Bergaptol, a Major Furocoumarin in Citrus: Pharmacological Properties and Toxicity
Abstract
1. Introduction
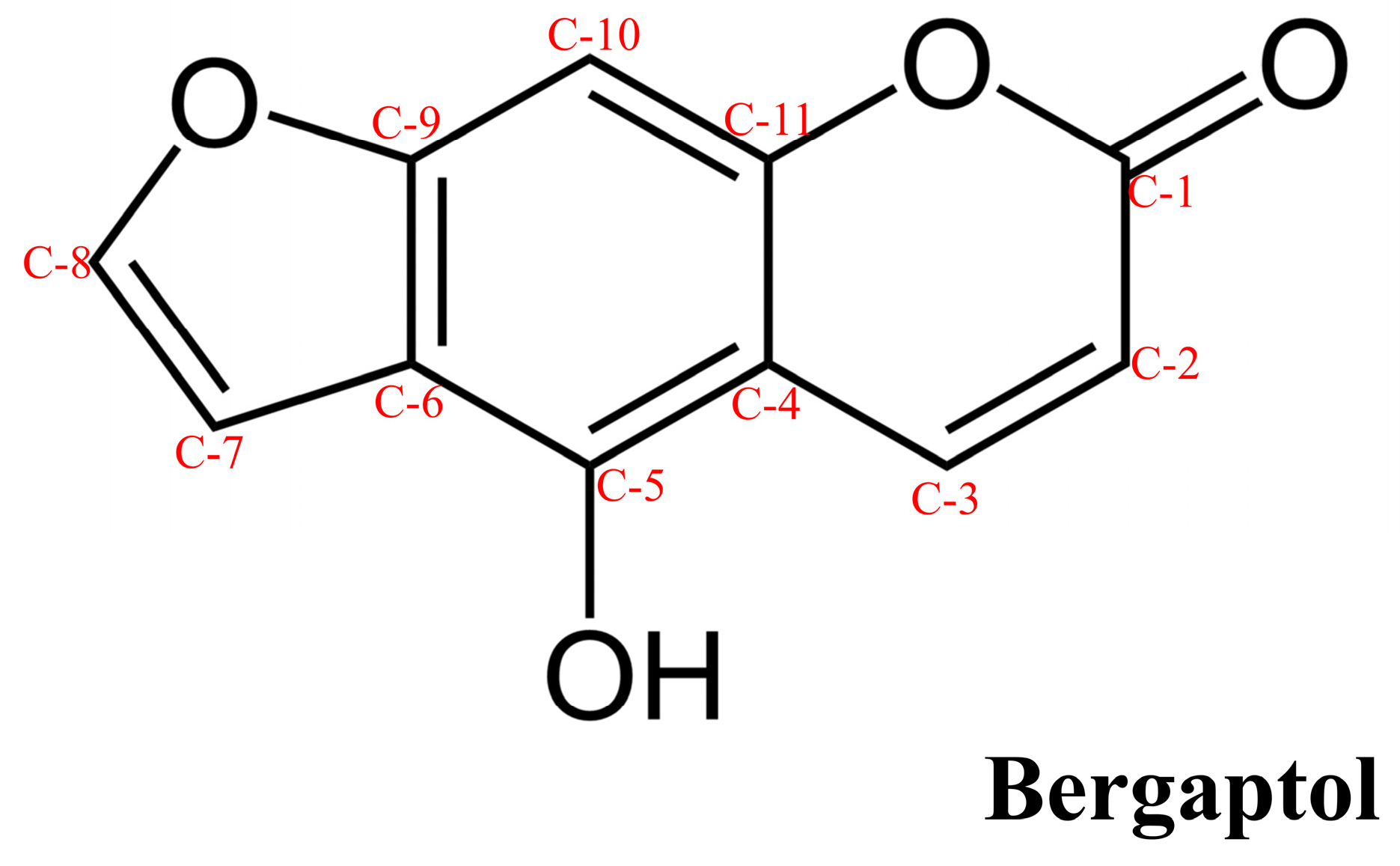
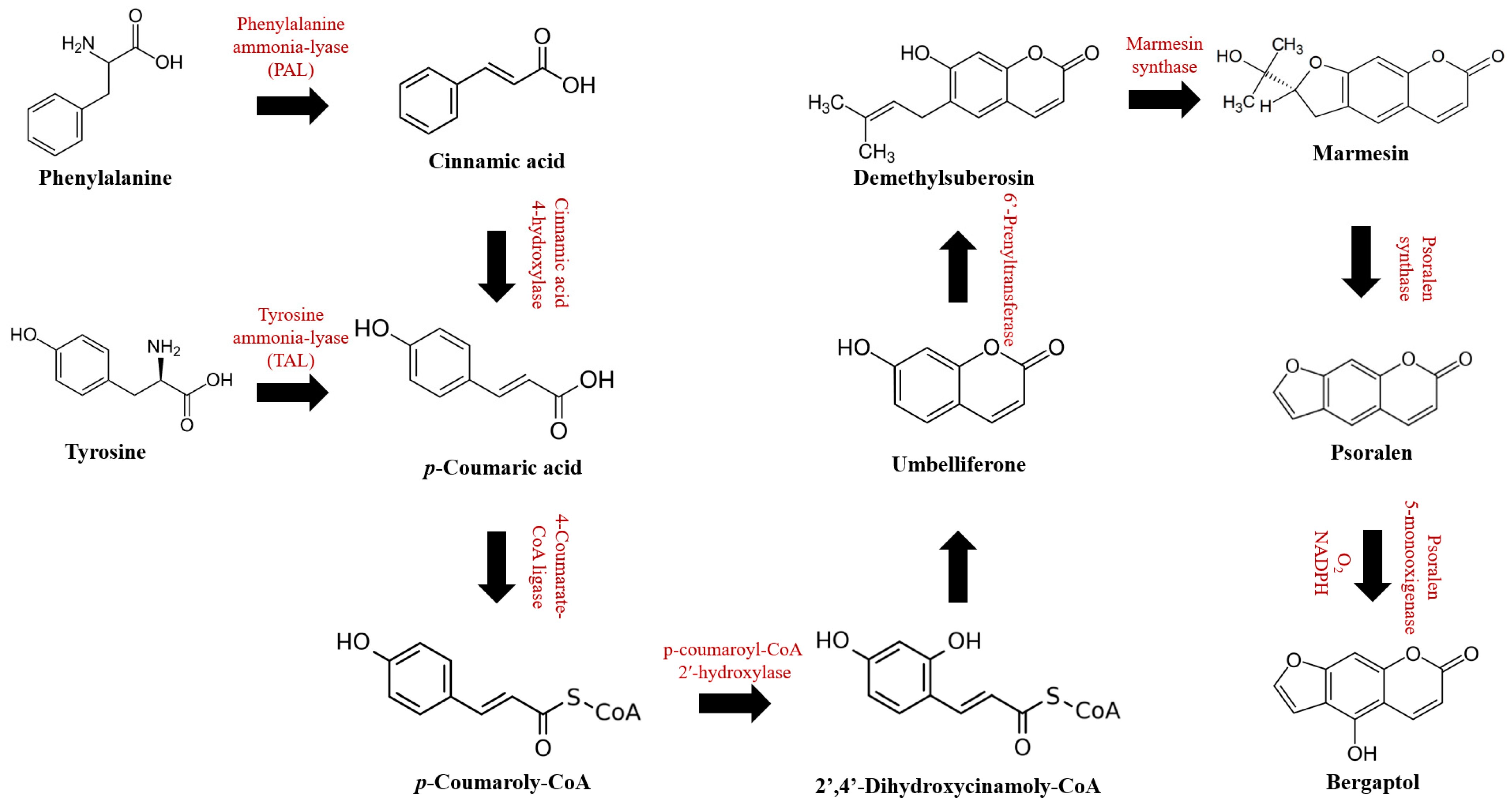
2. Chemical Properties, Bioavailability, and Pharmacokinetics
3. Pharmacological Properties
3.1. Anti-Inflammatory Effects
3.2. Antioxidative Effects
3.3. Anti-Cancer Effects
3.4. Anti-Osteoporosis Effects
3.5. Antilipidemic Effect
3.6. Antimicrobial
3.7. Neuroprotective Effect
4. Toxicity
5. Methodology
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kai, K.; Mizutani, M.; Kawamura, N.; Yamamoto, R.; Tamai, M.; Yamaguchi, H.; Sakata, K.; Shimizu, B. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 2008, 55, 989–999. [Google Scholar] [CrossRef]
- Vialart, G.; Hehn, A.; Olry, A.; Ito, K.; Krieger, C.; Larbat, R.; Paris, C.; Shimizu, B.; Sugimoto, Y.; Mizutani, M.; et al. A 2-oxoglutarate-dependent dioxygenase from Ruta graveolens L. exhibits p-coumaroyl CoA 2′-hydroxylase activity (C2′H): A missing step in the synthesis of umbelliferone in plants. Plant J. 2012, 70, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A. Biosynthesis of Coumarin and Herniarin in Lavender. Science 1962, 137, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Hamerski, D.; Matern, U. Elicitor-induced biosynthesis of psoralens in Ammi majus L. suspension cultures. Eur. J. Biochem. 1988, 171, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ji, D.; Liu, J.; Hu, M.; Jin, Z. A New Approach to the Synthesis of Bergapten. Chem. Res. Chin. Univ. 2022, 38, 1492–1496. [Google Scholar] [CrossRef]
