Abstract
Pesticides have become an integral part of modern agricultural practices, but their widespread use poses a significant threat to human health. As such, there is a pressing need to develop effective methods for detecting pesticides in food and environmental samples. Traditional chromatography methods and common rapid detection methods cannot satisfy accuracy, portability, long storage time, and solution stability at the same time. In recent years, photoelectrochemical (PEC) sensing technology has gained attention as a promising approach for detecting various pesticides due to its salient advantages, including high sensitivity, low cost, simple operation, fast response, and easy miniaturization, thus becoming a competitive candidate for real-time and on-site monitoring of pesticide levels. This review provides an overview of the recent advancements in PEC methods for pesticide detection and their applications in ensuring food and environmental safety, with a focus on the categories of photoactive materials, from single semiconductor to semiconductor–semiconductor heterojunction, and signaling mechanisms of PEC sensing platforms, including oxidation of pesticides, steric hindrance, generation/decrease in sacrificial agents, and introduction/release of photoactive materials. Additionally, this review will offer insights into future prospects and confrontations, thereby contributing novel perspectives to this evolving domain.
1. Introduction
Pesticides are a general term for chemicals for preventing, eliminating, and controlling diseases caused by pests, insects, and weeds [1]. The application of pesticides significantly contributes to the advancement of agricultural production to meet the world’s increasing population [2]. Based on their effects, pesticides include several types, like herbicides, insecticides, fungicides, and rodenticides [3]. According to their chemical structures, pesticides can be classified into carbamate, neonicotinoid, organophosphate, organochlorine, and pyrethroid [4]. Most of the pesticides are hazardous, and their excessive or improper use may cause pesticide residue in crops, the atmosphere, soil, and water resources, posing a severe threat to the environmental system. Moreover, pesticide residue can enter the human body through the food chain to cause chronic or acute poisoning [5,6,7,8,9], manifesting as an increased risk of cancer, debilitating illnesses, and severe or even fatal symptoms [10,11,12,13,14]. In recent years, pesticide residues have aroused global concern as a significant issue in environmental and food safety [15]. Many countries have established policies in terms of their national conditions to stipulate the maximum residue limits of pesticides in agricultural products and acceptable daily intake from food [16,17]. Therefore, effective detection of pesticides in food and environmental samples is a crucial step in implementing these policies and ensuring the health of human beings [18,19].
To date, a variety of methods have been developed for pesticide detection. Chromatography methods, including gas chromatography (GC) [20], high-performance liquid chromatography (HPLC) [21], and chromatography-mass spectrometry (LC-MS, HPLC-MS), exhibit high accuracy and sensitivity and are regarded as the gold standard detection approach to the analysis of pesticide residue levels [22,23]. However, the demand for sophisticated and expensive instruments, laborious sample pretreatment, and professional operators limits their on-site application [24]. Common rapid detection methods such as capillary electrophoresis [25], fluorescence [26], enzyme-linked immunosorbent assay [27], and surface-enhanced Raman scattering [28] have satisfactory sensitivity and selectivity but still suffer from short storage times and solution instability [19,29]. As a consequence, there still need to be simple, low-cost, highly accurate, and sensitive methods for the sake of real-time and on-site monitoring of pesticide levels [30,31].
Photoelectrochemical (PEC) sensing is a newly emerged technique developed on the basis of electrochemical sensing via a systematic integration of light excitation and electrochemical analysis [32,33,34,35]. By using electrochemical instruments to output signals, PEC sensing technology inherits the salient features of low cost, simple operation, fast response, capability of real-time monitoring, and easy miniaturization of instruments from the traditional electrochemical detection method [36,37,38]. Moreover, due to the complete separation of light excitation and signal output, the PEC sensing method has much lower background signals than those of the conventional electrochemical method [39,40,41]. Also, the strong redox ability of photogenerated charges reduces the dependence on the applied potential, thus endowing PEC sensing superior performance compared to electrochemical analysis [42]. Owing to these advantages, PEC sensing has been widely used in many fields such as disease diagnosis [43,44,45], health monitoring [46,47,48], and medical science [49,50]. Meanwhile, it meets the demand for rapid, convenient, sensitive, and accurate detection of pesticide residues in food and environmental samples [41,51,52].
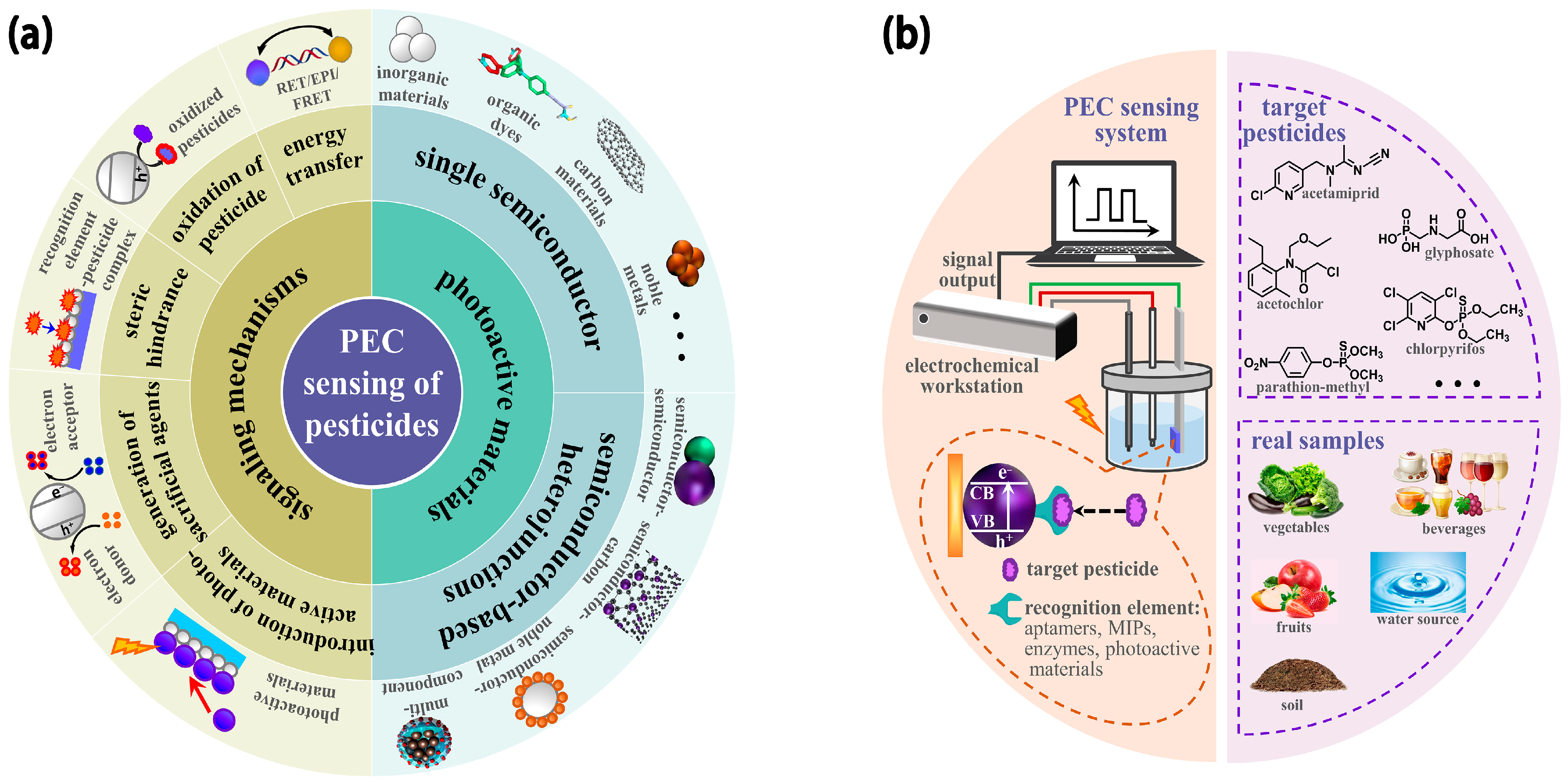
Currently, several review reports have been published focusing on the advancement of PEC sensing technologies and their applications to multifarious target analytes [34,35,36,37,41]. Nevertheless, an integral overview of PEC sensors concentrated on pesticide analysis for food and environmental safety is rare. We have witnessed inspiring progress in the PEC sensing of pesticides with high accuracy and sensitivity based on various efficient photoactive materials and flexible recognition mechanisms in recent years. Thus, this work presents a systematic and comprehensive review of the achievements in PEC sensing of pesticides. As displayed in Figure 1a, we intend to provide the readers with a clear view of the PEC sensors for pesticide detection, ranging from photoactive materials to signaling mechanisms. At the same time, the challenges and prospects in this area are also put forward.
Figure 1.
Schematic illustration of the building blocks of PEC sensing of pesticides: (a) photoactive materials and signaling mechanisms; (b) PEC sensing systems, targets, and real samples.
2. Principles of the PEC Sensing System
A PEC sensing system converts light energy to electrical signals (Figure 1b), and its detection principle can be generally described as follows: Under illumination with higher energy than its band gap, the photoactive material on the photoelectrode absorbs photons and produces photogenerated electron-hole (e−-h+) pairs. For n-type semiconductors, electrons in the conduction band (CB) migrate to the photoelectrode, while holes in the valence band (VB) are neutralized by an electron donor in the electrolyte, generating an anode photocurrent. For p-type semiconductors, electrons donated from the photoelectrode neutralize the holes in the VB, while CB electrons transfer to the electron acceptor in the electrolyte, producing a cathode photocurrent. When recognition elements in the PEC sensing system interact with the target analyte, the PEC response of the photoactive material changes and exhibits a certain correlation with the concentration of the analyte [53,54,55,56]. There are two types of recognition elements for PEC sensing of pesticides: the first type includes aptamers, enzymes, and molecular imprinted polymers (MIPs), which are fixed on the photoelectrode surface of the photoactive materials via chemisorption, noncovalent/covalent binding, and affinity binding; the other type is the photoactive materials mobilized on the photoelectrode surface, which can directly react with pesticide to form a complex or cause a redox reaction.
3. Photoactive Materials
Photoactive material selection and material properties are key elements that impact the performance of the PEC sensor [57,58]. Almost all the PEC sensing platforms for different analyte detections (e.g., biomolecule monitoring, antibiotic detection, pesticide sensing) follow the same principle: photoactive material with high e−-h+ separation efficiency displays excellent PEC conversion ability, which will contribute to the high sensitivity of the PEC sensor [59,60]. Up to now, various semiconductors with photoactivity have been developed and employed in the construction of PEC sensing systems. This section will provide an overview of the categories of photoactive materials, including single semiconductors and semiconductor-based heterojunctions, along with an introduction to their PEC properties, which is a precondition for developing effective strategies for the detection of pesticides.
3.1. Single Semiconductor
Semiconductor element-based photoactive materials have been widely investigated as PEC transducers. Some of them have wide band gaps and are ultraviolet (UV) sensitive, such as TiO2, ZnO, ZnS, BiOCl, etc. [61,62,63,64]. Due to the damaging effect on organic targets and biomolecules caused by high-energy UV illumination, some semiconductors with narrow band gaps and visible-responsive properties are being developed for PEC sensing, including CdX (X = S, Se, Te), PbS, Bi2S3, BiVO4, etc. [65,66,67,68,69]. Noble metals such as gold and silver with various nanostructures are also regarded as photoactive materials owing to their discrete band gaps and size-dependent PEC properties [70,71]. Inspired by organic dye-sensitized solar cells (DSSCs), some organic dyes with rational-designed structures, for example, porphyrin [72], triphenylamine [73], and ruthenium bipyridyl derivatives [74], have been employed as both photoactive materials and recognition elements in PEC sensors. Recently, two-dimensional layered materials including graphene, graphitic phase carbon nitride (g-C3N4), MXenes, metal-organic frameworks (MOFs), and covalent organic frameworks (COFs) have become research hotspots for designing PEC sensors due to their large specific surface areas, excellent charge mobility, and outstanding stability [48,75,76,77,78,79,80].
