The Iodine/Iodide/Starch Supramolecular Complex
Abstract
1. Introduction
2. General Considerations on the Reaction of Starch with Iodine
3. Dependence on the Nature of the Organic (Bio)Polymer
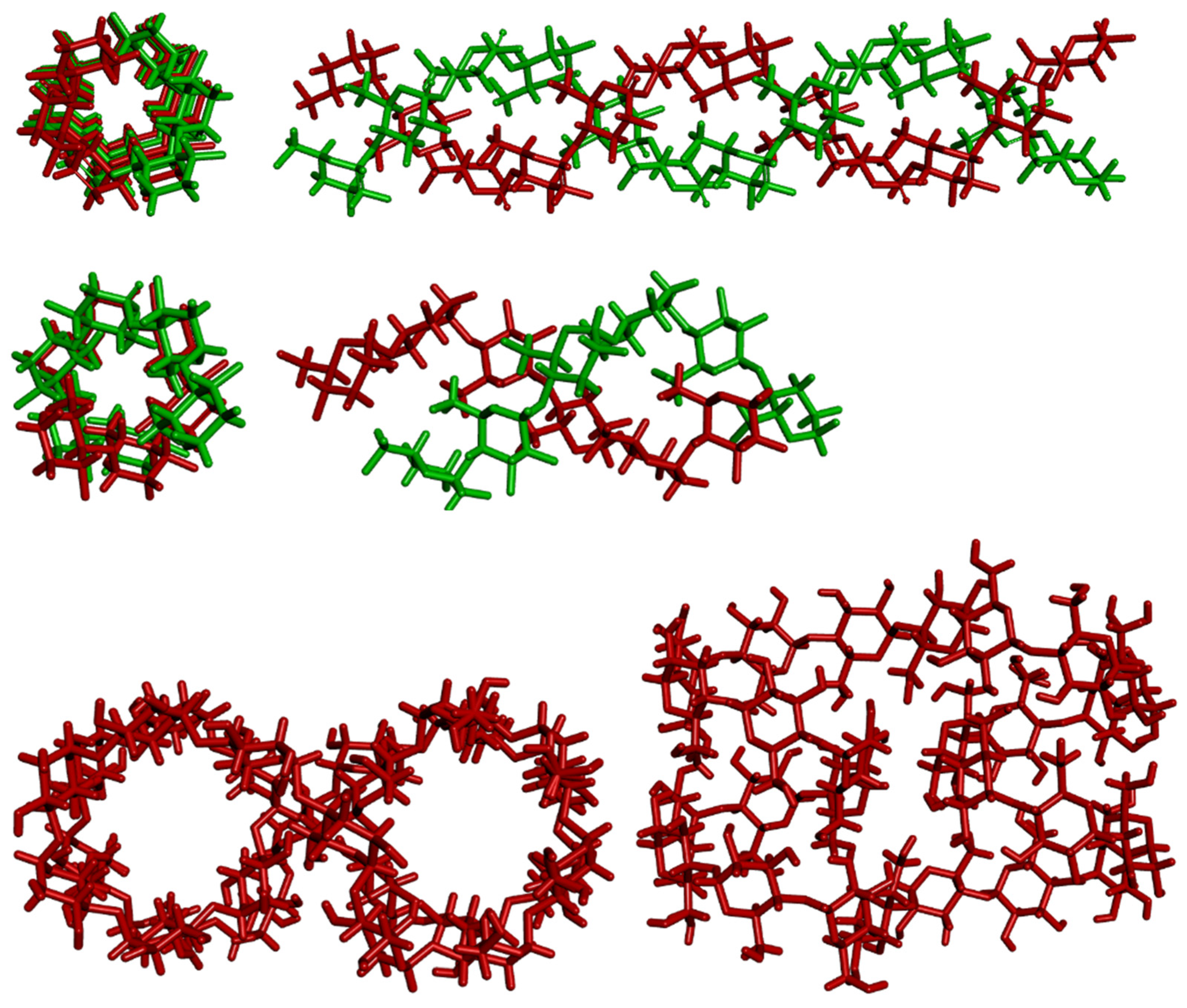
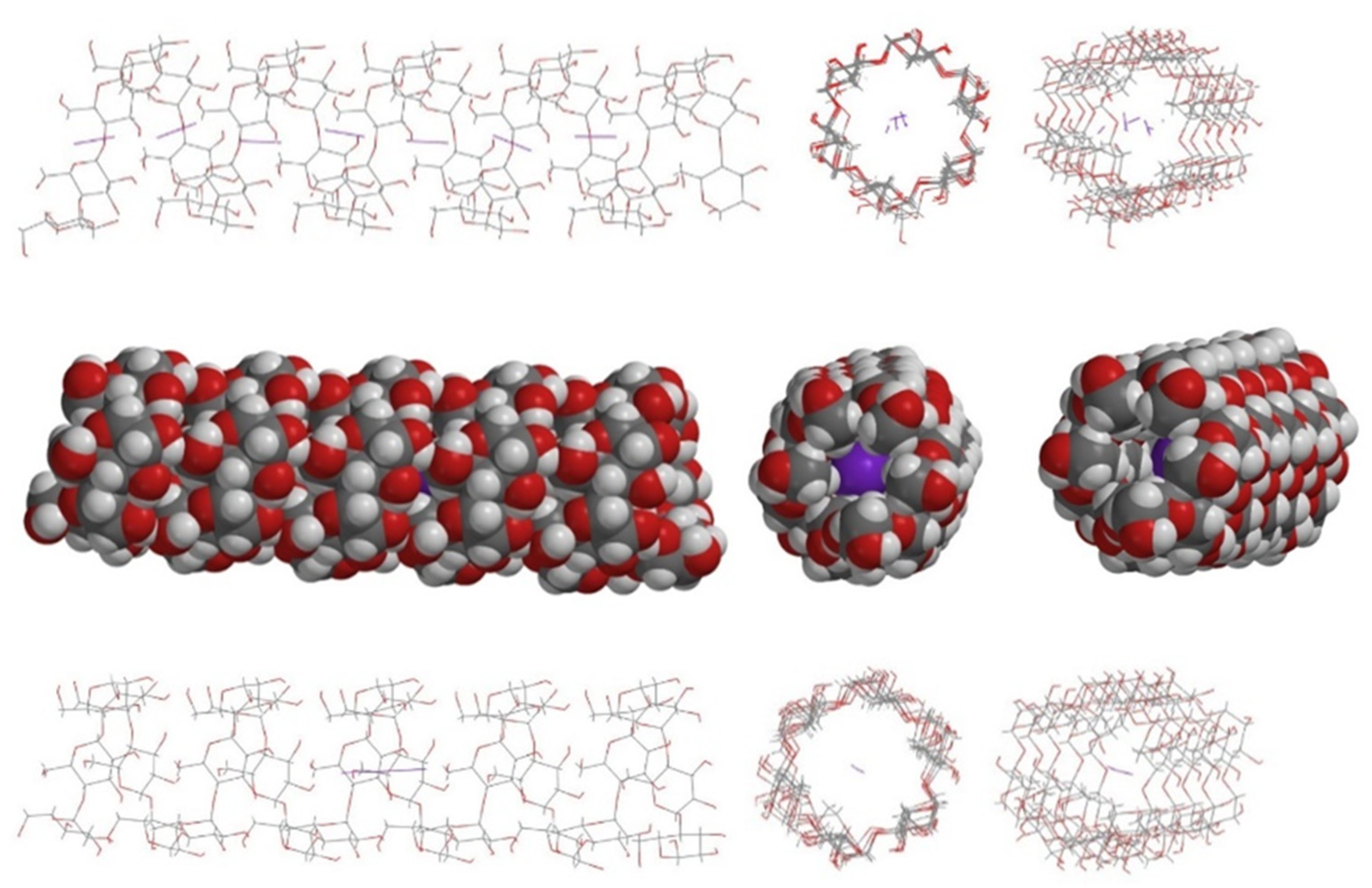
4. Geometrical Data
5. The I2-Only Hypothesis
6. Poly-Iodine Anions as Candidates
7. Structural Role of the Solvent
8. The I5−-I2 Hypothesis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M. Principles of Biochemistry. In Principles of Biochemistry, 5th ed.; W.H. Freeman & Company: New York, NY, USA, 2008. [Google Scholar]
- Wang, K.; Vilaplana, F.; Wu, A.; Hasjim, J.; Gilbert, R.G. The Size Dependence of the Average Number of Branches in Amylose. Carbohydr. Polym. 2019, 223, 115134. [Google Scholar] [CrossRef]
- Green, M.M.; Blankenhorn, G.; Hart, H. Which Starch Fraction Is Water-Soluble, Amylose or Amylopectin? J. Chem. Educ. 1975, 52, 729. [Google Scholar] [CrossRef]
- Nouri, A.; Khoee, S. Preparation of Amylose-Poly(Methyl Methacrylate) Inclusion Complex as a Smart Nanocarrier with Switchable Surface Hydrophilicity. Carbohydr. Polym. 2020, 246, 116662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Guan, L.; Wang, S.; Zhang, J.; Tan, L.; Kong, L.; Zhang, H. Lipophilization and Amylose Inclusion Complexation Enhance the Stability and Release of Catechin. Carbohydr. Polym. 2021, 269, 118251. [Google Scholar] [CrossRef]
- Prasher, P.; Fatima, R.; Sharma, M. Therapeutic Delivery with V-Amylose. Drug Dev. Res. 2021, 82, 727–729. [Google Scholar] [CrossRef]
- Katz, J.R. Abhandlungen Zur Physikalischen Chemie Der Stärke Und Der Brotbereitung. Z. Phys. Chem. 1930, 150A, 37–59. [Google Scholar] [CrossRef]
- Katz, J.R.; Derksen, J.C. Abhandlungen Zur Physikalischen Chemie Der Stärke Und Der Brotbereitung. Z. Phys. Chem. 1933, 167A, 129–136. [Google Scholar] [CrossRef]
- Bear, R.S. The Significance of the “V” X-Ray Diffraction Patterns of Starches. J. Am. Chem. Soc. 1942, 64, 1388–1392. [Google Scholar] [CrossRef]
- Meyer, K.H.; Bernfeld, P.; Wolf, E. Recherches Sur l’amidon III. Fractionnement et Purification de l’amylose de Maïs Naturel. Helv. Chim. Acta 1940, 23, 854–864. [Google Scholar] [CrossRef]
- Sarko, A.; Zugenmaier, P. Fiber Diffraction Methods. Am. Chem. Soc. 1980, 141, 459–482. [Google Scholar]
- Rappenecker, G.; Zugenmaier, P. Detailed refinement of the crystal structure of Vh-amylose. Carbohydr Res. 1981, 89, 11–19. [Google Scholar] [CrossRef]
- Murphy, V.G.; Zaslow, B.; French, A.D. The structure of V amylose dehydrate: A combined X-ray and stereochemical approach. Biopolymers 1975, 14, 1487–1501. [Google Scholar] [CrossRef]
- Brisson, J.; Chanzy, H.; Winter, W.T. The crystal and molecular structure of VH amylose by electron diffraction analysis. Int. J. Biol. Macromol. 1991, 13, 31–39. [Google Scholar] [CrossRef]
- Veregin, R.P.; Fyfe, C.A.; Marchessault, R.H. Investigation of the crystalline “V” amylose complexes by high-resolution carbon-13 CP/MAS NMR spectroscopy. Macromolecules 1987, 20, 3007–3012. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bociek, S.M. Carbon-13 CP/MAS NMR studies of amylose inclusion complexes, cyclodextrins, and the amorphous phase of starch granules: Relationships between glycosidic linkage conformation and solid-state carbon-13 chemical shifts. J. Am. Chem. Soc. 1988, 110, 3820–3829. [Google Scholar] [CrossRef]
- Imberty, A.; Chanzy, H.; Pérez, S.; Bulèon, A.; Tran, V. The Double-Helical Nature of the Crystalline Part of A-Starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Sarko, A.; Wu, H.-C.H. The Crystal Structures of A-, B- and C-Polymorphs of Amylose and Starch. Starch-Starke 1978, 30, 73–78. [Google Scholar] [CrossRef]
