Porous Organic Materials in Tissue Engineering: Recent Advances and Applications for Severed Facial Nerve Injury Repair
Abstract
1. Introduction
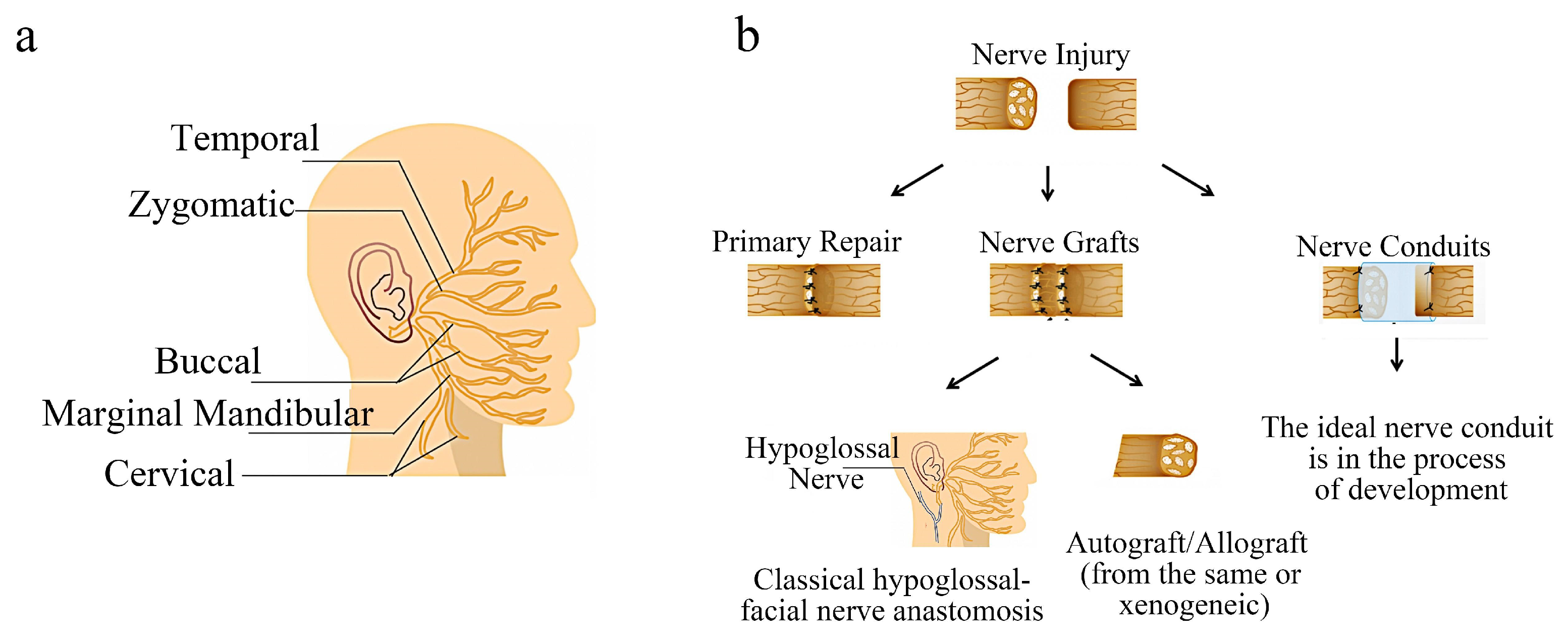
2. Facial Nerve Injury and Repair
Current Common Treatment Methods of Facial Nerve Injury
3. Tissue Engineering Nerve Conduits
3.1. Requirements of the Tissue Engineering Nerve Conduits
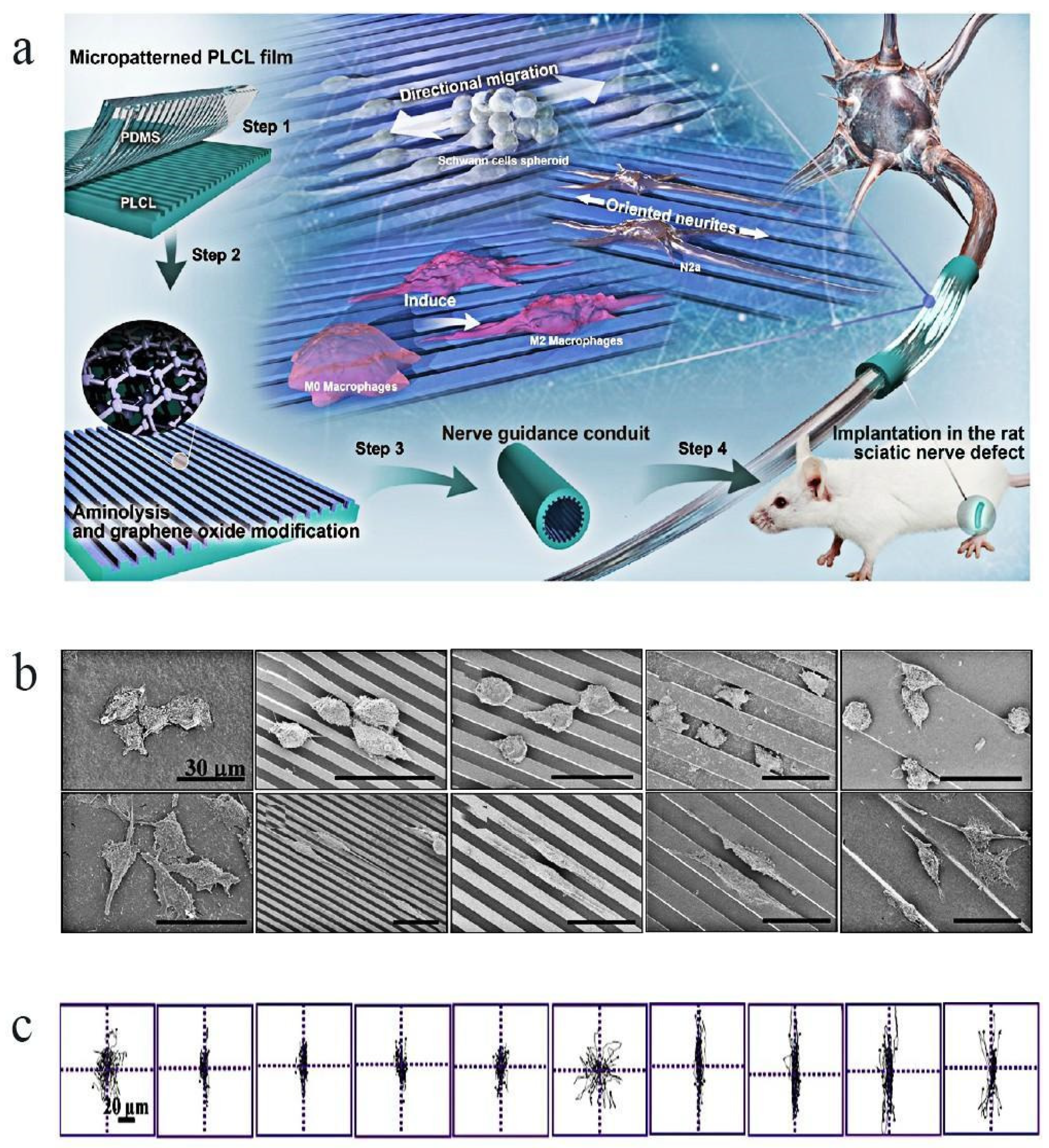
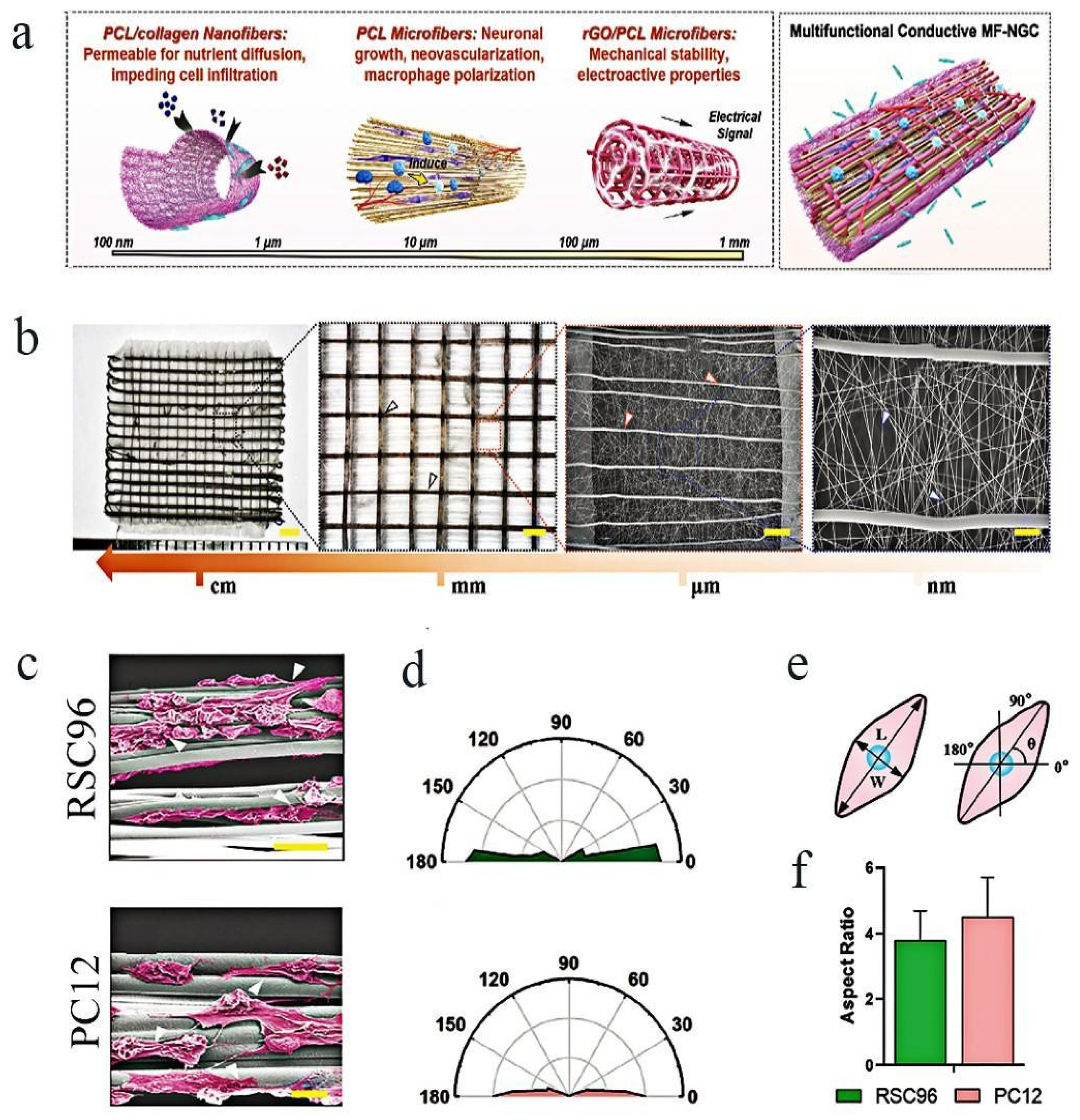
3.2. Development and Prospect of Tissue Engineering Nerve Conduit
4. Porous Organic Materials in Facial Nerve Injury
4.1. Natural Porous Organic Materials
4.1.1. Collagen
4.1.2. Chitosan
4.1.3. Bacterial Cellulose (BC)
4.2. Synthetic Porous Organic Materials
4.2.1. PCL
4.2.2. PLA
4.2.3. PGA
4.2.4. PLGA
4.3. Copolymer or a Blend of Polymers
5. Prospects
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owusu, J.A.; Stewart, C.M.; Boahene, K. Facial Nerve Paralysis. Med. Clin. N. Am. 2018, 102, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Thielker, J.; Wahdan, A.; Buentzel, J.; Kaftan, H.; Boeger, D.; Mueller, A.H.; Wittig, A.; Schultze-Mosgau, S.; Ernst, T.; Guntinas-Lichius, O. Long-Term Facial Nerve Outcome in Primary Parotid Cancer Surgery: A Population-Based Analysis. Laryngoscope 2021, 131, 2694–2700. [Google Scholar] [CrossRef]
- Kawata, R.; Kinoshita, I.; Omura, S.; Kawata, R.; Kinoshita, I.; Omura, S.; Higashino, M.; Nishikawa, S.; Terada, T.; Haginomori, S.; et al. Risk Factors of Postoperative Facial Palsy for Benign Parotid Tumors: Outcome of 1,018 Patients. Laryngoscope 2021, 131, E2857–E2864. [Google Scholar] [CrossRef] [PubMed]
- Ruijs, A.C.; Jaquet, J.B.; Kalmijn, S.; Giele, H.; Hovius, S.E. Median and Ulnar Nerve Injuries: A Meta-analysis of Predictors of Motor and Sensory Recovery after Modern Microsurgical Nerve Repair. Plast. Reconstr. Surg. 2005, 116, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, I.R.; Brenner, M.J.; Singham, J.; Rickman, S.R.; Hunter, D.A.; Mackinnon, S.E. Effect of Tension on Nerve Regeneration in Rat Sciatic Nerve Transection Model. Ann. Plast. Surg. 2004, 53, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, M.S.; Jeon, J.; Lee, D.H.; Yang, K.; Cho, S.W.; Han, I.; Yang, H.S. Biodegradable Nerve Guidance Conduit with Microporous and Micropatterned Poly(lactic-co-glycolic acid)-Accelerated Sciatic Nerve Regeneration. Macromol. Biosci. 2018, 18, e1800290. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Kannan, S.; Cao, T.; Fuh, J.Y.H.; Sriram, G.; Lu, W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019, 7, 266. [Google Scholar] [CrossRef]
- Subbiah, R.; Guldberg, R.E. Materials Science and Design Principles of Growth Factor Delivery Systems in Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2019, 8, e1801000. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, M.; Zhao, J.; Chai, R.; Kang, J. Looking into the Future: Toward Advanced 3D Biomaterials for Stem-Cell-Based Regenerative Medicine. Adv. Mater. 2018, 30, e1705388. [Google Scholar] [CrossRef]
- Takezawa, K.; Townsend, G.; Ghabriel, M. The Facial Nerve: Anatomy and Associated Disorders for Oral Health Professionals. Odontology 2018, 106, 103–116. [Google Scholar] [CrossRef]
- Lyford-Pike, S.; Helwig, N.E.; Sohre, N.E.; Guy, S.J.; Hadlock, T.A. Predicting Perceived Disfigurement from Facial Function in Patients with Unilateral Paralysis. Plast. Reconstr. Surg. 2018, 142, 722e–728e. [Google Scholar] [CrossRef]
- Bovenzi, C.D.; Ciolek, P.; Crippen, M.; Curry, J.M.; Krein, H.; Heffelfinger, R. Reconstructive Trends and Complications Following Parotidectomy: Incidence and Predictors in 11,057 Cases. J. Otolaryngol. Head Neck Surg. 2019, 48, 64. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, R.H.; Liebman, E.; Jiménez, D.F.; Buchheit, W.A.; Andrews, D.W. Facial Reanimation after Facial Nerve Injury. Neurosurgery 1991, 29, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Jia, X.H.; Xu, J.; Yu, J.; Wang, J.; Zhao, W.D.; Chi, F.L.; Dai, C.F.; Li, H.W.; Zhong, P.; et al. Neurorrhaphy for Facial Reanimation with Interpositional Graft: Outcome in 23 Patients and the Impact of Timing on the Outcome. World Neurosurg. 2019, 126, e688–e693. [Google Scholar] [CrossRef] [PubMed]
- Sahovaler, A.; Yeh, D.; Yoo, J. Primary Facial Reanimation in Head and Neck Cancer. Oral Oncol. 2017, 74, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.; Faibisoff, B.; Williams, B. The Nerve Gap: Suture under Tension vs. Graft. Plast. Reconstr. Surg. 1975, 56, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.R.; Callahan, N.; Miloro, M. Management of Traumatic Trigeminal and Facial Nerve Injuries. Oral. Maxillofac. Surg. Clin. N. Am. 2021, 33, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.L.; Stramiello, J.A.; Orosco, R.K.; Greene, J.J. Advances in Facial Nerve Management in the Head and Neck Cancer Patient. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 235–240. [Google Scholar] [CrossRef]
