 3 g/kg lincomycin for ten days. CMC solutions were administered to the control groups. The ZO extraction group was orally administered 100 or 300 mg/kg lincomycin for ten days.
3.5. Immunoblotting
Proteins from mouse small intestines were extracted in radioimmunoprecipitation assay buffer containing a phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), 1 mM dithiothreitol (Wako, Tokyo, Japan), and a Complete™ Mini Protease Inhibitor Cocktail (Roche Diagnostics Corp., Indianapolis, IN, USA). The protein solution was centrifuged at 4 °C and 13,000 rpm for 20 min to separate the proteins from the intestinal tissues. After separating the proteins by SDS-PAGE, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was incubated with 5% skim milk overnight. Subsequently, specific antibodies were diluted in TBS with Tween 20 (0%) and applied to the membrane. The membrane was washed three times with TBS-T buffer. The membrane was incubated with a secondary antibody linked to horseradish peroxidase (HRP) at room temperature for 2 h. The protein signals were visualized with the Super Signal West Femto Substrate (Thermo Fisher, Emeryville, CA, USA) using the Fusion Solo Chemiluminescence System (Vilber Lourmat, Paris, France).
3.6. RT-qPCR
Mouse small intestines tissues were extracted using BioMasher (TaKaRa, Shiga, Japan) and filtrated using a QIAshredder (Qiagen, Hilden, Germany). Total RNA from the intestinal tissues was isolated and purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany), followed by cDNA synthesis using the RevertAid First-Strand cDNA synthesis kit (Fermentas, MA, USA). The qRT-PCR quantification was performed using Mm02524428_g1(α-defensin-1), Mm01228299_m1 (lysozyme), and Mm01342184_m1 (claudin1) TaqMan primer sets (Applied Biosystems, Foster City, CA, USA). The amplification conditions were determined using the Quant 3 PCR system (Applied Biosystems).
3.7. Determination of Short-Chain Fatty Acids
To evaluate the SCFA content in the mouse cecum, we ground the tissue using the BioMasher (TaKaRa, Shiga, Japan) in an 80% methanol solution. The ground solution was then centrifuged at 13,000 rpm for 10 min at 4 °C, and the supernatant was filtered using ADVANTEC’s 0.45 μm syringe filter. Subsequently, analysis was performed using a flame ionization detector (HP-5890/5971, Hewlett Packard, Palo Alto, CA, USA) and GC column (DB-FFAP 123-3253, Agilent Technologies, Inc., Santa Clara, CA, USA, 50 mm × 0.32 mm × 0.50 μm). The SCFA levels were quantified using acetic acid, propionic acid, and butyric acid as standards. The content of SCFAs in the cecum was determined using a calibration curve based on the respective standards. Acetic acid, propionic acid, and butyric acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.8. Statistical Analysis
The results from the experiments were analyzed for significance using GraphPad Prism 5.02. The data are presented as the mean and standard deviation (SD) of triplicate experiments for all measured parameters. Significant differences between samples were determined using one-way ANOVA followed by Tukey’s multiple comparison analyses.
4. Conclusions
Currently, treatments such as the antibiotics metronidazole and vancomycin, and the anti-diarrheal agent loperamide, are used as therapeutic agents for antibiotic-associated diarrhea [50]. These treatments are known to have side effects such as allergic reactions, decreased appetite, development of resistant bacterial strains, and intestinal microbial imbalance [51,52,53,54]. Natural products are known to be relatively safe and have few side effects, and research is being conducted on the development of medicines using natural products. Currently utilized herbal medicines such as Glycyrrhiza glabra, Allium sativum, Poria cocos, and Atractylodes reportedly induce effects including alleviation of intestinal inflammation, antimicrobial effects, anti-diarrheal properties, and enhancement of digestive function [55,56,57,58].
This study analyzed the effects of ZO extract on AAD induced by lincomycin. Administration of a hot water extract of ZO was shown to improve diarrhea status, weight loss, and water intake in the AAD mouse model. Moreover, the expressions of lysozyme and α-defensin-1, the antimicrobial peptides of the small intestine, which were reduced due to AAD, were increased. An increase in the expression of claudin-1, the TJ protein, was also observed. Furthermore, the use of ZO extract improved the concentrations of intestinal SCFAs such as acetic and butyric acid, which were reduced due to AAD.
The majority of polysaccharides generated through natural hot water extraction of ZO are pectins [59]. These polysaccharides primarily consist of homogalacturonan with a structure incorporating bound rhamnogalacturonan [60]. The pharmacological activity of pectin is largely attributed to subtle structural differences in rhamnogalacturonan. In this study, we conducted chemical and monosaccharide composition analyses of ZO extracts. Further research is warranted to elucidate the active components of ZO and undertake structural analyses of ZO polysaccharides through additional purification processes. To summarize, the hot water extract of ZO can be used for the prevention and treatment of AAD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29030732/s1, Figure S1: HPLC chromatogram of ZO monosaccaharide composition.