- Filer, C.N.; Rodgers, T. Synthesis of [7-14C]bergapten. J. Label. Compd. Radiopharm. 2014, 57, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Row, E.; Brown, S.; Stachulski, A.; Lennard, M. Design, synthesis and evaluation of furanocoumarin monomers as inhibitors of CYP3A4. Org. Biomol. Chem. 2006, 4, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Uesawa, Y.; Mohri, K. The Use of Heat Treatment to Eliminate Drug Interactions Due to Grapefruit Juice. Biol. Pharm. Bull. 2006, 29, 2274–2278. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Medicinal Herbs: Herbal Reference Library; Taylor & Francis: Abingdon, UK, 2001. [Google Scholar]
- Sethna, S.M.; Shah, N.M. The Chemistry of Coumarins. Chem. Rev. 1945, 36, 1–62. [Google Scholar] [CrossRef]
- Stanley, W.L.; Vannier, S.H. Chemical Composition of Lemon Oil. I. Isolation of a Series of Substituted Coumarins. J. Am. Chem. Soc. 1957, 79, 3488–3491. [Google Scholar] [CrossRef]
- Phucharoenrak, P.; Muangnoi, C.; Trachootham, D. Metabolomic Analysis of Phytochemical Compounds from Ethanolic Extract of Lime (Citrus aurantifolia) Peel and Its Anti-Cancer Effects against Human Hepatocellular Carcinoma Cells. Molecules 2023, 28, 2965. [Google Scholar] [CrossRef]
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef]
- Myung, K.; Manthey, J.A.; Narciso, J.A. Biotransformations of 6′,7′-dihydroxybergamottin and 6′,7′-epoxybergamottin by the citrus-pathogenic fungi diminish cytochrome P450 3A4 inhibitory activity. Bioorganic Med. Chem. Lett. 2012, 22, 2279–2282. [Google Scholar] [CrossRef]
- CaymanChemical. Product Information: Bergaptol. Available online: https://www.caymanchem.com/product/38742 (accessed on 31 October 2023).
- ChemSpider. Bergaptol. Available online: http://www.chemspider.com/Chemical-Structure.4444066.html (accessed on 11 August 2023).
- SelleckChemical. Bergaptol: Technical Data. Available online: https://www.selleckchem.com/datasheet/bergaptol-S944200-DataSheet.html (accessed on 31 October 2023).