- Saenger, W. Inclusion Compounds; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Academic Press: London, UK, 1984; Volume 2. [Google Scholar]
- Harata, K. Comprehensive Supramolecular Chemistry; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Pergamon: Oxford, UK, 1996; Volume 3. [Google Scholar]
- Cohen, R.; Orlova, Y.; Kovalev, V.; Ungar, Y.; Shimoni, E. Structural and Functional Properties of Amylose Complexes with Genistein. J. Agric. Food Chem. 2008, 56, 4212–4218. [Google Scholar] [CrossRef]
- Pesek, S.; Lehene, M.; Brânzanic, A.M.V.; Silaghi-Dumitrescu, R. On the Origin of the Blue Color in The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2022, 27, 8974. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Ali, U. Degree-Based Topological Indices of Polysaccharides: Amylose and Blue Starch-Iodine Complex. J. Chem. 2021, 2021, 6652014. [Google Scholar] [CrossRef]
- Landolt, H. Uber Die Zeitdauer Der Reaction Zwischen Jodsaure Und Schwefliger Saure. Ber. Dtsch. Chem. Ges. 1886, 19, 1317–1365. [Google Scholar] [CrossRef]
- Gilbert, G.A.; Marriott, J.V.R. Starch-Iodine Complexes. Part I. Trans. Faraday Soc. 1948, 44, 84–93. [Google Scholar] [CrossRef]
- Thoma, J.A.; French, D. The Starch-Iodine-Iodide Interaction. Part I. Spectrophotometric Investigations 1. J. Am. Chem. Soc. 1960, 82, 4144–4147. [Google Scholar] [CrossRef]
- Stein, R.S.; Rundle, R.E. On the Nature of the Interaction between Starch and Iodine. J. Chem. Phys. 1948, 16, 195–207. [Google Scholar] [CrossRef]
- Hiromi, K.; Shibaoka, T.; Ono, S. Kinetic Studies of Amylose-Iodine-Iodide Reaction by Stopped-Flow Method. J. Biochem. 1970, 68, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Nishimura, T.; Ishii, T.; Handa, T. Effect of Concentration of Iodide on the Bound Species of I2/I−3 in the Amylose-Iodine Complex. Carbohydr. Res. 1987, 163, 155–167. [Google Scholar] [CrossRef]
- Cronan, C.L.; Schneider, F.W. Cooperativity and Composition of the Linear Amylose-Iodine-Iodide Complex. J. Phys. Chem. 1969, 73, 3990–4004. [Google Scholar] [CrossRef]
- Cramer, F.; Herbst, W. Die Lichtabsorption von Jodkettenmolekeln. Naturwissenschaften 1952, 39, 256. [Google Scholar] [CrossRef]
- Bersohn, R.; Isenberg, I. Metallic Nature of the Starch-Iodine Complex. J. Chem. Phys. 1961, 35, 1640–1643. [Google Scholar] [CrossRef]
- Rundle, R.E.; Baldwin, R.R. The Configuration of Starch and Starch-Iodine Complex. I. The Dichroism of Flow of Starch-Iodine Solutions. J. Am. Chem. Soc. 1943, 65, 554–558. [Google Scholar] [CrossRef]
- Rundle, R.E. The Configuration of Starch in the Starch-Iodine Complex. V. Fourier Projections from X-ray Diagrams. J. Am. Chem. Soc. 1947, 69, 1769–1772. [Google Scholar] [CrossRef]
- Noltemeyer, M.; Saenger, W. X-Ray Studies of Linear Polyiodide Chains in α-Cyclodextrin Channels and a Model for the Starch-Iodine Complex. Nature 1976, 259, 629–632. [Google Scholar] [CrossRef]
- Bluhm, T.L.; Zugenmaier, P. Detailed Structure of the Vh-Amylose-Iodine Complex: A Liner Polyiodine Chain. Carbohydr. Res. 1981, 89, 1–10. [Google Scholar] [CrossRef]
- Handa, T.; Yajima, H. Conformation of Amylose-Iodine-Iodide Complex in Aqueous Solution. Biopolymers 1981, 20, 2051–2072. [Google Scholar] [CrossRef]
- Mould, D.L.; Synge, R.M. Separations of Polysaccharides Related to Starch by Electrokinetic Ultrafiltration in Collodion Membranes. Biochem. J. 1954, 58, 571–600. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Tsuchihashi, S.; Kuge, T. On the Starch-Iodine Complex. J. Am. Chem. Soc. 1953, 75, 3601–3602. [Google Scholar] [CrossRef]