- Scaramella, L.F. Cross-face Facial Nerve Anastomosis: Historical Notes. Ear Nose Throat J. 1996, 75, 343–354. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhu, W.D.; Tremp, M.; Chen, G.; Wang, Z.Y.; Wu, H.; Wang, W. Facial Reanimation with Interposition Nerve Graft or Masseter Nerve Transfer: A Comparative Retrospective Study. Neural Regen. Res. 2022, 17, 1125–1130. [Google Scholar] [CrossRef]
- Pinkiewicz, M.; Dorobisz, K.; Zatoński, T.A. Comprehensive Approach to Facial Reanimation: A Systematic Review. J. Clin. Med. 2022, 11, 2890. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, N.; Matsumine, H.; Fujii, K.; Osaki, H.; Ueta, Y.; Kamei, W.; Niimi, Y.; Miyata, M.; Sakurai, H. Facial Nerve Regeneration with Bioabsorbable Collagen Conduits Filled with Collagen Filaments: An Experimental Study. Regen. Ther. 2021, 18, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, C.S.; Park, C.H. Harnessing Nanotopography of Electrospun Nanofibrous Nerve Guide Conduits (NGCs) for Neural Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1078, 395–408. [Google Scholar] [PubMed]
- Xue, W.; Shi, W.; Kong, Y.; Kuss, M.; Duan, B. Anisotropic Scaffolds for Peripheral Nerve and Spinal Cord Regeneration. Bioact. Mater. 2021, 6, 4141–4160. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Lee, J.H. Fabrication and Characterization of Hydrophilized Porous PLGA Nerve Guide Conduits by a Modified Immersion Precipitation Method. J. Biomed. Mater. Res. Part A 2007, 80, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, Y.; Quan, X.; Lu, L.; Xia, B.; Gao, J.; Qi, F.; Li, S.; Zhao, L.; Mei, L.; et al. Oxygen Carrier in Core-Shell Fibers Synthesized by Coaxial Electrospinning Enhances Schwann Cell Survival and Nerve Regeneration. Theranostics 2020, 10, 8957–8973. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Li, Z.; Bai, Y.; Liao, G.; Pan, J.; Zhang, C. Collagen/Nano-Sized β-Tricalcium Phosphate Conduits Combined with Collagen Filaments and Nerve Growth Factor Promote Facial Nerve Regeneration in Miniature Swine: An in Vivo Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 472–478. [Google Scholar] [CrossRef]
- Behtaj, S.; St John, J.A.; Ekberg, J.A.K.; Rybachuk, M. Neuron-Fibrous Scaffold Interfaces in the Peripheral Nervous System: A Perspective on the Structural Requirements. Neural Regen. Res. 2022, 17, 1893–1897. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Y.; Duan, Y.; Yu, X.; Shi, H.; Nakkala, J.R.; Zuo, X.; Hong, L.; Mao, Z.; Gao, C. Surface-Anchored Graphene Oxide Nanosheets on Cell-Scale Micropatterned Poly(d,l-lactide-co-caprolactone) Conduits Promote Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 7915–7930. [Google Scholar] [CrossRef]
- Raimundo, R.D.; Landim, F.S.; Gomes, A.C.; Castro, C.B.; Junior, V.A.; Vasconcelos, B.C. Morphofunctional Effect of Stem Cells on the Regeneration of the Facial Nerve in a Rat Model. J. Oral Maxillofac. Surg. 2019, 77, 2168.e1–2168.e12. [Google Scholar]
- Abbas, O.L.; Borman, H.; Uysal, Ç.A.; Gönen, Z.B.; Aydin, L.; Helvacioğlu, F.; Ilhan, Ş.; Yazici, A.C. Adipose-Derived Stem Cells Enhance Axonal Regeneration through Cross-Facial Nerve Grafting in a Rat Model of Facial Paralysis. Plast. Reconstr. Surg. 2016, 138, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Han, U.G.; Ye, S.; Kang, S.; Yoo, J.; Cho, Y.S.; Jung, Y. Effect of Electrical Stimulation on Nerve-Guided Facial Nerve Regeneration. ACS Biomater. Sci. Eng. 2023, 9, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Gerth, D.J.; Tashiro, J.; Thaller, S.R. Clinical Outcomes for Conduits and Scaffolds in Peripheral Nerve Repair. World J. Clin. Cases 2015, 3, 141–147. [Google Scholar] [CrossRef]
- Sang, S.; Cheng, R.; Cao, Y.; Yan, Y.; Shen, Z.; Zhao, Y.; Han, Y. Biocompatible Chitosan/Polyethylene Glycol/Multi-Walled Carbon Nanotube Composite Scaffolds for Neural Tissue Engineering. J. Zhejiang Univ. Sci. B 2022, 23, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA Nanofibrous Scaffolds Coated with Graphene Oxide for Promoting Neural Cell Growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, C.; Li, Z.; Zhang, S.; Liu, J.; Bai, Y.; Pan, J.; Zhang, C. Collagen/β-TCP Nerve Guidance Conduits Promote Facial Nerve Regeneration in Mini-Swine and the Underlying Biological Mechanism: A Pilot in Vivo Study. J. Biomed. Mater. Res. Prat B Appl. Biomater. 2019, 107, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Bengur, F.B.; Stoy, C.; Binko, M.A.; Nerone, W.V.; Fedor, C.N.; Solari, M.G.; Marra, K.G. Facial Nerve Repair: Bioengineering Approaches in Preclinical Models. Tissue Eng. Part B Rev. 2022, 28, 364–378. [Google Scholar] [CrossRef]
