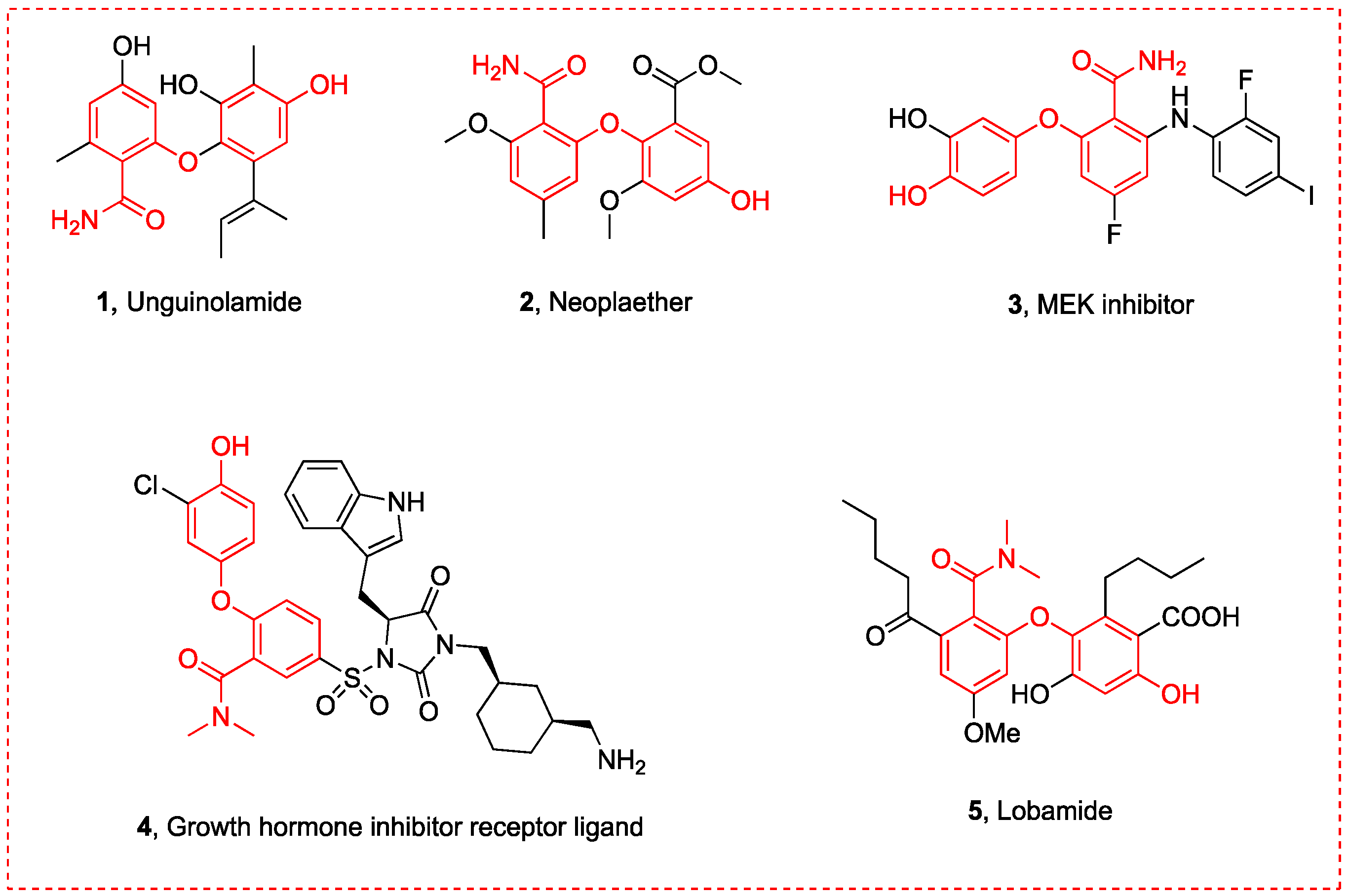
3. Experimental Section
1H-NMR and
13C-NMR spectra were recorded on a 500 MHz instrument (125 MHz for
13C NMR, Bruker Bioscience, Billerica, MA, USA) at 25 °C. Chemical shift values were reported in ppm, and with tetramethylsilane (TMS) as the internal reference, set at 0.00 ppm. The multiplicity of the signals is denoted as follows: s (singlet); d (doublet), t (triplet), q (quadruplet), qui (quintuplet), m (multiplet), dd (doublet of doublets), and dt (doublet of triplets). Coupling constants (J) are given in hertz (Hz). High-resolution mass spectra (HRMS) data were acquired on a Q-TOF microspectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Melting points were determined using a standard micro-melting point apparatus (SGWX-4, Shenzhen, China) and were reported without correction. Flash chromatography was performed using silica gel (200–300 mesh) as the stationary phase, with a mobile phase consisting of a mixture of methanol (MeOH), ethyl acetate (EA), and petroleum ether (PE). Thin-layer chromatography (TLC) was carried out on glass-backed plates pre-coated with silica gel 60 GF254, developed with standard visualization agents, and the spots were visualized under ultraviolet (UV) light. All reagents and solvents were purchased from commercial sources and were used without further purification. Various substituted 2-(methyl(phenyl)amino)benzoic acids and 2-(methyl(phenyl)amino)benzamide were synthesized in-house. All
1H NMR,
13C NMR, and HR-MS spectra are available in
Supplementary Materials.