- Moulay, S. Molecular Iodine/Polymer Complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Séne, M.; Thévenot, C.; Prioul, J.L. Simultaneous Spectrophotometric Determination of Amylose and Amylopectin in Starch from Maize Kernel by Multi-Wavelength Analysis. J. Cereal Sci. 1997, 26, 211–221. [Google Scholar] [CrossRef]
- Sashio, M.; Tanaka, M. Thermal Reaction of Poly(Vinyl Alcohol)-Iodine Complex Membranes. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 905–909. [Google Scholar] [CrossRef]
- Dintzis, F.R. Instability of Solutions of Amylose-Iodine Complex in Concentrated Calcium Chloride. Starch-Stärke 1974, 26, 56–58. [Google Scholar] [CrossRef]
- Tashiro, K.; Gakhutishvili, M. Crystal Structure of Cellulose-Iodine Complex. Polymer 2019, 171, 140–148. [Google Scholar] [CrossRef]
- Konishi, T.; Tanaka, W.; Kawai, T.; Fujikawa, T. Iodine L-Edge XAFS Study of Linear Polyiodide Chains in Amylose and α-Cyclodextrin. J. Synchrotron Radiat. 2001, 8, 737–739. [Google Scholar] [CrossRef]
- Knutson, C.A. Evaluation of Variations in Amylose–Iodine Absorbance Spectra. Carbohydr. Polym. 2000, 42, 65–72. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Ishii, T.; Endo, R. Effect of Molecular Weight of Amylose on the Iodine Coloring Species Responsible for the Optical Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1989, 46, 537–544. [Google Scholar] [CrossRef][Green Version]
- SenGupta, U.K.; MuKherjee, A.K.; SenGupta, K.K. Spectrophotometric Studies on Amylose-Iodine and Amylopectin-Iodine Complexes. Kolloid-Z. Z. Polym. 1966, 208, 32–34. [Google Scholar] [CrossRef]
- Sakajiri, T.; Kikuchi, T.; Simon, I.; Uchida, K.; Yamamura, T.; Ishii, T.; Yajima, H. Molecular Dynamics Approach to Study the Discrepancies in the Thermal Behavior of Amylose and Chitosan Conformations. J. Mol. Struct. THEOCHEM 2006, 764, 133–140. [Google Scholar] [CrossRef]
- Szejtli, J.; Augustat, S.; Richter, M. Molecular Configuration of Amylose and Its Complexes in Aqueous Solutions. Part III. Investigation of the DP Distribution of Helical Segments in Amylose-Iodine Complexes. Biopolymers 1967, 5, 17–26. [Google Scholar] [CrossRef]
- McMullan, R.K.; Saenger, W.; Fayos, J.; Mootz, D. Topography of Cyclodextrin Inclusion Complexes. Carbohydr. Res. 1973, 31, 211–227. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Bear, R.S.; Rundle, R.E. The Relation of Starch—Iodine Absorption Spectra to the Structure of Starch and Starch Components 1. J. Am. Chem. Soc. 1944, 66, 111–115. [Google Scholar] [CrossRef]
- Davis, H.; Khan, A. Determining the Chromophore in the Amylopectin–Iodine Complex by Theoretical and Experimental Studies. J. Polym. Sci. A Polym. Chem. 1994, 32, 2257–2265. [Google Scholar] [CrossRef]
- Rendleman, J.A. The Reaction of Starch with Iodine Vapor. Determination of Iodide-Ion Content of Starch–Iodine Complexes. Carbohydr. Polym. 2003, 51, 191–202. [Google Scholar] [CrossRef]
- Yu, X.; Houtman, C.; Atalla, R.H. The Complex of Amylose and Iodine. Carbohydr. Res. 1996, 292, 129–141. [Google Scholar] [CrossRef]
- Abe, T. The Visible and Ultraviolet Absorption Spectra of Cellulose- and Amylose-Iodine Complexes. Bull. Chem. Soc. Jpn. 1958, 31, 661–662. [Google Scholar] [CrossRef]
- Takahashi, Y.J. Binding Properties of Alginic Acid and Chitin. Inclus. Phenom. 1978, 5, 525–534. [Google Scholar] [CrossRef]
- Yajima, H.; Morita, M.; Hashimoto, M.; Sashiwa, H.; Kikuchi, T.; Ishii, T. Complex Formation of Chitosan with Iodine and Its Strucutre and Spectroscopic Properties—Molecular Assembly and Thermal Hysteresis Behavior. Int. J. Thermophys. 2001, 22, 1265–1283. [Google Scholar] [CrossRef]
- Gunasekaran, M. Physiological Studies on Phymatotrichum Omnivorum II. Physiocochemical Properties of Glycogen. Arch. Mikrobiol. 1972, 84, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Roman, A.; Khan, A. Chromophore and Spectrum of the Glycogen-Iodine Complex. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2975–2980. [Google Scholar] [CrossRef]