- Sasaki, R.; Watanabe, Y.; Yamato, M.; Okamoto, T. Tissue-Engineered Nerve Guides with Mesenchymal Stem Cells in the Facial Nerve Regeneration. Neurochem. Int. 2021, 148, 105062. [Google Scholar] [CrossRef]
- Sasaki, R.; Watanabe, Y.; Yamato, M.; Aoki, S.; Okano, T.; Ando, T. Surgical Anatomy of the Swine Face. Lab. Anim. 2010, 44, 359–363. [Google Scholar] [CrossRef]
- Sasaki, R.; Matsumine, H.; Watanabe, Y.; Yamato, M.; Ando, T. Anesthesia for Research on Reconstructive Facial Surgery in Rats. J. Reconstr. Microsurg. 2013, 29, 209–210. [Google Scholar]
- Sasaki, R.; Aoki, S.; Yamato, M.; Uchiyama, H.; Wada, K.; Okano, T.; Ogiuchi, H. Tubulation with Dental Pulp Cells Promotes Facial Nerve Regeneration in Rats. Tissue Eng. Part A 2008, 14, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Aoki, S.; Yamato, M.; Uchiyama, H.; Wada, K.; Ogiuchi, H.; Okano, T.; Ando, T. PLGA Artificial Nerve Conduits with Dental Pulp Cells Promote Facial Nerve Regeneration. J. Tissue Eng. Regen. Med. 2011, 5, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Matsumine, H.; Watanabe, Y.; Takeuchi, Y.; Yamato, M.; Okano, T.; Miyata, M.; Ando, T. Electrophysiologic and Functional Evaluations of Regenerated Facial Nerve Defects with a Tube Containing Dental Pulp Cells in Rats. Plast. Reconstr. Surg. 2014, 134, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, H.; Takeuchi, Y.; Sasaki, R.; Kazama, T.; Kano, K.; Matsumoto, T.; Sakurai, H.; Miyata, M.; Yamato, M. Adipocyte-Derived and Dedifferentiated Fat Cells Promoting Facial Nerve Regeneration in a Rat Model. Plast. Reconstr. Surg. 2014, 134, 686–697. [Google Scholar] [CrossRef]
- Shimizu, M.; Matsumine, H.; Osaki, H.; Ueta, Y.; Tsunoda, S.; Kamei, W.; Hashimoto, K.; Niimi, Y.; Watanabe, Y.; Miyata, M.; et al. Adipose-Derived Stem Cells and the Stromal Vascular Fraction in Polyglycolic Acid-Collagen Nerve Conduits Promote Rat Facial Nerve Regeneration. Wound Repair. Regen. 2018, 26, 446–455. [Google Scholar] [CrossRef]
- Oatari, M.; Uehara, M.; Shimizu, F. Evaluation of the Effects of a Polyglycolic Acid-Collagen Tube in the Regeneration of Facial Nerve Defects in Rats. Int. J. Artif. Organs 2018, 41, 664–669. [Google Scholar] [CrossRef]
- Fujimaki, H.; Matsumine, H.; Osaki, H.; Ueta, Y.; Kamei, W.; Shimizu, M.; Hashimoto, K.; Fujii, K.; Kazama, T.; Matsumoto, T.; et al. Dedifferentiated Fat Cells in Polyglycolic Acid-Collagen Nerve Conduits Promote Rat Facial Nerve Regeneration. Regen. Ther. 2019, 11, 240–248. [Google Scholar] [CrossRef]
- Matsumine, H.; Sasaki, R.; Tabata, Y.; Matsui, M.; Yamato, M.; Okano, T.; Sakurai, H. Facial Nerve Regeneration Using Basic Fibroblast Growth Factor-Impregnated Gelatin Microspheres in a Rat Model. J. Tissue Eng. Regen. Med. 2016, 10, E559–E567. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wang-Bennett, L.T.; Coker, N.J. Facial Nerve Regeneration in the Silicone Chamber: The Influence of Nerve Growth Factor. Exp. Neurol. 1989, 103, 52–60. [Google Scholar] [CrossRef]
- Spector, J.G.; Lee, P.; Derby, A.; Frierdich, G.E.; Neises, G.; Roufa, D.G. Rabbit Facial Nerve Regeneration in NGF-Containing Silastic Tubes. Laryngoscope 1993, 103, 548–558. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, H.; Zhu, T.; Liu, Y.; Pan, H.; Fan, C.; Zhao, X.; Lu, W.W. Bioinspired Multichannel Nerve Guidance Conduit Based on Shape Memory Nanofibers for Potential Application in Peripheral Nerve Repair. ACS Nano 2020, 14, 12579–12595. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, C.; Liu, Z.; Ko, J.; Chen, L.; Zhang, T.; Xiong, Z.; Zhang, L.; Sun, W. 3D Printed Conductive Multiscale Nerve Guidance Conduit with Hierarchical Fibers for Peripheral Nerve Regeneration. Adv. Sci. 2023, 10, e2205744. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.C.; Chon, J.; Jung, J.; Kim, S.S.; Bae, S.; Kim, S.H.; Yeo, S.G. Potential Therapeutic Strategies and Substances for Facial Nerve Regeneration Based on Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 4926. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Liu, W.; Jesural, N.; Li, X.; Hunter, D.; Xia, Y. Nerve Guidance Conduits Based on Double-Layered Scaffolds of Electrospun Nanofibers for Repairing the Peripheral Nervous System. ACS Appl. Mater. Interfaces 2014, 6, 9472–9480. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef]