- Valussi, M.; Donelli, D.; Firenzuoli, F.; Antonelli, M. Bergamot Oil: Botany, Production, Pharmacology. Encyclopedia 2021, 1, 152–176. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Zhang, L.; Zhang, Y.-B.; Yang, X.-W. Simultaneous assessment of absorption characteristics of coumarins from Angelicae Pubescentis Radix: In vitro transport across Caco-2 cell and in vivo pharmacokinetics in rats after oral administration. J. Chromatogr. B 2017, 1060, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Melough, M.M.; Vance, T.M.; Lee, S.G.; Provatas, A.A.; Perkins, C.; Qureshi, A.; Cho, E.; Chun, O.K. Furocoumarin Kinetics in Plasma and Urine of Healthy Adults Following Consumption of Grapefruit (Citrus paradisi Macf.) and Grapefruit Juice. J. Agric. Food Chem. 2017, 65, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Myung, K.; Manthey, J.A.; Narciso, J.A. Aspergillus niger metabolism of citrus furanocoumarin inhibitors of human cytochrome P450 3A4. Appl. Microbiol. Biotechnol. 2008, 78, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Messer, A.; Nieborowski, A.; Strasser, C.; Lohr, C.; Schrenk, D. Major furocoumarins in grapefruit juice I: Levels and urinary metabolite(s). Food Chem. Toxicol. 2011, 49, 3224–3231. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Goldeck, D.; Derhovanessian, E. Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 2014, 29, 23–28. [Google Scholar] [CrossRef]
- Tang, S.Y.; Cheah, I.K.; Wang, H.; Halliwell, B. Notopterygium forbesii Boiss Extract and Its Active Constituent Phenethyl Ferulate Attenuate Pro-Inflammatory Responses to Lipopolysaccharide in RAW 264.7 Macrophages. A “Protective” Role for Oxidative Stress? Chem. Res. Toxicol. 2009, 22, 1473–1482. [Google Scholar] [CrossRef]
- Dhara, A.K.; Nayak, A.K. Chapter 1—Introduction to herbal biomolecules. In Herbal Biomolecules in Healthcare Applications; Mandal, S.C., Nayak, A.K., Dhara, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–19. [Google Scholar]
- Uto, T.; Tung, N.H.; Taniyama, R.; Miyanowaki, T.; Morinaga, O.; Shoyama, Y. Anti-inflammatory Activity of Constituents Isolated from Aerial Part of Angelica acutiloba Kitagawa. Phytother. Res. 2015, 29, 1956–1963. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Wang, T.-X.; Jiang, J.-G.; Huang, C.-L.; Zhu, W. Bergaptol from blossoms of Citrus aurantium L. var. amara Engl inhibits LPS-induced inflammatory responses and ox-LDL-induced lipid deposition. Food Funct. 2020, 11, 4915–4926. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S. Chapter 9—Molecular Imaging of Rheumatoid Arthritis and Osteoarthritis. In Arthritis in Color; Bruno, M.A., Mosher, T.J., Gold, G.E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 193–213. [Google Scholar]
- Alcaraz, M.J.; Fernández, P.; Guillén, M.I. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef]
- Lee, Y.; Hyun, C.-G. Anti-Inflammatory Effects of Psoralen Derivatives on RAW264.7 Cells via Regulation of the NF-κB and MAPK Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 5813. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Xie, Q.; He, X.; Guo, Z.; Zheng, B.; Wang, S.; Yang, Q.; Du, C. Bergaptol Alleviates LPS-Induced Neuroinflammation, Neurological Damage and Cognitive Impairment via Regulating the JAK2/STAT3/p65 Pathway. J. Inflamm. Res. 2022, 15, 6199–6211. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Girennavar, B.; Jayaprakasha, G.K.; Jadegoud, Y.; Nagana Gowda, G.A.; Patil, B.S. Radical scavenging and cytochrome P450 3A4 inhibitory activity of bergaptol and geranylcoumarin from grapefruit. Bioorganic Med. Chem. 2007, 15, 3684–3691. [Google Scholar] [CrossRef]
- Milanović, Ž.; Antonijević, M.; Jovanović, J.Đ.; Avdović, E.; Milenković, D.; Marković, Z. Influence of Nonpolar Medium on Antioxidant Capacity of Bergaptol and Xanthotoxol—Kinetic DFT Study. Chem. Proc. 2021, 3, 91. [Google Scholar] [CrossRef]
- Tang, J.; You, G.; Ruan, L.; Lu, Y.; Wen, B.; Wu, S. Antioxidant Behavior Affected by Polarity in the Olive Oil: Experimental and Molecular Simulation Investigations. ACS Omega 2021, 6, 7119–7126. [Google Scholar] [CrossRef]
- Milanović, Ž.; Antonijević, M.; Đorović Jovanović, J.; Milenković, D. Comparative Antiradical Activity and Molecular Docking Study of Bergaptol and Xanthotoxol. J. Serb. Soc. Comput. 2020, 13, 71–84. [Google Scholar] [CrossRef]
- Jialal, I.; Fuller, C.J. Oxidized LDL and antioxidants. Clin. Cardiol. 1993, 16, I6–I9. [Google Scholar] [CrossRef]
- Prince Ahad, M.; Shreya, M.; Ashish, V.; Nishant, K.; Manisha, A.; Navneet, N. In-vitro Antioxidant and Anti-inflammatory Potential of Ficus infectoria Fruits. harmacognosy Res. 2022, 14, 153–157. [Google Scholar] [CrossRef]
- Mohamed, T.K. Chemical constituents and antioxidant activity of Citrus paradisi (star-ruby red grapefruit) and Citrus sinensis (blood sweet orange) Egyptian cultivars. Asian J. Chem. 2004, 16, 1753–1764. [Google Scholar]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical Profile and Antioxidant and Anti-Inflammatory Activities of the Enriched Polyphenol Fractions Isolated from Bergamot Fruit and Leave. Antioxidants 2021, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xiang, S.; Pan, Y.; Long, X.; Cheng, Y.; Han, L.; Zhao, X. Effects of Cold-Pressing and Hydrodistillation on the Active Non-volatile Components in Lemon Essential Oil and the Effects of the Resulting Oils on Aging-Related Oxidative Stress in Mice. Front. Nutr. 2021, 8, 689094. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International. Worldwide Cancer Data. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/#:~:text=Find%20information%20about%20world%20cancer,and%208.8%20million%20in%20women (accessed on 5 September 2023).
