Boosted Electrocatalytic Degradation of Levofloxacin by Chloride Ions: Performances Evaluation and Mechanism Insight with Different Anodes
Abstract
1. Introduction
2. Results and Discussion
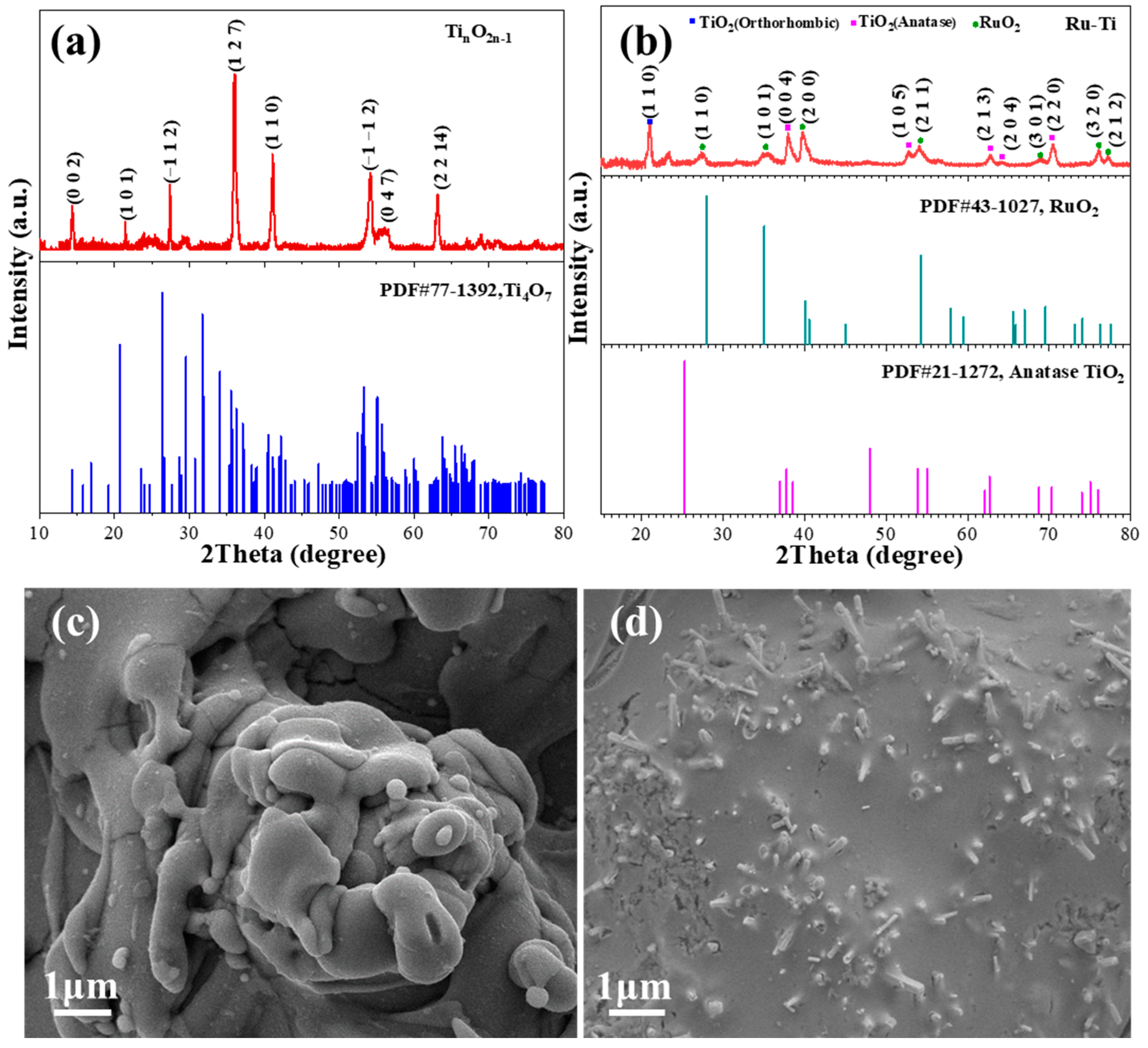
2.1. Characterization of Catalytic Electrodes
2.2. Electrochemical Properties of Catalytic Electrodes
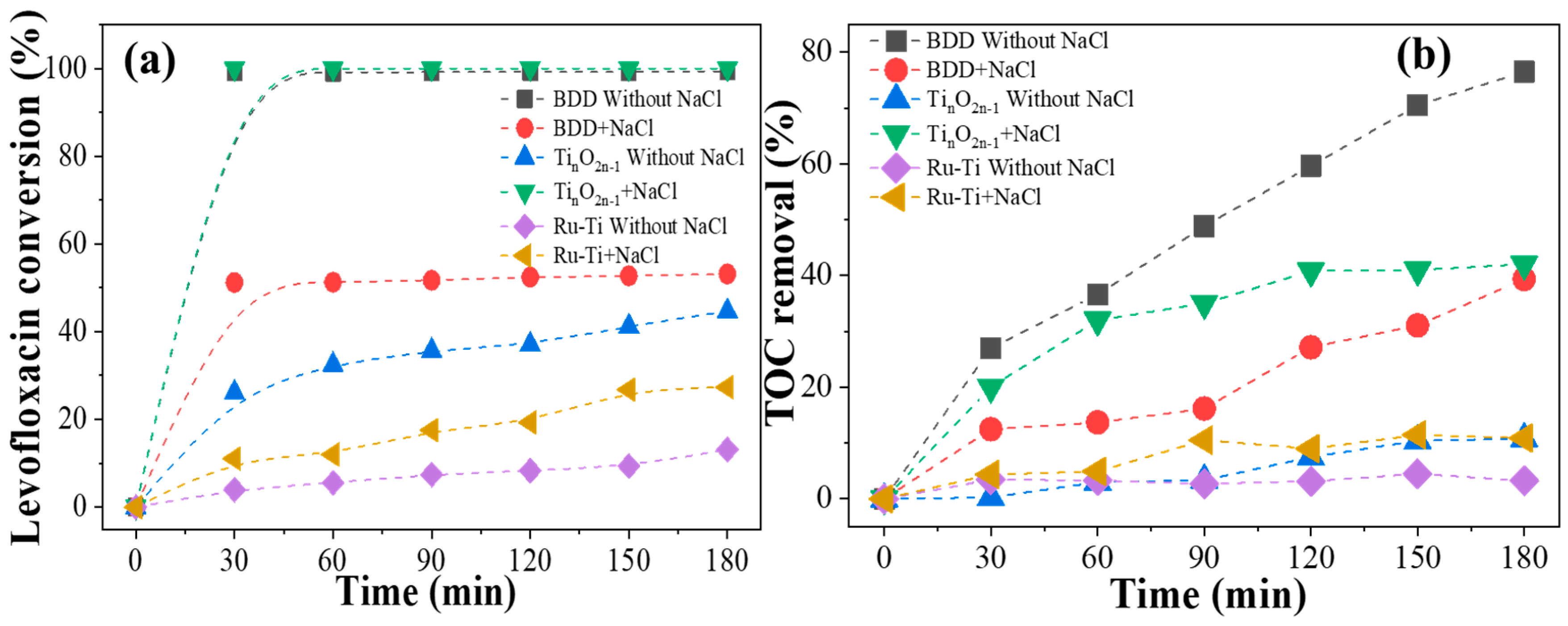
2.3. Effects of Chloride Ions on Electrochemical Properties
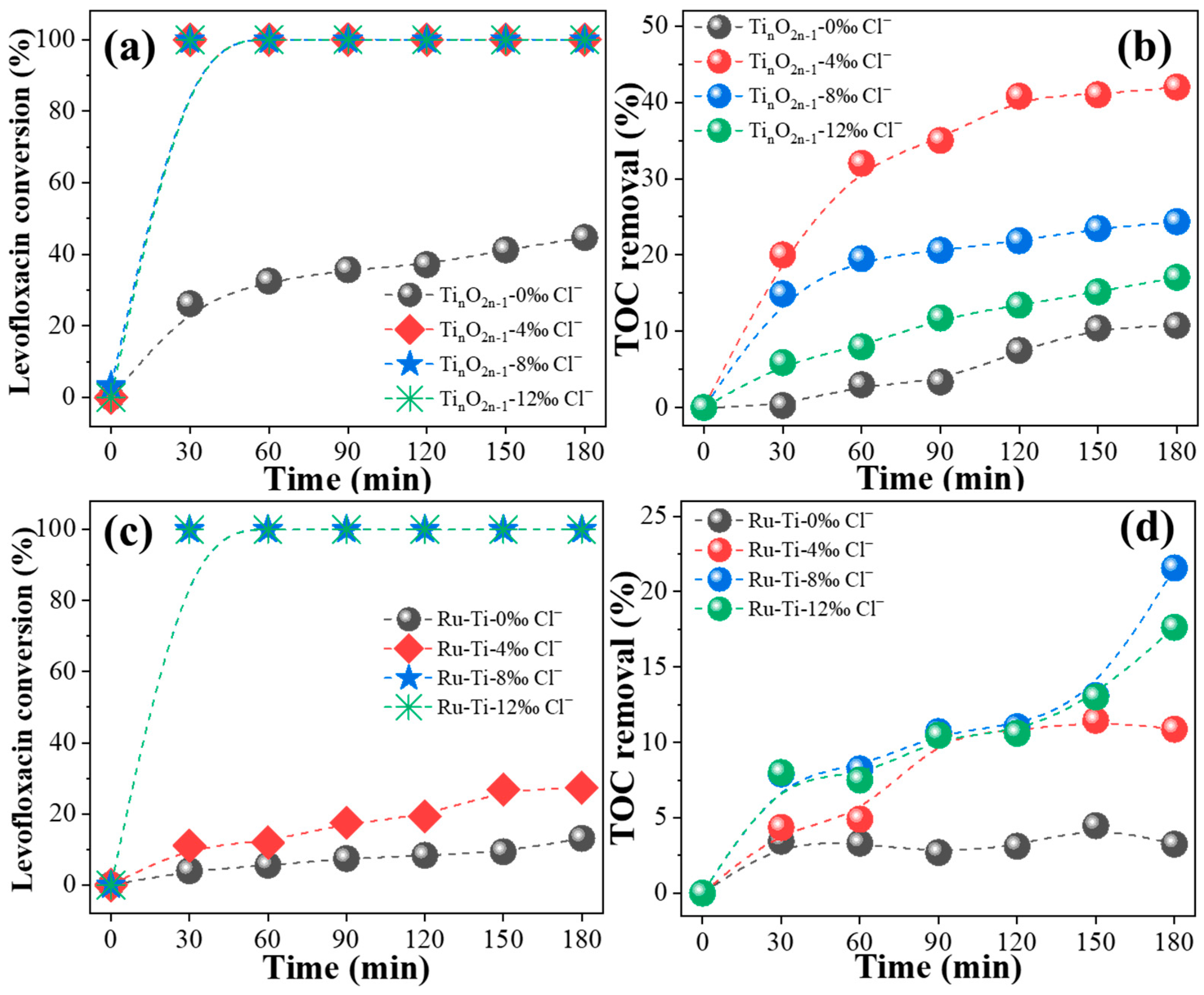
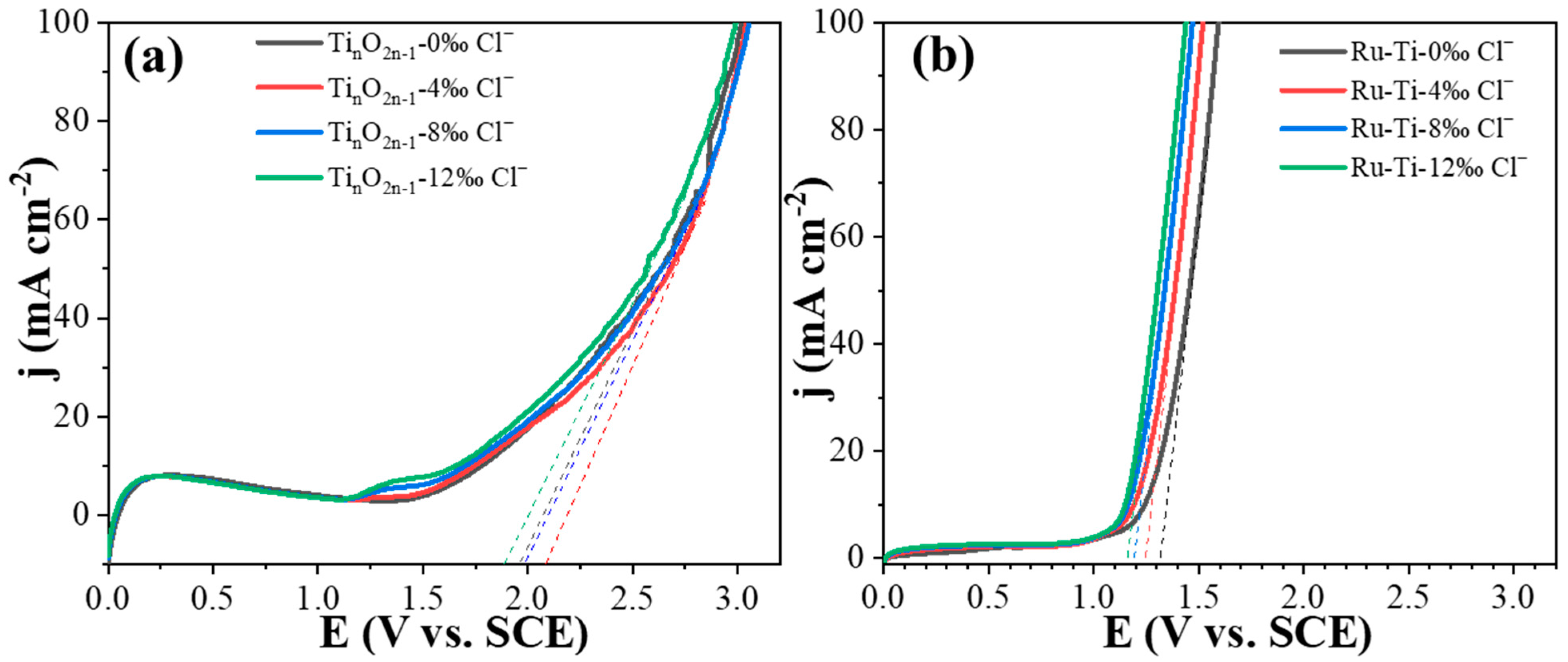
2.4. Effect of Different Chloride Concentrations on Electrochemical Properties
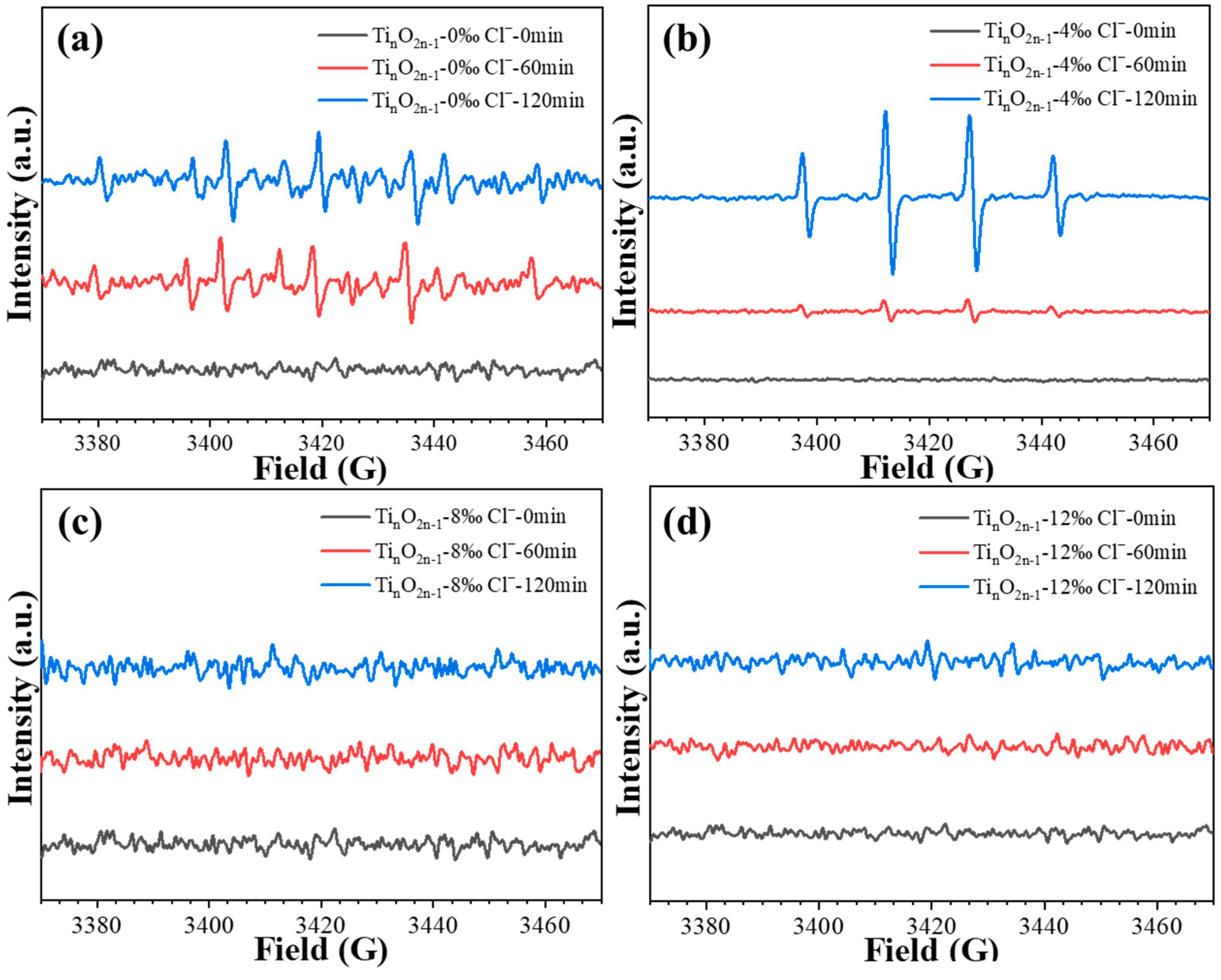
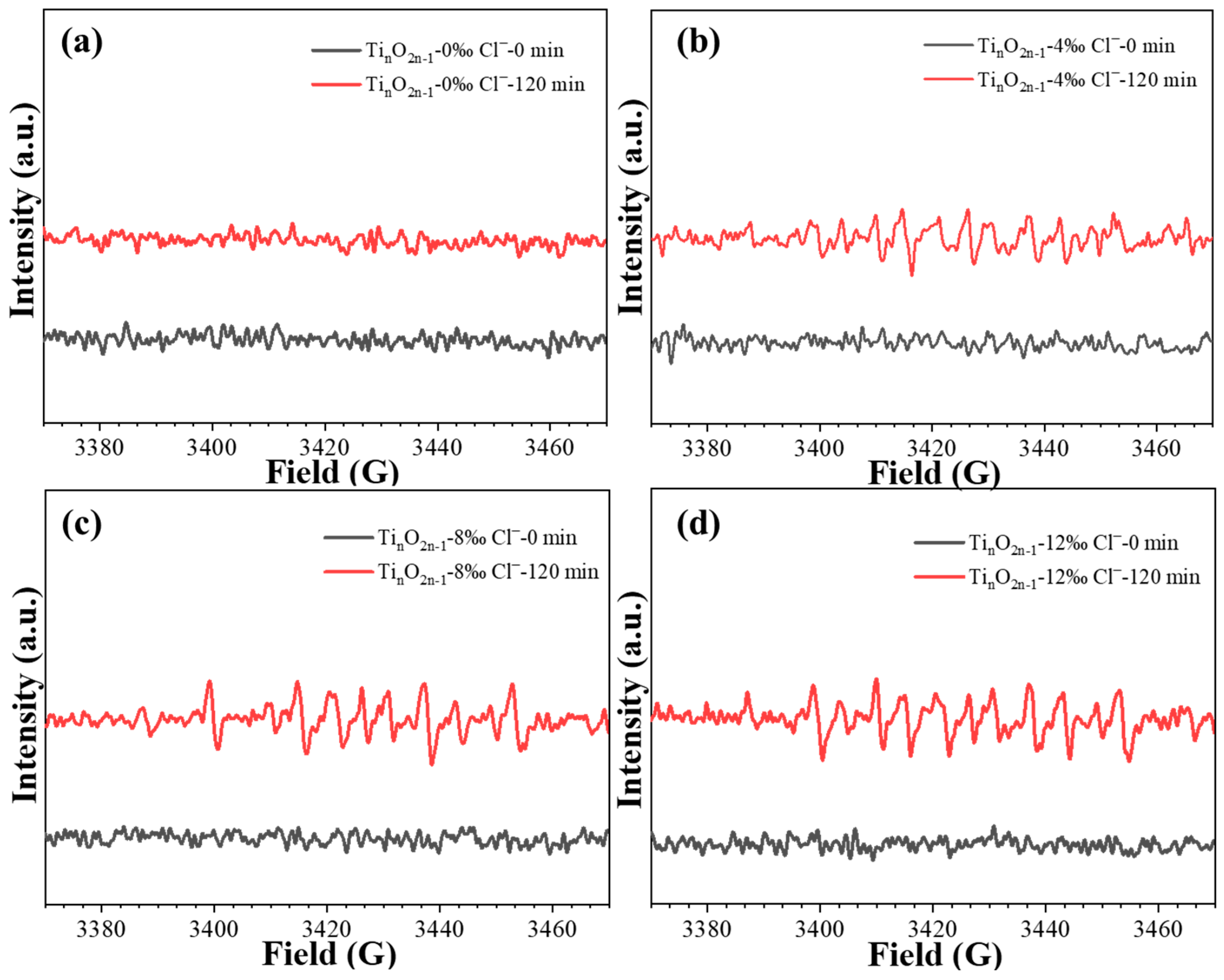
2.5. Chlorine Free Radical Test
2.6. Electrochemical Testing
2.7. Exploration of Reaction Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Electrode Preparation
3.3. Experimental Instruments
3.4. Material Characterizations
3.5. Electrocatalytic Degradation of Levofloxacin
3.6. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Mesdaghinia, A. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci. Total Environ. 2018, 619–620, 446–459. [Google Scholar] [CrossRef]
- Sinha, R.K.; Biswas, P. Structural elucidation of Levofloxacin and Ciprofloxacin using density functional theory and Raman spectroscopy with inexpensive lab-built setup. J. Mol. Struct. 2020, 1222, 128946. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Wang, J.; Wang, J.; Zhu, L.; Ge, W. Macrolide- and quinolone-resistant bacteria and resistance genes as indicators of antibiotic resistance gene contamination in farmland soil with manure application. Ecol. Indic. 2019, 106, 105456. [Google Scholar] [CrossRef]
- Haenni, M.; Dagot, C.; Chesneau, O.; Bibbal, D.; Labanowski, J.; Vialette, M.; Bouchard, D.; Martin-Laurent, F.; Calsat, L.; Nazaret, S.; et al. Environmental contamination in a high-income country (France) by antibiotics, antibiotic-resistant bacteria, and antibiotic resistance genes: Status and possible causes. Environ. Int. 2022, 159, 107047. [Google Scholar] [CrossRef]
- Yan, N.; Xia, S.; Xu, L.; Zhu, J.; Zhang, Y.; Rittmann, B.E. Internal loop photobiodegradation reactor (ILPBR) for accelerated degradation of sulfamethoxazole (SMX). Appl. Microbiol. Biotechnol. 2012, 94, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.; Kolvenbach, B.A.; Nunes, O.C.; Corvini, P.F.X. Biodegradation of antibiotics: The new resistance determinants—Part II. New Biotechnol. 2020, 54, 13–27. [Google Scholar] [CrossRef]
