Phenolic Compounds from Apples: From Natural Fruits to the Beneficial Effects in the Digestive System
Abstract
1. Introduction
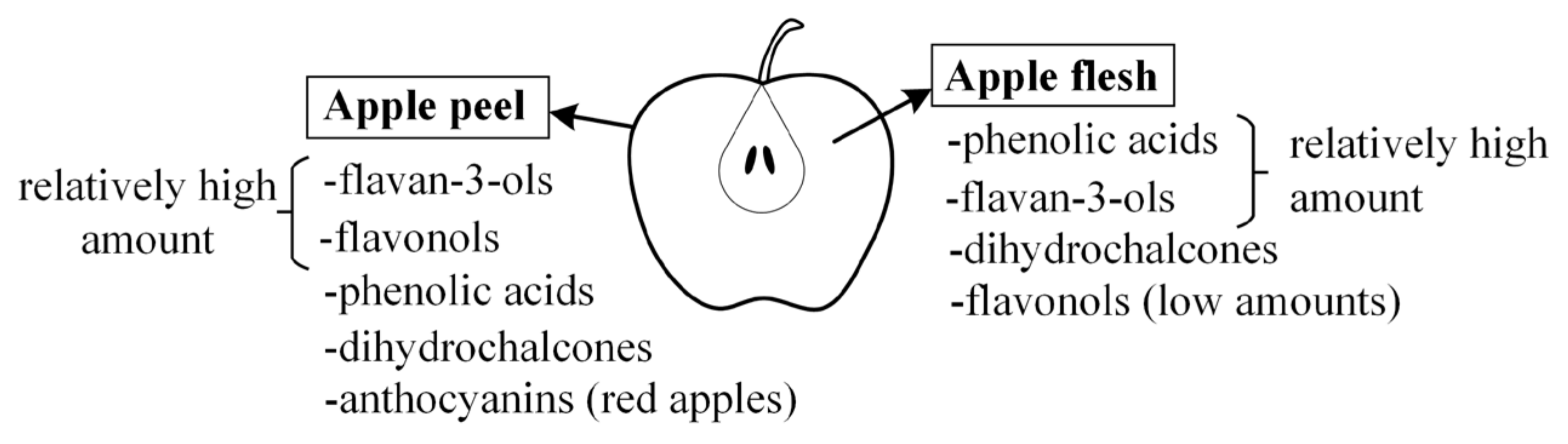
2. Apples
3. Phenolic Compounds from Apples
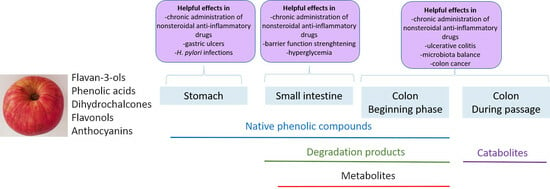
4. Phenolic Compounds from Apples in the Digestive Tract
4.1. The Forms of Phenolic Compounds in the Digestive Tract
4.1.1. Mouth and Stomach
4.1.2. Small Intestine
4.1.3. Colon
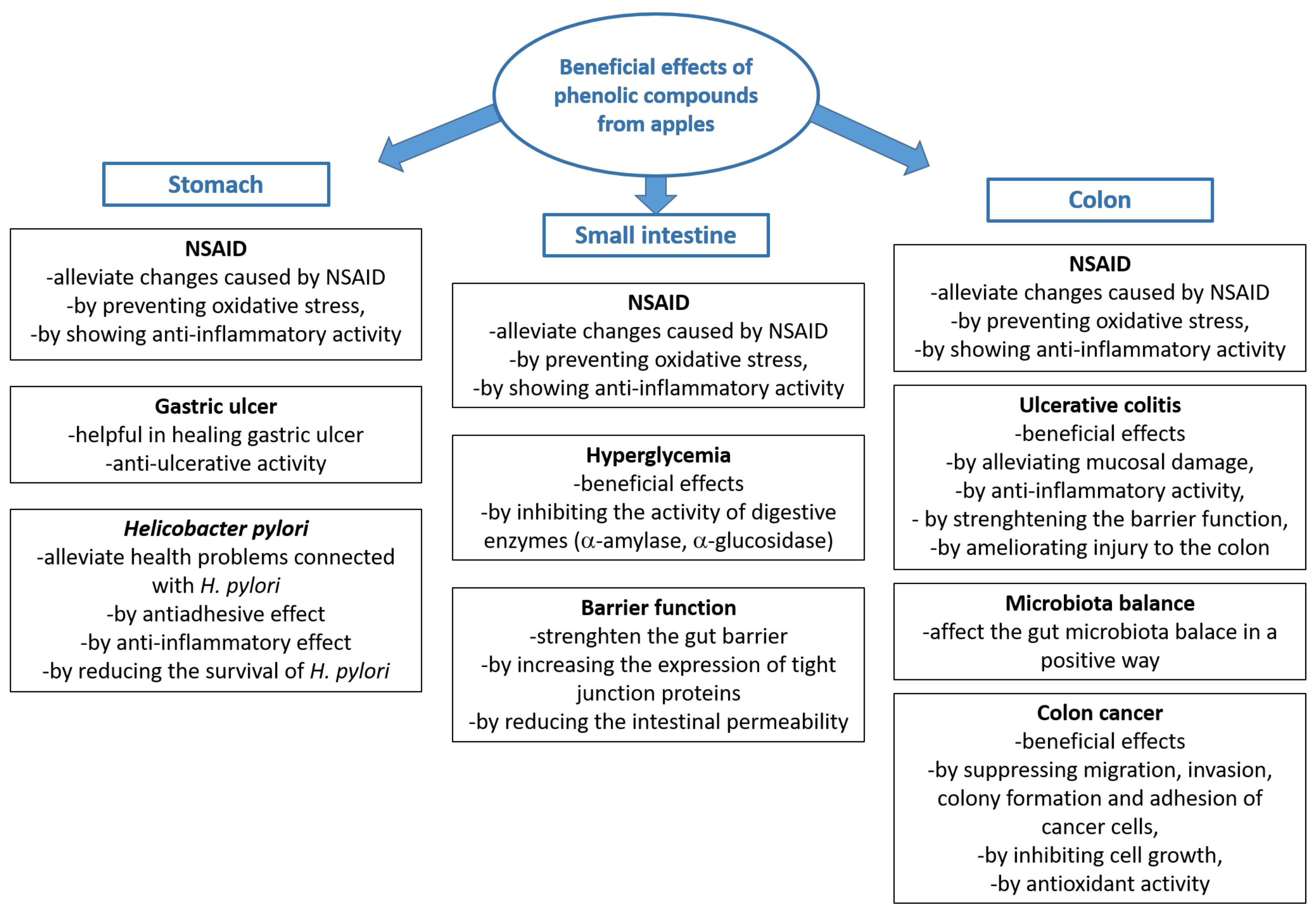
5. Beneficial Effects of Phenolic Compounds from Apples in the Digestive Tract
5.1. Beneficial Effects in the Stomach
5.2. Beneficial Effects in the Small Intestine
5.3. Beneficial Effects in the Colon
6. The Influence of the Food Matrix
7. Conclusions
8. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ji, Y.; Guo, Y.; Wang, H.; Wu, Z.; Li, H.; Wang, H. Dietary supplementation of apple phlorizin attenuates the redox state related to gut microbiota homeostasis in C57BL/6J mice fed with a high-fat diet. J. Agric. Food Chem. 2021, 69, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Qiu, D.; Wu, Z.; Yang, H.; Xie, Y.; Li, S.; Yin, Y.; Li, X. Apple polyphenol extract alleviates DSS-induced ulcerative colitis and linked behavioral disorders via regulating the gut-brain axis. Food Biosci. 2023, 53, 102720. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Tenore, G.C.; Campiglia, P.; Ritieni, A.; Novellino, E. In vitro bioaccessibility, bioavailability and plasma protein interaction of polyphenols from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013, 141, 3519–3524. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Clifford, M.N.; Polyviou, T.; Ludwig, I.A.; Alfheeaid, H.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Plasma pharmacokinetics of (poly)phenol metabolites and catabolites after ingestion of orange juice by endurance trained men. Free Radical Biol. Med. 2020, 160, 784–795. [Google Scholar] [CrossRef]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent advances on dietary polyphenol’s potential roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225. [Google Scholar] [CrossRef]
- Van Duynhoven, J.; van Velzen, E.; Jacobs, D.M. Nutrikinetic assessment of polyphenol exposure. Curr. Opin. Food Sci. 2017, 16, 88–95. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.J.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Šavikin, K.; Živković, J.; Zdunić, G.; Gođevac, D.; Ðorđević, B.; Dojčinović, B.; Ðorđević, N. Phenolic and mineral profiles of four Balkan indigenous apple cultivars monitored at two different maturity stages. J. Food Compos. Anal. 2014, 35, 101–111. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Antunes, C.; Ramos, A.; Serra, A.T.; Figueira, M.E.; Duarte, C.M.M.; de Carvalho, A.; Bronze, M.R. Characterization of traditional and exotic apple varieties from Portugal. Part 1.—Nutritional, phytochemical and sensory evaluation. J. Funct. Food 2010, 2, 35–45. [Google Scholar] [CrossRef]