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Bai, Y.; Li, D.; Zhou, T.; Qin, N.; Li, Z.; Yu, Z.; Hua, H. Coumarins from the roots of Angelica dahurica with antioxidant and antiproliferative activities. J. Funct. Foods 2016, 20, 453–462. [Google Scholar] [CrossRef]
- Ge, Z.-C.; Qu, X.; Yu, H.-F.; Zhang, H.-M.; Wang, Z.-H.; Zhang, Z.-T. Antitumor and apoptotic effects of bergaptol are mediated via mitochondrial death pathway and cell cycle arrest in human breast carcinoma cells. Bangladesh J. Pharmacol. 2016, 11, 489–494. [Google Scholar] [CrossRef]
- Sadasivam, M.; Kumarasamy, C.; Thangaraj, A.; Govindan, M.; Kasirajan, G.; Vijayan, V.; Devadasan, V.; Chia-Her, L.; Madhusudhanan, G.R.; Ramaraj, T.; et al. Phytochemical constituents from dietary plant Citrus hystrix. Nat. Prod. Res. 2018, 32, 1721–1726. [Google Scholar] [CrossRef]
- Connolly, P.; Garcia-Carpio, I.; Villunger, A. Cell-Cycle Cross Talk with Caspases and Their Substrates. Cold Spring Harb. Perspect. Biol. 2020, 12, a036475. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, P.E.; Chen, Z.J.; Pang, Y.C.; Xu, Z.W.; Tan, J.; Jiang, Z.H.; Yang, B.B.; Zhan, R.; Xu, H.; et al. Ethanol Extract of Citrus grandis ‘Tomentosa’ Exerts Anticancer Effects by Targeting Skp2/p27 Pathway in Non-Small Cell Lung Cancer. Mol. Nutr. Food Res. 2023, 67, e2300061. [Google Scholar] [CrossRef]
- Cai, Z.; Moten, A.; Peng, D.; Hsu, C.-C.; Pan, B.-S.; Manne, R.; Li, H.-y.; Lin, H.-K. The Skp2 Pathway: A Critical Target for Cancer Therapy. Semin. Cancer Biol. 2020, 67, 16–33. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. The Multifaced Role of STAT3 in Cancer and Its Implication for Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhang, C.; Martincuks, A.; Herrmann, A.; Yu, H. STAT proteins in cancer: Orchestration of metabolism. Nat. Rev. Cancer 2023, 23, 115–134. [Google Scholar] [CrossRef]
- Chang, N.-S.; To, K.K.; Liou, Y.-C.; Li, Y.-J. Editorial: The role of STAT3 signaling pathway in tumor progression. Front. Oncol. 2023, 13, 1151862. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef]
- Yap, T.A.; Omlin, A.; de Bono, J.S. Development of therapeutic combinations targeting major cancer signaling pathways. J. Clin. Oncol. 2013, 31, 1592–1605. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Ohnishi, A.; Matsuo, H.; Yamada, S.; Takanaga, H.; Morimoto, S.; Shoyama, Y.; Ohtani, H.; Sawada, Y. Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by Caco-2 cells and on the activity of cytochrome P450 3A4. Br. J. Pharmacol. 2000, 130, 1369–1377. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine, and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.D.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef]
- Wacher, V.J.; Wu, C.Y.; Benet, L.Z. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: Implications for drug delivery and activity in cancer chemotherapy. Mol. Carcinog. 1995, 13, 129–134. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Yuan, S.; Su, Y.; Jia, Y.; Zhang, Y.; Duan, X. Study of Active Phytochemicals and Mechanisms of Cnidii Fructus in Treating Osteoporosis Based on HPLC-Q-TOF-MS/MS and Network Pharmacology. Comb. Chem. High. Throughput Screen. 2023, 27, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, S.; Liu, J.; Liu, Y.; Liang, Q. Vitamin K2 stimulates MC3T3-E1 osteoblast differentiation and mineralization through autophagy induction. Mol. Med. Rep. 2019, 19, 3676–3684. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Yang, R.S.; Chien, M.Y.; Chen, C.C.; Fu, W.M. Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and ERK-dependent pathway in osteoblasts. Eur. J. Pharmacol. 2008, 579, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-S.; Xu, X.-X.; Shi, Y.-Y.; Chen, Y.; Li, Y.-Q.; Jiang, S.-Q.; Wang, T.; Li, P.; Li, F. System pharmacology analysis to decipher the effect and mechanism of active ingredients combination from herb couple on rheumatoid arthritis in rats. J. Ethnopharmacol. 2022, 288, 114969. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vasc. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef]
- Kong, F.; Ding, Z.; Zhang, K.; Duan, W.; Qin, Y.; Su, Z.; Bi, Y. Optimization of extraction flavonoids from Exocarpium Citri Grandis and evaluation its hypoglycemic and hypolipidemic activities. J. Ethnopharmacol. 2020, 262, 113178. [Google Scholar] [CrossRef]
- Ansari, B.; Singh, M.; Sharma, S.; Choudhary, B.; Mohseen, M. Preclinical Antihyperlipidemic Effect of Herbalism against Lipid Elevating Agents: A Review. Biomed. Pharmacol. J. 2020, 13, 1695–1707. [Google Scholar] [CrossRef]
- Rathi, P.; Nath, R.; Pant, K.; Dixit, R.; Pal, R.; Kumar, R. Evaluation of Hypolipidemic and TNF-α Lowering Effect of Ficus Religiosa in Dyslipidemic Wistar Rats. Curre Res. Diabetes Obes. J. 2019, 10, 555799. [Google Scholar] [CrossRef]