3.1. Preparation of Methyl 2-Iodobenzoate (12)
The intermediate methyl 2-iodobenzoate (
12) was prepared according to the literature [
20]. To the solution of 2-iodobenzoic acid (15 g, 0.06 mol) in methanol (200 mL) was added concentrated sulfuric acid (9 mL), and the reaction mixture was stirred at room temperature under a nitrogen atmosphere for 7 h. The mixture was then cooled to room temperature, and part of the organic solvent was removed by evaporation. Acetate ester (80 mL) was added, and the organic phase was sequentially washed with 10% aqueous Na
2CO
3 (30 mL × 3), 1 M hydrochloric (30 mL × 3), and water (15 mL × 3). The organic phase was dried over anhydrous MgSO
4 and concentrated to yield methyl 2-iodobenzoate (
12), which was used directly for the next step without further purification (15.26 g, 96.3%).
3.2. Preparation of Methyl 2-Phenoxybenzoate (13)
To the solution of methyl 2-iodobenzoate (12, 4.0 g, 15.26 mmol) and phenol (1.72 g, 18.32 mmol) in toluene (40 mL), Cs2CO3 (7.46 g, 22.90 mmol) and copper(I) iodine (2.91 g, 15.26 mmol) were added; the reaction mixture was stirred at reflux under nitrogen atmosphere for 4 h. The mixture was then cooled to room temperature, filtered through celite, and washed an with acetate ester (15 mL × 3). The organic phase was sequentially washed with saturated saline (15 mL × 3) and water (15 mL × 3), dried over anhydrous MgSO4, filtered, and concentrated to yield the crude product, which was purified by flash chromatography (PE:EA = 50:1) to obtain methyl 2-phenoxybenzoate (13, 2.90 g, 83.3%). 1H NMR (500 MHz, DMSO-d6) δ 7.86 (dd, J = 7.8, 1.8 Hz, 1H), 7.61 (ddd, J = 8.2, 7.4, 1.8 Hz, 1H), 7.42–7.34 (m, 2H), 7.30 (td, J = 7.6, 1.1 Hz, 1H), 7.13 (tt, J = 7.3, 1.1 Hz, 1H), 7.05 (dd, J = 8.3, 1.1 Hz, 1H), 6.98–6.91 (m, 2H), 3.73 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.90, 157.72, 155.54, 134.52, 131.85, 130.44, 124.50, 123.63, 123.56, 121.40, 118.25, 52.56.
3.3. General Procedure for Preparation of Intermediates 14a–14r
To the solution of methyl 2-phenoxybenzoate (13, 1.0 g, 4.38 mmol) and varied amines (1.0 mL) in methanol (40 mL), sodium methoxide (0.47 g, 8.70 mmol) was added. The reaction solution was stirred at reflux under a nitrogen atmosphere for 6 h. The mixture was then cooled to room temperature, and water (35 mL) was added. The precipitate solid was filtered, washed with water (10 mL × 3), and dried to yield the desired product as a white solid.
2-Phenoxybenzamide (14a): 91.3%; m.p. 122–126 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.76 (dd, J = 7.7, 1.8 Hz, 1H), 7.65 (s, 1H), 7.57 (s, 1H), 7.50–7.38 (m, 3H), 7.23 (td, J = 7.5, 1.1 Hz, 1H), 7.18 (t, J = 7.4 Hz, 1H), 7.08–7.03 (m, 2H), 6.90 (dd, J = 8.2, 1.1 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 167.11, 156.84, 154.37, 132.40, 130.81, 130.51, 127.96, 124.20, 124.03, 119.48, 119.25.