- Cao, X.; Wang, C.; Pei, H.; Sun, B. Separation and identification of polyphenols in apple pomace by high-speed counter-current chromatography and high-performance liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2009, 1216, 4268–4274. [Google Scholar] [CrossRef] [PubMed]
- Giomaro, G.; Karioti, A.; Bilia, A.R.; Bucchini, A.; Giamperi, L.; Ricci, D.; Fraternale, D. Polyphenols profile and antioxidant activity of skin and pulp of a rare apple from Marche region (Italy). Chem. Cent. J. 2014, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Khanizadeh, S.; Tsao, R.; Rekika, D.; Yang, R.; Charles, M.T.; Rupasinghe, H.P.V. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J. Food Compos. Anal. 2008, 21, 396–401. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Zhao, T.; Gong, T.; Zou, L.; Guo, Y. Young apple polyphenols postpone starch digestion in vitro and in vivo. J. Funct. Food 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Landi, M.; Massai, R.; Remorini, D.; Conte, G.; Guidi, L. Ancient apple cultivars from Garfagnana (Tuscany, Italy): A potential sourcefor ‘nutrafruit’ production. Food Chem. 2019, 294, 518–525. [Google Scholar] [CrossRef]
- Stracke, B.A.; Rűfer, C.E.; Weibel, F.P.; Bub, A.; Watzl, B. Three-year comparison of the polyphenol contents and antioxidant capacities in organically and conventionally produced apples (Malus domestica Bork. Cultivar Golden Delicious). J. Agric. Food Chem. 2009, 57, 4598–4605. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, L.; Wang, Z.; Nisar, T.; Gong, T.; Li, D.; Niu, P.; Guo, Y. The antioxidant property and α-amylase inhibition activity of young apple polyphenols are related with apple varieties. LWT-Food Sci. Technol. 2019, 111, 252–259. [Google Scholar] [CrossRef]
- Faramarzi, S.; Pacifico, S.Y.A.; Lettieri, A.; Nocera, P.; Piccolella, S. Red-fleshed apples: Old autochthonous fruits as a novel source of anthocyanin antioxidants. Plant Food Hum. Nutr. 2015, 70, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Ištuk, J.; Matić, P.; Skendrović Babojelić, M. Interactions of polyphenols from traditional apple varieties ‘Bobovac’, ‘Ljepocvjetka’ and ‘Crvenka’ with β-Glucan during in vitro simulated digestion. Food Chem. 2021, 363, 130283. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Ištuk, J.; Barron, A.; Matić, P. Bioactive phenolic compounds from apples during simulated in vitro gastrointestinal digestion: Kinetics of their release. Appl. Sci. 2023, 13, 8434. [Google Scholar] [CrossRef]
- Graziani, G.; Gaspari, A.; Di Vaio, C.; Cirillo, A.; Ronca, C.L.; Grosso, M.; Ritieni, A. Assessment of in vitro bioaccessibility of polyphenols from Annurca, Limoncella, Red Delicious, and Golden Delicious apples using a sequential enzymatic digestion model. Antioxidants 2021, 10, 541. [Google Scholar] [CrossRef]