- Lecker, D.N.; Kumari, S.; Khan, A. Iodine Binding Capacity and Iodine Binding Energy of Glycogen. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1409–1412. [Google Scholar] [CrossRef]
- Aterman, K. A Historical Note on the Iodine-Sulphuric Acid Reaction of Amyloid. Histochemistry 1976, 49, 131–143. [Google Scholar] [CrossRef]
- Dzwolak, W. Insulin Amyloid Fibrils Form an Inclusion Complex with Molecular Iodine: A Misfolded Protein as a Nanoscale Scaffold. Biochemistry 2007, 46, 1568–1572. [Google Scholar] [CrossRef]
- Pritchard, J.G.; Serra, F.T. Complexation of Polyvinyl Acetate with Iodine. Talanta 1973, 20, 541–546. [Google Scholar] [CrossRef]
- Hughes, J. Analytical Behaviour of Poly(Vinyl Acetate) and Its Hydrolysis Products with Iodine. Talanta 1979, 26, 1161–1163. [Google Scholar] [CrossRef]
- Schulz, R.C.; Fleischer, D.; Henglein, A.; Bössler, H.M.; Trisnadi, J.; Tanaka, H. Addition Compounds and Complexes with Polymers and Models. Pure Appl. Chem. 1974, 38, 227–247. [Google Scholar] [CrossRef]
- Chernov’yants, M.S.; Burykin, I.V.; Pisanov, R.V.; Shalu, O.A. Synthesis and Antimicrobial Activity of Poly(N-Methyl-4-Vinylpyridinium Triiodide). Pharm. Chem. J. 2010, 44, 61–63. [Google Scholar] [CrossRef]
- Pal, S.K.; Krishnan, A.; Das, P.K.; Samuelson, A.G. Schiff Base Linked Ferrocenyl Complexes for Second-Order Nonlinear Optics. J. Organomet. Chem. 2000, 604, 248–259. [Google Scholar] [CrossRef]
- Liu, W.-J.; Xiong, G.-X.; Zeng, D.-H. Synthesis and Electrical Properties of Three Novel Poly (Ferrocenyl-Schiff Bases) and Their Charge Transfer Complexes with Iodine. J. Inorg. Organomet. Polym. 2010, 20, 97–103. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.F.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH)x. J. Chem. Soc. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Bakueva, L.; Matheson, D.; Musikhin, S.; Sargent, E.H. Luminescence of Pure and Iodine Doped PPV: Internal Energetic Structure Revealed through Spectral Signatures. Synth. Met. 2002, 126, 207–211. [Google Scholar] [CrossRef]
- Fasahat, P.; Rahman, S.; Ratnam, W. Genetic Controls on Starch Amylose Content in Wheat and Rice Grains. J. Genet. 2014, 93, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Ashogbon, A.O.; Akintayo, E.T.; Oladebeye, A.O.; Oluwafemi, A.D.; Akinsola, A.F.; Imanah, O.E. Developments in the Isolation, Composition, and Physicochemical Properties of Legume Starches. Crit. Rev. Food Sci. Nutr. 2021, 61, 2938–2959. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Zhao, J.; Li, C.; Wu, H.; Zhao, H.; Wu, Q. Granule-Bound Starch Synthase in Plants: Towards an Understanding of Their Evolution, Regulatory Mechanisms, Applications, and Perspectives. Plant Sci. 2023, 336, 111843. [Google Scholar] [CrossRef]
- Guo, K.; Liang, W.; Wang, S.; Guo, D.; Liu, F.; Persson, S.; Herburger, K.; Petersen, B.L.; Liu, X.; Blennow, A.; et al. Strategies for Starch Customization: Agricultural Modification. Carbohydr. Polym. 2023, 321, 121336. [Google Scholar] [CrossRef]
- Chiaramonte, E.; Rhazi, L.; Aussenac, T.; White, D.R. Amylose and Amylopectin in Starch by Asymmetric Flow Field-Flow Fractionation with Multi-Angle Light Scattering and Refractive Index Detection (AF4–MALS–RI). J. Cereal Sci. 2012, 56, 457–463. [Google Scholar] [CrossRef]
- Ulbrich, M.; Scholz, F.; Flöter, E. Chromatographic Study of High Amylose Corn Starch Genotypes—Investigation of Molecular Properties after Specific Enzymatic Digestion. Starch-Stärke 2022, 74, 2100303. [Google Scholar] [CrossRef]