2-(m-Tolyloxy)benzamide (14b): 84.0%; m.p. 100–102 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.76 (dd, J = 7.7, 1.8 Hz, 1H), 7.61 (s, 1H), 7.56 (s, 1H), 7.46 (ddd, J = 8.7, 7.4, 1.8 Hz, 1H), 7.29 (t, J = 7.8 Hz, 1H), 7.22 (td, J = 7.5, 1.1 Hz, 1H), 6.99 (d, J = 7.5 Hz, 1H), 6.92–6.86 (m, 2H), 6.83 (dd, J = 8.1, 2.5 Hz, 1H), 2.31 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 167.05, 156.78, 154.46, 140.23, 132.41, 130.82, 130.22, 127.78, 124.94, 123.93, 119.76, 119.47, 116.28, 21.43.
2-(3-Bromophenoxy)benzamide (14c): 90.8%; m.p. 105–107 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.72 (dd, J = 7.7, 1.8 Hz, 1H), 7.68 (s, 1H), 7.55–7.46 (m, 2H), 7.40–7.33 (m, 2H), 7.29 (td, J = 7.5, 1.1 Hz, 1H), 7.24–7.20 (m, 1H), 7.06–6.97 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 167.13, 158.31, 153.19, 132.43, 132.13, 130.67, 128.95, 126.69, 124.91, 122.60, 121.56, 120.46, 117.77.
2-(3-Chlorophenoxy)benzamide (14d): 74.5%; m.p. 93–95 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.72 (dd, J = 7.7, 1.8 Hz, 1H), 7.68 (s, 1H), 7.55–7.47 (m, 2H), 7.42 (t, J = 8.1 Hz, 1H), 7.30 (td, J = 7.5, 1.1 Hz, 1H), 7.21 (ddd, J = 8.0, 2.0, 0.9 Hz, 1H), 7.09 (t, J = 2.2 Hz, 1H), 7.02 (dd, J = 8.2, 1.1 Hz, 1H), 6.98 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 167.13, 158.30, 153.17, 134.36, 132.42, 131.82, 130.67, 128.97, 124.92, 123.78, 120.49, 118.74, 117.36.
5-Methyl-2-phenoxybenzamide (14e): 72.2%; m.p. 99–102 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.60–7.55 (m, 2H), 7.51 (s, 1H), 7.43–7.34 (m, 2H), 7.28 (ddd, J = 8.4, 2.4, 0.8 Hz, 1H), 7.14 (tt, J = 7.4, 1.1 Hz, 1H), 7.03–6.97 (m, 2H), 6.84 (d, J = 8.3 Hz, 1H), 2.34 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 167.06, 157.39, 151.91, 133.42, 132.88, 131.04, 130.41, 127.84, 123.79, 120.10, 118.67, 20.67.
5-Methyl-2-(m-tolyloxy)benzamide (14f): 88.3%; m.p. 100–103 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.58 (d, J = 2.3 Hz, 1H), 7.55 (s, 1H), 7.52 (s, 1H), 7.30–7.21 (m, 2H), 6.95 (d, J = 7.5 Hz, 1H), 6.86–6.81 (m, 2H), 6.79 (dd, J = 8.1, 2.5 Hz, 1H), 2.33 (s, 3H), 2.29 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 167.02, 157.33, 152.00, 140.11, 133.33, 132.92, 131.06, 130.13, 127.65, 124.54, 120.11, 119.16, 115.72, 21.44, 20.67.
5-Chloro-2-(m-tolyloxy)benzamide (14g): 84.8%; m.p. 126–128 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.75–7.67 (m, 3H), 7.50 (dd, J = 8.8, 2.8 Hz, 1H), 7.30 (t, J = 7.8 Hz, 1H), 7.01 (ddt, J = 7.6, 1.7, 0.9 Hz, 1H), 6.94–6.88 (m, 2H), 6.86 (dd, J = 8.1, 2.5 Hz, 1H), 2.31 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.79, 156.48, 153.41, 140.37, 131.98, 130.30, 130.03, 129.55, 127.67, 125.30, 121.26, 119.92, 116.46, 21.41.
2-([1,1′-Biphenyl]-3-yloxy)-5-chlorobenzamide (14h): 55.2%; m.p. 130–133 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.82 (s, 1H), 7.72 (s, 2H), 7.67 (d, J = 7.7 Hz, 2H), 7.49 (q, J = 8.4, 7.6 Hz, 5H), 7.39 (d, J = 6.5 Hz, 2H), 7.06 (d, J = 6.5 Hz, 1H), 7.01 (d, J = 8.8 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 165.90, 157.14, 153.21, 142.76, 139.75, 131.99, 131.11, 130.04, 129.89, 129.49, 128.39, 127.87, 127.27, 122.88, 121.42, 118.32, 117.63.