Author Contributions
Conceptualization, M.-S.S. and Y.-K.C.; methodology, S.J.K. and M.-S.S.; validation, S.J.K.; formal analysis, S.J.K. and M.-S.S.; investigation, S.J.K.; resources, M.-S.S. and Y.-K.C.; data curation, S.J.K.; writing—original draft preparation, S.J.K.; writing—review and editing, M.-S.S.; project administration, Y.-K.C.; funding acquisition, Y.-K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Research Foundation of Korea (NRF, NRF-2021R1F1A1062854) with grants supported by the Korean government (MSIP).
Institutional Review Board Statement
All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Gachon University (Approval No: GU1-2022-IA0050-00).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cohen, M.L. Changing patterns of infectious disease. Nature 2000, 406, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharmacol. 2017, 133, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Nam, J.P.; Kim, J.H.; Kim, Y.M.; Nah, J.W.; Jang, M.K. Antimicrobial action of water-soluble β-chitosan against clinical multi-drug resistant bacteria. Int. J. Mol. Sci. 2015, 16, 7995–8007. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Xiao, N.; Peng, M.; Tan, Z. The effect of qiweibaizhu powder crude polysaccharide on antibiotic-associated diarrhea mice is associated with restoring intestinal mucosal bacteria. Front. Nutr. 2022, 9, 952647. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liu, C.; Ye, C.; Sun, J.; Tan, X.; Zhang, C.; Qu, Q.; Shi, D.; Guo, S. Structural modulation of gut microbiota during alleviation of antibiotic-associated diarrhea with herbal formula. Int. J. Biol. Macromol. 2017, 105, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, P.; Wang, J. High-fat diet alters the intestinal microbiota in streptozotocin-induced type 2 diabetic mice. Microorganisms 2019, 7, 176. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, B.; Xu, J.; Liu, Y.; Qiu, E.; Li, Z.; Li, Z.; He, Y.; Zhou, H.; Bai, Y.; et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018, 9, 1040. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Q.; Huang, J.; Liu, D.; Lan, Y.; Yuan, L.; Chen, Q. Xianglian Pill ameliorates antibiotic-associated diarrhea by restoring intestinal microbiota and attenuating mucosal damage. J. Ethnopharmacol. 2021, 264, 113377. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef]
- Park, D.H.; Han, B.; Shin, M.S.; Hwang, G.S. Enhanced intestinal immune response in mice after oral administration of Korea red ginseng-derived polysaccharide. Polymers 2020, 12, 2186. [Google Scholar] [CrossRef]
- Ahn, H.-R.; Park, D.H.; Shin, M.-S.; Nguyen, Q.N.; Park, J.Y.; Kim, D.-W.; Kang, K.S.; Lee, H.L. The ameliorating effect of Lizhong-Tang on antibiotic-associated imbalance in the gut microbiota in mouse. Appl. Sci. 2022, 12, 6943. [Google Scholar] [CrossRef]
- Ma, Z.J.; Wang, H.J.; Ma, X.J.; Li, Y.; Yang, H.J.; Li, H.; Su, J.R.; Zhang, C.E.; Huang, L.Q. Modulation of gut microbiota and intestinal barrier function during alleviation of antibiotic-associated diarrhea with rhizoma Zingiber officinale (Ginger) extract. Food Funct. 2020, 11, 10839–10851. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-S.; Yu, K.-W.; Shin, K.-S.; Lee, H. Enhancement of immunological activity in mice with oral administration of cell wall components of Bifidobacterium bifidum. Food Sci. Biotechnol. 2004, 13, 85–89. [Google Scholar]
- Min, S.J.; Kim, S.J.; Park, J.Y.; Seo, C.S.; Choi, Y.K. Preparation of herbal extracts for intestinal immune modulation activity based on in vitro screening and in vivo evaluation of Zingiber officinale Rosc. Extracts. Molecules 2023, 28, 6743. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.H.; Ni, Z.J.; Zhu, Y.Y.; Thakur, K.; Zhang, F.; Zhang, Y.Y.; Hu, F.; Zhang, J.G.; Wei, Z.J. A recent update on the multifaceted health benefits associated with ginger and its bioactive components. Food Funct. 2021, 12, 519–542. [Google Scholar] [CrossRef]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Connell DW, Sutherland MD A re-examination of gingerol, shogaol, and zingerone the pungent principles of ginger (Zingiber officinale Roscoe). Aust. J. Chem. 1969, 22, 1033–1043. [CrossRef]
- Lee BS, Ko MS, Kim HJ, Kwak IS, Kim DH, Chung BW Separation of 6-gingerol from ginger (Zingiber officinale Roscoe) and antioxidative activity. Korean J. Biotechnol. Bioeng. 2006, 21, 484–488.