- Lai, H.T.; Wang, T.S.; Chou, C.C. Implication of light sources and microbial activities on degradation of sulfonamides in water and sediment from a marine shrimp pond. Bioresour. Technol. 2011, 102, 5017–5023. [Google Scholar] [CrossRef]
- Dorival-Garcia, N.; Zafra-Gomez, A.; Navalon, A.; Gonzalez, J.; Vilchez, J.L. Removal of quinolone antibiotics from wastewaters by sorption and biological degradation in laboratory-scale membrane bioreactors. Sci. Total Environ. 2013, 442, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Minale, M.; Guadie, A.; Li, Y.; Meng, Y.; Wang, X.; Zhao, J. Enhanced removal of oxytetracycline antibiotics from water using manganese dioxide impregnated hydrogel composite: Adsorption behavior and oxidative degradation pathways. Chemosphere 2021, 280, 130926. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, Y.; Zou, B.; Lou, Q.; Zhang, W.; Zhong, J.; Lu, L.; Dai, G. Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J. Hazard. Mater. 2016, 307, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Pirom, T.; Sunsandee, N.; Wongsawa, T.; Ramakul, P.; Pancharoen, U.; Nootong, K. The effect of temperature on mass transfer and thermodynamic parameters in the removal of amoxicillin via hollow fiber supported liquid membrane. Chem. Eng. J. 2015, 265, 75–83. [Google Scholar] [CrossRef]
- Sahar, E.; Messalem, R.; Cikurel, H.; Aharoni, A.; Brenner, A.; Godehardt, M.; Jekel, M.; Ernst, M. Fate of antibiotics in activated sludge followed by ultrafiltration (CAS-UF) and in a membrane bioreactor (MBR). Water Res. 2011, 45, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.L.; Tonetti, A.L.; Vidal, C.; Montagner, C.C.; Nogueira, R.F.P. Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation. Appl. Catal. B Environ. 2018, 224, 761–771. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Ge, R.; Yang, S.; Zhai, Y.; Hua, T.; Ondon, B.S.; Zhou, Q.; Li, F. Microbial electro-Fenton: A promising system for antibiotics resistance genes degradation and energy generation. Sci. Total Environ. 2020, 699, 134160. [Google Scholar] [CrossRef]