5-Fluoro-2-phenoxybenzamide (14i): 76.0%; m.p. 122–124 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.73 (s, 1H), 7.68 (s, 1H), 7.52 (dd, J = 9.0, 3.3 Hz, 1H), 7.44–7.37 (m, 2H), 7.33 (ddd, J = 9.0, 7.9, 3.3 Hz, 1H), 7.19–7.12 (m, 1H), 7.05–7.02 (m, 1H), 7.02–6.97 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.84, 159.25, 157.32, 150.22, 150.20, 130.49, 130.02, 124.04, 122.22, 122.15, 119.15, 118.97, 118.68, 116.91, 116.71.
5-Fluoro-2-(m-tolyloxy)benzamide (14j): 84.2%; m.p. 124–127 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.69 (d, J = 11.5 Hz, 2H), 7.52 (dd, J = 9.0, 3.3 Hz, 1H), 7.36–7.22 (m, 2H), 7.02–6.91 (m, 2H), 6.86 (t, J = 2.1 Hz, 1H), 6.80 (dd, J = 8.2, 2.5 Hz, 1H), 2.30 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.80, 159.20, 157.28, 157.25, 150.32, 150.30, 140.23, 130.20, 129.83, 129.77, 124.80, 122.18, 122.12, 119.20, 119.17, 118.99, 116.88, 116.69, 115.72, 21.42.
2-([1,1′-Biphenyl]-3-yloxy)-5-fluorobenzamide (14k): 53.1%; m.p. 164–166 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.79 (s, 1H), 7.70 (s, 1H), 7.68–7.62 (m, 2H), 7.53 (dd, J = 8.9, 3.2 Hz, 1H), 7.51–7.45 (m, 4H), 7.42–7.31 (m, 3H), 7.09 (dd, J = 9.0, 4.5 Hz, 1H), 7.03–6.98 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 165.93, 159.32, 157.90, 157.41, 150.08, 142.67, 139.86, 131.00, 130.16, 129.49, 128.36, 127.25, 122.39, 119.17, 118.98, 117.54, 116.92, 116.72.
N-Butyl-2-phenoxybenzamide (14l): 85.6%; m.p. 158–160 °C; 1H NMR (500 MHz, DMSO-d6) δ 8.17 (t, J = 5.9 Hz, 1H), 7.66 (dd, J = 7.6, 1.8 Hz, 1H), 7.50–7.43 (m, 1H), 7.43–7.35 (m, 2H), 7.24 (td, J = 7.5, 1.1 Hz, 1H), 7.15 (tt, J = 7.4, 1.1 Hz, 1H), 7.05–6.98 (m, 2H), 6.94 (dd, J = 8.2, 1.1 Hz, 1H), 3.20 (q, J = 6.6 Hz, 2H), 1.42–1.36 (m, 2H), 1.28–1.20 (m, 2H), 0.83 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.49, 157.12, 153.86, 131.98, 130.46, 130.40, 129.02, 124.19, 123.94, 119.83, 118.86, 39.12, 31.60, 19.97, 14.16.
N-Methyl-2-phenoxy-N-phenylbenzamide (14m): 85.6%; m.p. 161–163 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.38 (t, J = 7.7 Hz, 3H), 7.25–7.14 (m, 7H), 7.00 (t, J = 7.6 Hz, 1H), 6.78 (d, J = 5.0 Hz, 2H), 6.55 (d, J = 8.4 Hz, 1H), 3.36 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 167.50, 156.23, 152.90, 143.94, 130.72, 130.42, 129.81, 129.25, 127.38, 127.12, 124.34, 123.17, 119.60, 117.42, 37.15.
N-Cyclohexyl-2-phenoxybenzamide (14n): 50.0%; m.p. 97–99 °C; 1H NMR (500 MHz, DMSO-d6) δ 8.00 (d, J = 8.0 Hz, 1H), 7.64 (dd, J = 7.6, 1.8 Hz, 1H), 7.47 (td, J = 7.7, 1.8 Hz, 1H), 7.43–7.35 (m, 2H), 7.25 (td, J = 7.5, 1.1 Hz, 1H), 7.17–7.10 (m, 1H), 7.03–6.98 (m, 2H), 6.97 (dd, J = 8.2, 1.1 Hz, 1H), 3.71–3.64 (m, 1H), 1.71–1.61 (m, 4H), 1.53 (dt, J = 13.2, 3.8 Hz, 1H), 1.31–1.05 (m, 5H). 13C NMR (126 MHz, DMSO-d6) δ 164.61, 157.24, 153.62, 131.98, 130.45, 130.41, 129.30, 124.36, 123.82, 120.18, 118.53, 48.28, 32.58, 25.67, 24.89.