- Pan, Y.; Tan, J.; Long, X.; Yi, R.; Zhao, X.; Park, K.-Y. Anti-obesity effect of fermented lemon peel on high-fat diet-induced obese mice by modulating the inflammatory response. J. Food Biochem. 2022, 46, e14200. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. The role of TNF alpha in adipocyte metabolism. Semin. Cell Dev. Biol. 1999, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Manderfeld, M.M.; Schafer, H.W.; Davidson, P.M.; Zottola, E.A. Isolation and identification of antimicrobial furocoumarins from parsley. J. Food Prot. 1997, 60, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Girennavar, B. Grapefruit-Drug Interaction: Isolation, Synthesis, and Biological Activities of Furocoumarins and Their Variation Due to Pre-and Post–Harvest Factors. PhD Dissertation, Texas A&M University, College Station, TX, USA, 2007. [Google Scholar]
- DeLisa, M.P.; Bentley, W.E. Bacterial autoinduction: Looking outside the cell for new metabolic engineering targets. Microb. Cell Fact. 2002, 1, 5. [Google Scholar] [CrossRef]
- Mok, K.C.; Wingreen, N.S.; Bassler, B.L. Vibrio harveyi quorum sensing: A coincidence detector for two autoinducers controls gene expression. Embo J. 2003, 22, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, D.; Mansor, M.; Yan, G.O.; Md Yusof, M.Y.; Hassan, H.; Sekaran, S.D. Detection of quorum sensing signal molecules and identification of an autoinducer synthase gene among biofilm-forming clinical isolates of Acinetobacter spp. PLoS ONE 2012, 7, e36696. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef]
- Persson, T.; Givskov, M.; Nielsen, J. Quorum Sensing Inhibition: Targeting Chemical Communication in Gram-negative Bacteria. Curr. Med. Chem. 2005, 12, 3103–3115. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Bondar, A.; Konovalova, S.; Sultanov, R.; Manzhulo, I. N-Docosahexanoylethanolamine Reduces Microglial Activation and Improves Hippocampal Plasticity in a Murine Model of Neuroinflammation. Int. J. Mol. Sci. 2020, 21, 9703. [Google Scholar] [CrossRef]
- Galvani, G.; Mottolese, N.; Gennaccaro, L.; Loi, M.; Medici, G.; Tassinari, M.; Fuchs, C.; Ciani, E.; Trazzi, S. Inhibition of microglia overactivation restores neuronal survival in a mouse model of CDKL5 deficiency disorder. J. Neuroinflamm. 2021, 18, 155. [Google Scholar] [CrossRef]
- Moyse, E.; Krantic, S.; Djellouli, N.; Roger, S.; Angoulvant, D.; Debacq, C.; Leroy, V.; Fougere, B.; Aidoud, A. Neuroinflammation: A Possible Link Between Chronic Vascular Disorders and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 827263. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Liao, Y.-R.; Hung, H.-Y.; Chuang, C.-W.; Hwang, T.-L.; Huang, S.-C.; Shiao, Y.-J.; Kuo, D.-H.; Wu, T.-S. Anti-inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis. Molecules 2017, 22, 967. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Habermeyer, M.; Schrenk, D.; Eisenbrand, G. Update of the toxicological assessment of furanocoumarins in foodstuffs (Update of the SKLM statement of 23/24 September 2004)—Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol. Nutr. Food Res. 2011, 55, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Rice, M.S.; Park, M.K.; Chun, O.K.; Melough, M.M.; Nan, H.; Willett, W.C.; Li, W.-Q.; Qureshi, A.A.; Cho, E. Intake of Furocoumarins and Risk of Skin Cancer in 2 Prospective US Cohort Studies. J. Nutr. 2020, 150, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Lee, W. Dietary assessment methods. In Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings; FAO, Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Messer, A.; Raquet, N.; Lohr, C.; Schrenk, D. Major furocoumarins in grapefruit juice II: Phototoxicity, photogenotoxicity, and inhibitory potency vs. cytochrome P450 3A4 activity. Food Chem. Toxicol. 2012, 50, 756–760. [Google Scholar] [CrossRef]
- Jiang, T.; Cheng, T.; Li, J.; Zhou, M.; Tan, R.; Yang, X.; Wang, Y.; Li, W.; Zheng, J. Bergaptol, a mechanism-based inactivator of CYP2C9. Med. Chem. Res. 2020, 29, 1230–1237. [Google Scholar] [CrossRef]
- Klein, K.; Zanger, U.M. Pharmacogenomics of Cytochrome P450 3A4: Recent Progress Toward the “Missing Heritability” Problem. Front. Genet. 2013, 4, 12. [Google Scholar] [CrossRef]
- Miners, J.O.; Birkett, D.J. Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol. 1998, 45, 525–538. [Google Scholar] [CrossRef]
- Girennavar, B.; Jayaprakasha, G.K.; Patil, B.S. Potent Inhibition of Human Cytochrome P450 3A4, 2D6, and 2C9 Isoenzymes by Grapefruit Juice and Its Furocoumarins. J. Food Sci. 2007, 72, C417–C421. [Google Scholar] [CrossRef]
- Hanley, M.J.; Cancalon, P.; Widmer, W.W.; Greenblatt, D.J. The effect of grapefruit juice on drug disposition. Expert. Opin. Drug Metab. Toxicol. 2011, 7, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Row, E.C.; Brown, S.A.; Stachulski, A.V.; Lennard, M.S. Synthesis of 8-geranyloxypsoralen analogues and their evaluation as inhibitors of CYP3A4. Bioorg. Med. Chem. 2006, 14, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, M.; Al Rihani, S.B.; Arwood, M.J.; Darakjian, L.; Dow, P.; Turgeon, J.; Michaud, V. Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice. Pharmaceutics 2020, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.B.; Kawano, D.F.; Carvalho, I.; da Conceição, E.C.; de Freitas, O.; da Silva, C.H. Psoralen and Bergapten: In Silico Metabolism and Toxicophoric Analysis of Drugs Used to Treat Vitiligo. J. Pharm. Pharm. Sci. 2009, 12, 378–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
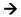 indicates activation,
indicates activation,  indicates inhibition.
indicates inhibition.