- Gupta, P.; Verma, N. Evaluation of degradation and mineralization of glyphosate pollutant in wastewater using catalytic wet air oxidation over Fe-dispersed carbon nanofibrous beads. Chem. Eng. J. 2021, 417, 128029. [Google Scholar] [CrossRef]
- Segura, Y.; Cruz Del Alamo, A.; Munoz, M.; Alvarez-Torrellas, S.; Garcia, J.; Casas, J.A.; De Pedro, Z.M.; Martinez, F. A comparative study among catalytic wet air oxidation, Fenton, and Photo-Fenton technologies for the on-site treatment of hospital wastewater. J. Environ. Manag. 2021, 290, 112624. [Google Scholar] [CrossRef]
- Qian, X.; Chen, Z.; Yang, X.; Zhao, W.; Liu, C.; Sun, T.; Zhou, D.; Yang, Q.; Wei, G.; Fan, M. Perovskite cesium lead bromide quantum dots: A new efficient photocatalyst for degrading antibiotic residues in organic system. J. Clean. Prod. 2020, 249, 119335. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L. Enhanced photocatalytic oxidation degradability for real cyanide wastewater by designing photocatalyst GO/TiO2/ZSM-5: Performance and mechanism research. Chem. Eng. J. 2022, 428, 131257. [Google Scholar] [CrossRef]
- Teng, J.; You, S.; Ma, F.; Chen, X.; Ren, N. Enhanced electrochemical decontamination and water permeation of titanium suboxide reactive electrochemical membrane based on sonoelectrochemistry. Ultrason. Sonochem. 2020, 69, 105248. [Google Scholar] [CrossRef]
- Pei, S.; Shi, H.; Zhang, J.; Wang, S.; Ren, N.; You, S. Electrochemical removal of tetrabromobisphenol A by fluorine-doped titanium suboxide electrochemically reactive membrane. J. Hazard. Mater. 2021, 419, 126434. [Google Scholar] [CrossRef]
- Yang, X.; Jiuji, G.; Zhaowu, Z.; Zhang, H.; Qi, T. Doping effects on the electro-degradation of phenol on doped titanium suboxide anodes. Chin. J. Chem. Eng. 2018, 26, 830–837. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, H.; Teng, J.; You, S. Electrochemical degradation of perfluorooctanoic acid by macro-porous titanium suboxide anode in the presence of sulfate. Chem. Eng. J. 2019, 371, 7–14. [Google Scholar] [CrossRef]