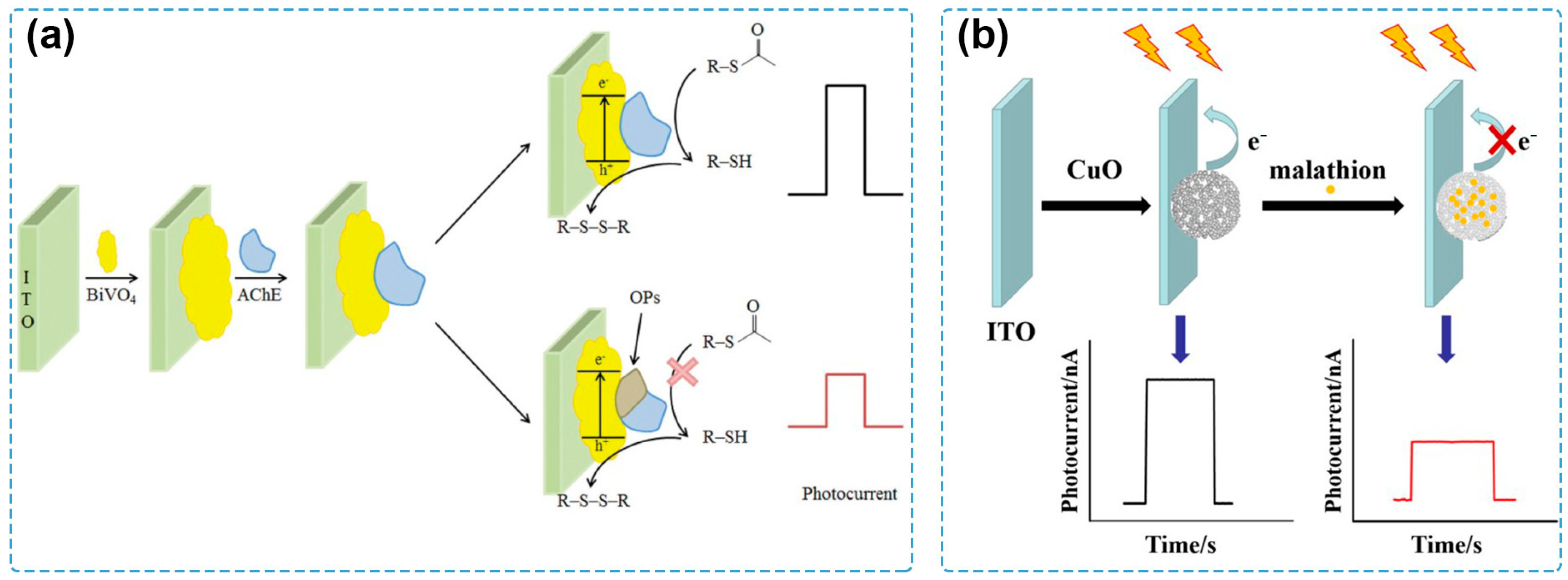
In the early stages of the development of PEC sensing, classical single-component semiconductors were used as photoactive materials for the tentative exploration of PEC sensing technology. For example, a PEC sensing system for detecting acetylcholinesterase (AChE) activity and its inhibitor by employing CdS quantum dots (QDs) to construct the photoelectrode is one of the pioneering works in the history of PEC sensing [81]. Afterwards, PEC sensors based on CdSe QDs, CdTe QDs, ruthenium bipyridyl derivatives, and porphyrin derivatives were used for the detection of several kinds of target analytes, including small biomolecules, cells, and heavy metal ions [66,82,83,84]. With the progress in material preparation and photoelectrode modification, other semiconductors were synthesized and applied for PEC sensing of pesticides. A PEC sensor based on polythiophene derivative film (PS2TTz) via a one-step electropolymerization process was constructed by Xu et al. [85], where PS2TTz serves both as a photoactive material and a recognition element for chlorpyrifos, displaying a detection range from 1 to 218.92 μg/L and a limit of detection (LOD) of 0.36 μg/L. Crossed BiOI nanoflake arrays were immobilized on an indium tin oxide (ITO) glass substrate via successive ionic layer adsorption and reaction approaches as a photocathode to detect methyl parathion, a model organophosphate pesticide [86]. By coupling with AChE as the recognition element, the fabricated PEC system showed an LOD of 0.04 ng/mL and fine applicability for the detection of spiked food samples, including garlic, apple, and cabbage. Miao and coworkers investigated the effect of treatment temperature on the photocurrent response of BiVO4 and reported a sensitive PEC biosensor for pesticide detection by using the ITO/BiVO4 photoelectrode [87] (Figure 2a). Based on the best PEC performance obtained after the treatment at 300 °C, the PEC sensor could detect chlorpyrifos with an LOD of 0.25 pM and showed satisfactory recovery values for the analysis of spiked real-life samples, including grape, strawberry, spinach, and water samples. By calcination of Cu-benzene-1,3,5-tricarboxylic acid (Cu-BTC) MOF at 300 °C, a hierarchical CuO material was obtained and modified on an ITO electrode [88] (Figure 2b), possessing a high PEC conversion efficiency under visible-light irradiation. Based on the formation of the CuO–malathion complex, the as-prepared PEC sensor had an LOD of 8.6 × 10−11 M and a successful application for the analysis of spiked Chinese cabbage samples.
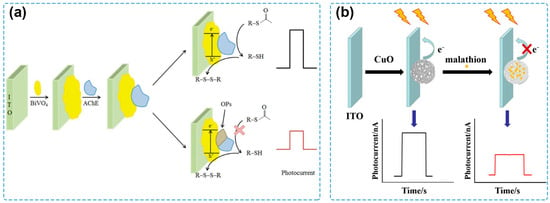
Figure 2.
(a) Schematic of a PEC sensor for chlorpyrifos detection by using an ITO/BiVO4 photoelectrode [87]. Copyright 2021 Taylor & Francis. (b) Schematic of a PEC sensor for malathion detection based on Cu-BTC MOF-derived CuO material [88]. Copyright 2019 Springer Nature.
3.2. Semiconductor-Based Heterojunctions
Despite some single-component photoactive materials that have been reported, the drawbacks of limited light-absorption ability, easy recombination of photogenerated e−-h+ pairs, or serious photo-corrosion have impeded their PEC conversion efficiency and confined their practical application to a great extent [35,38,41]. To improve the PEC properties of the photoactive materials and to enhance the performance of the PEC sensors, heterojunctions are favorable for the construction of PEC sensing systems.
3.2.1. Semiconductor–Semiconductor Heterojunction
It has been considered an effective approach to constructing heterostructures based on two semiconductors with different band gaps to acquire photoelectrodes with the desired PEC properties [29]. The most typical heterojunction used in PEC sensing is composed of two kinds of inorganic semiconductors with suitable energy levels for e−-h+ pair separation. TiO2 is one of the classical photoactive materials used in PEC sensing due to its good biocompatibility and photochemical stability [89]. However, its wide band gap results in limited excitation only under UV light, which is known for harming biological substances and having a low proportion in natural light [90,91]. Zhou and coworkers synthesized a nanocomposite of g-C3N4 and TiO2 nanotube arrays (TNA/g-C3N4) by in situ generating g-C3N4 on the surface of TNA [92]. Due to the relatively narrow band gap of g-C3N4 and the suitable energy level matching between TNA and g-C3N4, TNA/g-C3N4 showed a photocurrent response in the visible-light region. By utilizing TNA/g-C3N4 as a photoelectrode, a PEC sensor for acetamiprid detection was fabricated with the combination of λ exonuclease-assisted recycling amplification and DNAzyme-catalyzed precipitation, showing a low LOD of 0.025 pM and application for the analysis of spiked tomato, cucumber, and bitter gourd samples. Lyu and coworkers developed an n–n heterojunction by immobilizing BiOI on the surface of TiO2, which had a heterophase junction of anatase/rutile (AR-TiO2) [93] (Figure 3a). The different functions of AR-TiO2 built an internal electric field to promote carrier transfer across the heterophase, and the three-dimensional BiOI had a loose porous structure consisting of abundant small and thin nanosheets, which contributed to enhanced light absorption and separation of photogenerated e−-h+ pairs. The PEC sensor showed good performance toward detecting chlorpyrifos with an LOD of 0.24 pg/mL and a potential application value for detecting spiked lettuce, pitaya, and water samples. Bi2S3@g-C3N4, a p–n heterojunction photoactive composite, was prepared by Lei et al. to promote charge transfer and suppress e−-h+ pair recombination [94]. The obtained Bi2S3@g-C3N4/ITO photoelectrode was used to detect chlorpyrifos based on the affinity between chlorpyrifos and the Bi (III) surface, presenting a linear range from 50 ng/mL to 0.1 mg/mL and an LOD of 0.03 ng/mL. Furthermore, the novel PEC sensing platform could support fast and effective monitoring of spiked green vegetable samples.
Organic molecule-based photovoltaic materials have attracted much attention for their high visible-light absorption, precise molecular structures, and controllable assembling process. Therefore, the organic–inorganic heterojunction can lay a solid foundation for PEC sensing and pesticide detection. Poly(3-hexylthiophene) (P3HT) is one of the excellent photoelectric materials with high charge carrier mobility and strong absorption in the visible region [95]. Hu’s group used P3HT as a sensitizer of TiO2 to prepare P3HT/TiO2 nanocomposites for the PEC detection of chlorpyrifos [96]. Later, they reported a TiO2-P3HT-ionic liquid (IL) nanocomposite film by blending TiO2 nanoparticles (NPs) and P3HT at room temperature IL [97]. The improved ionic conductivity resulted from IL was beneficial for promoted charge transfer and higher PEC efficiency, and the proposed PEC sensor could detect acetochlor with an LOD of 0.2 nM. The results of spiked water samples were in acceptable agreement with those of the GC-MS method. Song and coworkers synthesized a di-branched di-anchoring dye, T(TA)2, to sensitize TiO2 NPs for the highly sensitive PEC sensing of organophosphate pesticides [98] (Figure 3b). The unique D-(π-A)2 structure endows the T(TA)2-TiO2 nanocomposites with higher PEC conversion efficiency and superior photo-stability in aqueous solutions, even better than two kinds of organic dye-sensitized TiO2 nanocomposites mostly used in DSSCs or PEC sensing fields. The PEC sensor showed a wide linear range of 2 × 10−12–4 × 10−6 g/mL, a low LOD of 5.6 × 10−13 g/mL, and acceptable accuracy for the detection of spiked apple juice samples.
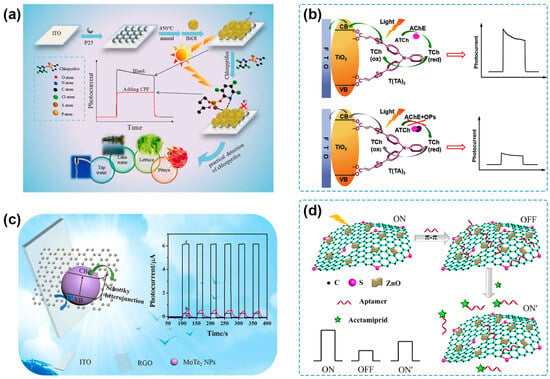
Figure 3.
(a) Schematic illustration of the PEC sensing platform for the detection of chlorpyrifos based on BiOI/AR-TiO2 heterojunction [93]. Copyright 2023 Elsevier. (b) Schematic illustration of PEC analysis of parathion based on T(TA)2-TiO2 modified photoelectrode [98]. Copyright 2018 Elsevier. (c) PEC performance enhancement mechanism diagram and photocurrent of RGO-curve a, MoTe2 NPs-curve b, and MoTe2 NPs/RGO-curve c based on MoTe2 NPs/RGO heterostructure [99]. Copyright 2021, Elsevier. (d) Schematic representation for PEC acetamiprid aptasensor based on t-SG/ZnO heterojunction [100]. Copyright 2017 Elsevier.
Figure 3.
(a) Schematic illustration of the PEC sensing platform for the detection of chlorpyrifos based on BiOI/AR-TiO2 heterojunction [93]. Copyright 2023 Elsevier. (b) Schematic illustration of PEC analysis of parathion based on T(TA)2-TiO2 modified photoelectrode [98]. Copyright 2018 Elsevier. (c) PEC performance enhancement mechanism diagram and photocurrent of RGO-curve a, MoTe2 NPs-curve b, and MoTe2 NPs/RGO-curve c based on MoTe2 NPs/RGO heterostructure [99]. Copyright 2021, Elsevier. (d) Schematic representation for PEC acetamiprid aptasensor based on t-SG/ZnO heterojunction [100]. Copyright 2017 Elsevier.
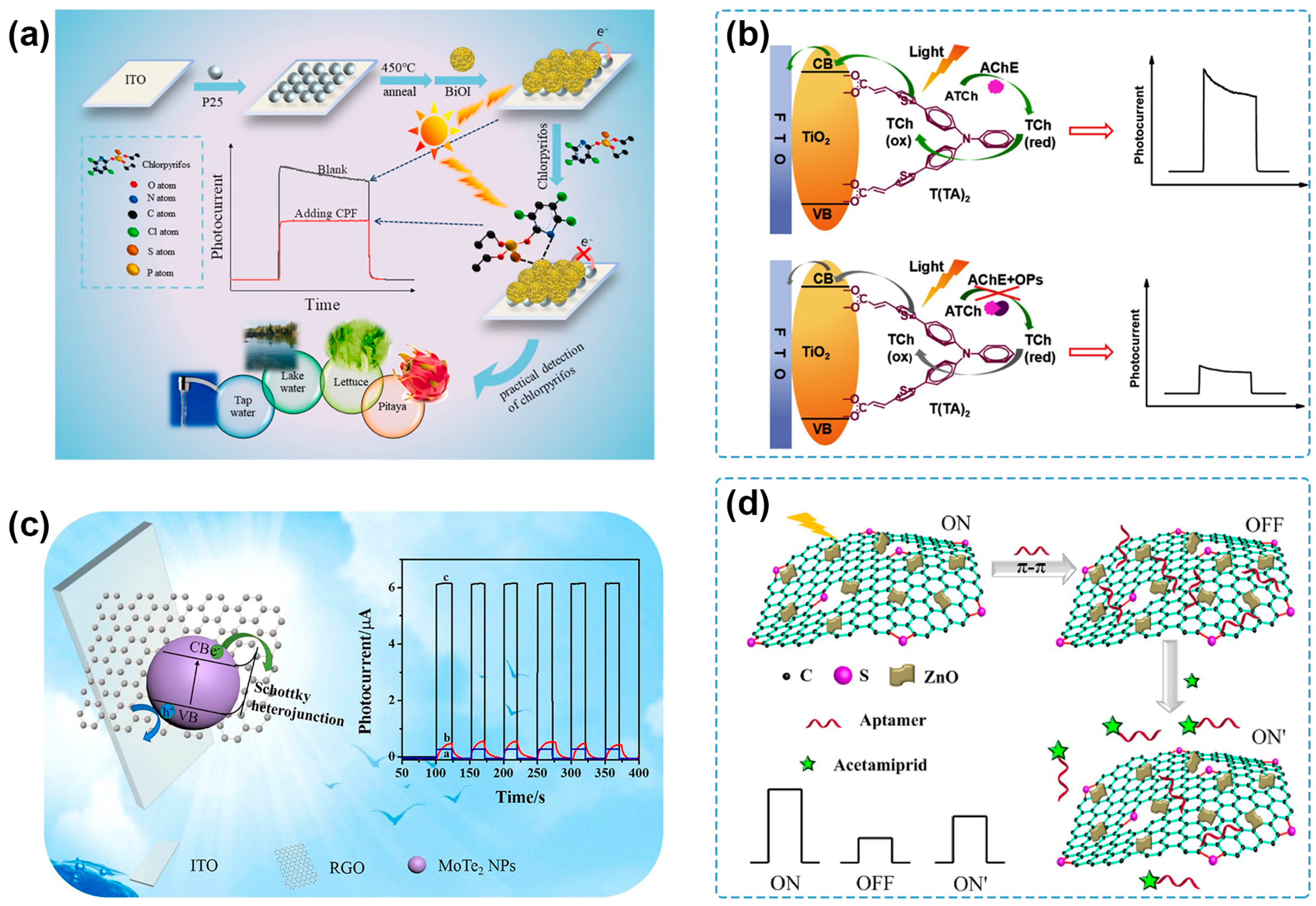
3.2.2. Semiconductor–Carbon Heterojunction
Carbon-based materials have diverse electronic structures of C elements (sp, sp2, and sp3 hybridization) and various nanostructures, including nanosheets (NSs), nanofibers (NFs), and nanotubes (NTs) [101,102,103]. Due to their inherent merits, such as good chemical stability, fast electron mobility, excellent biocompatibility, and large specific surface area, carbon materials have been extensively employed for coupling with semiconductors to obtain heterojunctions for developing PEC sensors. They are usually considered ideal electron transfer media to accelerate the transfer of photogenerated electrons and promote the separation of e−-h+ pairs [38,41]. Additionally, a large surface area can also provide abundant active sites to absorb electroactive species and biomolecules via covalent bonding, π–π packing, and hydrogen bonding [104]. Therefore, semiconductor–carbon heterojunction usually contributes to the better PEC properties of photoactive materials and endows PEC sensors with superior performance.