(2-Phenoxyphenyl)(pyrrolidin-1-yl)methanone (14o): 88.6%; m.p. 94–97 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.44–7.35 (m, 4H), 7.21 (td, J = 7.4, 1.1 Hz, 1H), 7.16 (tt, J = 7.4, 1.1 Hz, 1H), 7.04–6.98 (m, 2H), 6.94 (dd, J = 8.3, 1.0 Hz, 1H), 3.36 (t, J = 6.8 Hz, 2H), 3.25 (t, J = 6.5 Hz, 2H), 1.87–1.72 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ 165.89, 156.85, 152.90, 130.96, 130.46, 130.37, 128.79, 124.21, 124.17, 119.22, 47.91, 45.63, 25.93, 24.46.
(2-Phenoxyphenyl)(piperidin-1-yl)methanone (14p): 52.4%; m.p. 91–93 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.40 (ddd, J = 10.3, 5.7, 2.1 Hz, 3H), 7.34 (dd, J = 7.6, 1.7 Hz, 1H), 7.22 (td, J = 7.4, 1.1 Hz, 1H), 7.16 (t, J = 7.4 Hz, 1H), 7.04–6.98 (m, 2H), 6.91 (dd, J = 8.3, 1.0 Hz, 1H), 3.54 (td, J = 7.6, 5.0 Hz, 2H), 3.22 (t, J = 5.5 Hz, 2H), 1.61–1.40 (m, 6H). 13C NMR (126 MHz, DMSO-d6) δ 165.89, 156.85, 152.75, 130.77, 130.52, 129.34, 128.73, 124.27, 124.14, 119.10, 118.89, 47.89, 42.18, 26.41, 25.77, 24.46.
N-Butyl-5-methyl-2-(m-tolyloxy)benzamide (14q): 62.4%; m.p. 89–92 °C; 1H NMR (500 MHz, DMSO-d6) δ 8.13–8.06 (m, 1H), 7.46 (d, J = 2.5 Hz, 1H), 7.29–7.20 (m, 2H), 6.93 (d, J = 7.5 Hz, 1H), 6.86 (d, J = 8.4 Hz, 1H), 6.79 (t, J = 2.1 Hz, 1H), 6.74 (dd, J = 8.2, 2.5 Hz, 1H), 3.17 (q, J = 6.9 Hz, 2H), 2.33 (s, 3H), 2.28 (s, 3H), 1.39–1.33 (m, 2H), 1.25–1.18 (m, 2H), 0.82 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.46, 157.60, 151.45, 139.94, 133.49, 132.44, 130.68, 130.01, 128.79, 124.26, 120.42, 118.80, 115.37, 39.09, 31.62, 21.45, 20.67, 19.97, 14.16.
N-Cyclohexyl-5-fluoro-2-phenoxybenzamide (14r): 45.1%; m.p. 103–105 °C; 1H NMR (500 MHz, DMSO-d6) δ 8.09 (d, J = 7.9 Hz, 1H), 7.45–7.30 (m, 4H), 7.12 (tt, J = 7.3, 1.1 Hz, 1H), 7.06 (dd, J = 9.0, 4.5 Hz, 1H), 7.00–6.93 (m, 2H), 3.68–3.61 (m, 1H), 1.70–1.59 (m, 4H), 1.53 (dt, J = 13.0, 3.7 Hz, 1H), 1.30–1.04 (m, 5H). 13C NMR (126 MHz, DMSO-d6) δ 163.37, 159.47, 157.68, 157.55, 149.46, 149.44, 131.33, 131.28, 130.39, 123.66, 122.89, 122.82, 118.69, 118.51, 117.96, 116.70, 116.50, 48.40, 32.46, 25.62, 24.85.
3.4. General Procedure Preparation of Product 15a–15r
To the solution of substituted 2-phenoxybenzaide (14a–14r, 1.0 mmol) in TFA (10 mL) was added KOH (0.11 g, 2.0 mmol) and iodobenzene (0.33 g, 1.5 mmol). The reaction solution was stirred at room temperature for 4 h. 10% aqueous Na2CO3 (30 mL) was added, then was extracted by acetate ester (15 mL × 3). The organic phase was sequentially washed with saturated saline (15 mL × 3), and dried over anhydrous MgSO4, filtered, and concentrated to yield the crude product, which was purified by flash chromatography (THF:EA = 1:2) to obtain the products 15a–15r.