- Yu, S.; Gao, Y.; Khan, R.; Liang, P.; Zhang, X.; Huang, X. Electrospun PAN-based graphene/SnO2 carbon nanofibers as anodic electrocatalysis microfiltration membrane for sulfamethoxazole degradation. J. Membr. Sci. 2020, 614, 118368. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Bedford, N.M.; Han, Z.; Thomsen, L.; Smith, S.; Amal, R.; Lu, X. Direct insights into the role of epoxy groups on cobalt sites for acidic H2O2 production. Nat. Commun. 2020, 11, 4181. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Duan, X.; Li, Y.; Zhao, X.; Chang, L. Degradation of polyvinyl chloride microplastics via electrochemical oxidation with a CeO2–PbO2 anode. J. Clean. Prod. 2023, 432, 139668. [Google Scholar] [CrossRef]
- Wang, S.; Lv, H.; Jin, C.; Wang, Y.; Wei, H.; Sun, C.; Yu, L.; Ma, L.; Li, X.; Liu, X. Electrocatalytic degradation of levofloxacin wastewater by Ru-Ti-Ni/CNT electrodes. Catal. Commun. 2023, 182, 106756. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Niu, Y.; Yin, Y.; Xu, R.; Yang, Z.; Wang, J.; Xu, D.; Yuan, Y.; Han, J.; Wang, H. Electrocatalytic oxidation of low concentration cefotaxime sodium wastewater using Ti/SnO2–RuO2 electrode: Feasibility analysis and degradation mechanism. Chemosphere 2022, 297, 134146. [Google Scholar] [CrossRef]
- Hamze, M.; Rezaei, M.; Tabaian, S.H. Cobalt ferrite coated on Ti/Ni/PbOx with enhanced electrocatalytic stability for chloride ions-contained water splitting. J. Electroanal. Chem. 2023, 948, 117823. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T. Enhanced hydrogen generation via overall water splitting using novel MoS2-BN nanoflowers assembled TiO2 ternary heterostructures. Int. J. Hydroen Energy 2023, 48, 22044–22059. [Google Scholar] [CrossRef]
- Marwat, M.A.; Humayun, M.; Afridi, M.W.; Zhang, H.; Abdul Karim, M.R.; Ashtar, M.; Usman, M.; Waqar, S.; Ullah, H.; Wang, C.; et al. Advanced Catalysts for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2021, 4, 12007–12031. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T.A.-O.X. Decorating Thermodynamically Stable (101) Facets of TiO(2) with MoO(3) for Multifunctional Sustainable Hydrogen Energy and Ammonia Gas Sensing Applications. Inorg. Chem. 2024, 63, 304–315. [Google Scholar] [CrossRef]
- Ye, Z.; Guelfi, D.R.V.; Álvarez, G.; Alcaide, F.; Brillas, E.; Sirés, I. Enhanced electrocatalytic production of H2O2 at Co-based air-diffusion cathodes for the photoelectro-Fenton treatment of bronopol. Appl. Catal. B Environ. 2019, 247, 191–199. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Xu, W.; Zhang, Z.; Pang, H.; Yang, J.; Chen, K.; Zhao, H.; Lu, J. Synergistic oxidation of simazine in electro-peroxone system with enhanced in-situ electrogenerated H2O2 activity electrode: Performance and mechanism. J. Clean. Prod. 2023, 404, 136967. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, S.; Song, Z.; Ma, L.; Liu, H.; Fang, M.; Zhang, H.; Ye, X.; Wu, Z. Electrocatalytic degradation of octadecylamine and 4-dodecylmorpholine by the Ti/SnO2-Sb/β-PbO2 anode at high salinity conditions: Activity and mechanism insights. Sep. Purif. Technol. 2024, 330, 125271. [Google Scholar] [CrossRef]
- Bian, X.; Shi, F.; Li, J.; Liang, J.; Bao, C.; Zhang, H.; Jia, J.; Li, K. Highly selective electrocatalytic reduction of nitrate to nitrogen in a chloride ion-free system by promoting kinetic mass transfer of intermediate products in a novel Pd–Cu adsorption confined cathode. J. Environ. Manag. 2022, 324, 116405. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Pan, S.; Zhao, X.; Liu, N. Mn doping improves in-situ H2O2 generation and activation in electro-Fenton process by Fe/Mn@CC cathode using high-temperature shock technique. Chemosphere 2022, 307, 136074. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Li, L.; Wang, Y.; Huang, Q. Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes. Water Res. 2020, 170, 115254. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Liang, S.; Wang, C.; Wang, Y.; Jin, F.; Luo, Q.; Huang, Q. Development of macroporous Magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances. Chem. Eng. J. 2018, 354, 1058–1067. [Google Scholar] [CrossRef]
- Tan, X.; Ma, L.; Han, P.; Wei, H.; Wei, Q.; Sun, C. Fabrication of boron-doped diamond films electrode for efficient electrocatalytic degradation of cresols. Chemosphere 2020, 246, 125786. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Lin, X.; Ganiyu, S.O.; Kamaraj, S.-K.; Thiam, A.; Liu, D. Insight into BDD electrochemical oxidation of florfenicol in water: Kinetics, reaction mechanism, and toxicity. Chemosphere 2022, 288, 132433. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Q.; Du, X.; Xue, T.; Yan, Z.; Liu, Z.; Zhang, H.; Qi, T. The fabrication of Ti4O7 particle composite modified PbO2 coating electrode and its application in the electrochemical oxidation degradation of organic wastewater. J. Alloys Compd. 2022, 897, 162742. [Google Scholar] [CrossRef]