Graphene (GR) and reduced graphene oxide (RGO) are the most common carbon materials used for constructing semiconductor–carbon heterojunctions in PEC sensing of pesticides. BiPO4-functionalized RGO nanocomposites (BiPO4-RGO) were synthesized and showed enhanced PEC response compared with the pure BiPO4 NPs. Because the photogenerated electrons can be stored by RGO NSs, the accelerated electron transfer reduced the recombination rate of the photogenerated e−-h+ pairs [105]. The as-developed PEC sensor for chlorpyrifos detection exhibited a broad linear range from 0.05 to 80 ng/mL, a low LOD of 0.02 ng/mL, and satisfactory results according to the determination of spiked wastewater samples. Ding and coworkers fabricated a MoTe2 NPs/RGO heterostructure via a one-step hydrothermal synthesis process [99] (Figure 3c). Due to the suitable Schottky barrier, the obtained heterostructure exhibited a photocurrent intensity of 21.8 times that of RGO and 10.5 times that of MoTe2 NPs, respectively. Then, a label-free PEC aptasensor was successfully constructed to determine profenofos in concentrations ranging from 10−9 to 10−2 g/L with an LOD of 3.3 × 10−10 g/L and was successfully conducted in spiked vegetable samples. p-type BiOBr nanoplates decorated with n-type N-doped GR (BiOBr-NGR) composites were prepared to construct a p–n heterojunction for the separation of photogenerated e−-h+ pairs and enhanced PEC response [106]. Based on the improved photocurrent signal, a PEC sensor was developed for the detection of chlorpyrifos, presenting a low LOD of 1.67 pg/mL and offering a general approach to chlorpyrifos detection in food and environmental samples. Yan and coworkers fabricated a thiophene-sulfur-doped GR/ZnO nanocomposite (t-SG/ZnO) [100] (Figure 3d), where the t-SG/ZnO had a narrower band gap, stronger visible-light absorption, and better photo-stability than that of the pure ZnO nanoplates because of the facilitated interfacial charge transfer. Meanwhile, t-SG/ZnO also exhibited a PEC response of about 1.5 times and 2.6 times that of N-doped GR/ZnO and GR/ZnO, respectively. On this basis, an “on-off-on” PEC aptasensor was constructed for the detection of acetamiprid, showing an LOD of 0.33 ng/mL and analytical applicability for the detection of spiked cucumber samples.
3.2.3. Semiconductor–Noble Metal Heterojunction
Noble metals, including gold, silver, and platinum, have excellent conductivity and strong electron trapping abilities, so they can serve as electron traps to accelerate the transportation of charge carriers and suppress the recombination of the photogenerated e−-h+ pairs [107,108,109]. Generally, there are two possible paths for the electron transfer in a semiconductor–noble metal heterojunction [110]: in one situation, the photoexcited electrons from the semiconductor will transfer to the noble metals, forming an energy transfer process; in the other situation, when the heterojunction is composed of nanostructured noble metal and wide band gap semiconductor, hot electrons induced by the surface plasmon resonance (SPR) effect will transfer from the noble metal to the semiconductor, along with enhanced visible-light absorption at the same time.
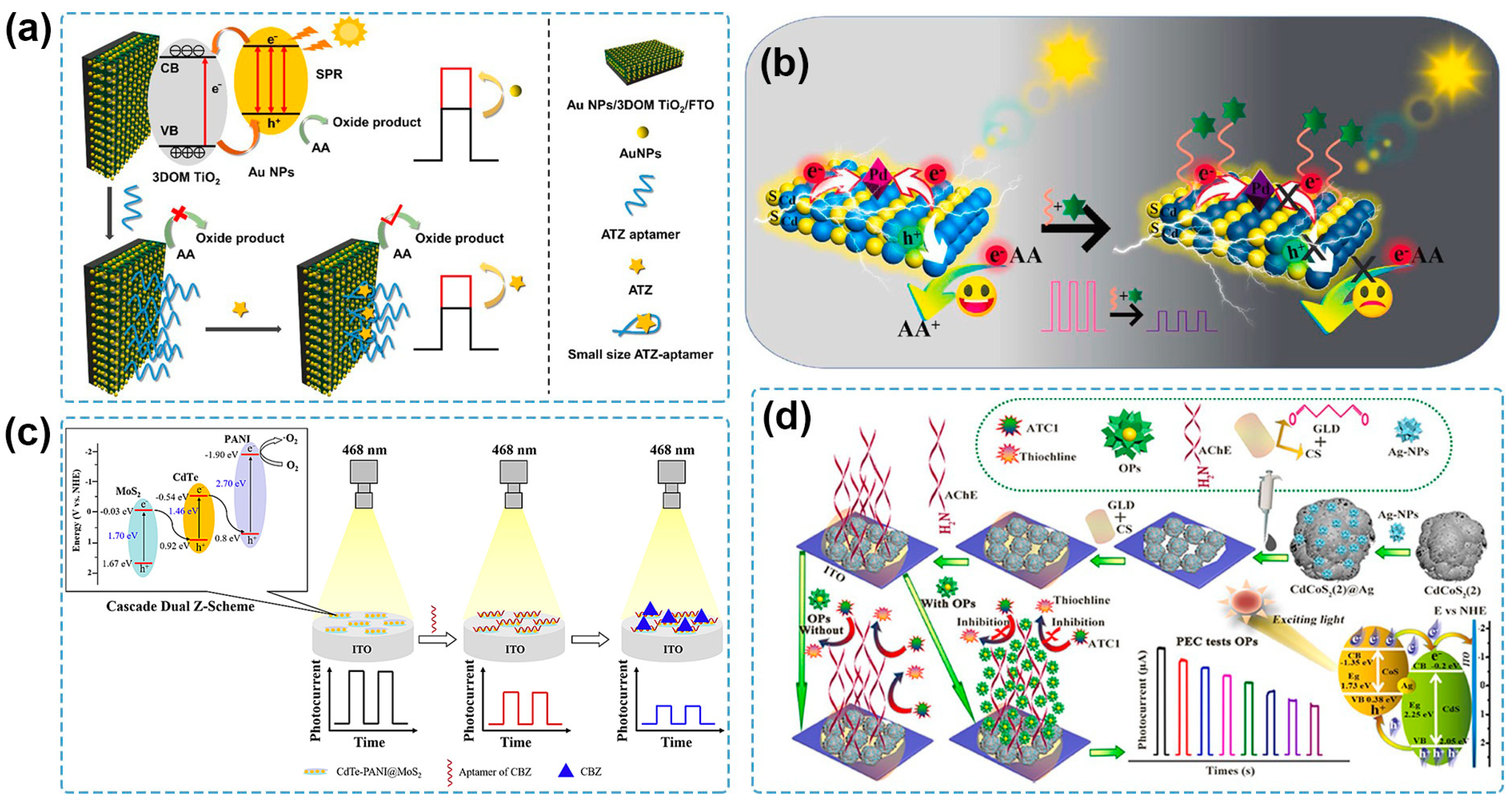
Recently, Au NPs have been frequently coupled with nanostructured TiO2 to establish PEC sensing systems for pesticide detection. A PEC aptasensor was fabricated for the detection of diazinon by using Au NPs/TiO2 nanocomposite as the photoactive material [111]. The use of Au NPs not only improved the PEC properties of the Au NPs/TiO2 nanocomposite with a higher photocurrent response compared to the pure TiO2, but also provided active sites for the aptamer conjugating to the Au NPs/TiO2 through S-Au bonds. The as-fabricated PEC sensor showed high sensitivity, selectivity, reproducibility, and stability for the quantitative determination of diazinon and obtained satisfactory results for the detection of diazinon in water and biological samples. Zhang and coworkers developed a PEC sensing platform based on Au NPs modified with a three-dimensionally ordered macroporous (3DOM) TiO2 nanostructure frame for trace detection of atrazine [112] (Figure 4a). Owing to multi-signal amplification of the 3DOM structure of TiO2 and the SPR effect of Au NPs, the Au NPs/3DOM TiO2 photoanode showed improved PEC performance under visible light. Meanwhile, its orderly nanostructure contributed to a large packing density of aptamers by Au-S bond with dominant spatial orientation for the specific recognition of atrazine, thus providing the PEC aptasensor with a low LOD of 0.167 ng/L and a great application prospect based on analysis of real water samples. Besides Au NPs, Pd NPs are another plasmonic nanostructured noble metal used to construct semiconductor–noble metal heterojunctions for pesticide determination. Wen and coworkers prepared Pd NPs decorated CdS microsphere (Pd NPs/CdS) for the PEC aptasensing of carbendazim [113] (Figure 4b). Based on the SPR effect of Pd NPs and the Schottky junction between Pd NPs and the CdS microsphere, the photocurrent obtained from Pd NPs/CdS was 7.7 times higher than that of the bare CdS microsphere. The as-fabricated PEC aptasensor possessed excellent performance with a wide linear range of 1.0 × 10−12–1.0 × 10−6 M and a low LOD of 3.3 × 10−13 M. Furthermore, the PEC aptasensor was employed for the analysis of spiked lettuce samples with satisfactory results.
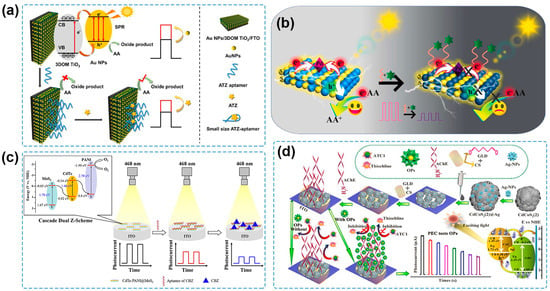
Figure 4.
(a) Schematic illustration of an atrazine PEC sensor based on an Au NPs/3DOM TiO2 photoanode [112]. Copyright 2023 Elsevier. (b) Schematic illustration of the carbendazim PEC aptasensor based on Pd NPs/CdS/ITO [113]. Copyright 2022, Elsevier. (c) Schematic illustration of PEC aptasensor for the detection of carbendazim based on cascade dual Z-scheme CdTe-PANI@MoS2 [114]. Copyright 2023 Elsevier. (d) Schematic illustration of a PEC biosensor for chlorpyrifos detection based on a CdCoS2(2)@Ag photoelectrode [115]. Copyright 2022 Elsevier.
3.2.4. Multicomponent Heterojunction
A multicomponent heterojunction is composed of semiconductors, carbon materials, or noble metals with more than two components. It involves interactions between several materials and usually consists of more than one heterojunction, thus combining the advantages of different types of heterojunctions and providing the opportunity to elaborately match the band levels of each semiconductor for an optimized electron transfer path [36]. For example, a TiO2/S-BiVO4@Ag2S heterojunction with cascade band-edge levels can accelerate charge transfer and promote separation of the charge carriers effectively, presenting a photocurrent intensity about 2.1 times higher than that obtained from the TiO2/S-BiVO4 electrode [116]. A CuO/Pd NPs nanocomposite coupled with CdS QDs can exhibit a synergistic effect through Schottky junction and sensitization, therefore suppressing efficient charge recombination and light absorption to a great extent [117].
Recently, considerable efforts have been devoted to designing and constructing multicomponent heterojunctions for PEC sensing of pesticides. Zhou and coworkers prepared a BiOI-Bi/short carbon nanotubes (BiOI-Bi/s-CNTs) composite that contained a semiconductor–metal heterojunction and a semiconductor–carbon heterojunction [118]. An intensive photocurrent response was achieved due to the active SPR effect of Bi NPs and the high charge mobility of s-CNTs, and the PEC sensor was established for chlorpyrifos detection, showing an LOD of 1.89 pg/mL and satisfactory recoveries of chlorpyrifos in spiked cabbage and Chinese cabbage samples. A PEC aptasensor for carbendazim detection was constructed based on the CdTe-polyaniline@MoS2 heterostructure [114] (Figure 4c). The cascade Z-scheme electron transfer resulted in a significantly increased photocurrent, which was 2.7-fold higher than that at CdTe/MoS2 for the facilitated separation of photogenerated e−-h+ pairs. The fabricated PEC aptasensor possessed a linear range of 0.1–100 ng/mL with an LOD of 0.033 ng/mL, and it was successfully applied for spiked tomato sample analysis. Zheng and coworkers synthesized a porous hollow CdCoS2(2) microsphere consisting of a CdS-CoS heterojunction with well-matched energy bands and outstanding PEC properties, which was further improved by compositing CdCoS2(2) with the conductive Ag NPs [115] (Figure 4d). The PEC sensor was used to monitor chlorpyrifos based on its inhibition effect on AChE and achieved a wide linear response range (0.001 to 270 μg/mL), a low LOD (0.57 ng/mL), and successful application for the chlorpyrifos determination in spiked river water samples.
4. Signaling Mechanisms
Almost all PEC sensors achieve selective detection of the target based on specific recognition between the target and the recognition element. As an important application area of PEC sensing, PEC sensors for pesticide detection share common recognition element categories and signaling transduction strategies with PEC sensing platforms for the detection of other small molecules. For pesticide detection, recognition elements include aptamers [92], enzymes [98,115], MIPs [119], and photoactive materials [88,93], and their chemical compositions and molecular structures are usually selected or designed according to the specific target pesticides. The reactions between the recognition element and the target pesticide will subsequently trigger physical or chemical reactions and cause the photocurrent change of the PEC sensor via a series of signaling mechanisms. This section will provide an overview of the signaling mechanisms for PEC sensing of pesticides, which are important for the design of recognition strategies for the PEC sensors.