- Kahle, K.; Huemmer, W.; Kempf, M.; Scheppach, W.; Erk, T.; Richling, E. Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J. Agric. Food Chem. 2007, 55, 10605–10614. [Google Scholar] [CrossRef]
- Veeriah, S.; Hofmann, T.; Glei, M.; Dietrich, H.; Will, F.; Schreier, P.; Knaup, S.; Pool-Zobel, B.L. Apple polyphenols and products formed in the gut differently inhibit survival of human cell lines derived from colon adenoma (LT97) and carcinoma (HT29). J. Agric. Food Chem. 2007, 55, 2892–2900. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Balderas, C.; Sánchez-Moreno, C.; De Ancos, B. Impact of an in vitro dynamic gastrointestinal digestion on phenolic compounds and antioxidant capacity of apple treated by high-pressure processing. Innov. Food Sci. Emerg. 2020, 66, 102486. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef] [PubMed]
- Hagl, S.; Deusser, H.; Soyalan, B.; Janzowski, C.; Will, F.; Dietrich, H.; Albert, F.W.; Rohner, S.; Richling, E. Colonic availability of polyphenols and D-(-)-quinic acid after apple smoothie consumption. Mol. Nutr. Food Res. 2011, 55, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Speisky, H.; Brunser, O.; Pastene, E.; Gotteland, M. Apple peel polyphenols protect against gastrointestinal mucosa alterations induced by indomethacin in rats. J. Agric. Food Chem. 2011, 59, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
- Pastene, E.; Spiesky, H.; García, A.; Moreno, J.; Troncoso, M.; Figueroa, G. In vitro and in vivo effects of apple polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2010, 58, 7172–7179. [Google Scholar] [CrossRef]
- Pollini, L.; Juan-García, A.; Blasi, F.; Mañes, J.; Cossignani, L.; Juan, C. Assessing bioaccessibility and bioavailability in vitro of phenolic compounds from freeze-dried apple pomace by LC-Q-TOF-MS. Food Biosci. 2022, 48, 101799. [Google Scholar] [CrossRef]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Fritzsche Rodrigues, R.; Maróstica Junior, M.R.; de Souza Fonseca, B.; Ragagnin de Menezes, C.; et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Food 2020, 65, 103714. [Google Scholar] [CrossRef]
- Bellion, P.; Hofmann, T.; Pool-Zebel, B.L.; Will, F.; Dietrich, H.; Knaup, B.; Richling, E.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of phenolic apple juice extracts and their gut fermentation products in the human colon carcinoma cell line Caco-2. J. Agric. Food Chem. 2008, 56, 6310–6317. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 2006, 136, 1192–1197. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Esterase activity able to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, S.; De Noni, I.; Caracciolo, F.; Molinari, F.; Parini, C.; Mora, D. Bacterial cinnamoyl esterase activity screening for the production of a novel functional food product. Appl. Environ. Microbiol. 