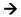 indicates activation,
indicates activation,  indicates inhibition.
indicates inhibition.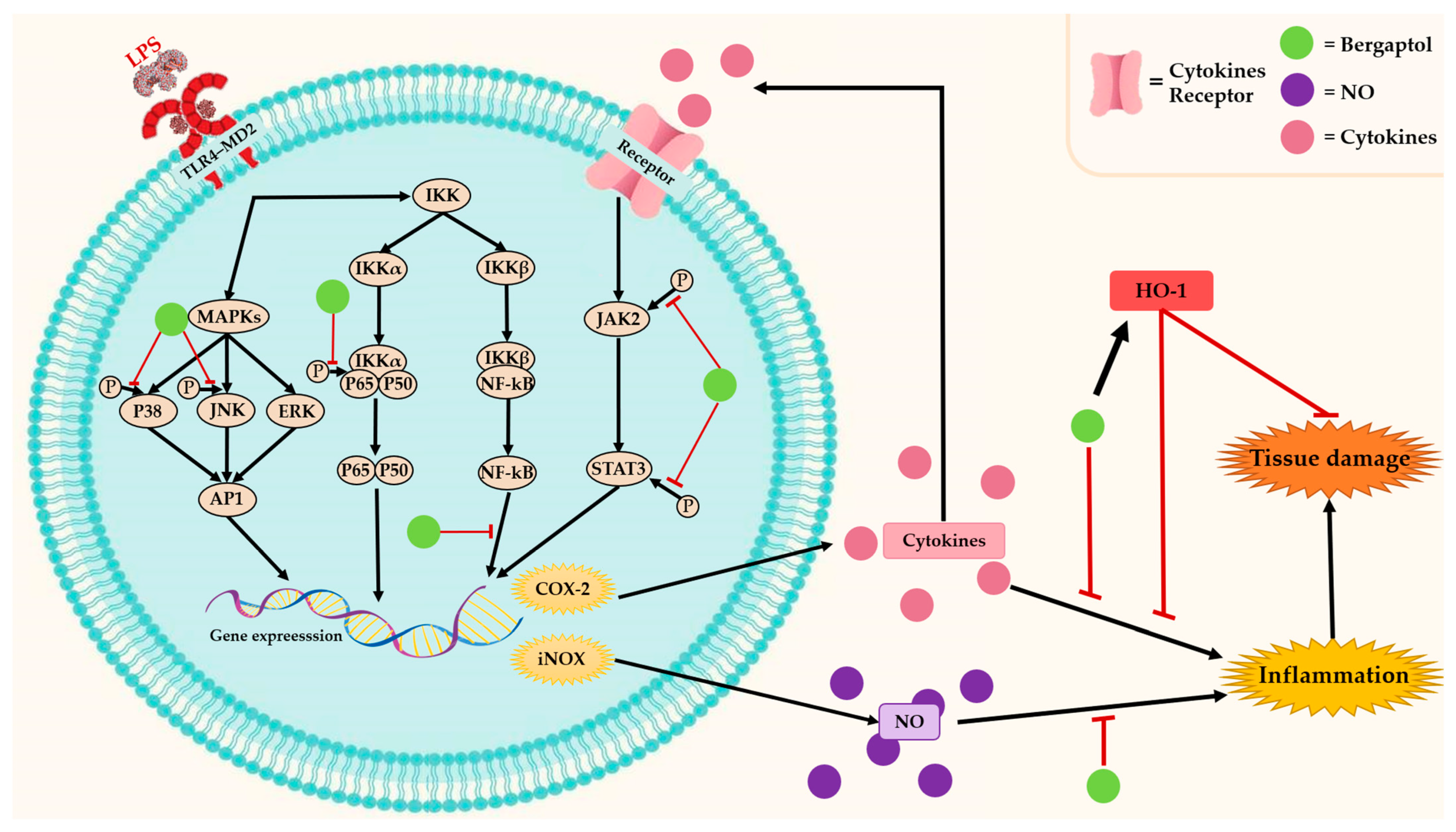
 indicates activation,
indicates activation,  indicates inhibition, and
indicates inhibition, and  indicates possibility of inhibition.
indicates possibility of inhibition.
 indicates activation,
indicates activation,  indicates inhibition, and
indicates inhibition, and  indicates possibility of inhibition.