2-(4-Hydroxyphenoxy) benzamide (15a): 72.9%; m.p. 155–159 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.41 (s, 1H), 7.76 (dd, J = 7.7, 1.8 Hz, 1H), 7.63 (s, 1H), 7.60–7.57 (m, 10H), 7.39 (ddd, J = 8.4, 7.3, 1.8 Hz, 1H), 7.13 (td, J = 7.5, 1.1 Hz, 1H), 6.99–6.92 (m, 2H), 6.85–6.78 (m, 2H), 6.73 (dd, J = 8.3, 1.1 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 167.06, 156.40, 154.69, 147.82, 132.38, 130.92, 126.03, 122.71, 121.71, 117.17, 116.76; HRMS [M + H]+ (C13H12NO3): Calculated: 230.0739; Found: 230.0796.
2-(4-Hydroxy-3-methylphenoxy)benzamide (15b): 75.2%; m.p. 198–200 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.30 (s, 1H), 7.77 (dd, J = 7.7, 1.8 Hz, 1H), 7.59 (d, J = 12.5 Hz, 2H), 7.39 (ddd, J = 8.6, 7.3, 1.9 Hz, 1H), 7.12 (td, J = 7.5, 1.1 Hz, 1H), 6.88 (d, J = 2.9 Hz, 1H), 6.82 (d, J = 8.6 Hz, 1H), 6.80–6.71 (m, 2H), 2.13 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 167.01, 156.53, 152.76, 147.53, 132.42, 130.94, 126.06, 125.80, 122.78, 122.62, 118.67, 117.23, 115.83, 16.55. HRMS [M + H]+ (C14H14NO3): Calculated: 244.0895; Found: 244.0969.
2-(3-Bromo-4-hydroxyphenoxy)benzamide (15c): 54.8%; m.p. 213–216 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.20 (s, 1H), 7.72 (dd, J = 7.7, 1.8 Hz, 1H), 7.65 (s, 1H), 7.57 (s, 1H), 7.42 (ddd, J = 8.3, 7.3, 1.8 Hz, 1H), 7.29 (d, J = 2.5 Hz, 1H), 7.17 (td, J = 7.5, 1.1 Hz, 1H), 7.03–6.95 (m, 2H), 6.81 (dd, J = 8.3, 1.0 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 167.16, 155.49, 151.44, 148.59, 132.34, 130.77, 126.98, 124.61, 123.37, 120.71, 117.88, 117.26, 109.79. HRMS [M + H]+ (C13H11BrNO3): Calculated: 307.9844; Found: 307.9912.
2-(3-Chloro-4-hydroxyphenoxy)benzamide (15d): 54.4%; m.p. 210–214 °C; 1H NMR (500 MHz, DMSO-d6) δ 10.12 (s, 1H), 7.72 (dd, J = 7.7, 1.8 Hz, 1H), 7.64 (s, 1H), 7.56 (s, 1H), 7.42 (ddd, J = 8.4, 7.3, 1.8 Hz, 1H), 7.21–7.14 (m, 2H), 7.01 (d, J = 8.9 Hz, 1H), 6.93 (dd, J = 8.8, 2.9 Hz, 1H), 6.82 (dd, J = 8.3, 1.1 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 167.15, 155.44, 150.39, 148.43, 132.33, 130.77, 127.01, 123.40, 121.79, 120.49, 120.06, 117.94, 117.66. HRMS [M + H]+ (C13H11ClNO3): Calculated: 264.0349; Found: 264.0420.
2-(4-Hydroxyphenoxy)-5-methylbenzamide (15e): 60.0%; m.p. 160–163 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.37 (s, 1H), 7.61–7.52 (m, 3H), 7.21 (dd, J = 8.5, 2.4 Hz, 1H), 6.95–6.88 (m, 2H), 6.83–6.76 (m, 2H), 6.67 (d, J = 8.4 Hz, 1H), 2.30 (s, 3H); 13C NMR (126 MHz, DMSO-d6) δ 167.03, 154.41, 154.09, 148.43, 132.87, 131.95, 131.14, 125.82, 121.21, 117.80, 116.68, 20.55. HRMS [M + H]+ (C13H14NO3): Calculated: 244.0895; Found: 244.0966.