- Guo, S.; Xu, Z.; Hu, W.; Yang, D.; Wang, X.; Xu, H.; Xu, X.; Long, Z.; Yan, W. Progress in Preparation and Application of Titanium Sub-Oxides Electrode in Electrocatalytic Degradation for Wastewater Treatment. Catalysts 2022, 12, 618. [Google Scholar] [CrossRef]
- Sun, J.; Liu, L.; Yang, F. Electro-enhanced chlorine-mediated ammonium nitrogen removal triggered by an optimized catalytic anode for sustainable saline wastewater treatment. Sci. Total Environ. 2021, 776, 146035. [Google Scholar] [CrossRef]
- Li, C.; Feng, G.; Sun, M.; Pan, Z.; Yan, X.; Fan, X.; Song, C.; Wang, T. Preparation and application of high-performance and acid-tolerant TiO2/carbon electrocatalytic membrane for organic wastewater treatment. Chemosphere 2022, 296, 134017. [Google Scholar] [CrossRef]
- Mertah, O.; Gómez-Avilés, A.; Kherbeche, A.; Belver, C.; Bedia, J. Peroxymonosulfate enhanced photodegradation of sulfamethoxazole with TiO2@CuCo2O4 catalysts under simulated solar light. J. Environ. Chem. Eng. 2022, 10, 108438. [Google Scholar] [CrossRef]
- Pang, D.; Liu, Y.; Song, H.; Chen, D.; Zhu, W.; Liu, R.; Yang, H.; Li, A.; Zhang, S. Trace Ti3+- and N-codoped TiO2 nanotube array anode for significantly enhanced electrocatalytic degradation of tetracycline and metronidazole. Chem. Eng. J. 2021, 405, 126982. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Zhang, Q.; Cui, R.; Hassan, M.; Dong, L.; Li, J.; He, Y. Single Mn atom anchored on N-doped porous carbon as highly efficient Fenton-like catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2020, 279, 119363. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Cai, M.; Yang, F.; Liu, Y.; Chen, J.; Zhang, P.; Li, X.; Chen, X. Facile fabrication of TaON/Bi2MoO6 core–shell S-scheme heterojunction nanofibers for boosting visible-light catalytic levofloxacin degradation and Cr(VI) reduction. Chem. Eng. J. 2022, 428, 131158. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.-y.; Lin, L. Fabrication of a SnO2-Sb nano-pin array anode for efficient electrocatalytic oxidation of bisphenol A in wastewater. J. Hazard. Mater. 2023, 444, 130444. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Yang, Y.; Hoffmann, M.R. Activation of Peroxymonosulfate by Oxygen Vacancies-Enriched Cobalt-Doped Black TiO2 Nanotubes for the Removal of Organic Pollutants. Environ. Sci. Technol. 2019, 53, 6972–6980. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Shang, Y.; Gao, B.; Xu, X. Co3O4 anchored in N, S heteroatom co-doped porous carbons for degradation of organic contaminant: Role of pyridinic N-Co binding and high tolerance of chloride. Appl. Catal. B Environ. 2021, 282, 119484. [Google Scholar] [CrossRef]