- Tutorskii, I.A.; Sokolova, L.V. Mechanism of the Reaction of Polybutadiene with Molecular Iodine. Polym. Sci. U.S.S.R. 1977, 19, 176–183. [Google Scholar] [CrossRef]
- Sreeja, R.; Najidha, S.; Remya Jayan, S.; Predeep, P.; Mazur, M.; Sharma, P.D. Electro-Optic Materials from Co-Polymeric Elastomer–Acrylonitrile Butadiene Rubber (NBR). Polymer 2006, 47, 617–623. [Google Scholar] [CrossRef]
- Vippa, P.; Rajagopalan, H.; Thakur, M. Electrical and Optical Properties of a Novel Nonconjugated Conductive Polymer, Poly (β-pinene). J. Polym. Sci. Part B Polym. Phys. 2005, 43, 3695–3698. [Google Scholar] [CrossRef]
- Nagelli, W. Beitrage Zur Naheren Kenntniss Der Starkegruppe. Ann. Chem. 1874, 173, 218–227. [Google Scholar] [CrossRef]
- Helbert, W.; Chanzy, H. The Ultrastructure of Starch from Ultrathin Sectioning in Melamine Resin. Starch-Starke 1996, 48, 185–188. [Google Scholar] [CrossRef]
- Atkin, N.J.; Abeysekera, R.M.; Cheng, S.L.; Robards, A.W. An Experimentally-Based Predictive Model for the Separation of Amylopectin Subunits during Starch Gelatinization. Carbohydr. Polym. 1998, 36, 173–192. [Google Scholar] [CrossRef]
- Baker, A.A.; Miles, M.J.; Helbert, W. Internal Structure of the Starch Resistant Granule Revealed by AFM. Carbohydr. Res. 2001, 330, 249–256. [Google Scholar] [CrossRef]
- Baldwin, P.M.; Adler, J.; Davies, M.; Melia, D. High Resolution Imaging of Starch Granule Surfaces by Atomic Force Microscopy. J. Cereal Sci. 1998, 27, 255–265. [Google Scholar] [CrossRef]
- Dang, J.M.C.; Copeland, L. Imaging Rice Grains Using Atomic Force Microscopy. J. Cereal Sci. 2003, 37, 165–170. [Google Scholar] [CrossRef]
- Ohtani, T.; Yoshimo, T.; Hagiwara, S.; Maekawa, T. High-Resolution Imaging of Starch Granule Structure Using Atomic Force Microscopy. Starch-Starke 2000, 52, 150–153. [Google Scholar] [CrossRef]
- Park, H.; Xu, S.; Seetharaman, S. A Novel in Situ Atomic Force Microscopy Imaging Technique to Probe Surface Morphological Features of Starch Granules. Carbohydr. Res. 2011, 346, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.J.; Gunning, A.P.; Parker, M.L.; Wilson, R.H.; Morris, V.J. Using AFM to Image the Internal Structure of Starch Grsnules. Carbohydr. Polym. 2002, 50, 123–132. [Google Scholar] [CrossRef]
- Szymonska, J.; Krok, F. Potato Starch Granule Nanostructure Studied by Highresolution Non-Contact AFM. Int. J. Biol. Macromol. 2003, 33, 1–7. [Google Scholar] [CrossRef]
- Waduge, R.N.; Xu, S.; Seetharaman, S. Iodine Absorption Properties and Its Effect on the Crystallinity of Developing Wheat Starch Granules. Carbohydr. Polym. 2010, 82, 786–794. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of Starch: Evidence of a New Level of Granule Organization. Carbohydr. Polym. 1997, 32, 177–191. [Google Scholar] [CrossRef]
- Doutch, J.; Gilbert, E.P. Characterisation of Large Scale Structures in Starch Granules via Small-Angle Neutron and X-ray Scattering Techniques. Carbohydr. Polym. 2013, 91, 444–451. [Google Scholar] [CrossRef]
- Barrett, A.J.; Barrett, K.L.; Khan, A. Effects of Acetone, Ethanol, Isopropanol, and Dimethyl Sulfoxide on Amylose-Iodine Complex. J. Macromol. Sci. Part A 1998, 35, 711–722. [Google Scholar] [CrossRef]
- Fonslick, J.; Khan, A. Thermal Stability and Composition of the Amylose–Iodine Complex. J. Polym. Sci. A Polym. Chem. 1989, 27, 4161–4167. [Google Scholar] [CrossRef]
- Rundle, R.E.; French, D. The Configuration of Starch and the Starch—Iodine Complex. II. Optical Properties of Crystalline Starch Fractions 1. J. Am. Chem. Soc. 1943, 65, 558–561. [Google Scholar] [CrossRef]
- Rundle, R.E.; French, D. The Configuration of Starch in the Starch—Iodine Complex. III. X-ray Diffraction Studies of the Starch—Iodine Complex 1. J. Am. Chem. Soc. 1943, 65, 1707–1710. [Google Scholar] [CrossRef]