indicates possibility of inhibition.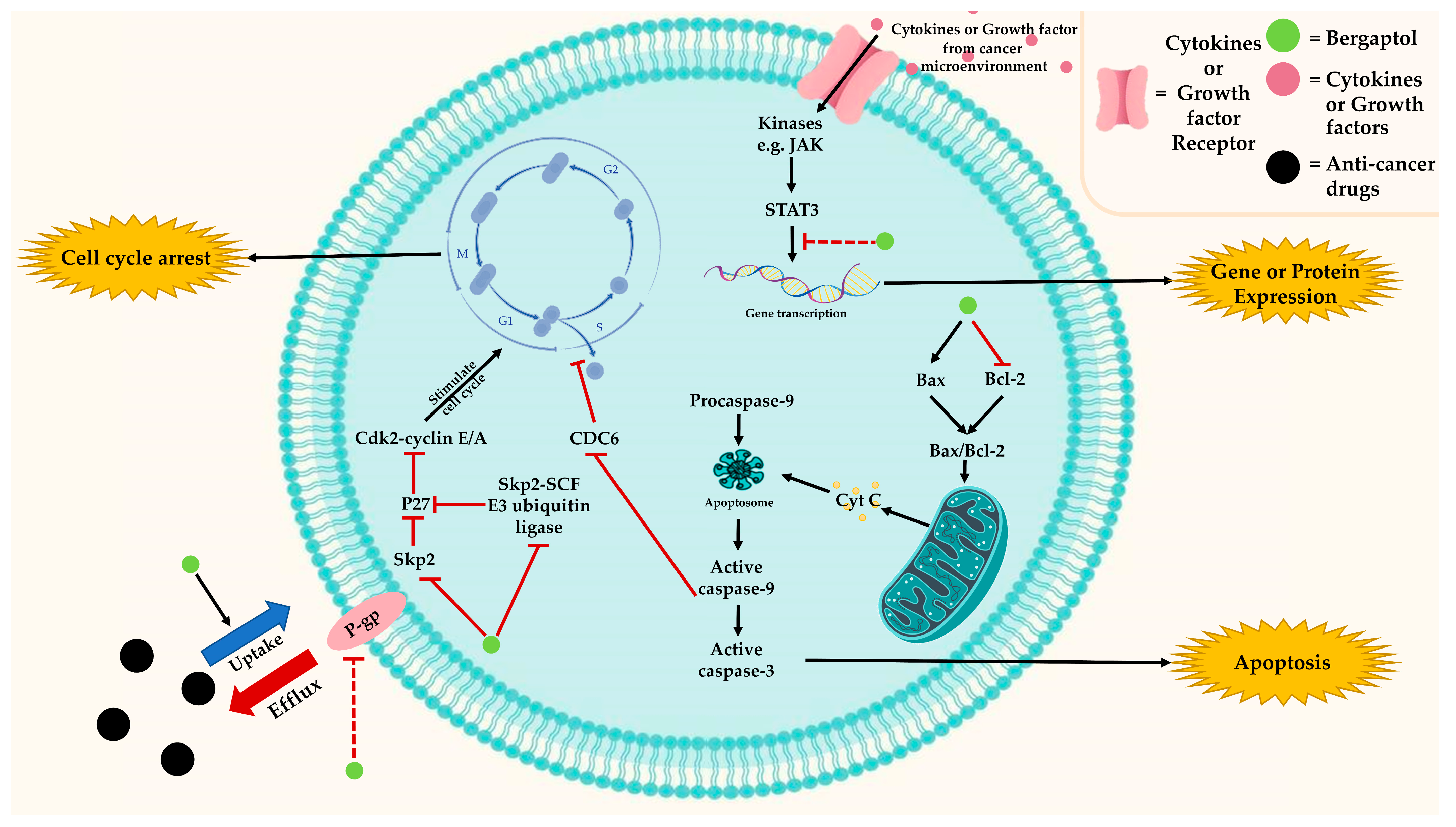
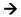 indicates activation,
indicates activation,  indicates inhibition.
indicates inhibition.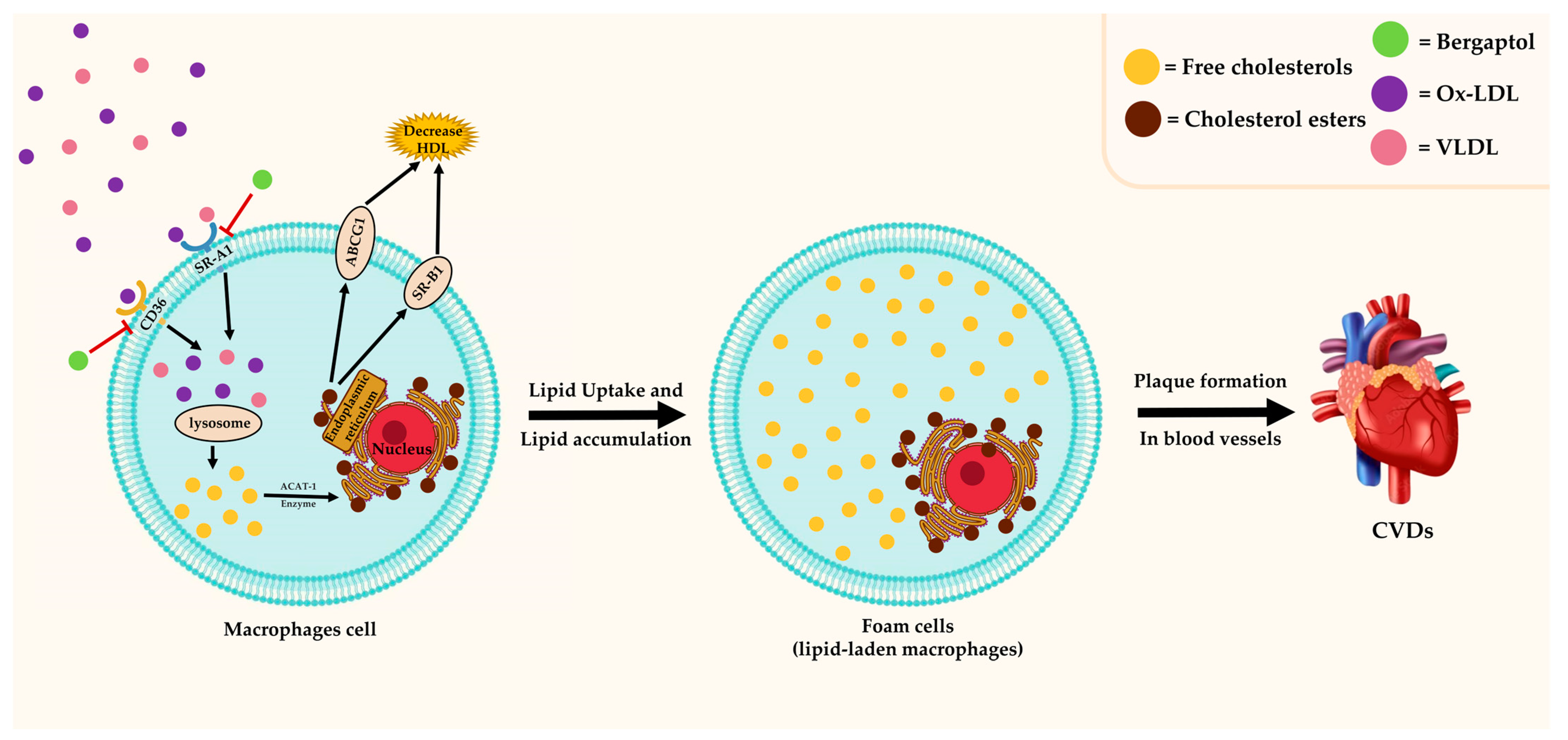
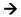 indicates activation,
indicates activation,  indicates inhibition.