4.1. Oxidation of Pesticide
During the PEC sensing process, photoactive materials are excited under illumination to generate e−-h+ pairs, and the holes with strong oxidative power can directly oxidize some target pesticides or oxidize some hole mediators to form free radical moieties, which further induce the oxidation of the target pesticides. Such interaction between the photogenerated holes and the pesticides is beneficial for the neutralization of the holes and the separation of the photogenerated e−-h+ pairs, thus improving the PEC conversion efficiency and enhancing the photocurrent response [120].
Wang and coworkers fabricated a PEC sensor based on Au NPs and poly(o-phenylenediamine) (PoPD)-modified TiO2 NTs for the detection of lindane [119]. PoPD not only served as an MIP to specifically capture lindane molecules but also acted as a photoactive material to generate charge carriers under visible-light excitation. The electrons transferred from the lowest unoccupied molecular orbital of PoPD to the CB of the TiO2 NTs, while the holes of PoPD were consumed by taking part in the oxidation of the lindane, promoting the separation of the photogenerated e−-h+ pairs and the amplification of the photocurrent response. A similar work was proposed by the same research group for the PEC sensing of chlorpyrifos, in which a PoPD-AuNPs/TiO2 NTs composite was proposed by using Au NPs to improve the interfacial charge transfer efficiency [121]. Based on the oxidation of the chlorpyrifos by photogenerated holes, the photocurrent increased proportionally to chlorpyrifos levels ranging from 0.05 to 10 μM, with an LOD of 0.96 nM. Furthermore, the PEC sensor was successfully applied to the determination of spiked green vegetables.
When a hole mediator exists in the PEC sensing system, it can be oxidized by the photogenerated holes to form free radicals with superior oxidative abilities, which can subsequently oxidize the pesticides. H2O is the most commonly used hole mediator, as PEC sensing measurements are often carried out in aqueous solutions. For example, a PEC sensor for chlorpyrifos determination was fabricated based on a 1D TiO2−x/3D nitrogen-doped graphene hydrogel (NGH) heterostructure [122]. Under light excitation, the photogenerated holes were clustered on the VB of TiO2 and could oxidize H2O to produce hydroxyl radicals (•OH). Then, •OH and holes induced the oxidation of chlorpyrifos, leading to an amplified photocurrent response. The constructed PEC sensor possessed high reliability and accuracy for chlorpyrifos detection and exhibited good performance for practical determination in wastewater samples. Wen and coworkers developed a PEC sensing platform by employing Blue-TiO2 (B-TiO2) and N-hydroxyphthalimide (NHPI) as the photoactive material and the hole mediator, respectively [123] (Figure 5a). Photogenerated holes transferred from the VB of B-TiO2 to NHPI and oxidized NHPI to phthalimide-N-oxyl radical, which could oxidize the diazinon captured by the aptamer immobilized on the photoelectrode surface. The as-fabricated PEC aptasensor exhibited a linear range for diazinon detection from 0.1 to 1000 nM with an LOD of 0.03 nM and showed food recovery results for spiked apple samples.
4.2. Steric Hindrance
Steric hindrance resulting from molecular recognition reactions is another signaling strategy in the construction of PEC sensing platforms. For pesticide detection, the common recognition reactions consist of biocatalytic precipitation and binding interactions between the pesticide and the recognition elements. The introduction of steric hindrance may lead to diffusion suppression of electron donor/acceptor to photogenerated carriers and transfer inhibition of the photogenerated carriers to the photoactive materials, thereby resulting in a significant decrease in photocurrent response [55].
Biocatalytic precipitation (BCP) is an effective approach to inducing steric hindrance to the PEC sensing systems. Horseradish peroxidase (HRP) can facilitate the oxidation of 4-chloro-1-naphthol (4-CN) by H2O2 to generate benzo-4-chlorohexadienone (4-CD), an insoluble and insulating precipitate, on photoelectrode surfaces [124]. Based on this principle, some DNAzymes or nanozymes have been designed to simulate the activity of HRP for a BCP reaction [92,125,126,127]. Zhou and coworkers developed a PEC aptasensor for acetamiprid detection. Combining aptasensing and λ exonuclease-assisted recycling amplification, an HRP-mimicking hemin/G-quadruplex DNAzyme formed and biocatalytically oxidized 4-CN to generate 4-CD, inhibiting electron donor diffusion to the surface of the photoanode and reducing the photocurrent response [92]. A PEC aptasensor was constructed by using CeO2-Au nanozyme as a signal amplification platform to conduct a BCP reaction and quench the photocurrent signals [127] (Figure 5b). The recognition of target acetamiprid caused the release of CeO2-Au from the photoelectrode, thus weakening the quenching effect and recovering the photocurrent signal. The obtained PEC aptasensor exhibited an LOD of 0.05 pM over a linear range of 0.1 pM–10 μM acetamiprid and a high acetamiprid detection accuracy in spiked cucumber samples.
The specific binding of the recognition element to the pesticide is another strategy to form steric hindrance on the photoelectrode surface. For example, when a target pesticide is captured by its aptamer, or MIP, the formation of an aptamer–pesticide complex or MIP–pesticide complex will increase the steric hindrance and decrease the conductivity for electron transfer [128,129,130,131,132,133,134]. A PEC sensor was constructed by Cao and coworkers based on a hierarchically porous Cu-BTC/g-C3N4 nanosheet (Cu-BTC/CN-NS) as both a photoactive material and the recognition element for glyphosate [135] (Figure 5c). During the PEC sensing process, the Cu metal center coordinated with glyphosate to form Cu–glyphosate complexes, resulting in an increased steric hindrance to block electron transfer and an obvious decrease in photocurrent. The constructed PEC sensor can achieve the detection of glyphosate with a low LOD (1.3 × 10−13 M) and a wide detection range (1.0 × 10−12–1.0 × 10−8 M and 1.0 × 10−8–1.0 × 10−3 M), and it also showed great analytical performance in PEC sensing of glyphosate in spiked soybean samples. A similar strategy was utilized for the PEC sensing of malathion, exhibiting high sensitivity and potential application in real-life sample analysis [88]. Besides Cu-containing photoactive materials, some photoactive materials, including Bi and Co-based semiconductors, can also form similar photoactive material–pesticide complexes, which contribute to increased steric hindrance, suppressed charge transfer, and reduced photocurrent response [93,105,106,135,136,137,138,139,140,141].
For PEC sensing platforms based on the steric hindrance strategy, the conductivity of the photoelectrode interface decreases as the pesticide concentration increases. Meanwhile, long operation times might promote the formation of recognition element–target pesticide complexes and contribute to the steric hindrance. To achieve high sensitivity, some researchers optimized the incubation or adsorption time until the binding reactions reached saturation [128,131]. As the steric hindrance is caused by the formation of recognition element–target pesticide complexes, most of the sensing photoelectrodes are not reusable due to the irreversible binding reactions between the recognition elements and the target pesticides. Only some PEC sensors using MIPs as recognition elements mentioned that the molecularly imprinted electrode could be reused several times by eluting the templates to regenerate the MIPs [132,142,143].
4.3. Generation/Decrease in Sacrificial Agents
Regulating the generation of the sacrificial agents is a straightforward approach to designing the signaling strategy of a PEC sensor. On one hand, the in situ generated electron donor/acceptor can react with excited holes/electrons, thus suppressing the recombination of the photogenerated e−-h+ pairs to obtain increased and stable photocurrent signals [37]. A simple example is the hydrolyzation of parathion-methyl in alkaline conditions, and the hydrolysate, p-nitrophenol, which served as the electron donor, is oxidized by the holes to amplify the photocurrent response [144,145]. On the other hand, the inhibition of the generation of sacrificial agents will in turn decline the PEC signal.
Enzymatic reactions are commonly utilized to establish the signaling mechanism for the PEC sensing of organophosphate pesticides. AChE is a typical enzyme used in a PEC sensor for pesticide detection due to the catalytical hydrolysis of acetylthiocholine (ATCh) to generate thiocholine (TCh) as electron donors, resulting in an enhanced photocurrent response [98]. Organophosphate pesticides can inhibit the bioactivity of AChE, and the photocurrent signal will decrease due to the reduced production of TCh [115]. Upon this method, some PEC sensing platforms for organophosphate pesticides have been developed by collaborating photoactive materials with high PEC conversion efficiency [98,115,146,147]. For example, a self-powered PEC sensor based on ZnO nanorod/3D graphene aerogel-sensitized structure and inhibition effects of parathion-methyl on AChE was fabricated (Figure 5d), showing the detection of parathion-methyl with a linear range of 0.1 ng/mL to 0.1 μg/mL and a LOD of 0.03 ng/mL. Cheng and coworkers proposed a visible-light-driven and self-powered PEC sensor based on AChE-immobilized N-doped carbon QDs/TiO2/ITO photoelectrode [147], which can achieve the detection of chlorpyrifos with high sensitivity (a LOD of 0.07 ng/mL), board detection range (0.001–1.5 μg/mL), and accurate determination of spiked lake water and Chinese cabbage samples. It should be noticed that once removed from the pesticide solution, the AChE-modified photoelectrodes cannot regain their enzymatic activity due to the irreversible inhibition of pesticides, and thus the photoelectrodes are single-use items in theory. The inhibited AChE can be reactivated when using nucleophilic compounds such as pralidoxime iodide. For example, Du and coworkers have observed that an AChE-modified electrode inhibited by malathion could recover more than 90% of its original activity after immersing in a pralidoxime iodide solution for several minutes [148], which might be helpful to obtain reusable photoelectrodes. Apart from AChE, other enzymes, including alkaline phosphatase and glucose oxidase, have been employed on account of the catalytical hydrolysis of their substrates to in situ generate ascorbic acid and H2O2, serving as electron donors to enhance the PEC conversion efficiency and amplify the photocurrent response [149,150].
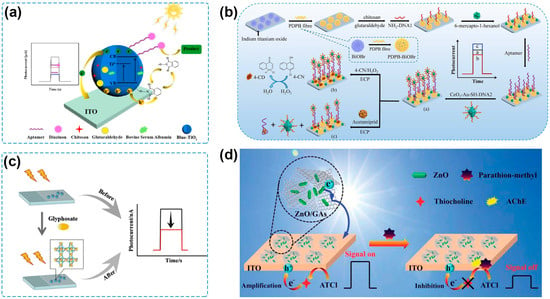
Figure 5.
(a) Schematic illustration of a PEC aptasensor based on /B-TiO2/ITO with NHPI as the hole mediator [123]. Copyright 2022, Elsevier. (b) Schematic illustration of the PEC aptasensor based on poly (diphenylbutadiene)-BiOBr heterojunction and Au-modified CeO2 octahedrons [127]. Copyright 2022, Elsevier. (c) Schematic illustration of a Cu-BTC/CN-NS-based PEC sensor for glyphosate detection [135]. Copyright 2019 Elsevier. (d) Schematic illustration of the PEC biosensor with ZnO/GAs nanocomposites based on the inhibition effect of AChE [146]. Copyright 2021, Royal Society of Chemistry.
Figure 5.
(a) Schematic illustration of a PEC aptasensor based on /B-TiO2/ITO with NHPI as the hole mediator [123]. Copyright 2022, Elsevier. (b) Schematic illustration of the PEC aptasensor based on poly (diphenylbutadiene)-BiOBr heterojunction and Au-modified CeO2 octahedrons [127]. Copyright 2022, Elsevier. (c) Schematic illustration of a Cu-BTC/CN-NS-based PEC sensor for glyphosate detection [135]. Copyright 2019 Elsevier. (d) Schematic illustration of the PEC biosensor with ZnO/GAs nanocomposites based on the inhibition effect of AChE [146]. Copyright 2021, Royal Society of Chemistry.
4.4. Introduction/Release of Photoactive Materials
The introduction/release of photoactive species is an effective method for PEC signaling. By using the recognition event to introduce/release the photoactive materials on/from the photoelectrode, the PEC properties, including light absorption properties, e−-h+ generation, and charge transfer, can be regulated, thereby leading to the change of PEC signals [37,38].
Zeng and coworkers reported a signal-on PEC sensor to determine malathion based on the in situ formation of DNA-templated Ag2S photoactive materials [151] (Figure 6a). The target-induced hybridization chain reaction (HCR) caused the exposure of rich “C” strands to assemble multiple silver ions by C-Ag+-C chelation. Afterwards, the Ag2S was generated in situ through the reaction between S2− and Ag+ ions, presenting a desirable PEC signal. This signaling strategy dispensed with the pre-synthesis or immobilization of the photoactive materials, so it had nearly zero background noise, which was desirable for a high sensitivity with an LOD of 2 pg/mL. Meanwhile, the PEC sensor was also successfully applied to detect malathion in real samples. In another work, a target (malathion)-induced HCR can produce a long DNA concatemer containing the sequence of the G-quadruplex structure, then obtain HRP-like activity via the formation of hemin/G-quadruplex complexes and binding with manganese porphyrin [152]. With the aid of H2O2, the HRP-mimicking DNA concatemer catalyzed S2O32− to generate S2−, which could react with BiOBr nanoflowers to in situ form a BiOBr/Bi2S3 heterostructures with enhanced PEC conversion efficiency. The proposed PEC sensor achieved a wide linear range of 0.001–1000 ng/mL and an LOD of 0.12 pg/mL for malathion determination, and its application potential was verified by using it for the detection of spiked milk samples.