- Kitahara, A.K.; Nishimura, Y.; Shimizu, Y.; Endo, K. Facial Nerve Repair Accomplished by the Interposition of a Collagen Nerve Guide. J. Neurosurg. 2000, 93, 113–120. [Google Scholar] [CrossRef]
- Vasconcelos, B.C.; Gay-Escoda, C. Facial Nerve Repair with Expanded Polytetrafluoroethylene and Collagen Conduits: An Experimental Study in the Rabbit. J. Oral Maxillofac. Surg. 2000, 58, 1257–1262. [Google Scholar] [CrossRef]
- Ma, F.; Wang, H.; Yang, X.; Wu, Y.; Liao, C.; Xie, B.; Li, Y.; Zhang, W. Controlled Release of Ciliary Neurotrophic Factor from Bioactive Nerve Grafts Promotes Nerve Regeneration in Rats with Facial Nerve Injuries. J. Biomed. Mater. Res. Part A 2022, 110, 788–796. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, Z.; Jin, W.; Chen, B.; Meng, D.; Ding, W.; Han, S.; Hou, X.; Zhu, T.; Yuan, B.; et al. Induction of Rat Facial Nerve Regeneration by Functional Collagen Scaffolds. Biomaterials 2013, 34, 1302–1310. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sasaki, R.; Matsumine, H.; Yamato, M.; Okano, T. Undifferentiated and Differentiated Adipose-derived Stem Cells Improve Nerve Regeneration in a Rat Model of Facial Nerve Defect. J. Tissue Eng. Regen. Med. 2017, 11, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xu, F.; Li, R.; Li, R.; Zheng, Y.; Wang, F.; Wei, N.; Zhong, J.; Tang, Q.; Zhu, T.; et al. Sustained Delivery of Glial Cell-derived Neurotrophic Factors in Collagen Conduits for Facial Nerve Regeneration. Acta Biomater. 2018, 69, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, H.; Numakura, K.; Climov, M.; Watanabe, Y.; Giatsidis, G.; Orgill, D.P. Facial-nerve Regeneration Ability of a Hybrid Artificial Nerve Conduit Containing Uncultured Adipose-derived Stromal Vascular Fraction: An Experimental Study. Microsurgery 2017, 37, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xu, H.; Xu, Y.P.; Liu, H.H.; Lang, J.T.; Chen, X.P.; Xu, W.H.; Deng, Y.; Fan, J.P. Olfactory Ensheathing Cells Promote Nerve Regeneration and Functional Recovery after Facial Nerve Defects. Neural Regen. Res. 2019, 14, 124–131. [Google Scholar] [PubMed]
- Ma, F.; Zhu, T.; Xu, F.; Wang, Z.; Zheng, Y.; Tang, Q.; Chen, L.; Shen, Y.; Zhu, J. Neural Stem/progenitor Cells on Collagen with Anchored Basic Fibroblast Growth Factor as Potential Natural Nerve Conduits for Facial Nerve Regeneration. Acta Biomater. 2017, 50, 188–197. [Google Scholar] [CrossRef]
- Şahin, M.M.; Cayonu, M.; Dinc, S.K.; Ozkocer, E.; Ilhan, M.; Uzunoğlu, E.; Elmas, C.; Yılmaz, M.; Akkol, E. Effects of Chitosan and Platelet-Rich Plasma on Facial Nerve Regeneration in an Animal Model. Eur. Arch. Otorhinolaryngol. 2022, 279, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Liu, H.; Yang, S.; Li, Y.; Xiang, L.; Hu, M.; Wang, X. Chitosan Tubes Inoculated with Dental Pulp Stem Cells and Stem Cell Factor Enhance Facial Nerve-Vascularized Regeneration in Rabbits. ACS Omega 2022, 7, 18509–18520. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wen, W.; Hu, M.; Bi, W.; Chen, L.; Liu, S.; Chen, P.; Tan, X. Chitosan Conduits Combined with Nerve Growth Factor Microspheres Repair Facial Nerve Defects. Neural Regen. Res. 2013, 8, 3139–3147. [Google Scholar]
- Wu, H.; Zhang, J.; Luo, Y.; Wan, Y.; Sun, S. Mechanical Properties and Permeability of Porous Chitosan-poly(p-dioxanone)/Silk Fibroin Conduits Used for Peripheral Nerve Repair. J. Mech. Behav. Biomed. Mater. 2015, 50, 192–205. [Google Scholar] [CrossRef]
- Li, G.; Xue, C.; Wang, H.; Yang, X.; Zhao, Y.; Zhang, L.; Yang, Y. Spatially Featured Porous Chitosan Conduits with Micropatterned Inner Wall and Seamless Sidewall for Bridging Peripheral Nerve Regeneration. Carbohydr. Polym. 2018, 194, 225–235. [Google Scholar] [CrossRef]
- Chen, C.; Ding, W.; Zhang, H.; Zhang, L.; Huang, Y.; Fan, M.; Yang, J.; Sun, D. Bacterial Cellulose-based Biomaterials: From Fabrication to Application. Carbohydr. Polym. 2022, 278, 118995. [Google Scholar] [CrossRef] [PubMed]