indicates inhibition.
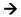 indicates activation,
indicates activation,  indicates inhibition.
indicates inhibition.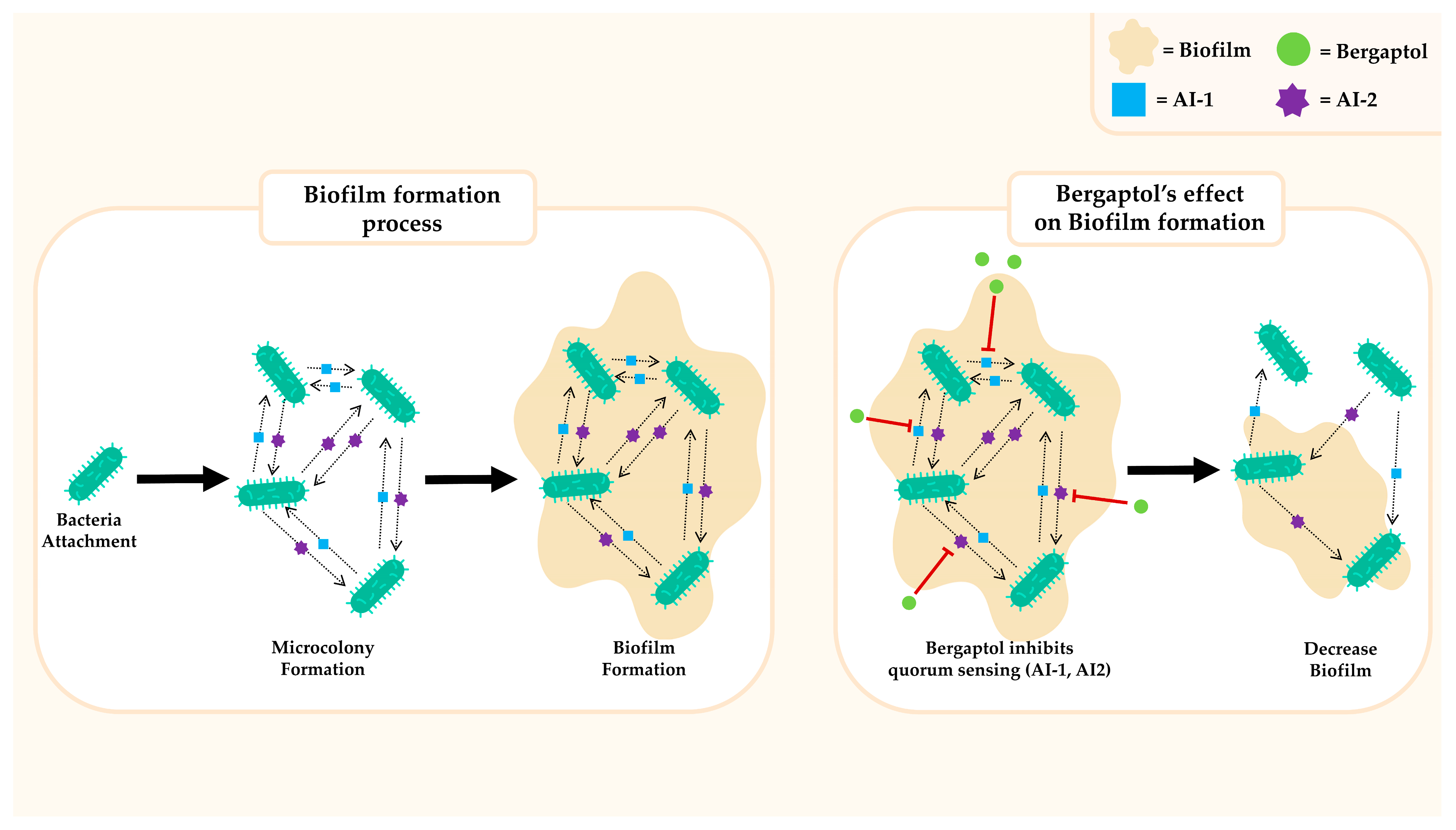
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phucharoenrak, P.; Trachootham, D. Bergaptol, a Major Furocoumarin in Citrus: Pharmacological Properties and Toxicity. Molecules 2024, 29, 713. https://doi.org/10.3390/molecules29030713
Phucharoenrak P, Trachootham D. Bergaptol, a Major Furocoumarin in Citrus: Pharmacological Properties and Toxicity. Molecules. 2024; 29(3):713. https://doi.org/10.3390/molecules29030713
Chicago/Turabian StylePhucharoenrak, Pakkapong, and Dunyaporn Trachootham. 2024. "Bergaptol, a Major Furocoumarin in Citrus: Pharmacological Properties and Toxicity" Molecules 29, no. 3: 713. https://doi.org/10.3390/molecules29030713
APA StylePhucharoenrak, P., & Trachootham, D. (2024). Bergaptol, a Major Furocoumarin in Citrus: Pharmacological Properties and Toxicity. Molecules, 29(3), 713. https://doi.org/10.3390/molecules29030713