Inhibiting the generation of photoactive materials or releasing photoactive materials from the photoelectrode will result in a drop in the PEC signal. For example, a PEC sensor for asulam detection was developed based on the inhibitory effect on HRP enzyme activity and the lower production of S2− from the conversion of S2O32− [153] (Figure 6b). Consequently, the amount of in situ-generated CdS reduced, and the photocurrent decreased with the concentration of asulam in a range from 0.02 to 2.0 ng/mL. The PEC sensor showed an LOD of 4.1 pg/mL and good accuracy in the analysis of spiked environmental water samples. Qin and coworkers proposed a PEC sensing platform for paraoxon detection by using a dissociable photoelectrode based on CdS nanocrystal-functionalized MnO2 NSs [154]. TCh can effectively etch MnO2 NSs, causing the dissociation of MnO2-CdS from the photoelectrode and the decline of the photocurrent response. With the presence of organophosphate pesticides, the generation of TCh and the dissociation of MnO2 NSs were prevented due to the inhibition of AChE, thus recovering the PEC signals. Upon this signaling strategy, rolling circle amplification was introduced to assemble a large number of butyrylcholinesterase (BChE) for increased production of TCh and promoted dissociation of MnO2-CdS [155]. Based on the inhibition of BChE by malathion, the PEC sensor possessed high sensitivity for malathion with a low LOD of 0.68 pg/mL and favorable accuracy in the analysis of spiked red wine and milk samples.
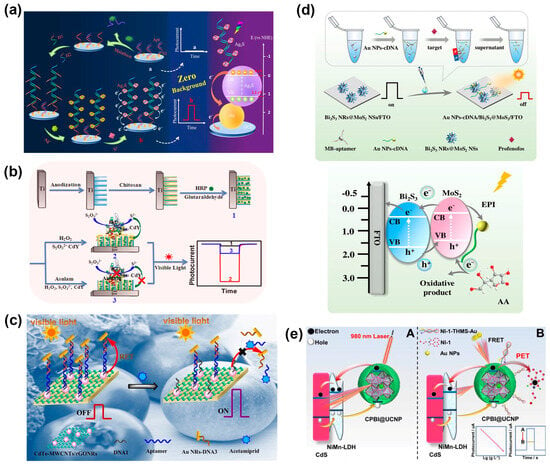
Figure 6.
(a) Schematic illustration of the PEC sensor based on HCR and in situ generation of Ag2S photosensitive material for the detection of malathion [151]. Copyright 2021 Springer Nature. (b) Schematic illustration of the construction of a PEC sensor for asulam detection based on the in situ synthesis of CdS QDs [153]. Copyright 2018 Elsevier. (c) Schematic illustration of the PEC acetamiprid aptasensor based on the RET from CdTe QDs to Au NRs [156]. Copyright 2016 Elsevier. (d) Schematic illustration of the split-type PEC sensor for profenofos detection based on magnetic-assisted exciton–plasmon interactions [157]. Copyright 2023 Springer Nature. (e) Schematic illustration of the PEC sensor based on the cascade sensitization effect and synergetic quenching effect of FRET/PET [158]. Copyright 2023 American Chemical Society.
Figure 6.
(a) Schematic illustration of the PEC sensor based on HCR and in situ generation of Ag2S photosensitive material for the detection of malathion [151]. Copyright 2021 Springer Nature. (b) Schematic illustration of the construction of a PEC sensor for asulam detection based on the in situ synthesis of CdS QDs [153]. Copyright 2018 Elsevier. (c) Schematic illustration of the PEC acetamiprid aptasensor based on the RET from CdTe QDs to Au NRs [156]. Copyright 2016 Elsevier. (d) Schematic illustration of the split-type PEC sensor for profenofos detection based on magnetic-assisted exciton–plasmon interactions [157]. Copyright 2023 Springer Nature. (e) Schematic illustration of the PEC sensor based on the cascade sensitization effect and synergetic quenching effect of FRET/PET [158]. Copyright 2023 American Chemical Society.
4.5. Energy Transfer
Energy transfer between the photoactive materials (namely, energy donor and acceptor) is a powerful approach to modulating the PEC properties of the PEC sensor. For PEC sensing of pesticides, the energy transfer strategy can be designed based on the recognition events, and the induced varying charge transfer behaviors will change the PEC conversion efficiency of the photoactive materials as well as the PEC signal [37].
Liu and coworkers reported a resonance energy transfer (RET) system from CdTe QDs to Au nanorods (NRs) for PEC aptasensing of acetamiprid [156]. Multi-walled carbon nanotubes/reduced graphene oxide nanoribbons (MWCNTs/rGONRs) served as the PEC signal amplifier and support for CdTe QDs loading, and the proposed CdTe-MWCNTs/rGONRs exhibited the typical fluorescence emission overlapped with the UV-vis absorption spectrum of Au NRs, leading to a RET process and photocurrent decrease. The binding of acetamiprid with aptamer resulted in the disassembly of the aptamer and low loading of Au NRs at the photoelectrode surface, thus inhibiting the RET process and recovering the photocurrent response (Figure 6c). The proposed PEC aptasensor was able to detect acetamiprid concentrations in the range of 0.5 pM to 10 μM with an LOD of 0.2 pM and was successfully applied to the direct detection of spiked apple and tomato samples. A split-type PEC sensor was designed for the detection of profenofos based on magnetic-assisted exciton–plasmon interactions (EPIs) [157] (Figure 6d). The target-triggered DNA double-stranded structure opening event released Au NPs-cDNA, which had significant overlap in the spectra of the Bi2S3/MoS2 substrate. The simultaneous excitation of both excitons and plasmons led to EPI between Bi2S3/MoS2 and Au NPs induced by SPR, which effectively suppressed the photocurrent generation. The PEC sensor exhibited good performance with a wide linear range (1.0 pg/mL–1.0 μg/mL), a low LOD (0.23 pg/mL), and satisfactory results in the analysis of spiked milk, cucumber, wastewater, and soil samples. Wang and coworkers designed a perovskite/upconversion lab-on-paper PEC device for the sensitive detection of malathion based on CsPbBr2I@NaYF4:Yb,Tm/NiMn-layered double hydroxide/CdS/Au NPs functionalized working electrode and entropy-driven strand displacement amplification with a triple helix molecules switch strategy [158] (Figure 6e). A synergistic quenching effect including fluorescence energy resonance transfer (FRET) and photoinduced electron transfer (PET) was customized to efficiently decrease the photocurrent, achieving sensitive detection of malathion with a linear range from 0.01 ng/L to 5 μg/L and an LOD of 4.8 fg/L. The applicability of the PEC device was verified by determining malathion in spiked water, cabbage juice, spinach juice, and soil samples.
A summary of the different categories of photoactive materials and signaling mechanisms-based PEC sensors for pesticide sensing in food and environmental samples is recorded in Table 1. Combining various advanced photoactive materials with well-designed recognition and signaling strategies, some of the reported PEC sensors can detect pesticides with a wide linear range of several orders of magnitude and high sensitivity down to the picomole scale. The applicabilities of these PEC sensing platforms have been demonstrated by real sample analysis of food and environmental samples, such as vegetables, fruits, beverages, water, and soil samples. Meanwhile, the employed light source is necessary and important for the PEC conversion process. It can be seen that most of the PEC sensing systems utilized xenon (Xe) lamps, halogen lamps, and light-emitting diode (LED) energy-saving lamps as light sources to simulate natural sunlight, and some of the light sources were equipped with monochromators or UV cut-off filters to achieve visible-light excitation and avoid the harmful effects caused by UV irradiation. The applied bias provides driving forces for charge transfer from photoactive materials to photoelectrodes. Most of the PEC sensors in Table 1 used relatively low applied biases, for example, around 0 V. Compared to traditional electrochemical methods, PEC sensing methods are less dependent on the applied potential, which is not only energy-saving but also beneficial for a lower background and higher sensitivity.

Table 1.
A summary of the different categories of photoactive materials and signaling mechanisms-based PEC sensors for pesticide sensing in food and environmental samples.
5. Conclusions and Perspectives
PEC sensing has emerged as a promising method for the detection of pesticide residue levels in food and environmental samples. Its advantages, such as high sensitivity and selectivity, portability, rapid analysis, and user-friendly operation, make it a futuristic option for pesticide analysis. In recent years, there has been a significant development of novel PEC sensing systems and new sensing protocols for pesticide determination. This review provides a comprehensive summary of the progress in the development of PEC sensors for pesticide analysis, focusing on PEC sensing principles, categories of photoactive materials, PEC signaling mechanisms, and their applications in food and environmental media analysis. The integration of photoactive materials with high PEC conversion efficiency and signaling mechanisms with wise transduction strategies has significantly enhanced the sensitivity, selectivity, and application performance of PEC sensors.
In spite of advances, significant work still remains in the research on PEC sensing of pesticides. On one hand, the PEC sensing method still has some common limitations: (1) The inherent background signal of the detection method remains challenging for current PEC sensors. Self-referencing PEC sensors based on different voltages or wavelengths with dual channels have been proposed to detect several types of targets, and additional efforts should be put into constructing ratiometric PEC sensors for pesticide analysis with high sensitivity. (2) Most of the PEC sensing systems contain lamps as light sources, bulky photo-to-electrochemical signal conversion equipment, and external power sources. To meet the requirements of on-site pesticide analysis, utilizing sunlight as a light source, replacing conventional electrochemical workstations with miniaturized signaling devices, and using screen-printing electrodes or transparent paper-based conductive electrodes with high light transmittance as working electrodes may improve portability and practicality. (3) Almost all reported PEC sensors are just carried out in laboratory research. Future efforts should be put into combining electronic information, micro-nanofabrication, and mass manufacturing to develop integrated detection devices with practicability.
On the other hand, as to the PEC sensing of pesticides, the following aspects should be considered besides the above limitations: Firstly, the coexistence of multiple toxic elements has led to a notable increase in threats to food and environmental safety. Most of the currently developed PEC sensing systems focus on only one pesticide and are not able to meet current needs. The construction of a PEC sensing platform that simultaneously detects multiple pesticides can better satisfy real needs. Secondly, the applicability of proposed PEC sensors for pesticide detection is mostly verified through the analysis of spiked samples or spiked sample extracts at present, and the application of real-life samples is still rare. Overall, applying the developed PEC sensors on the user end is the final goal in this field. As the aforementioned requirements are continuously met, the PEC sensing technique is poised to play a pivotal role in pesticide detection for food and environmental safety.
Author Contributions
J.S.: Conceptualization, formal analysis, investigation, writing original draft preparation. Y.C. and L.L.: Formal analysis, investigation. M.T.: supervision, writing—review and editing. W.S.: Conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Foundation of Education Administration of Liaoning Province (LJKQZ20222392; XLYC2203091), and the Open Foundation of the State Key Laboratory of Marine Food Processing and Safety Control (SKL202309).