- Raut, M.P.; Asare, E.; Syed Mohamed, S.M.D.; Amadi, E.N.; Roy, I. Bacterial Cellulose-Based Blends and Composites: Versatile Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2023, 24, 986. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, F.; Babaeipour, V.; Bakhtiari, S. Bacterial Cellulose-based Composites for Nerve Tissue Engineering. Int. J. Biol. Macromol. 2022, 217, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Binnetoglu, A.; Demir, B.; Akakin, D.; Kervancioglu, D.E.; Batman, C. Bacterial Cellulose Tubes as a Nerve Conduit for Repairing Complete Facial Nerve Transection in a Rat Model. Eur. Arch. Otorhinolaryngol. 2020, 277, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Delgado, M.E.; Mora-Galindo, J.; Gómez-Pinedo, U.; Feria-Velasco, A.; Castro-Castañeda, S.; López-Dellamary, T.F.A.; Luquin-De, A.S.; García-Segura, L.M.; García-Estrada, J. Facial Nerve Regeneration Through Progesterone-Loaded Chitosan Prosthesis. A Preliminary Report. J. Biomed. Mater. Res. Prat B Appl. Biomater. 2003, 67, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Moon, C.; Maharajan, N.; Ang, M.J.; Kim, M.; Jang, C.H. Effect of Pre-Induced Mesenchymal Stem Cell-Coated Cellulose/Collagen Nanofibrous Nerve Conduit on Regeneration of Transected Facial Nerve. Int. J. Mol. Sci. 2022, 23, 7638. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Cobianchi, S.; Heimann, C.; Phillips, J.B.; Udina, E.; Navarro, X. Stabilization, Rolling, and Addition of Other Extracellular Matrix Proteins to Collagen Hydrogels Improve Regeneration in Chitosan Guides for Long Peripheral Nerve Gaps in Rats. Neurosurgery 2017, 80, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeon, J.; Kim, B.; Lee, M.S.; Park, S.; Lim, J.; Yi, J.; Lee, H.; Yang, H.S.; Lee, J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2003759. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. Nerve Guide Conduits for Peripheral Nerve Injury Repair: A Review on Design, Materials and Fabrication Methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Long, Q.; Wu, B.; Yang, Y.; Wang, S.; Shen, Y.; Bao, Q.; Xu, F. Nerve Guidance Conduit Promoted Peripheral Nerve Regeneration in Rats. Artif. Organs 2021, 45, 616–624. [Google Scholar] [CrossRef]
- Jang, C.H.; Lee, H.; Kim, M.; Kim, G. Effect of Polycaprolactone/collagen/hUCS Microfiber Nerve Conduit on Facial Nerve Regeneration. Int. J. Biol. Macromol. 2016, 93, 1575–1582. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A Biodegradable Porous Composite Scaffold of PGA/Beta-TCP for Bone Tissue Engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef]
- Park, K.E.; Kang, H.K.; Lee, S.J.; Min, B.M.; Park, W.H. Biomimetic Nanofibrous Scaffolds: Preparation and Characterization of PGA/Chitin Blend Nanofibers. Biomacromolecules 2006, 7, 635–643. [Google Scholar] [CrossRef]
- Pereira, L.V.; Bento, R.F.; Cruz, D.B.; Marchi, C.; Salomone, R.; Oiticicca, J.; Costa, M.P.; Haddad, L.; Mingroni-Netto, R.C.; Costa, H.J.Z.R. Stem Cells from Human Exfoliated Deciduous Teeth (SHED) Differentiate in vivo and Promote Facial Nerve Regeneration. Cell Transplant. 2019, 28, 55–64. [Google Scholar] [CrossRef]
- Costa, H.J.; Bento, R.F.; Salomone, R.; Azzi-Nogueira, D.; Zanatta, D.B.; Costa, M.P.; Silva, C.F.; Strauss, B.E.; Haddad, L.A. Mesenchymal Bone Marrow Stem Cells within Polyglycolic Acid Tube Observed in vivo after Six Weeks Enhance Facial Nerve Regeneration. Brain Res. 2013, 1510, 10–21. [Google Scholar] [CrossRef]
- Xu, W.; Xu, X.; Yao, L.; Xue, B.; Xi, H.; Cao, X.; Piao, G.; Lin, S.; Wang, X. VEGFA-modified DPSCs Combined with LC-YE-PLGA NGCs Promote Facial Nerve Injury Repair in Rats. Heliyon 2023, 9, e14626. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, M.; Li, M.; Song, X.; Chen, D.; Zhu, M.; Zheng, H.; Chen, S. The Bridging Effect of Controlled-Release Glial Cell-Derived Neurotrophic Factor Microcapsules within Nerve Conduits on Rat Facial Nerve Regeneration. Dis. Markers 2022, 2022, 8942985. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, C.; Meng, D.; Xiao, Z.; Hou, X.; Ding, W.; Kou, D.; Yao, Y.; Chen, B.; Zhang, Z.; et al. Collagen Scaffolds Modified with CNTF and bFGF Promote Facial Nerve Regeneration in Minipigs. Biomaterials 2014, 35, 7819–7827. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Yang, Y.; Liu, J. Construction of Tissue Engineered Nerve Grafts and Their Application in Peripheral Nerve Regeneration. Prog. Neurobiol. 2011, 93, 204–230. [Google Scholar] [CrossRef]
- Lee, S.Y.; Thow, S.Y.; Abdullah, S.; Ng, M.H.; Haflah, N.H.M. Advancement of Electrospun Nerve Conduit for Peripheral Nerve Regeneration: A Systematic Review (2016-2021). Int. J. Nanomed. 2022, 17, 6723–6758. [Google Scholar] [CrossRef]
- Galla, T.J.; Vedecnik, S.V.; Halbgewachs, J.; Steinmann, S.; Friedrich, C.; Stark, G.B. Fibrin/Schwann Cell Matrix in Poly-epsilon-caprolactone Conduits Enhances Guided Nerve Regeneration. Int. J. Artif. Organs 2004, 27, 127–136. [Google Scholar] [CrossRef]