2008, 74, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Hamauzu, Y.; Irie, M.; Kondo, M.; Fujita, T. Antiulcerative properties of crude polyphenols and juice of apple, and Chinese quince extracts. Food Chem. 2008, 108, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Hamauzu, Y.; Inno, T.; Kume, C.; Irie, M.; Hiramatsu, K. Antioxidant and antiulcerative properties of phenolics from chinese quince, quince, and apple fruits. J. Agric. Food Chem. 2006, 54, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.E.; Mohanad, M.; Ahmed, A.A.E.; Aboulhoda, B.E.; El-Awdan, S.A. Mechanistic insights into the protective effects of chlorogenic acid against indomethacin-induced gastric ulcer in rats: Modulation of the cross talk between autophagy and apoptosis signaling. Life Sci. 2021, 275, 119370. [Google Scholar] [CrossRef] [PubMed]
- Pastene, E.; Speisky, H.; Troncoso, M.; Alarcon, J.; Figueroa, G. In vitro inhibitory effect of apple peel extract on the growth of Helicobacter pylori and respiratory burst induced on human neutrophils. J. Agric. Food Chem. 2009, 57, 7743–7749. [Google Scholar] [CrossRef]
- Pastene, E.; Troncoso, M.; Fiġueroa, G.; Alarcón, J.; Speisky, H. Association between polymerization degree of apple peel polyphenols and inhibition of Helicobacter pylori urease. J. Agric. Food Chem. 2009, 57, 416–424. [Google Scholar] [CrossRef]
- Peri, L.; Pietraforte, D.; Scorza, G.; Napolitano, A.; Fogliano, V.; Minetti, M. Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: A new biological function for polyphenols with a catechol group? Free Radical Biol. Med. 2005, 39, 668–681. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W.; Meng, Y.; Yang, X.; Yuan, L.; Guo, Y. Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J.; Guo, Y.; Miao, M. Mechanism of binding interactions between young apple polyphenols and porcine pancreatic α-amylase. Food Chem. 2019, 283, 468–474. [Google Scholar] [CrossRef]
- Gong, T.; Yang, X.; Bai, F.; Li, D.; Zhao, T.; Zhang, J.; Sun, L.; Guo, Y. Young apple polyphenols as natural α-glucosidase inhibitors: In vitro and in silico studies. Bioorg Chem. 2020, 96, 103625. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Yamada, M.; Miura, T.; Nagashima, K.; Ogura, K.; Inagaki, N.; Maeda-Yamamoto, M. Chronic administration of apple polyphenols ameliorates hyperglycaemia in high-normal and borderline subjects: A randomised, placebo-controlled trial. Diabetes Res. Clin. Pract. 2017, 129, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Gotteland, M.; Speisky, H. Apple peel polyphenol extract protects against indomethacin-induced damage in Caco-2 cells by preventing mitochondrial complex I inhibition. J. Agric. Food Chem. 2011, 59, 11501–11508. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Hidalgo-Liberona, N.; Gonzaález-Domínguez, R.; Garcia-Aloy, M.; Guglielmetti, S.; Bernardi, S.; Kirkup, B.; Kroon, P.A.; Cherubini, A.; Riso, P.; et al. Exploring the molecular pathways behind the effects of nutrients and dietary polyphenols on gut microbiota and intestinal permeability: A perspective on the potential of metabolomics and future clinical applications. J. Agr. Food Chem. 2020, 68, 1780–1789. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, J.; Qi, J.; Yue, T.; Yuan, Y.; Shi, Y. In vitro and in vivo evaluation of chlorogenic acid-encapsulated lignin on patulin adsorption and alleviation of patulin-induced colonic damage. J. Agric. Food Chem. 2023, 71, 11217–11227. [Google Scholar] [CrossRef]
- D’Argenio, G.; Mazzone, G.; Tuccillo, C.; Ribecco, M.T.; Graziani, G.; Gravina, A.G.; Caserta, S.; Guido, S.; Fogliano, V.; Caporaso, N.; et al. Apple polyphenols extract (APE) improves colon damage in a rat model of colitis. Digest Liver Dis. 2012, 44, 555–562. [Google Scholar] [CrossRef]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Cui, Y.; Yin, Y.; Li, S.; Li, X. Apple polyphenols extracts ameliorate high carbohydrate diet-induced body weight gain by regulating the gut microbiota and appetite. J. Agric. Food Chem. 2022, 70, 196–210. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple polyphenol extract improves high-fat diet-induced hepatic steatosis by regulating bile acid synthesis and gut microbiota in C57BL/6 Male Mice. J. Agric. Food Chem. 2021, 69, 6829–6841. [Google Scholar] [CrossRef]
- Elkahoui, S.; Levin, C.E.; Bartley, G.E.; Yokoyama, W.; Friedman, M. Levels of fecal procyanidins and changes in microbiota and metabolism in mice fed a high-fat diet supplemented with apple peel. J. Agric. Food Chem. 2019, 67, 10352–10360. [Google Scholar] [CrossRef]
- Sembries, S.; Dongowski, G.; Mehrlander, K.; Will, F.; Dietrich, H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. J. Agric. Food Chem. 2006, 54, 10269–10280. [Google Scholar] [CrossRef]
- Huang, T.; Che, Q.; Chen, X.; Chen, D.; Yu, B.; He, J.; Chen, H.; Yan, H.; Zheng, P.; Luo, Y.; et al. Apple polyphenols improve intestinal antioxidant capacity and barrier function by activating the Nrf2/Keap1 signaling pathway in a pig model. J. Agric. Food Chem. 2022, 70, 7576–7585. [Google Scholar] [CrossRef] [PubMed]
- Koseoğlu, A.; Al-Taie, A. The potential chemo-preventive roles of Malus domestica against the risk of colorectal cancer: A suggestive insight into clinical application. Clin. Nutr. 2022, 52, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Martínez-Bardají, A.; Macià, A.; Motilva, M.J.; Piñol-Felis, C. Consumption evaluation of one apple flesh a day in the initial phases prior to adenoma/adenocarcinoma in an azoxymethane rat colon carcinogenesis model. J. Nutr. Biochem. 2020, 83, 108418. [Google Scholar] [CrossRef] [PubMed]