- Koo, M.S.; Chen, X.; Cho, K.; An, T.; Choi, W. In Situ Photoelectrochemical Chloride Activation Using a WO3 Electrode for Oxidative Treatment with Simultaneous H2 Evolution under Visible Light. Environ. Sci. Technol. 2019, 53, 9926–9936. [Google Scholar] [CrossRef]
- Ni, T.; Feng, H.; Tang, J.; Wang, J.; Yu, J.; Yi, Y.; Wu, Y.; Guo, Y.; Tang, L. A novel electrocatalytic system with high reactive chlorine species utilization capacity to degrade tetracycline in marine aquaculture wastewater. Chemosphere 2022, 300, 134449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, Y.; Zhang, C. Generation and application of reactive chlorine species by electrochemical process combined with UV irradiation: Synergistic mechanism for enhanced degradation performance. Sci. Total Environ. 2020, 712, 136501. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Causserand, C.; Oturan, M.A. Efficiency of plasma elaborated sub-stoichiometric titanium oxide (Ti4O7) ceramic electrode for advanced electrochemical degradation of paracetamol in different electrolyte media. Sep. Purif. Technol. 2019, 208, 142–152. [Google Scholar] [CrossRef]
- Misal, S.N.; Lin, M.-H.; Mehraeen, S.; Chaplin, B.P. Modeling electrochemical oxidation and reduction of sulfamethoxazole using electrocatalytic reactive electrochemical membranes. J. Hazard. Mater. 2020, 384, 121420. [Google Scholar] [CrossRef]
- Bu, J.; Jiang, H.; Li, T.; Zhou, C.; Zhong, S. Electro-assisted activation of peroxymonosulfate in the presence of chloride ion for degradation of sulfamethoxazole. J. Clean. Prod. 2023, 405, 136914. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, C.; Wang, P.; Guo, R.; Liu, X.; Tian, Y. Electrochemical degradation of methylene blue by Pb modified porous SnO2 anode. Chemosphere 2022, 305, 135447. [Google Scholar] [CrossRef]
- Hui, H.; Wang, H.; Mo, Y.; Yin, Z.; Li, J. Optimal design and evaluation of electrocatalytic reactors with nano-MnOx/Ti membrane electrode for wastewater treatment. Chem. Eng. J. 2019, 376, 120190. [Google Scholar] [CrossRef]