2-(4-Hydroxy-3-methylphenoxy)-5-methylbenzamide (15f): 55.7%; m.p. 192–195 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.26 (s, 1H), 7.59 (d, J = 2.3 Hz, 1H), 7.55 (d, J = 9.4 Hz, 2H), 7.20 (dd, J = 8.4, 2.4 Hz, 1H), 6.83 (d, J = 2.9 Hz, 1H), 6.79 (d, J = 8.6 Hz, 1H), 6.73 (dd, J = 8.6, 3.0 Hz, 1H), 6.67 (d, J = 8.4 Hz, 1H), 2.29 (s, 3H), 2.12 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 166.98, 154.21, 152.48, 148.14, 132.92, 131.85, 131.15, 125.95, 125.60, 122.30, 118.17, 117.86, 115.76, 20.55, 16.54. HRMS [M + H]+ (C14H14NO3): Calculated: 258.1052; Found: 258.1121.
5-Chloro-2-(4-hydroxy-3-methylphenoxy)benzamide (15g): 68.2%; m.p. 210–212 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.35 (s, 1H), 7.71 (q, J = 3.5 Hz, 3H), 7.43 (dd, J = 8.9, 2.8 Hz, 1H), 6.90 (d, J = 2.8 Hz, 1H), 6.86–6.77 (m, 2H), 6.75 (d, J = 8.8 Hz, 1H), 2.13 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.75, 155.39, 153.00, 147.31, 131.94, 130.07, 127.62, 126.39, 126.18, 122.82, 119.08, 118.75, 115.87, 16.53. HRMS [M + H]+ (C14H13ClNO3): Calculated: 278.0506; Found: 278.0578.
5-Chloro-2-((6-hydroxy-[1,1′-biphenyl]-3-yl)oxy)benzamide (15h): 56.9%; m.p. 200–202 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.65 (s, 1H), 7.86–7.64 (m, 3H), 7.58 (d, J = 7.1 Hz, 2H), 7.47–7.38 (m, 3H), 7.32 (t, J = 7.3 Hz, 1H), 7.09 (d, J = 2.8 Hz, 1H), 7.00 (qd, J = 8.7, 2.8 Hz, 2H), 6.87 (d, J = 8.9 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 165.87, 155.03, 151.81, 148.08, 138.18, 131.93, 130.03, 129.54, 129.33, 128.50, 128.15, 127.43, 126.62, 122.31, 120.58, 119.35, 117.64. HRMS [M + H]+ (C14H15ClNO3): Calculated: 340.0662; Found: 340.0727.
5-Fluoro-2-(4-hydroxyphenoxy)benzamide (15i): 89.5%; m.p. 193–195 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.41 (s, 1H), 7.72 (s, 2H), 7.51 (dd, J = 9.0, 3.3 Hz, 1H), 7.26 (ddd, J = 9.1, 7.8, 3.3 Hz, 1H), 6.97–6.90 (m, 2H), 6.80 (dt, J = 9.0, 2.3 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.80, 158.45, 156.55, 154.60, 152.36, 148.39, 127.93, 127.88, 121.24, 119.72, 119.65, 119.06, 118.87, 116.87, 116.76, 116.67. HRMS [M + H]+ (C13H11FNO3): Calculated: 248.0645; Found: 248.0713.
5-Fluoro-2-(4-hydroxy-3-methylphenoxy)benzamide (15j): 75.6%; m.p. 221–223 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.29 (s, 1H), 7.70 (d, J = 12.4 Hz, 2H), 7.52 (dd, J = 9.1, 3.3 Hz, 1H), 7.26 (td, J = 8.4, 3.3 Hz, 1H), 6.86 (d, J = 2.9 Hz, 1H), 6.84–6.78 (m, 2H), 6.75 (dd, J = 8.7, 2.9 Hz, 1H), 2.12 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.75, 165.69, 158.40, 156.50, 152.68, 152.49, 148.10, 127.63, 126.08, 122.33, 119.74, 119.68, 119.12, 118.93, 118.20, 116.85, 116.65, 115.82, 16.53. HRMS [M + H]+ (C14H13FNO3): Calculated: 262.0801; Found: 262.0870.
5-Fluoro-2-((6-hydroxy-[1,1′-biphenyl]-3-yl)oxy)benzamide (15k): 61.2%; m.p. 196–198 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.59 (s, 1H), 7.75 (d, J = 11.2 Hz, 2H), 7.60–7.55 (m, 2H), 7.51 (dd, J = 9.0, 3.3 Hz, 1H), 7.41 (t, J = 7.7 Hz, 2H), 7.35–7.25 (m, 2H), 7.05 (d, J = 2.9 Hz, 1H), 6.99 (d, J = 8.7 Hz, 1H), 6.96–6.91 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.89, 158.56, 156.65, 152.07, 151.46, 148.87, 138.26, 129.53, 129.24, 128.50, 127.39, 121.74, 119.99, 119.07, 118.88, 117.57, 116.85, 116.66. HRMS [M + H]+ (C19H15FNO3): Calculated: 324.0958; Found: 324.1016.