- Cramer, F.; Windel, H. Über Einschlußverbindungen, X. Mitteil.: Die Blauen Jodverbindungen Der Cumarine Und Anderer Verwandter Verbindungen. Chem. Ber. 1956, 89, 354–365. [Google Scholar] [CrossRef]
- Saenger, W. The Structure of the Blue Starch-Iodine Complex. Naturwissenschaften 1984, 71, 31–36. [Google Scholar] [CrossRef]
- Immel, S.; Lichtenthaler, F.W. The Hydrophobic Topographies of Amylose and Its Blue Iodine Complex. Starch-Stärke 2000, 52, 1–8. [Google Scholar] [CrossRef]
- Minick, M.; Fotta, K.; Khan, A. Polyiodine Units in Starch-Iodine Complex: INDO CI Study of Spectra and Comparison with Experiments. Biopolymers 1991, 31, 57–63. [Google Scholar] [CrossRef]
- Zaslow, B.; Miller, R.L. Hydration of the “V” Amylose Helix 1. J. Am. Chem. Soc. 1961, 83, 4378–4381. [Google Scholar] [CrossRef]
- Hirai, M.; Hirai, T.; Ueki, T. Effect of Branching of Amylopectin on Complexation with Iodine as Steric Hindrance. Polymer 1994, 35, 2222–2225. [Google Scholar] [CrossRef]
- Dintzis, F.R.; Beckwith, A.C.; Babcock, G.E.; Tobin, R. Amylose-Iodine Complex. I. Sedimentation Behavior. Macromolecules 1976, 9, 471–478. [Google Scholar] [CrossRef]
- Moulik, S.P.; Gupta, S. Effects of Solvents on the Spectrophotometric and Hydrodynamic Behavior of Amylose and Its Iodine Complex. Carbohydr. Res. 1980, 81, 131–143. [Google Scholar] [CrossRef]
- Senior, M.B.; Hamori, E. Investigation of the Effect of Amylose/Iodine Complexation on the Conformation of Amylose in Aqueous Solution. Biopolymers 1973, 12, 65–78. [Google Scholar] [CrossRef]
- Vladimirov, A.V.; Volkova, T.V.; Agafonov, A.V. Temperature Dependence of Stability Constants of the Iodine-Iodide-Amylose Complexes. Russ. J. Phys. Chem. A 2003, 77, 612–615. [Google Scholar]
- Zhang, Q.; Lu, Z.; Hu, H.; Yang, W.; Marszalek, P.E. Direct Detection of the Formation of V-Amylose Helix by Single Molecule Force Spectroscopy. J. Am. Chem. Soc. 2006, 128, 9387–9393. [Google Scholar] [CrossRef] [PubMed]
- Dintzis, F.R.; Tobin, R.; Beckwith, A.C. Amylose-Iodine Complex. II. Molecular Weight Estimates. Macromolecules 1976, 9, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Mikus, F.F.; Hixon, R.M.; Rundle, R.E. The Complexes of Fatty Acids with Amylose 1. J. Am. Chem. Soc. 1946, 68, 1115–1123. [Google Scholar] [CrossRef]
- Calabrese, V.T.; Khan, A. Amylose-Iodine Complex Formation without KI: Evidence for Absence of Iodide Ions within the Complex. J. Polym. Sci. A Polym. Chem. 1999, 37, 2711–2717. [Google Scholar] [CrossRef]
- Cesaro, A.; Benegas, J.C.; Ripoll, D.R. Molecular Model of the Cooperative Amylose-Iodine-Triiodide Complex. J. Phys. Chem. 1986, 90, 2787–2791. [Google Scholar] [CrossRef]
- Kuge, T.; Ono, S. Amylose-Iodine Complex. III. Potentiometric and Spectrophotometric Studies. Bull. Chem. Soc. Jpn. 1960, 33, 1273–1278. [Google Scholar] [CrossRef]
- Schulz, W.; Sklenar, H.; Hinrichs, W.; Saenger, W. The Structure of the Left-Handed Antiparallel Amylose Double Helix: Theoretical Studies. Biopolymers 1993, 33, 363–375. [Google Scholar] [CrossRef]
- Moulik, S.P.; Gupta, S. Environment-Induced, Physicochemical Behavior of Amylose-Iodine Complexes. Carbohydr. Res. 1979, 71, 251–264. [Google Scholar] [CrossRef]
- Szejtli, J.; Richter, M.; Augustat, S. Molecular Configuration of Amylose and Its Complexes in Aqueous Solutions. Part IV. Determination OfDP of Amylose by Measuring the Concentration of Free Iodine in Solution of Amylose-Iodine Complex. Biopolymers 1968, 6, 27–41. [Google Scholar] [CrossRef]
- Peng, Q.-J.; Perlin, A.S. Observations on N.M.R. Spectra of Starches in Dimethyl Sulfoxide, Iodine-Complexing, and Solvation in Water-Di-Methyl Sulfoxide. Carbohydr. Res. 1987, 160, 57–72. [Google Scholar] [CrossRef]