- Pooshidani, Y.; Zoghi, N.; Rajabi, M.; Haghbin Nazarpak, M.; Hassannejad, Z. Fabrication and Evaluation of Porous and Conductive Nanofibrous Scaffolds for Nerve Tissue Engineering. J. Mater. Sci. Mater. Med. 2021, 32, 46. [Google Scholar] [CrossRef]
- Chen, J.; Ge, J.; Guo, B.; Gao, K.; Ma, P.X. Nanofibrous Polylactide Composite Scaffolds with Electroactivity and Sustained Release Capacity for Tissue Engineering. J. Mater. Chem. B 2016, 4, 2477–2485. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Matsumine, H.; Sasaki, R.; Yamato, M.; Okano, T.; Sakurai, H. A Polylactic Acid Non-woven Nerve Conduit for Facial Nerve Regeneration in Rats. J. Tissue Eng. Regen. Med. 2014, 8, 454–462. [Google Scholar] [CrossRef]
- Inada, Y.; Hosoi, H.; Yamashita, A.; Morimoto, S.; Tatsumi, H.; Notazawa, S.; Kanemaru, S.; Nakamura, T. Regeneration of Peripheral Motor Nerve Gaps with a Polyglycolic Acid-collagen Tube: Technical Case Report. Neurosurgery 2007, 61, E1105–E1107. [Google Scholar] [CrossRef]
- Kiyotani, T.; Teramachi, M.; Takimoto, Y.; Nakamura, T.; Shimizu, Y.; Endo, K. Nerve Regeneration Across a 25-mm Gap Bridged by a Polyglycolic Acid-collagen Tube: A Histological and Electrophysiological Evaluation of Regenerated Nerves. Brain Res. 1996, 740, 66–74. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ohnishi, K.; Kiyotani, T.; Sekine, T.; Ueda, H.; Nakamura, T.; Endo, K.; Shimizu, Y. Peripheral Nerve Regeneration Across an 80-mm Gap Bridged by a Polyglycolic Acid (PGA)-collagen Tube Filled with Laminin-coated Collagen Fibers: A Histological and Electrophysiological Evaluation of Regenerated Nerves. Brain Res. 2000, 868, 315–328. [Google Scholar] [CrossRef]
- Tsujimoto, G.; Sunada, K.; Nakamura, T. Effect of Cervical Sympathetic Ganglionectomy on Facial Nerve Reconstruction Using Polyglycolic Acid-collagen Tubes. Brain Res. 2017, 1669, 79–88. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Trifanova, E.M.; Khvorostina, M.A.; Mariyanats, A.O.; Sochilina, A.V.; Nikolaeva, M.E.; Khaydukov, E.V.; Akasov, R.A.; Popov, V.K. Natural and Synthetic Polymer Scaffolds Comprising Upconversion Nanoparticles as a Bioimaging Platform for Tissue Engineering. Molecules 2022, 27, 6547. [Google Scholar] [CrossRef]
- Moore, M.J.; Friedman, J.A.; Lewellyn, E.B.; Mantila, S.M.; Krych, A.J.; Ameenuddin, S.; Knight, A.M.; Lu, L.; Currier, B.L.; Spinner, R.J.; et al. Multiple-channel Scaffolds to Promote Spinal Cord Axon Regeneration. Biomaterials 2006, 27, 419–429. [Google Scholar] [CrossRef]
- Zhou, L.; Du, H.D.; Tian, H.B.; Li, C.; Tian, J.; Jiang, J.J. Experimental Study on Repair of the Facial Nerve with Schwann Cells Transfected with GDNF Genes and PLGA Conduits. Acta Otolaryngol. 2008, 128, 1266–1272. [Google Scholar] [CrossRef]
- Lin, C.C.; Chang, J.J.; Yung, M.C.; Huang, W.C.; Chen, S.Y. Spontaneously Micropatterned Silk/gelatin Scaffolds with Topographical, Biological, and Electrical Stimuli for Neuronal Regulation. ACS Biomater. Sci. Eng. 2020, 6, 1144–1153. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Campora, S.; Notarbartolo, M.; Ghersi, G. Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen. Molecules 2023, 28, 1152. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, W.; Zhu, C.; Zhang, X.; Ye, D.; Zhang, W.; Zhou, Y.; Jiang, X.; Zhang, Z. Sciatic Nerve Regeneration in Rats by a Promising Electrospun Collagen/poly(ε-caprolactone) Nerve Conduit with Tailored Degradation Rate. BMC Neurosci. 2011, 12, 68. [Google Scholar] [CrossRef]
- Wang, Y.L.; Gu, X.M.; Kong, Y.; Feng, Q.L.; Yang, Y.M. Electrospun and Woven Silk Fibroin/Poly(lactic-co-glycolic acid) Nerve Guidance Conduits for Repairing Peripheral Nerve Injury. Neural Regen. Res. 2015, 10, 1635–1642. [Google Scholar]
- Amagat, J.; Su, Y.; Svejsø, F.H.; Le Friec, A.; Sønderskov, S.M.; Dong, M.; Fang, Y.; Chen, M. Self-snapping Hydrogel-based Electroactive Microchannels as Nerve Guidance Conduits. Mater. Today Bio 2022, 16, 100437. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Z.; Chen, S.; Zhang, F.; Rao, Z.; Zhao, C.; Quan, D.; Bai, Y.; Shen, J. Nanofibrous Nerve Guidance Conduits Decorated with Decellularized Matrix Hydrogel Facilitate Peripheral Nerve Injury Repair. Theranostics 2021, 11, 2917–2931. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeon, E.Y.; Nam, J.J.; Park, J.H.; Choi, I.C.; Kim, S.H.; Chung, J.J.; Lee, K.; Park, J.W.; Jung, Y. Development of a Regenerative Porous PLCL Nerve Guidance Conduit with Swellable Hydrogel-based Microgrooved Surface Pattern via 3D Printing. Acta Biomater. 2022, 141, 219–232. [Google Scholar] [CrossRef]
- Zhu, W.; George, J.K.; Sorger, V.J.; Zhang, L.G. 3D Printing Scaffold Coupled with Low Level Light Therapy for Neural Tissue Regeneration. Biofabrication 2017, 9, 025002. [Google Scholar] [CrossRef]