Data Availability Statement
The authors declare that all data generated or analyzed during this study are included in the published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rosenthal, T. Pesticides: Health, Safety, and the Environment. J. Rural Health 2007, 23, 188. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence Detection of Pesticides Using Quantum Dot Materials—A Review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What Are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kumar, P.S.; Vo, D.-V.N.; Rajamohan, N.; Saravanan, R. Microbial Degradation of Recalcitrant Pesticides: A Review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; Silva, C.C.G.; De Souza, D. Pesticides Determination in Foods and Natural Waters Using Solid Amalgam-Based Electrodes: Challenges and Trends. Talanta 2020, 212, 120756. [Google Scholar] [CrossRef]
- Bini Dhouib, I.; Annabi, A.; Jallouli, M.; Marzouki, S.; Gharbi, N.; Elfazaa, S.; Montassar Lasram, M. Carbamates Pesticides Induced Immunotoxicity and Carcinogenicity in Human: A Review. J. Appl. Biomed. 2016, 14, 85–90. [Google Scholar] [CrossRef]
- Hui, T.; Xiujuan, L.; Qifa, S.; Qiang, L.; Zhuang, K.; Yan, G. Evaluation of Drinking Water Quality Usingthe Water Quality Index (WQI), the SyntheticPollution Index (SPI) and Geospatial Toolsin Lianhuashan District, China. Pol. J. Environ. Stud. 2020, 30, 141–153. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Udeigwe, T.K.; Teboh, J.M.; Eze, P.N.; Hashem Stietiya, M.; Kumar, V.; Hendrix, J.; Mascagni, H.J.; Ying, T.; Kandakji, T. Implications of Leading Crop Production Practices on Environmental Quality and Human Health. J. Environ. Manag. 2015, 151, 267–279. [Google Scholar] [CrossRef]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The Global Distribution of Acute Unintentional Pesticide Poisoning: Estimations Based on a Systematic Review. BMC Public Health 2020, 20, 1875. [Google Scholar] [CrossRef]
- Thundiyil, J. Acute Pesticide Poisoning: A Proposed Classification Tool. Bull. World Health Organ. 2008, 86, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides: An Update of Human Exposure and Toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Beseler, C.L.; Stallones, L.; Hoppin, J.A.; Alavanja, M.C.R.; Blair, A.; Keefe, T.; Kamel, F. Depression and Pesticide Exposures among Private Pesticide Applicators Enrolled in the Agricultural Health Study. Environ. Health Perspect. 2008, 116, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M. Poisoning by Pesticides. Medicine 2020, 48, 214–217. [Google Scholar] [CrossRef]
- The Innovation Editorial Team, China. Editorial Innovation Focus in 2021. Innovation 2022, 3, 100203. [Google Scholar] [CrossRef]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide Regulations and Their Malpractice Implications on Food and Environment Safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A Review of the Global Pesticide Legislation and the Scale of Challenge in Reaching the Global Harmonization of Food Safety Standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, W.; Chen, L.; Shen, G.; Bai, B.; Zhou, C. Determination and Dietary Risk Assessment of 284 Pesticide Residues in Local Fruit Cultivars in Shanghai, China. Sci. Rep. 2021, 11, 9681. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Yang, Q.; Hou, X. Integration of Metallic Nanomaterials and Recognition Elements for the Specifically Monitoring of Pesticides in Electrochemical Sensing. Crit. Rev. Anal. Chem. 2023, 1–22. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, J.; Zhang, S.; An, Y.; Hao, L.; Yang, X.; Wang, C.; Wang, Z.; Wu, Q. Novel N-Riched Covalent Organic Framework for Solid-Phase Microextraction of Organochlorine Pesticides in Vegetable and Fruit Samples. Food Chem. 2022, 388, 133007. [Google Scholar] [CrossRef]
- Senosy, I.A.; Lu, Z.-H.; Zhou, D.-D.; Abdelrahman, T.M.; Chen, M.; Zhuang, L.-Y.; Liu, X.; Cao, Y.-W.; Li, J.-H.; Yang, Z.H. Construction of a Magnetic Solid-Phase Extraction Method for the Analysis of Azole Pesticides Residue in Medicinal Plants. Food Chem. 2022, 386, 132743. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Al-Natour, M.Q.; Al-Abboodi, A.R.; Alkarasneh, A.Y.; El Darra, N.; Khazaal, S.; Holley, R. Detection and Risk Associated with Organochlorine, Organophosphorus, Pyrethroid and Carbamate Pesticide Residues in Chicken Muscle and Organ Meats in Jordan. Food Control. 2023, 144, 109355. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Guo, L.-P.; Wang, C.-X.; Zhong, D.-B.; Shang, L.; Nian, H.-J.; Cui, X.-M.; Huang, S.-J. Rapid Determination and Dietary Intake Risk Assessment of 249 Pesticide Residues in Panax Notoginseng. Ecotoxicol. Environ. Saf. 2022, 233, 113348. [Google Scholar] [CrossRef] [PubMed]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A Review of Extraction, Analytical and Advanced Methods for Determination of Pesticides in Environment and Foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Ben Attig, J.; Latrous, L.; Zougagh, M.; Ríos, Á. Ionic Liquid and Magnetic Multiwalled Carbon Nanotubes for Extraction of N-Methylcarbamate Pesticides from Water Samples Prior Their Determination by Capillary Electrophoresis. Talanta 2021, 226, 122106. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, C.; He, J.; Zhang, H.; Xu, Z. Fluorescence Assay for Three Organophosphorus Pesticides in Agricultural Products Based on Magnetic-Assisted Fluorescence Labeling Aptamer Probe. Food Chem. 2020, 307, 125534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, L.; Zhang, Z.; Wu, J.; Zhang, L.; Jing, X.; Wang, X. Dispersive Liquid–liquid Microextraction Combined with Enzyme-Linked Immunosorbent Assay for the Analysis of Chlorpyrifos in Cereal Samples. Talanta 2023, 265, 124802. [Google Scholar] [CrossRef]
- Ma, L.; Xu, Q.; Yin, L.; Wu, W.; Han, E.; Wang, C.; Zhou, R.; Bai, J.; Cai, J. Simultaneous Detection of Acetamiprid and Carbendazim Based on Raman-Silent Spectral Window Tags-Mediated Surface-Enhanced Raman Scattering Aptasensor Coupled with Magnetic Separation. Sens. Actuators B Chem. 2024, 400, 134792. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Haruna, S.A.; Bei, Q.; Wei, W.; Hassan, M.M.; Chen, Q. Recent Progress in Photoelectrochemical Sensors to Quantify Pesticides in Foods: Theory, Photoactive Substrate Selection, Recognition Elements and Applications. TrAC Trends Anal. Chem. 2023, 164, 117108. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, J.; Melo, J.S.; Sandaka, B.P. Progressive Development in Biosensors for Detection of Dichlorvos Pesticide: A Review. J. Environ. Chem. Eng. 2021, 9, 105067. [Google Scholar] [CrossRef]
- Ding, R.; Li, Z.; Xiong, Y.; Wu, W.; Yang, Q.; Hou, X. Electrochemical (Bio)Sensors for the Detection of Organophosphorus Pesticides Based on Nanomaterial-Modified Electrodes: A Review. Crit. Rev. Anal. Chem. 2023, 53, 1766–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical Bioanalysis: The State of the Art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef]
- Qureshi, A.; Shaikh, T.; Niazi, J.H. Semiconductor Quantum Dots in Photoelectrochemical Sensors from Fabrication to Biosensing Applications. Analyst 2023, 148, 1633–1652. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, Z.; Zhao, C.; Shen, M.; Li, H.; Zhang, S.; Zhang, Z. Photoelectrochemical Biosensing Platforms for Tumor Marker Detection. Coord. Chem. Rev. 2022, 469, 214675. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, J.; Ju, Y.; Xue, H.; Pang, H. Current Advances in Semiconductor Nanomaterial-Based Photoelectrochemical Biosensing. Chem. Eur. J. 2018, 24, 14010–14027. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D. Recent Advances in Photoelectrochemical Biosensors for Analysis of Mycotoxins in Food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Qiu, Z.; Tang, D. Nanostructure-Based Photoelectrochemical Sensing Platforms for Biomedical Applications. J. Mater. Chem. B 2020, 8, 2541–2561. [Google Scholar] [CrossRef]
- Zhao, C.-Q.; Ding, S.-N. Perspective on Signal Amplification Strategies and Sensing Protocols in Photoelectrochemical Immunoassay. Coord. Chem. Rev. 2019, 391, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Gao, P.; Zhang, Y.; Ma, H.; Yang, J.; Du, B.; Wei, Q. A Signal-off Sandwich Photoelectrochemical Immunosensor Using TiO2 Coupled with CdS as the Photoactive Matrix and Copper (II) Ion as Inhibitor. Biosens. Bioelectron. 2015, 65, 97–102. [Google Scholar] [CrossRef]
- Bilge, S.; Sınağ, A. Current Trends and Strategies in the Development of Green MXene-Based Photoelectrochemical Sensing Application. TrAC Trends Anal. Chem. 2023, 163, 117059. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Q.; Hao, N.; Kun, W. Recent Developments of Photoelectrochemical Biosensors for Food Analysis. J. Mater. Chem. B 2019, 7, 7283–7300. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Zhao, D.; Geng, C.; Liu, X.; Jin, X.; Zhao, Z.; Liu, Y.; Alwarappan, S. Photoelectrochemical Detection of Superoxide Anions Released from Mitochondria in HepG2 Cells Based on the Synergistic Effect of MnO2@Co3O4 Core-Shell p-n Heterojunction. Biosens. Bioelectron. 2023, 237, 115368. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhu, R.; Du, C.; Zhang, X.; Chen, J. Highly Sensitive and Selective microRNA Photoelectrochemical Assay with Magnetic Electron Donor–Acceptor Covalent Organic Framework as Photoactive Material and ZnSe QDs as Photocurrent-Polarity-Switching Factor. Sens. Actuators B Chem. 2023, 380, 133403. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, X.; Lin, H.; Li, F. Perylene-Based Photoactive Material as a Double-Stranded DNA Intercalating Probe for Ultrasensitive Photoelectrochemical Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 16958–16964. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Yang, W.; Miao, F.; Zang, Y. Co3O4/ZnO/CNTs Photoelectrochemical Sensor for Sensitive and Selective LA Detection. IEEE Trans. Electron Devices 2023, 70, 6630–6636. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Song, J.; Cai, T.-X.; Jiang, R.-L.; Dai, J.; Xu, K.; Yang, X.-L.; Xie, M.-H. Construction of TiO2@ZnO Nanofibers with Beads-on-a-String Heterostructures for Photoelectrochemical Detection of Lactic Acid. J. Alloys Compd. 2023, 960, 170659. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.; Yu, K.; Chai, H.; Tian, M.; Qu, L.; Dong, H.; Zhang, X. Smartphone Light-Driven Zinc Porphyrinic MOF Nanosheets-Based Enzyme-Free Wearable Photoelectrochemical Sensor for Continuous Sweat Vitamin C Detection. Chem. Eng. J. 2023, 455, 140779. [Google Scholar] [CrossRef]
- Neven, L.; Shanmugam, S.T.; Rahemi, V.; Trashin, S.; Sleegers, N.; Carrión, E.N.; Gorun, S.M.; De Wael, K. Optimized Photoelectrochemical Detection of Essential Drugs Bearing Phenolic Groups. Anal. Chem. 2019, 91, 9962–9969. [Google Scholar] [CrossRef]
- Jacques, R.; Zhou, B.; Marhuenda, E.; Gorecki, J.; Das, A.; Iskratsch, T.; Krause, S. Photoelectrochemical Imaging of Single Cardiomyocytes and Monitoring of Their Action Potentials through Contact Force Manipulation of Organoids. Biosens. Bioelectron. 2023, 223, 115024. [Google Scholar] [CrossRef]
- Chen, X.; Wu, W.; Zeng, J.; Ibañez, E.; Cifuentes, A.; Mao, J.; Yu, L.; Wu, H.; Li, P.; Zhang, Z. A Smartphone-Powered Photoelectrochemical POCT via Z-Scheme Cu2O/Cu3SnS4 for Dibutyl Phthalate in the Environmental and Food. J. Hazard. Mater. 2023, 460, 132281. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yin, M.; Zhang, S.; Wei, J.; Jiao, T.; Chen, Q.; Chen, Q.; Chen, X.; Oyama, M.; Chen, X. A Paper-Based Photoelectrochemical Aptsensor Using near-Infrared Light-Responsive AgBiS2 Nanoflowers as Probes for the Detection of Staphylococcus Aureus in Pork. Talanta 2024, 266, 125128. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kang, J.; Wu, Y.; Pang, C.; Li, S.; Li, J.; Xiong, Y.; Luo, J.; Wang, M.; Xu, Z. Recent Advances in Metal/Covalent Organic Framework-Based Materials for Photoelectrochemical Sensing Applications. TrAC Trends Anal. Chem. 2022, 157, 116793. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Liu, X.; Tang, Y.; Zhou, Y.; Wong, D.K.Y. Detection Signal Amplification Strategies at Nanomaterial-Based Photoelectrochemical Biosensors. J. Mater. Chem. B 2020, 8, 7880–7893. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lei, J.; Ju, H. Principles and Applications of Photoelectrochemical Sensing Strategies Based on Biofunctionalized Nanostructures. Biosens. Bioelectron. 2017, 96, 8–16. [Google Scholar] [CrossRef]
- Tu, W.; Wang, Z.; Dai, Z. Selective Photoelectrochemical Architectures for Biosensing: Design, Mechanism and Responsibility. TrAC Trends Anal. Chem. 2018, 105, 470–483. [Google Scholar] [CrossRef]
- Jia, M.; Xiong, W.; Yang, Z.; Cao, J.; Zhang, Y.; Xiang, Y.; Xu, H.; Song, P.; Xu, Z. Metal-Organic Frameworks and Their Derivatives-Modified Photoelectrodes for Photoelectrochemical Applications. Coord. Chem. Rev. 2021, 434, 213780. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, H.; Ai, S. Recent Advances and Applications of Bi2S3-Based Composites in Photoelectrochemical Sensors and Biosensors. TrAC Trends Anal. Chem. 2023, 158, 116876. [Google Scholar] [CrossRef]