- Murdoch, K.A. The Amylose-Iodine Complex. Carbohydr. Res. 1992, 233, 161–174. [Google Scholar] [CrossRef]
- Knutson, C.A.; Cluskey, J.E.; Dintzis, F.R. Properties of Amylose-Iodine Complexes Prepared in the Presence of Excess Iodine. Carbohydr. Res. 1982, 101, 117–128. [Google Scholar] [CrossRef]
- Murakami, H. Electronic Structure of the Amylose-Iodine Complex. J. Chem. Phys. 1954, 22, 367–374. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Kubota, S.; Ishii, T.; Endo, R. Polymer Effect on the Iodine Coloring Species Responsible for the Spectroscopic Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1990, 47, 717–725. [Google Scholar] [CrossRef][Green Version]
- Nishimura, T.; Yajima, H.; Kubota, S.; Ishii, T.; Endo, R. Effect of I− Concentration on the Optical Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1988, 45, 945–952. [Google Scholar] [CrossRef][Green Version]
- Rawlings, P.K.; Schneider, F.W. Models for Competitive Cooperative Linear Adsorption. The Amylose–Iodine–Iodide Complex. J. Chem. Phys. 1970, 52, 946–952. [Google Scholar] [CrossRef]
- Kuge, T.; Ono, S. Advances in Carbohydrate Chemistry and Biochemistry. Bull. Chem. Sot. Jpn. 1960, 33, 1269–1272. [Google Scholar] [CrossRef]
- Teitelbaum, R.C.; Ruby, S.L.; Marks, T.J. A Resonance Raman/Iodine Moessbauer Investigation of the Starch-Iodine Structure. Aqueous Solution and Iodine Vapor Preparations. J. Am. Chem. Soc. 1980, 102, 3322–3328. [Google Scholar] [CrossRef]
- Cesàro, A.; Jerian, E.; Saule, S. Physicochemical Studies of Amylose and Its Derivatives in Aqueous Solutions: Thermodynamics of the Iodine-Triiodide Complex. Biopolymers 1980, 19, 1491–1506. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Ishii, T.; Endo, R. Study of the Bluing Mechanism of Amylose-Iodine Complexes by CD Stopped-Flow Method. Kobunshi Ronbunshu 1991, 48, 525–528. [Google Scholar] [CrossRef]
- Wolf, R.; Schulz, R.C. Optical Rotatory Dispersion of the Starch Iodine Complex. Part 2. J. Macromol. Sci. Part A Chem. 1968, 2, 821–832. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Vladimirov, A.V.; Volkova, T.V. The Concentration Dependences of the Stability Constants of Iodine-Iodide-Amylose Complexes in Aqueous Solutions of Electrolytes. Russ. J. Phys. Chem. A 2004, 78, 1584–1587. [Google Scholar]
- Yamamoto, M.; Sano, T.; Harada, S.; Yasunaga, T. Interaction of Amylose with Iodine. II. Kinetic Studies of the Complex Formation by the Temperature-Jump Method. Bull. Chem. Soc. Jpn. 1982, 55, 3702–3706. [Google Scholar] [CrossRef]
- Foster, J.F.; Zucker, D. Length of the Amylose–Iodine Complex as Determined by Streaming Dichroism. J. Phys. Chem. 1952, 56, 170–173. [Google Scholar] [CrossRef]
- Noltemeyer, M.; Saenger, W. Topography of Cyclodextrin Inclusion Complexes. 12. Structural Chemistry of Linear.Alpha.-Cyclodextrin-Polyiodide Complexes. X-ray Crystal Structures of (.Alpha.-Cyclodextrin)2.LiI3.I2.8H2O and (.Alpha.-Cyclodextrin)2.Cd0.5).I5.27H2O. Models for the Blue. J. Am. Chem. Soc. 1980, 102, 2710–2722. [Google Scholar] [CrossRef]
- Betzel, C.; Hingerty, B.; Noltemeyer, M.; Weber, G.; Saenger, W.; Hamilton, J.A. (β-Cyclodextrin)2 KI7 9 H2O. Spatial Fitting of a Polyiodide Chain to a given Matrix. J. Incl. Phenom. 1983, 1, 181–191. [Google Scholar] [CrossRef]
- Bowmaker, G. Bonding and Nuclear Quadrupole Coupling in Linear Pentaiodide Ions. Aust. J. Chem. 1978, 31, 2713. [Google Scholar] [CrossRef]