- Fini, L.; Selgrad, M.; Fogliano, V.; Graziani, G.; Romano, M.; Hotchkiss, E.; Daoud, Y.A.; De Vol, E.B.; Boland, C.R.; Ricciardiello, L. Annurca apple polyphenols have potent demethylating activity and can reactivate silenced tumor suppressor genes in colorectal cancer cells1,2. J. Nutr. 2007, 137, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Huang, C.C.; Hsu, L.S.; Kao, S.H.; Wang, C.J. Apple polyphenol inhibits colon carcinoma metastasis via disrupting Snail binding to focal adhesion kinase. J. Funct. Food 2015, 12, 80–91. [Google Scholar] [CrossRef]
- Barth, S.W.; Faehndrich, C.; Bub, A.; Watzl, B.; Will, F.; Dietrich, H.; Rechkemmer, G.; Briviba, K. Cloudy apple juice is more effective than apple polyphenols and an apple juice derived cloud fraction in a rat model of colon carcinogenesis. J. Agric. Food Chem. 2007, 55, 1181–1187. [Google Scholar] [CrossRef]
- Lhoste, E.F.; Bruneau, A.; Bensaada, M.; Cherbuy, C.; Philippe, C.; Bruel, S.; Sutren, M.; Rabot, S.; Guyot, S.; Duee, P.H.; et al. Apple proanthocyanidins do not reduce the induction of preneoplastic lesions in the colon of rats associated with human microbiota. J. Agric. Food Chem. 2010, 58, 4120–4125. [Google Scholar] [CrossRef]
- Kosmala, M.; Kozodziejczyk, K.; Zduńczyk, Z.; Juśkiewicz, J.; Boros, D. Chemical composition of natural and polyphenol-free apple pomace and the effect of this dietary ingredient on intestinal fermentation and serum lipid parameters in rats. J. Agric. Food Chem. 2011, 59, 9177–9185. [Google Scholar] [CrossRef]
- Licht, T.R.; Hansen, M.; Bergström, A.; Poulsen, M.; Krath, B.N.; Markowski, J.; Dragsted, L.O.; Wilcks, A. Effects of apples and specific apple components on the cecal environment of conventional rats: Role of apple pectin. BMC Microbiol. 2010, 10, 13. [Google Scholar] [CrossRef]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Rémésy, C.; Demigné, C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Sun, Y.; Xu, W.; Zheng, H.; Wang, Y.; Tang, Y.; Gao, X.; Song, C.; Long, Y.; et al. Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohy Polym. 2020, 230, 115726. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobek, L.; Matić, P. Phenolic Compounds from Apples: From Natural Fruits to the Beneficial Effects in the Digestive System. Molecules 2024, 29, 568. https://doi.org/10.3390/molecules29030568
Jakobek L, Matić P. Phenolic Compounds from Apples: From Natural Fruits to the Beneficial Effects in the Digestive System. Molecules. 2024; 29(3):568. https://doi.org/10.3390/molecules29030568
Chicago/Turabian StyleJakobek, Lidija, and Petra Matić. 2024. "Phenolic Compounds from Apples: From Natural Fruits to the Beneficial Effects in the Digestive System" Molecules 29, no. 3: 568. https://doi.org/10.3390/molecules29030568
APA StyleJakobek, L., & Matić, P. (2024). Phenolic Compounds from Apples: From Natural Fruits to the Beneficial Effects in the Digestive System. Molecules, 29(3), 568. https://doi.org/10.3390/molecules29030568