- Liu, K.; Yan, L.; Li, R.; Song, Z.; Ding, J.; Liu, B.; Chen, X. 3D Printed Personalized Nerve Guide Conduits for Precision Repair of Peripheral Nerve Defects. Adv. Sci. 2022, 9, e2103875. [Google Scholar] [CrossRef]
- Behtaj, S.; Ekberg, J.A.K.; John, J.A.S. Advances in Electrospun Nerve Guidance Conduits for Engineering Neural Regeneration. Pharmaceutics 2022, 14, 219. [Google Scholar] [CrossRef]
- Shiva, S.; Asuwin Prabu, R.G.; Bajaj, G.; John, A.E.; Chandran, S.; Kumar, V.V.; Ramakrishna, S. A review on the recent applications of synthetic biopolymers in 3D printing for biomedical applications. J. Mater. Sci. Mater. Med. 2023, 34, 62. [Google Scholar]
- Zhu, W.; Tringale, K.R.; Woller, S.A.; You, S.; Johnson, S.; Shen, H.; Schimelman, J.; Whitney, M.; Steinauer, J.; Xu, W.; et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater. Today 2018, 21, 951–959. [Google Scholar] [CrossRef]
- Gholap, A.D.; Rojekar, S.; Kapare, H.S.; Vishwakarma, N.; Raikwar, S.; Garkal, A.; Mehta, T.A.; Jadhav, H.; Prajapati, M.K.; Annapure, U. Chitosan scaffolds: Expanding horizons in biomedical applications. Carbohydr. Polym. 2024, 323, 121394. [Google Scholar] [CrossRef]
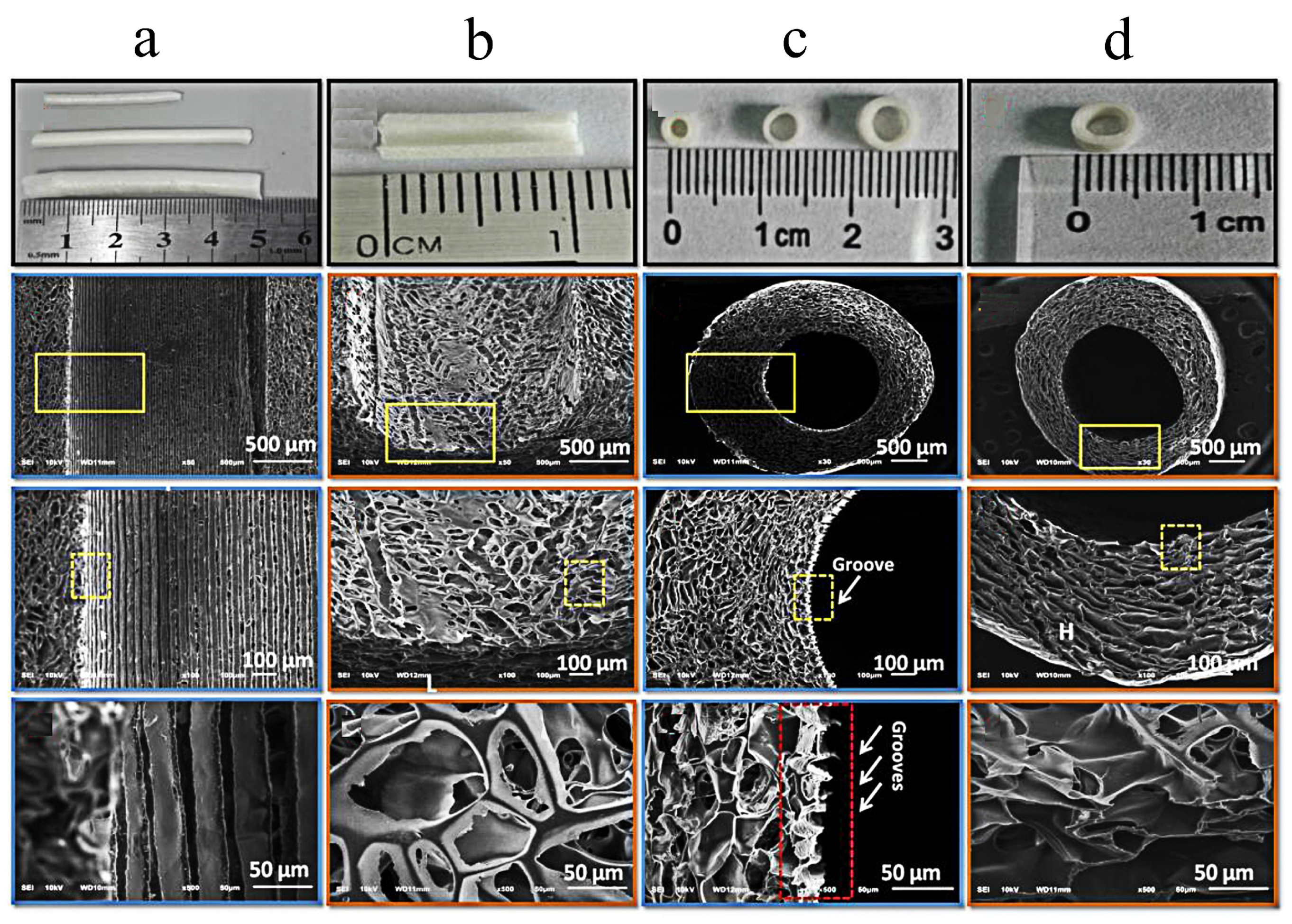
- Wan, T.; Wang, Y.L.; Zhang, F.S.; Zhang, X.M.; Zhang, Y.C.; Jiang, H.R.; Zhang, M.; Zhang, P.X. The Porous Structure of Peripheral Nerve Guidance Conduits: Features, Fabrication, and Implications for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 14132. [Google Scholar] [CrossRef]
- Vleggeert-Lankamp, C.L.A.M.; de Ruiter, G.C.W.; Wolfs, J.F.C.; Pego, A.P.; van den Berg, R.J.; Feirabend, H.K.P.; Malessy, M.J.A.; Lakke, E.A.J.F. Pores in synthetic nerve conduits are beneficial to regeneration. J. Biomed. Mater. Res. Part A 2007, 80, 965–982. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef]
- Bendella, H.; Rink, S.; Grosheva, M.; Sarikcioglu, L.; Gordon, T.; Angelov, D.N. Putative Roles of Soluble Trophic Factors in Facial Nerve Regeneration, Target Reinnervation, and Recovery of Vibrissal Whisking. Exp. Neurol. 2018, 300, 100–110. [Google Scholar] [CrossRef]
- Kong, L.; Gao, X.; Qian, Y.; Sun, W.; You, Z.; Fan, C. Biomechanical Microenvironment in Peripheral Nerve Regeneration: From Pathophysiological Understanding to Tissue Engineering Development. Theranostics 2022, 12, 4993–5014. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- de Assis, A.C.C.; Reis, A.L.S.; Nunes, L.V.; Ferreira, L.F.R.; Bilal, M.; Iqbal, H.M.N.; Soriano, R.N. Stem Cells and Tissue Engineering-Based Therapeutic Interventions: Promising Strategies to Improve Peripheral Nerve Regeneration. Cell Mol. Neurobiol. 2023, 43, 433–454. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Wang, D.; Liu, J. The Use of Stem Cells in Neural Regeneration: A Review of Current Opinion. Curr. Stem Cell Res. Ther. 2018, 13, 608–617. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Baker, J.T.; Rudraiah, S.; Arul, M.R.; Vella, A.T.; Domb, A.J.; Kumbar, S.G. Functional Polymeric Nerve Guidance Conduits and Drug Delivery Strategies for Peripheral Nerve Repair and Regeneration. J. Control. Release 2020, 317, 78–95. [Google Scholar] [CrossRef]
- Sarker, M.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol. J. 2018, 13, e1700635. [Google Scholar] [CrossRef]
- Gong, B.; Zhang, X.; Zahrani, A.A.; Gao, W.; Ma, G.; Zhang, L.; Xue, J. Neural tissue engineering: From bioactive scaffolds and in situ monitoring to regeneration. Exploration 2022, 2, 20210035. [Google Scholar] [CrossRef]


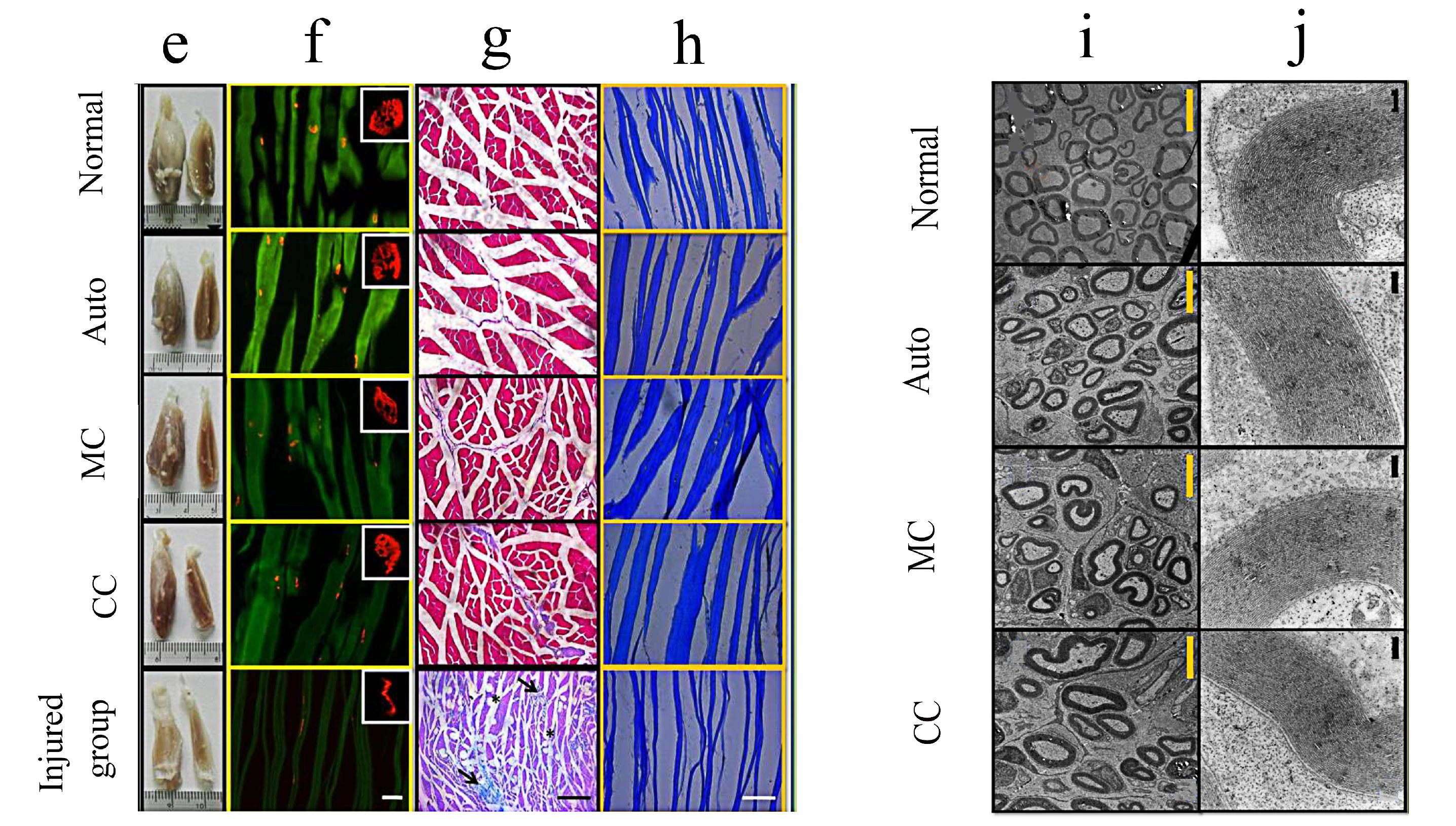
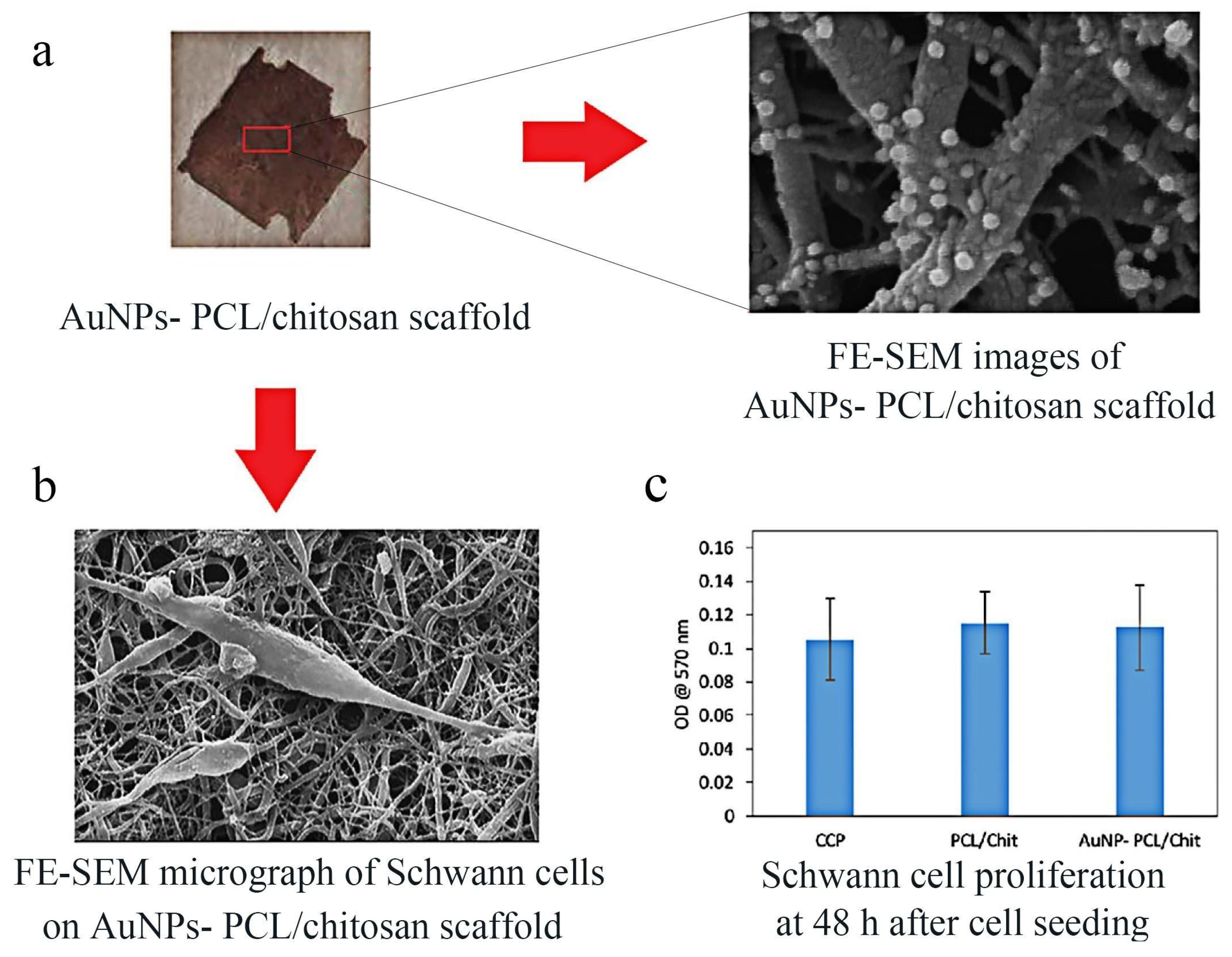

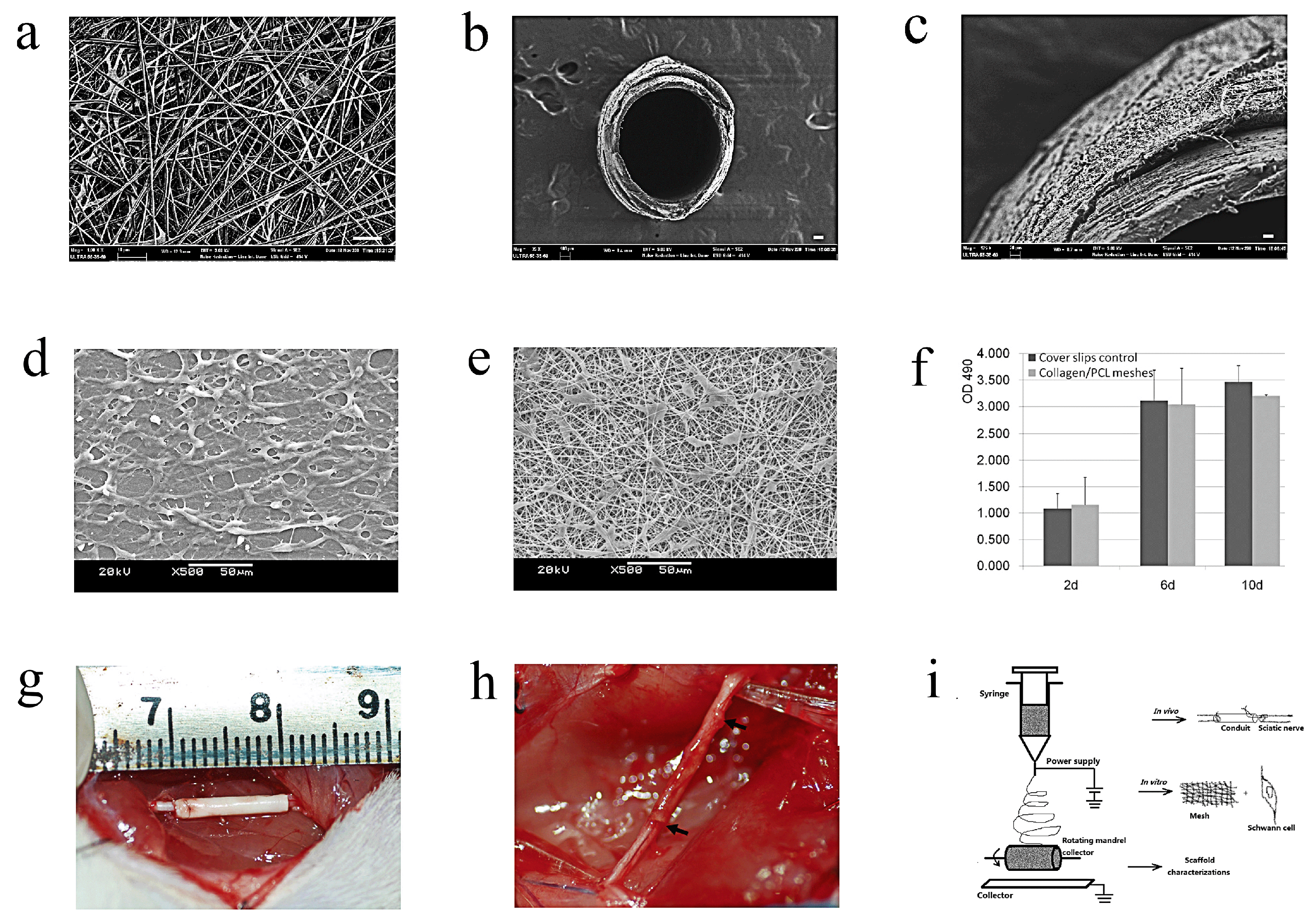
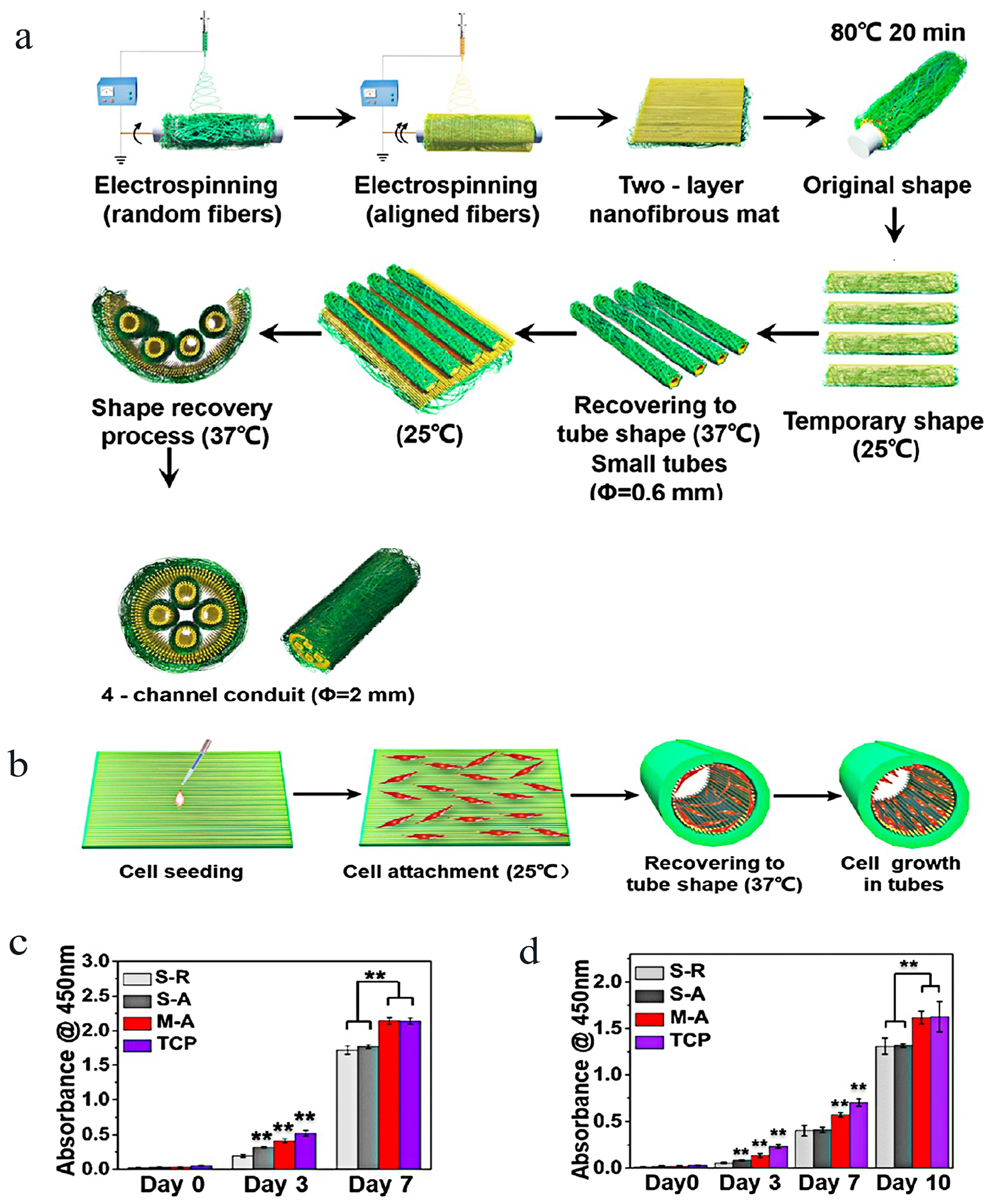
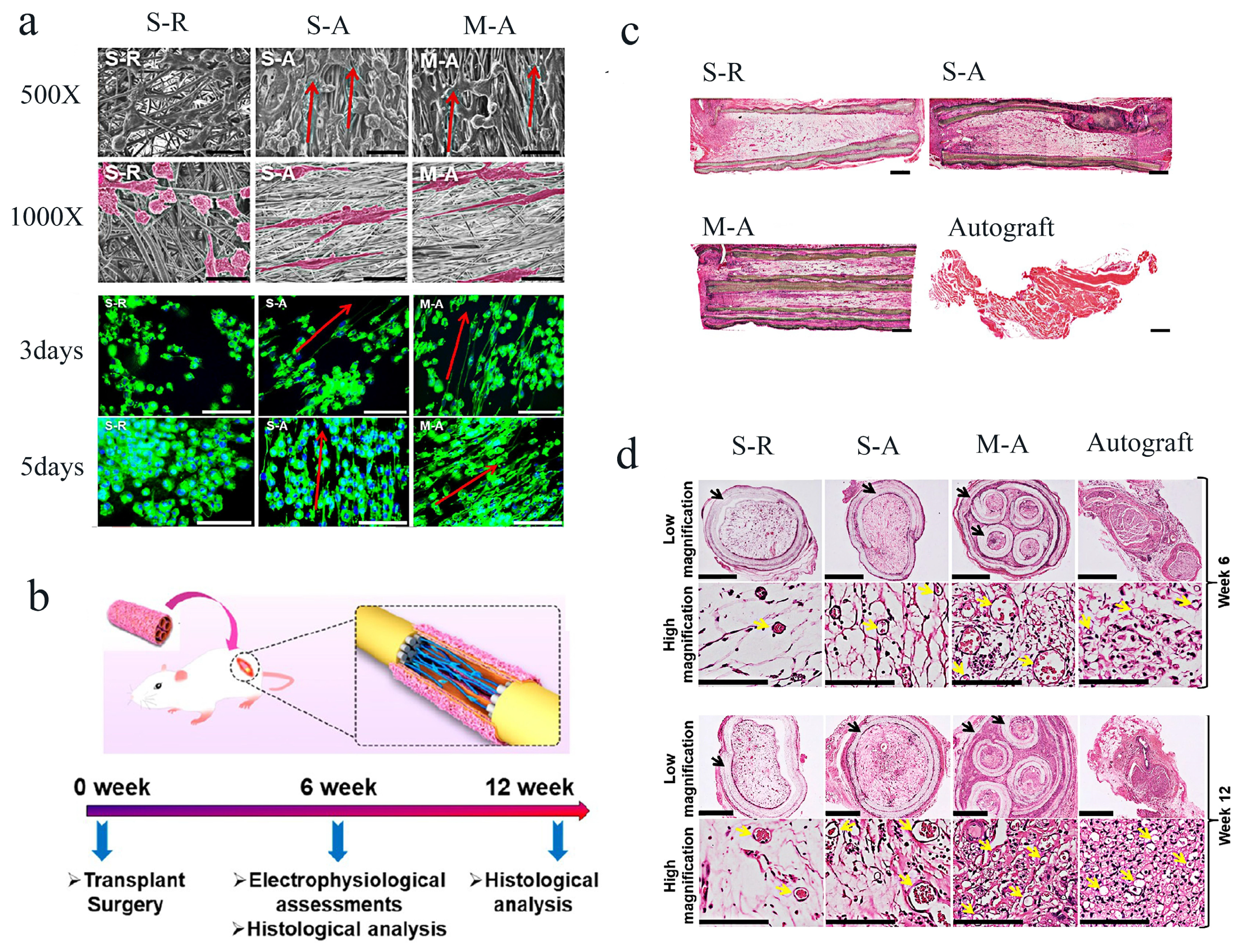
| Materials | Animals | Models | Results | Ref |
|---|---|---|---|---|
| Collagen | Rats | A 7 mm buccal branch defect | Collagen nerve conduit can effectively repair facial nerve injury, but the repair effect is not as good as that of autologous nerve. | [22] |
| Collagen | Swine | A 35 mm defect of the buccal branch | The functional COL/nb-TCP nerve conduit combined with NGF and COL filaments could promote facial nerve regeneration. | [29] |
| Collagen + PGA | Rats | A 10 mm defect in the marginal branch | The nerve function recovery effect of polyglycolate-collagen tube is not as good as that of autologous graft. | [43] |
| PGA | Wistar rats | A 7 mm buccal branch defect | DFAT cell-filled PGA conduits could promote nerve regenerate process. | [44] |