- Nimz, O.; Geßler, K.; Usón, I.; Laettig, S.; Welfle, H.; Sheldrick, G.M.; Saenger, W. X-Ray Structure of the Cyclomaltohexaicosaose Triiodide Inclusion Complex Provides a Model for Amylose–Iodine at Atomic Resolution. Carbohydr. Res. 2003, 338, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ziegast, G.; Pfannemüller, B. Resonance Raman Studies of Amaylose—Iodine Complexes. Int. J. Biol. Macromol. 1982, 4, 419–424. [Google Scholar] [CrossRef]
- Heyde, M.E.; Rimai, L.; Kilponen, R.G.; Gill, D. Resonance-Enhanced Raman Spectra of Iodine Complexes with Amylose and Poly(Vinyl Alcohol), and of Some Iodine-Containing Trihalides. J. Am. Chem. Soc. 1972, 94, 5222–5227. [Google Scholar] [CrossRef]
- Okuda, M.; Hiramatsu, T.; Yasuda, M.; Ishigaki, M.; Ozaki, Y.; Hayashi, M.; Tominaga, K.; Chatani, E. Theoretical Modeling of Electronic Structures of Polyiodide Species Included in α-Cyclodextrin. J. Phys. Chem. B 2020, 124, 4089–4096. [Google Scholar] [CrossRef]
- Mizuno, M.; Tanaka, J.; Harada, I. Electronic Spectra and Structures of Polyiodide Chain Complexes. J. Phys. Chem. 1981, 85, 1789–1794. [Google Scholar] [CrossRef]
- Teitelbaum, R.C.; Ruby, S.L.; Marks, T.J. On the Structure of Starch-Iodine. J. Am. Chem. Soc. 1978, 100, 3215–3217. [Google Scholar] [CrossRef]
- Hach, R.J.; Rundle, R.E. The Structure of Tetramethylammonium Pentaiodide 1,1a. J. Am. Chem. Soc. 1951, 73, 4321–4324. [Google Scholar] [CrossRef]
- Herbstein, F.H.; Kapon, M. Zigzag Chains of Alternating Molecules and Triiodide Ions in Crystalline (Phenacetin)2.HI5. Nat. Phys. Sci. 1972, 239, 153–154. [Google Scholar] [CrossRef]
- Haddock, A.; Steidemann, M.; Readnour, M. Polyiodide Equilibria in Aqueous Solutions of Iodine and Iodide. Synth. React. Inorg. Met. Org. Chem. 1979, 9, 39–56. [Google Scholar] [CrossRef]
- Ramette, R.W.; Sandford, R.W. Thermodynamics of Iodine Solubility and Triiodide Ion Formation in Water and in Deuterium Oxide. J. Am. Chem. Soc. 1965, 87, 5001–5005. [Google Scholar] [CrossRef]
- Sekine, T. Abstracts. Nippon Kagaku Zassi 1969, 90, 951–983. [Google Scholar] [CrossRef]
- Mould, D.L. Potentiometric and Spectrophotometric Studies of Complexes of Hydrolysis Products of Amylose with Iodine and Potassium Iodide. Biochem. J. 1954, 58, 593–600. [Google Scholar] [CrossRef]
- Bhide, S.V.; Kale, N.R. Ligand-Induced Structural Changes in Amylose Partially Complexed with Iodine. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1976, 444, 719–726. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Bernal-Uruchurtu, M.I.; Kerenskaya, G.; Janda, K.C. Structure, Spectroscopy and Dynamics of Halogen Molecules Interacting with Water. Int. Rev. Phys. Chem. 2009, 28, 223–265. [Google Scholar] [CrossRef]
- Kireev, S.V.; Shnyrev, S.L. Study of Molecular Iodine, Iodate Ions, Iodide Ions, and Triiodide Ions Solutions Absorption in the UV and Visible Light Spectral Bands. Laser Phys. 2015, 25, 075602. [Google Scholar] [CrossRef]
- Prasanna; Shrikanth, B.K.; Hegde, M.S. Formation and Structure of Iodine: Water (H2O-I2) Charge-Transfer Complex. J. Chem. Sci. 2021, 133, 51. [Google Scholar] [CrossRef]


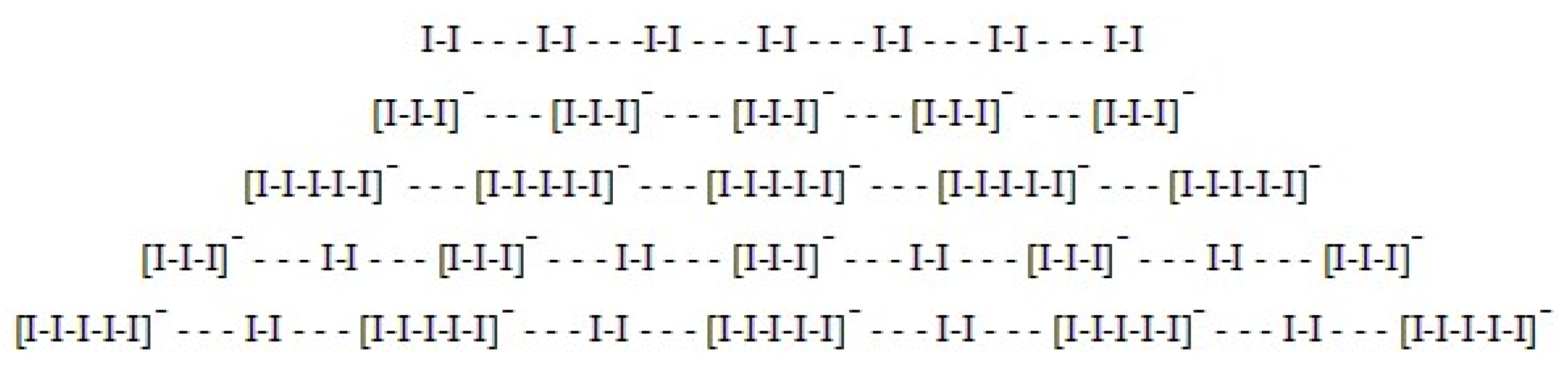
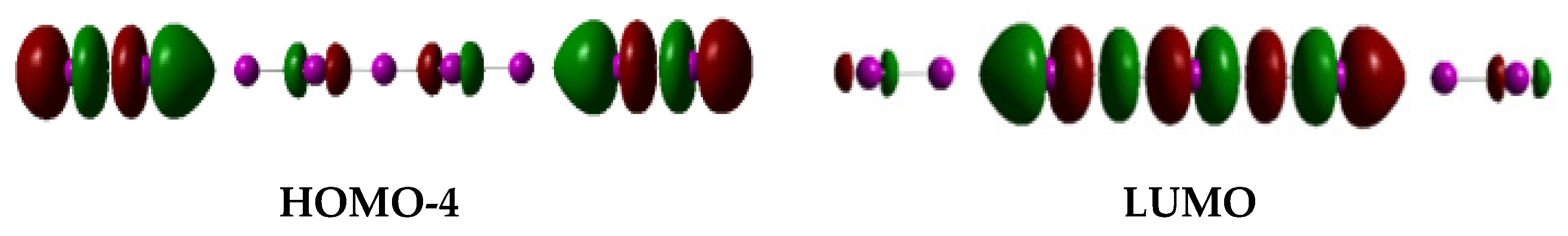
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesek, S.; Silaghi-Dumitrescu, R. The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2024, 29, 641. https://doi.org/10.3390/molecules29030641
Pesek S, Silaghi-Dumitrescu R. The Iodine/Iodide/Starch Supramolecular Complex. Molecules. 2024; 29(3):641. https://doi.org/10.3390/molecules29030641
Chicago/Turabian StylePesek, Szilard, and Radu Silaghi-Dumitrescu. 2024. "The Iodine/Iodide/Starch Supramolecular Complex" Molecules 29, no. 3: 641. https://doi.org/10.3390/molecules29030641
APA StylePesek, S., & Silaghi-Dumitrescu, R. (2024). The Iodine/Iodide/Starch Supramolecular Complex. Molecules, 29(3), 641. https://doi.org/10.3390/molecules29030641