- Moon, Y.S.; Lee, H.S.; Lee, S.E. Inhibitory effects of three monoterpenes from ginger essential oil on growth and aflatoxin production of Aspergillus flavus and their gene regulation in aflatoxin biosynthesis. Appl. Biol. Chem. 2018, 61, 243–250. [Google Scholar] [CrossRef]
- Shimoda, H.; Shan, S.J.; Tanaka, J.; Seki, A.; Seo, J.W.; Kasajima, N.; Murakami, N. Anti-inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. J. Med. Food 2010, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.B.; Mohsin, M.N.A.B.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar] [CrossRef]
- Zakaria-Runkat, F.; Prangdimurti, E. Antioxidant and immunoenhancement activities of ginger (Zingiber officinale Roscoe) extracts and compounds in vitro and in vivo mouse and human system. Nutraceuticals Foods 2003, 8, 96–104. [Google Scholar] [CrossRef]
- McCartney, F.N.; Allen, J.B.; Mizel, D.E.; Albina, J.E.; Xie, Q.W.; Nathan, C.F.; Wahl, S.M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 1993, 178, 749–754. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, H.; Shin, J.-Y.; Lee, S.J.; Yu, K.-W. The physiological activity of crude polysaccharide solvent extracted from herbal medicine mixture. Korean J. Food Nutr. 2021, 34, 36–46. [Google Scholar]
- Kim, H.W.; Shin, M.S.; Lee, S.J.; Park, H.R.; Jee, H.S.; Yoon, T.J.; Shin, K.S. Signaling pathways associated with macrophage-activating polysaccharides purified from fermented barley. Int. J. Biol. Macromol. 2019, 131, 1084–1091. [Google Scholar] [CrossRef]
- Kim, S.J.; Baek, S.H.; Kang, K.S.; Shin, M.S. Characterization of macrophage activation after treatment with polysaccharides from ginseng according to heat processing. Appl. Biol. Chem. 2023, 66, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Connell, D. The chemistry of the essential oil and oleoresin of ginger (Zingiber officinale Roscoe). Flavour Ind. 1970, 1, 677–693. [Google Scholar]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Deng, Z.; Hou, Y.; Zhang, G. Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2015, 2, 57. [Google Scholar] [CrossRef]
- El Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F.; et al. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500–5507. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; De Boeck, G.; Becker, K. Dietary roles of non-starch polysaccharides in human nutrition: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Pope, J.L.; Bhat, A.A.; Sharma, A.; Ahmad, R.; Krishnan, M.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 2014, 63, 622–634. [Google Scholar] [CrossRef]
- Hijova, E.; Chmelarova, A. Short chain fatty acids and colonic health. Bratisl. Lek. Listy 2007, 108, 354–358. [Google Scholar]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Kelly, P. Infectious diarrhoea. Medicine 2011, 39, 201–206. [Google Scholar] [CrossRef]
- Hanauer, S.B. The role of loperamide in gastrointestinal disorders. Rev. Gastroenterol. Disord. 2008, 8, 15–20. [Google Scholar] [PubMed]
- Cheung, R.P.; DiPiro, J.T. Vancomycin: An update. Pharmacotherapy 1986, 6, 153–169. [Google Scholar] [CrossRef]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M. Role of probiotics in antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, and recurrent Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol. 2008, 42 (Suppl. S2), S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ishihara, K.; Morota, T.; Takeda, S.; Aburada, M. Permeability of the flavonoids liquiritigenin and its glycosides in licorice roots and davidigenin, a hydrogenated metabolite of liquiritigenin, using human intestinal cell line Caco-2. J. Ethnopharmacol. 2003, 89, 285–289. [Google Scholar] [CrossRef]
- Olivas-Méndez, P.; Chávez-Martínez, A.; Santellano-Estrada, E.; Guerrero Asorey, L.; Sánchez-Vega, R.; Rentería-Monterrubio, A.L.; Chávez-Flores, D.; Tirado-Gallegos, J.M.; Méndez-Zamora, G. Antioxidant and antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers. Foods 2022, 11, 2018. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Jiang, Y.; Wu, J.; Chen, L.; Ding, Y.; Zhou, Y.; Deng, L.; Chen, X. Poria cocos polysaccharide ameliorated antibiotic-associated diarrhea in mice via regulating the homeostasis of the gut microbiota and intestinal mucosal barrier. Int. J. Mol. Sci. 2023, 24, 1423. [Google Scholar] [CrossRef]
- Han, K.; Kim, K.; Wang, J.; Kim, H. Effect of unfermented and fermented Atractylodes macrocephalae on gut permeability and lipopolysaccharide-induced inflammation. J. Soc. Korean Med. Obes. Res. 2013, 13, 24–32. [Google Scholar]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.; Albersheim, P.; Darvill, A. The pectic polysaccharides of primary cell walls. In Carbohydrates; Elsevier: Amsterdam, The Netherlands, 1990; Volume 2, pp. 415–441. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).