| Collagen | Cats | A 5 mm defect in the dorsal ramus | The collagen nerve guide has achieved good facial nerve regeneration effect. | [57] |
| Collagen | Rabbits | A 10 mm defect in buccal branch | Collagen and E-PTFE composite tubes are effective in the repair of peripheral nerves’ continuity defects. | [58] |
| Collagen | Rats | A 10 mm defect of the buccal branch | The extent of regeneration (of a large gap) approached that afforded by an autograft in both functional and histological aspects. | [59] |
| Collagen | Rats | A 4 mm defect in the trunk | The conduit can guide the axons’ orderly growth in the facial nerve transsection model and promote the recovery of nerve function. | [60] |
| Collagen | Rats | 7 mm facial nerve buccal branch defect | ASCs with different differentiation states may have therapeutic potential in facial nerve regeneration. | [61] |
| Collagen | Rats | A 8 mm defect in ficial buccal branch | The novel artificial nerve conduits formed by immobilizing GDNF in collagen conduits is beneficial to facial nerve repair. | [62] |
| Collagen | Rats | A 7 mm buccal branch defect | Optimal nerve regeneration was facilitated by the infusion of untreated-SVF into the nerve conduit. | [63] |
| Collagen | Rats | A 7 mm buccal branch defect | OECs promoted the facial nerve regeneration and the functional recovery of which. | [64] |
| Collagen | Rats | A 8 mm buccal branch defect | The scaffold effectively promoted the proliferation of NS/PCs in collagen scaffolds due to the sustained presentation of bFGF. | [65] |
| BC | Rats | A 3 mm defect in the main trunk in facial nerve | BC can be formed into a hollow tube that directs nerve axons, improving nerve regeneration following transection. | [74] |
| PCL | Rats | A 10 mm defect in the buccal branch | The 3-dimensional cell matrix composed of fibrin/Schwann improved both the amount and quality of peripheral nerve regeneration via PCL conduits. | [79] |
| PCL | Rats | A 4 mm defect in the main trunk | PCL/CoMF/UCS provides a beneficial environment for facial nerve regeneration. | [81] |
| PLA | Rats | A 7 mm defect in the buccal branch | Similar results have been obtained with the porous PLA non-woven fabric tube in stimulating peripheral nerve regeneration after autologous nerve transplantation. | [82] |
| PGA | Wistar rats | A 5 mm mandibular branch defect | In comparison with the autograft group, the regeneration was superior in the group treated with SHED linked to the PGA neurotube. | [83] |
| PGA | Rats | A 5 mm defect in the mandibular branch of the left facial | Both BMSC and Schwann-like cells within PGAt in rats could enhance facial nerve regeneration, but the Schwann-like cells worked better. | [84] |
| PGA | Beagle dogs | A 7 mm defect in buccal branch | PGA-c tubes Promote facial nerve regeneration and increase long-term blood flow. | [85] |
| PLGA | Rats | A 10 mm defect in the buccal branch | VEGFA-treated rDPSCs combined with LC-YE-PLGA NGCs are beneficial to facial nerve regeneration and functional recovery. | [86] |
| PLGA + Chitosan | Rats | The middle part of the nerve trunk (About 1 mm) | Stable and sustained-release GNDF microcapsules in biodegradable nerve conduits can reduce the dislocation of severed facial nerve. | [87] |
| Collagen | Minipigs | A 35 mm buccal branch defect | collagen scaffolds containing the neurocytokines bFGF and CNTF have a comparatively better therapeutic effect. | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Cao, W.; Pan, S.; He, L.; Ji, D.; Zheng, N.; Sun, X.; Wang, R.; Niu, Y. Porous Organic Materials in Tissue Engineering: Recent Advances and Applications for Severed Facial Nerve Injury Repair. Molecules 2024, 29, 566. https://doi.org/10.3390/molecules29030566
Sun J, Cao W, Pan S, He L, Ji D, Zheng N, Sun X, Wang R, Niu Y. Porous Organic Materials in Tissue Engineering: Recent Advances and Applications for Severed Facial Nerve Injury Repair. Molecules. 2024; 29(3):566. https://doi.org/10.3390/molecules29030566
Chicago/Turabian StyleSun, Jingxuan, Wenxin Cao, Shuang Pan, Lina He, Dongchao Ji, Nannan Zheng, Xiangyu Sun, Ranxu Wang, and Yumei Niu. 2024. "Porous Organic Materials in Tissue Engineering: Recent Advances and Applications for Severed Facial Nerve Injury Repair" Molecules 29, no. 3: 566. https://doi.org/10.3390/molecules29030566
APA StyleSun, J., Cao, W., Pan, S., He, L., Ji, D., Zheng, N., Sun, X., Wang, R., & Niu, Y. (2024). Porous Organic Materials in Tissue Engineering: Recent Advances and Applications for Severed Facial Nerve Injury Repair. Molecules, 29(3), 566. https://doi.org/10.3390/molecules29030566