- Jiang, T.; Guan, W.; Fu, M. Recovery of nickel from electroless nickel plating wastewater based on the synergy of electrocatalytic oxidation and electrodeposition technology. Water Environ. Res. 2022, 94, e10741. [Google Scholar] [CrossRef]
- Duan, X.; Ren, D.; Wang, S.; Zhang, M.; Sun, Y.; Sun, S.; Huo, Z.; Zhang, N. Carbon materials in electrocatalytic oxidation systems for the treatment of organic pollutants in wastewater: A review. Carbon Resour. Convers. 2023, 6, 262–273. [Google Scholar] [CrossRef]
- Meng, S.; Ling, Y.; Yang, M.; Zhao, X.; Osman, A.I.; Al-Muhtaseb, A.a.H.; Rooney, D.W.; Yap, P.-S. Recent research progress of electrocatalytic reduction technology for nitrate wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 109418. [Google Scholar] [CrossRef]
- Qin, S.; Lei, C.; Wang, X.; Chen, W.; Huang, B. Electrocatalytic activation of organic chlorides via direct and indirect electron transfer using atomic vacancy control of palladium-based catalyst. Cell Rep. Phys. Sci. 2022, 3, 100713. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Han, P.; Liu, Y.; Lv, H.; Chen, X.; Lei, Y.; Yu, L.; Ma, L.; Duan, P. Boosted Electrocatalytic Degradation of Levofloxacin by Chloride Ions: Performances Evaluation and Mechanism Insight with Different Anodes. Molecules 2024, 29, 662. https://doi.org/10.3390/molecules29030662
Yang K, Han P, Liu Y, Lv H, Chen X, Lei Y, Yu L, Ma L, Duan P. Boosted Electrocatalytic Degradation of Levofloxacin by Chloride Ions: Performances Evaluation and Mechanism Insight with Different Anodes. Molecules. 2024; 29(3):662. https://doi.org/10.3390/molecules29030662
Chicago/Turabian StyleYang, Keda, Peiwei Han, Yinan Liu, Hongxia Lv, Xiaofei Chen, Yihan Lei, Lian Yu, Lei Ma, and Pingzhou Duan. 2024. "Boosted Electrocatalytic Degradation of Levofloxacin by Chloride Ions: Performances Evaluation and Mechanism Insight with Different Anodes" Molecules 29, no. 3: 662. https://doi.org/10.3390/molecules29030662
APA StyleYang, K., Han, P., Liu, Y., Lv, H., Chen, X., Lei, Y., Yu, L., Ma, L., & Duan, P. (2024). Boosted Electrocatalytic Degradation of Levofloxacin by Chloride Ions: Performances Evaluation and Mechanism Insight with Different Anodes. Molecules, 29(3), 662. https://doi.org/10.3390/molecules29030662