- Liu, Q.; Huan, J.; Hao, N.; Qian, J.; Mao, H.; Wang, K. Engineering of Heterojunction-Mediated Biointerface for Photoelectrochemical Aptasensing: Case of Direct Z-Scheme CdTe-Bi2S3 Heterojunction with Improved Visible-Light-Driven Photoelectrical Conversion Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 18369–18376. [Google Scholar] [CrossRef]
- You, F.; Zhu, M.; Ding, L.; Xu, Y.; Wang, K. Design and Construction of Z-Scheme Bi2S3/Nitrogen-Doped Graphene Quantum Dots: Boosted Photoelectric Conversion Efficiency for High-Performance Photoelectrochemical Aptasensing of Sulfadimethoxine. Biosens. Bioelectron. 2019, 130, 230–235. [Google Scholar] [CrossRef]
- Yin, Z.; Zhi, J. A Photoelectrochemical Biosensor Based on the Direct Electron Transfer to Galactose Oxidase. J. Photochem. Photobiol. Chem. 2020, 397, 112560. [Google Scholar] [CrossRef]
- Xia, L.; Song, J.; Xu, R.; Liu, D.; Dong, B.; Xu, L.; Song, H. Zinc Oxide Inverse Opal Electrodes Modified by Glucose Oxidase for Electrochemical and Photoelectrochemical Biosensor. Biosens. Bioelectron. 2014, 59, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, X.; Wu, Y.; Di, J. ZnS Nanoparticles Electrodeposited onto ITO Electrode as a Platform for Fabrication of Enzyme-Based Biosensors of Glucose. Mater. Sci. Eng. C 2013, 33, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wan, X.; Wang, X.; Li, M.; Tang, D. Ultrathin Mesoporous BiOCl Nanosheets-Mediated Liposomes for Photoelectrochemical Immunoassay with In-Situ Signal Amplification. Biosens. Bioelectron. 2023, 239, 115628. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Pan, J.-B.; Lu, C.-Y.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Folding-Based Photoelectrochemical Biosensor: Binding-Induced Conformation Change of a Quantum Dot-Tagged DNA Probe for Mercury(II) Detection. Chem. Commun. 2014, 50, 12088–12090. [Google Scholar] [CrossRef]
- Wang, W.; Bao, L.; Lei, J.; Tu, W.; Ju, H. Visible Light Induced Photoelectrochemical Biosensing Based on Oxygen-Sensitive Quantum Dots. Anal. Chim. Acta 2012, 744, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Liu, K.-L.; Shu, J.-X.; Gu, T.-T.; Wu, X.-M.; Dong, Y.-M.; Li, Z.-J. A Novel Photoelectrochemical Sensor Based on Photocathode of PbS Quantum Dots Utilizing Catalase Mimetics of Bio-Bar-Coded Platinum Nanoparticles/G-Quadruplex/Hemin for Signal Amplification. Biosens. Bioelectron. 2015, 69, 106–112. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Z.; Guo, Y.; Wang, X.; Yin, H.; Ai, S. Visible-Light Induced Photoelectrochemical Biosensor for the Detection of microRNA Based on Bi2S3 Nanorods and Streptavidin on an ITO Electrode. Microchim. Acta 2015, 182, 241–248. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Miao, L.; Gao, Y.; Di, J. Photocurrent Switching Effect on BiVO4 Electrodes and Its Application in Development of Photoelectrochemical Glucose Sensor. J. Solid State Electrochem. 2020, 24, 411–420. [Google Scholar] [CrossRef]
- Dong, X.; Xu, C.; Yang, C.; Chen, F.; Manohari, A.G.; Zhu, Z.; Zhang, W.; Wang, R.; You, D.; Chen, J. Photoelectrochemical Response to Glutathione in Au-Decorated ZnO Nanorod Array. J. Mater. Chem. C 2019, 7, 5624–5629. [Google Scholar] [CrossRef]
- Yang, G.; Chen, X.; Pan, Q.; Liu, W.; Zhao, F. A Novel Photoelectrochemical Sensor for Thiamphenicol Based on Porous Three-Dimensional Imprinted Film. Int. J. Electrochem. Sci. 2017, 12, 7272–7286. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, Z.; He, H.; Ai, R.; Liu, X.; Lu, X. Photoelectrochemical Sensing for Hydroquinone Based on Porphyrin-Functionalized Au Nanoparticles on Graphene. Biosens. Bioelectron. 2013, 47, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Song, H.; Song, J.; He, C.; Ni, J.; Zhao, Y.; Wang, X. Development of Triphenylamine Functional Dye for Selective Photoelectrochemical Sensing of Cysteine. Anal. Chem. 2014, 86, 5922–5928. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tu, W.; Zhao, Y.; Wang, X.; Song, J.; Yang, X. Phosphonate-Substituted Ruthenium(II) Bipyridyl Derivative as a Photoelectrochemical Probe for Sensitive and Selective Detection of Mercury(II) in Biofluids. Anal. Chem. 2018, 90, 14423–14432. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, W.; Du, X.; Wen, G.; Fan, X. A Novel Photoelectrochemical Sensor Based on G-C3N4@CdS QDs for Sensitive Detection of Hg2+. Microchem. J. 2020, 152, 104259. [Google Scholar] [CrossRef]
- Ye, C.; Wu, Z.; Ma, K.; Xia, Z.; Pan, J.; Wang, M.; Ye, C. Ti3C2 MXene-Based Schottky Photocathode for Enhanced Photoelectrochemical Sensing. J. Alloys Compd. 2021, 859, 157787. [Google Scholar] [CrossRef]
- Kong, W.; Xiang, M.-H.; Xia, L.; Zhang, M.; Kong, R.-M.; Qu, F. In-Situ Synthesis of 3D Cu2O@Cu-Based MOF Nanobelt Arrays with Improved Conductivity for Sensitive Photoelectrochemical Detection of Vascular Endothelial Growth Factor 165. Biosens. Bioelectron. 2020, 167, 112481. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Li, J.; Yan, J.; Liu, P.; Fan, X.; Song, W. A Novel TAPP-DHTA COF Cathodic Photoelectrochemical Immunosensor Based on CRISPR/Cas12a-Induced Nanozyme Catalytic Generation of Heterojunction. Electrochim. Acta 2023, 441, 141771. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Zhang, H.; Wang, X.; Yang, C.; Zhao, Y. Shear-Flow-Induced Graphene Coating Microfibers from Microfluidic Spinning. Innovation 2022, 3, 100209. [Google Scholar] [CrossRef]
- Liu, X.; Verma, G.; Chen, Z.; Hu, B.; Huang, Q.; Yang, H.; Ma, S.; Wang, X. Metal-Organic Framework Nanocrystal-Derived Hollow Porous Materials: Synthetic Strategies and Emerging Applications. Innovation 2022, 3, 100281. [Google Scholar] [CrossRef]
- Pardo-Yissar, V.; Katz, E.; Wasserman, J.; Willner, I. Acetylcholine Esterase-Labeled CdS Nanoparticles on Electrodes: Photoelectrochemical Sensing of the Enzyme Inhibitors. J. Am. Chem. Soc. 2003, 125, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Jin, X.; Li, X. Aptamer Based Photoelectrochemical Cytosensor with Layer-by-Layer Assembly of CdSe Semiconductor Nanoparticles as Photoelectrochemically Active Species. Biosens. Bioelectron. 2011, 26, 3674–3678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, L.-H. Highly Sensitive and Selective Photoelectrochemical DNA Sensor for the Detection of Hg2+ in Aqueous Solutions. Biosens. Bioelectron. 2012, 37, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Nakasu, M.; Ogasawara, S.; Nakanishi, H.; Nakamura, M.; Kikuchi, J. Photoelectrochemical Sensor with Porphyrin-Deposited Electrodes for Determination of Nucleotides in Water. Org. Lett. 2009, 11, 1163–1166. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, H.; Zhang, J.; Li, Y.; Yang, Y.; Fang, Y.; Wu, Z.; Cui, B.; Hu, Q. One-Step Electropolymerization of Polythiophene Derivative Film for Photoelectrochemical Detection of Chlorpyrifos. J. Electrochem. Soc. 2022, 169, 106502. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; Li, X.; Wang, K. Highly Sensitive Visible Light Activated Photoelectrochemical Biosensing of Organophosphate Pesticide Using Biofunctional Crossed Bismuth Oxyiodide Flake Arrays. Biosens. Bioelectron. 2012, 38, 43–49. [Google Scholar] [CrossRef]
- Miao, L.; Li, Z.; Chen, Y.; Gao, Y.; Di, J. A Sensitive Photoelectrochemical Biosensor for Pesticide Detection Based on BiVO4. Int. J. Environ. Anal. Chem. 2023, 103, 5245–5258. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Su, D.; Liu, Y.; Hu, X. Photoelectrochemical Determination of Malathion by Using CuO Modified with a Metal-Organic Framework of Type Cu-BTC. Microchim. Acta 2019, 186, 481. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Wu, F.; Guan, Y.; Xu, G. TiO2 Nanomaterials in Photoelectrochemical and Electrochemiluminescent Biosensing. Top. Curr. Chem. 2020, 378, 28. [Google Scholar] [CrossRef]
- Liu, P.P.; Liu, X.; Huo, X.H.; Tang, Y.; Xu, J.; Ju, H. TiO2–BiVO4 Heterostructure to Enhance Photoelectrochemical Efficiency for Sensitive Aptasensing. ACS Appl. Mater. Interfaces 2017, 9, 27185–27192. [Google Scholar] [CrossRef]
- Fu, B.; Wu, W.; Gan, L.; Zhang, Z. Bulk/Surface Defects Engineered TiO2 Nanotube Photonic Crystals Coupled with Plasmonic Gold Nanoparticles for Effective in Vivo Near-Infrared Light Photoelectrochemical Detection. Anal. Chem. 2019, 91, 14611–14617. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, M.; Huang, W.; Tian, J.; Zhang, Y.; Lu, J. A Photoelectrochemical Aptasensor for Acetamiprid Determination Based on λ Exonuclease-Assisted Recycling Amplification and DNAzyme-Catalyzed Precipitation. J. Electrochem. Soc. 2021, 168, 037517. [Google Scholar] [CrossRef]
- Lyu, R.; Lei, Y.; Zhang, C.; Li, G.; Han, R.; Zou, L. An Ultra-Sensitive Photoelectrochemical Sensor for Chlorpyrifos Detection Based on a Novel BiOI/TiO2 n-n Heterojunction. Anal. Chim. Acta 2023, 1275, 341579. [Google Scholar] [CrossRef]
- Lei, Q.; Li, J.; Lei, J.; Hong, B.; Li, X.; Li, H.; Wan, Y. Photoelectrochemical Sensor Based on Bi2S3 @g-C3N4 Heterojunction for the Detection of Chlorpyrifos. Surf. Interfaces 2023, 39, 102913. [Google Scholar] [CrossRef]
- Zhu, Y.; Dan, Y. Photocatalytic Activity of Poly(3-Hexylthiophene)/Titanium Dioxide Composites for Degrading Methyl Orange. Sol. Energy Mater. Sol. Cells 2010, 94, 1658–1664. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Xu, Q.; Hu, X. Poly(3-Hexylthiophene)/TiO2 Nanoparticle-Functionalized Electrodes for Visible Light and Low Potential Photoelectrochemical Sensing of Organophosphorus Pesticide Chlopyrifos. Anal. Chem. 2011, 83, 9681–9686. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Xu, Q.; Wang, Y.; Hu, X. A Derivative Photoelectrochemical Sensing Platform for Herbicide Acetochlor Based on TiO2–Poly (3-Hexylthiophene)–Ionic Liquid Nanocomposite Film Modified Electrodes. Talanta 2014, 127, 169–174. [Google Scholar] [CrossRef]
- Song, J.; Wu, S.; Xing, P.; Zhao, Y.; Yuan, J. Di-Branched Triphenylamine Dye Sensitized TiO2 Nanocomposites with Good Photo-Stability for Sensitive Photoelectrochemical Detection of Organophosphate Pesticides. Anal. Chim. Acta 2018, 1001, 24–31. [Google Scholar] [CrossRef]
- Ding, L.; Wei, J.; Qiu, Y.; Wang, Y.; Wen, Z.; Qian, J.; Hao, N.; Ding, C.; Li, Y.; Wang, K. One-Step Hydrothermal Synthesis of Telluride Molybdenum/Reduced Graphene Oxide with Schottky Barrier for Fabricating Label-Free Photoelectrochemical Profenofos Aptasensor. Chem. Eng. J. 2021, 407, 127213. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Liu, Q.; Hao, N.; Mao, H.; Wang, K. A Facile Strategy to Construct Pure Thiophene-Sulfur-Doped Graphene/ZnO Nanoplates Sensitized Structure for Fabricating a Novel “on-off-on” Switch Photoelectrochemical Aptasensor. Sens. Actuators B Chem. 2017, 251, 99–107. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Chun, D.-M. Facile One-Step Deposition of ZnO-Graphene Nanosheets Hybrid Photoanodes for Enhanced Photoelectrochemical Water Splitting. J. Alloys Compd. 2021, 870, 159430. [Google Scholar] [CrossRef]
- Kumar, A.S.; Reddy, N.R.; Sai, K.N.S.; Reddy, G.R.; Dhananjaya, M.; Kim, J.S.; Joo, S.W. Design of P-CuS/n-CdFe2O4 Heterojunction Passivated with Carbon Nanofiber (CNF) for Impressive Performance in Supercapacitor and Photoelectrochemical Water Splitting. J. Alloys Compd. 2023, 968, 171795. [Google Scholar] [CrossRef]
- Umeyama, T.; Imahori, H. Carbon Nanotube-Modified Electrodes for Solar Energy Conversion. Energy Environ. Sci. 2008, 1, 120. [Google Scholar] [CrossRef]
- Qin, Q.; Bai, X.; Hua, Z. Electrochemical Synthesis of Well-Dispersed CdTe Nanoparticles on Reduced Graphene Oxide and Its Photoelectrochemical Sensing of Catechol. J. Electrochem. Soc. 2017, 164, H241–H249. [Google Scholar] [CrossRef]
- Qian, J.; Yang, Z.; Wang, C.; Wang, K.; Liu, Q.; Jiang, D.; Yan, Y.; Wang, K. One-Pot Synthesis of BiPO4 Functionalized Reduced Graphene Oxide with Enhanced Photoelectrochemical Performance for Selective and Sensitive Detection of Chlorpyrifos. J. Mater. Chem. A 2015, 3, 13671–13678. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Chen, D.; Li, Y.; Hao, N.; Qian, J.; Zhong, H.; You, T.; Wang, K. Facile Wet Chemical Method for Fabricating P-Type BiOBr/n-Type Nitrogen Doped Graphene Composites: Efficient Visible-Excited Charge Separation, and High-Performance Photoelectrochemical Sensing. Carbon 2016, 102, 10–17. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Hossain, M.A.; Lee, D.; Kim, H.-K.; Hwang, Y.-H. Pt-Coated TiO2 Nanorods for Photoelectrochemical Water Splitting Applications. Results Phys. 2016, 6, 373–376. [Google Scholar] [CrossRef]
- Wang, D.; Ding, Z.; Zhou, H.; Chen, L.; Feng, X. Au Nanoparticle-Decorated TiO2 Nanowires for Surface Plasmon Resonance-Based Photoelectrochemical Bioassays with a Solid–Liquid–Air Triphase Interface. ACS Appl. Nano Mater. 2021, 4, 9401–9408. [Google Scholar] [CrossRef]
- Zhang, N.; Li, M.; Tan, C.F.; Nuo Peh, C.K.; Sum, T.C.; Ho, G.W. Plasmonic Enhanced Photoelectrochemical and Photocatalytic Performances of 1D Coaxial Ag@Ag2S Hybrids. J. Mater. Chem. A 2017, 5, 21570–21578. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Zhang, L.; Zhu, L.-B.; Gao, Y.; Fan, G.-C.; Han, D.-M.; Chen, G.; Zhao, W.-W. Bismuth-Containing Semiconductors for Photoelectrochemical Sensing and Biosensing. Coord. Chem. Rev. 2019, 393, 9–20. [Google Scholar] [CrossRef]
- Deiminiat, B.; Rounaghi, G.H. Fabrication of a Novel Photoelectrochemical Aptasensor Using Gold Nanoparticle-Sensitized TiO2 Film for Quantitative Determination of Diazinon in Solutions. Electrocatalysis 2023, 14, 484–498. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, X.; Lu, G.; Du, B.; Liu, M. A Highly Sensitive and Selective Photoelectrochemical Aptasensor for Atrazine Based on Au NPs/3DOM TiO2 Photonic Crystal Electrode. J. Hazard. Mater. 2023, 451, 131132. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhu, W.; You, F.; Yuan, R.; Ding, L.; Hao, N.; Wei, J.; Wang, K. Ultrasensitive Photoelectrochemical Aptasensor for Carbendazim Detection Based on In-Situ Constructing Schottky Junction via Photoreducing Pd Nanoparticles onto CdS Microsphere. Biosens. Bioelectron. 2022, 203, 114036. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gong, Q.; Xu, X.; Meng, S.; Li, Y.; You, T. Photoelectrochemical Aptasensor Based on Cascade Dual Z-Scheme CdTe-polyaniline@MoS2 Heterostructure for the Sensitive Carbendazim Detection. J. Electroanal. Chem. 2023, 930, 117143. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, M.; Chen, Y.; Gao, W. In-Situ Preparation of Hollow CdCoS2 Heterojunction with Enhanced Photocurrent Response for Highly Photoelectrochemical Sensing of Organophosphorus Pesticides. Anal. Chim. Acta 2022, 1212, 339913. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Y.; Gao, Z.; Lv, H.; Zhang, X.; Fan, D.; Wei, Q. Visible-Light Driven Label-Free Photoelectrochemical Immunosensor Based on TiO2/S-BiVO4@Ag2S Nanocomposites for Sensitive Detection OTA. Biosens. Bioelectron. 2018, 99, 14–20. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Peng, J. Blue-Light Photoelectrochemical Aptasensor for Kanamycin Based on Synergistic Strategy by Schottky Junction and Sensitization. Sens. Actuators B Chem. 2021, 340, 129898. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Ling, Y.; Zeng, B.; Zhang, H.; Deng, K. Bismuth Oxyiodide-Bismuth Hybridized with Short Carbon Nanotubes as Cathode Photoelectrochemical Sensing Platform for Determination of Chlorpyrifos. Chin. J. Anal. Chem. 2022, 50, 253–262. [Google Scholar] [CrossRef]
- Wang, P.; Ge, L.; Li, M.; Li, W.; Li, L.; Wang, Y.; Yu, J. Photoelectrochemical Sensor Based on Molecularly Imprinted Polymer-Coated TiO2 Nanotubes for Lindane Specific Recognition and Detection. J. Inorg. Organomet. Polym. Mater. 2013, 23, 703–711. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Tang, Y.; Wang, C.; Zhao, F.; Zeng, B. Recent Advances in Bismuth Oxyhalide-Based Functional Materials for Photoelectrochemical Sensing. TrAC Trends Anal. Chem. 2020, 131, 116020. [Google Scholar] [CrossRef]
- Wang, P.; Dai, W.; Ge, L.; Yan, M.; Ge, S.; Yu, J. Visible Light Photoelectrochemical Sensor Based on Au Nanoparticles and Molecularly Imprinted Poly(o-Phenylenediamine)-Modified TiO2 Nanotubes for Specific and Sensitive Detection Chlorpyrifos. Analyst 2013, 138, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Sun, J.; Jiang, D.; Du, W. Non-Noble Metal Plasmonic Enhanced Photoelectrochemical Sensing of Chlorpyrifos Based on 1D TiO2-x/3D Nitrogen-Doped Graphene Hydrogel Heterostructure. Anal. Bioanal. Chem. 2021, 413, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Ding, L.; Zhu, W.; You, F.; Wang, T.; Hao, N.; Wei, J.; Wang, K. Enhanced Photoelectrochemical Aptasensing for Sensitive Detection of Diazinon Pesticide Used N-Hydroxyphthalimide as an Effective Hole Mediator. Sens. Actuators B Chem. 2022, 367, 132101. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Yu, P.-P.; Xu, J.-J.; Chen, H.-Y. Ultrasensitive Photoelectrochemical Biosensing Based on Biocatalytic Deposition. Electrochem. Commun. 2011, 13, 495–497. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.-M.; Xu, Y.-T.; Fan, G.-C.; Yu, X.-D.; Liang, Y.-Y.; Zhao, W.-W. Binding-Induced Formation of DNAzyme on an Au@Ag Nanoparticles/TiO2 Nanorods Electrode: Stimulating Biocatalytic Precipitation Amplification for Plasmonic Photoelectrochemical Bioanalysis. Biosens. Bioelectron. 2019, 134, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gao, L.; Tang, L.; Peng, B.; Huang, H.; Wang, J.; Yu, J.; Ouyang, X.; Tan, J. Ultrathin PtNi Nanozyme Based Self-Powered Photoelectrochemical Aptasensor for Ultrasensitive Chloramphenicol Detection. Biosens. Bioelectron. 2019, 146, 111756. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, S.; Yuan, J.; Qin, T.; Li, T.; Sun, Y.; Liu, X.; Wong, D.K.Y. Amplified Detection Signal at a Photoelectrochemical Aptasensor with a Poly(Diphenylbutadiene)-BiOBr Heterojunction and Au-Modified CeO2 Octahedrons. Biosens. Bioelectron. 2022, 197, 113742. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Peng, B.; Tang, L.; Feng, C.; Wang, J.; Yu, J.; Ouyang, X.; Zhu, X. Enhanced Photoelectric Conversion Efficiency: A Novel h-BN Based Self-Powered Photoelectrochemical Aptasensor for Ultrasensitive Detection of Diazinon. Biosens. Bioelectron. 2019, 142, 111546. [Google Scholar] [CrossRef]
- Li, H.; Qiao, Y.; Li, J.; Fang, H.; Fan, D.; Wang, W. A Sensitive and Label-Free Photoelectrochemical Aptasensor Using Co-Doped ZnO Diluted Magnetic Semiconductor Nanoparticles. Biosens. Bioelectron. 2016, 77, 378–384. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Zhou, L.; Li, H.; Wang, K. New Insights toward Efficient Charge-Separation Mechanism for High-Performance Photoelectrochemical Aptasensing: Enhanced Charge-Carrier Lifetime via Coupling Ultrathin MoS2 Nanoplates with Nitrogen-Doped Graphene Quantum Dots. Anal. Chem. 2017, 89, 4525–4531. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, L.; Wei, X.; Li, J. Chitosan-Based Molecularly Imprinted Photoelectric Sensor with ZnO/Bi2O3/Bi2S3 Sensing Layer for Thiamethoxam Determination. Microchim. Acta 2022, 189, 247. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Wei, X.; Li, J. Molecularly Imprinted Photoelectrochemical Sensor Based on AgBiS2/Bi2S3 for Determination of Propoxur. Chin. J. Anal. Chem. 2020, 48, 396–404. [Google Scholar] [CrossRef]
- Sun, X.; Gao, C.; Zhang, L.; Yan, M.; Yu, J.; Ge, S. Photoelectrochemical Sensor Based on Molecularly Imprinted Film Modified Hierarchical Branched Titanium Dioxide Nanorods for Chlorpyrifos Detection. Sens. Actuators B Chem. 2017, 251, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Q.; Xia, W.W.; Shu, Y.; Jin, D.; Zang, Y.; Hu, X. High Sensitive Visible Light Photoelectrochemical Sensor Based on In-Situ Prepared Flexible Sn3O4 Nanosheets and Molecularly Imprinted Polymers. Sens. Actuators B Chem. 2018, 271, 215–224. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Wang, C.; Hu, X.; Liu, Y.; Wang, G. Sensitive Detection of Glyphosate Based on a Cu-BTC MOF/g-C3N4 Nanosheet Photoelectrochemical Sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Luo, Y.; Mi, Y.; Tan, X.; Chen, Q.; Feng, D.; Ai, C. Ultrathin BiOCl Nanosheet Modified TiO2 for the Photoelectrochemical Sensing of Chlorpyrifos. Anal. Methods 2019, 11, 375–380. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, Y.; Hao, N.; Qian, J.; Li, L.; You, T.; Mao, H.; Wang, K. Nitrogen Functionlized Graphene Quantum Dots/3D Bismuth Oxyiodine Hybrid Hollow Microspheres as Remarkable Photoelectrode for Photoelectrochemical Sensing of Chlopyrifos. Sens. Actuators B Chem. 2018, 260, 1034–1042. [Google Scholar] [CrossRef]
- Wang, H.; Liang, D.; Xu, Y.; Liang, X.; Qiu, X.; Lin, Z. A Highly Efficient Photoelectrochemical Sensor for Detection of Chlorpyrifos Based on 2D/2D β-Bi2O3/g-C3N4 Heterojunctions. Environ. Sci. Nano 2021, 8, 773–783. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Zhao, F.; Zeng, B. One-Pot Synthesis of N-Graphene Quantum Dot-Functionalized I-BiOCl Z-Scheme Cathodic Materials for “Signal-Off” Photoelectrochemical Sensing of Chlorpyrifos. ACS Appl. Mater. Interfaces 2018, 10, 35281–35288. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Jiang, J.; Li, P.; Chen, X.; Zhang, S.; Gao, Y.; Hong, R.; Chen, G.; Mao, G.; et al. A Highly Sensitive and Visible-Light-Driven Photoelectrochemical Sensor for Chlorpyrifos Detection Using Hollow Co9S8@CdS Heterostructures. Sens. Actuators B Chem. 2021, 348, 130719. [Google Scholar] [CrossRef]
- Chen, S.-H.; Xiao, X.-Y.; Li, P.-H.; Li, Y.-X.; Yang, M.; Guo, Z.; Huang, X.-J. A Direct Z-Scheme ZnS/Co9S8 Heterojunction-Based Photoelectrochemical Sensor for the Highly Sensitive and Selective Detection of Chlorpyrifos. Environ. Sci. Nano 2020, 7, 753–763. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, J.; Sun, Y.; Liu, J.; Chen, W.; Xu, Y.; Yang, J.; Li, Y. A Photoelectrochemical Sensor Based on Z-Scheme TiO2@Au@CdS and Molecularly Imprinted Polymer for Uric Acid Detection. Microchim. Acta 2021, 188, 188. [Google Scholar] [CrossRef] [PubMed]
- Limthin, D.; Leepheng, P.; Tunhoo, B.; Onlaor, K.; Klamchuen, A.; Phromyothin, D.; Thiwawong, T. Preparation of Surface-Modified Electrode of Copper(II) Oxide Mixed with the Molecularly Imprinted Polymer for Enhancement of Melamine Detection with Photoelectrochemical Technique. RSC Adv. 2023, 13, 14729–14736. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Xu, Q.; Yang, Z.; Hu, X. A Derivative Photoelectrochemical Sensing Platform for 4-Nitrophenolate Contained Organophosphates Pesticide Based on Carboxylated Perylene Sensitized Nano-TiO2. Anal. Chim. Acta 2013, 766, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-J.; Wang, Y.; Meng, L.-R.; Zhu, J.-C.; Shu, R.-W.; He, J. Synthesis of rGO/TiO2/CdS Nanocomposites and Its Enhanced Photoelectrochemical Performance in Determination of Parathion-Methyl. Nano 2018, 13, 1850054. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Wang, Q.; Mao, H. A One-Step Hydrothermal Route to Fabricate a ZnO Nanorod/3D Graphene Aerogel-Sensitized Structure with Enhanced Photoelectrochemistry Performance and Self-Powered Photoelectrochemical Biosensing of Parathion-Methyl. RSC Adv. 2021, 11, 35644–35652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zheng, Z.; Yang, J.; Chen, M.; Yao, Q.; Chen, Y.; Gao, W. The Visible Light-Driven and Self-Powered Photoelectrochemical Biosensor for Organophosphate Pesticides Detection Based on Nitrogen Doped Carbon Quantum Dots for the Signal Amplification. Electrochim. Acta 2019, 296, 627–636. [Google Scholar] [CrossRef]
- Du, D.; Ding, J.; Tao, Y.; Chen, X. Application of Chemisorption/Desorption Process of Thiocholine for Pesticide Detection Based on Acetylcholinesterase Biosensor. Sens. Actuators B Chem. 2008, 134, 908–912. [Google Scholar] [CrossRef]
- Wang, M.; Hou, L.; Chen, X.; Lin, T. Homogeneous Photoelectrochemical Biosensor for Sensitive Detection of Omethoate via ALP-Mediated Pesticide Assay and Bi2S3@Bi2Sn2O7 Heterojunction as Photoactive Material. Anal. Bioanal. Chem. 2022, 414, 7277–7289. [Google Scholar] [CrossRef]
- Jin, D.; Gong, A.; Zhou, H. Visible-Light-Activated Photoelectrochemical Biosensor for the Detection of the Pesticide Acetochlor in Vegetables and Fruit Based on Its Inhibition of Glucose Oxidase. RSC Adv. 2017, 7, 17489–17496. [Google Scholar] [CrossRef]
- Zeng, Z.; Tang, J.; Zhang, M.; Pu, S.; Tang, D. Ultrasensitive Zero-Background Photoelectrochemical Biosensor for Analysis of Organophosphorus Pesticide Based on in Situ Formation of DNA-Templated Ag2S Photoactive Materials. Anal. Bioanal. Chem. 2021, 413, 6279–6288. [Google Scholar] [CrossRef]
- Li, J.; Xiong, P.; Tang, J.; Liu, L.; Gao, S.; Zeng, Z.; Xie, H.; Tang, D.; Zhuang, J. Biocatalysis-Induced Formation of BiOBr/Bi2S3 Semiconductor Heterostructures: A Highly Efficient Strategy for Establishing Sensitive Photoelectrochemical Sensing System for Organophosphorus Pesticide Detection. Sens. Actuators B Chem. 2021, 331, 129451. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Dong, J.; Huang, M.; Lu, J. Photoelectrochemical TiO2 Nanotube Arrays Biosensor for Asulam Determination Based on In-Situ Generation of Quantum Dots. Biosens. Bioelectron. 2018, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wu, Y.; Chen, G.; Jiao, L.; Hu, L.; Gu, W.; Zhu, C. Dissociable Photoelectrode Materials Boost Ultrasensitive Photoelectrochemical Detection of Organophosphorus Pesticides. Anal. Chim. Acta 2020, 1130, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, J.; Xiong, P.; Sun, Y.; Zeng, Z.; Tian, X.; Tang, D. Rolling Circle Amplification Promoted Magneto-Controlled Photoelectrochemical Biosensor for Organophosphorus Pesticides Based on Dissolution of Core-Shell MnO2 nanoflower@CdS Mediated by Butyrylcholinesterase. Microchim. Acta 2020, 187, 450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huan, J.; Dong, X.; Qian, J.; Hao, N.; You, T.; Mao, H.; Wang, K. Resonance Energy Transfer from CdTe Quantum Dots to Gold Nanorods Using MWCNTs/rGO Nanoribbons as Efficient Signal Amplifier for Fabricating Visible-Light-Driven “on-off-on” Photoelectrochemical Acetamiprid Aptasensor. Sens. Actuators B Chem. 2016, 235, 647–654. [Google Scholar] [CrossRef]
- Xu, B.-F.; Li, Q.; Qu, P.; Xin, X.-R.; Wang, A.-J.; Mei, L.-P.; Song, P.; Feng, J.-J. Magnetic-Assisted Exciton-Plasmon Interactions Modulated Bi2S3 nanorods@MoS2 Nanosheets Heterojunction: Towards a Split-Type Photoelectrochemical Sensing of Profenofos. Microchim. Acta 2023, 190, 350. [Google Scholar] [CrossRef]
- Wang, G.; Li, L.; Zheng, H.; Li, Q.; Huang, J.; Zhang, L.; Yang, H.; Cui, K.; Yu, J. Bifunctional Strategy toward Constructing Perovskite/Upconversion Lab-on-Paper Photoelectrochemical Device for Sensitive Detection of Malathion. ACS Nano 2023, 17, 13418–13429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).