N-Butyl-2-(4-hydroxyphenoxy)benzamide (15l): 66.7%; m.p. 141–144 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.39 (s, 1H), 8.16 (t, J = 5.7 Hz, 1H), 7.66 (dd, J = 7.7, 1.8 Hz, 1H), 7.38 (ddd, J = 8.3, 7.3, 1.8 Hz, 1H), 7.13 (td, J = 7.5, 1.1 Hz, 1H), 6.97–6.90 (m, 2H), 6.84–6.78 (m, 2H), 6.76 (dd, J = 8.3, 1.1 Hz, 1H), 3.26 (q, J = 6.6 Hz, 2H), 1.49–1.43(m, 2H), 1.34–1.26 (m, 2H), 0.87 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.55, 155.93, 154.56, 148.13, 131.90, 130.50, 127.07, 122.83, 121.44, 117.42, 116.69, 39.18, 31.65, 20.03, 14.17. HRMS [M + H]+ (C17H20NO3): Calculated: 286.1365; Found: 286.1436.
2-(4-Hydroxyphenoxy)-N-methyl-N-phenylbenzamide (15m): 45.1%; m.p. 168–170 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.39 (s, 1H), 7.36–7.08 (m, 7H), 6.92 (d, J = 7.6 Hz, 1H), 6.76 (d, J = 8.3 Hz, 2H), 6.59 (d, J = 7.9 Hz, 2H), 6.37 (d, J = 8.4 Hz, 1H), 3.38 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 167.78, 154.59, 154.46, 147.42, 144.04, 130.54, 129.60, 129.20, 128.09, 127.36, 127.04, 121.99, 121.68, 116.66, 115.35, 37.14. HRMS [M + H]+ (C20H18NO3): Calculated: 320.1208; Found: 320.1278.
N-Cyclohexyl-2-(4-hydroxyphenoxy)benzamide (15n): 55.6%; m.p. 168–170 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.37 (s, 1H), 8.00 (d, J = 7.9 Hz, 1H), 7.65 (dd, J = 7.7, 1.8 Hz, 1H), 7.39 (ddd, J = 8.6, 7.4, 1.8 Hz, 1H), 7.14 (td, J = 7.5, 1.1 Hz, 1H), 6.95–6.91 (m, 2H), 6.82–6.76 (m, 3H), 3.79–3.71 (m, 1H), 1.80–1.76(m, 2H), 1.68–1.64(m, 2H), 1.58–1.50 (m, 1H), 1.33–1.23 (m, 4H), 1.19–1.13 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.67, 155.76, 154.49, 148.28, 131.91, 130.47, 127.31, 123.00, 121.16, 117.76, 116.71, 48.24, 32.66, 25.68, 24.84. HRMS [M + H]+ (C19H22NO3): Calculated: 312.1521; Found: 312.1578.
(2-(4-Hydroxyphenoxy)phenyl)(pyrrolidin-1-yl)methanone (15o): 58.5%; m.p. 205–207 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.38 (s, 1H), 7.37–7.28 (m, 2H), 7.11 (td, J = 7.5, 1.0 Hz, 1H), 6.92–6.85 (m, 2H), 6.82–6.73 (m, 3H), 3.42 (t, J = 6.8 Hz, 2H), 3.26 (t, J = 6.5 Hz, 2H), 1.88–1.76 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ 166.24, 154.53, 148.09, 130.71, 129.23, 128.58, 122.97, 121.48, 116.99, 116.72, 47.84, 45.64, 25.95, 24.51. HRMS [M + H]+ (C17H18NO3): Calculated: 284.1208; Found: 284.1275.
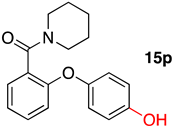
(2-(4-Hydroxyphenoxy)phenyl)(piperidin-1-yl)methanone (15p): 20.7%; m.p. 174–176 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.39 (s, 1H), 7.32 (ddd, J = 8.6, 7.4, 1.8 Hz, 1H), 7.28 (dd, J = 7.6, 1.8 Hz, 1H), 7.11 (td, J = 7.4, 1.0 Hz, 1H), 6.92–6.85 (m, 2H), 6.83–6.76 (m, 2H), 6.72 (dd, J = 8.3, 1.0 Hz, 1H), 3.58 (m, 2H), 3.23 (q, J = 6.3 Hz, 2H), 1.59 (m, 2H), 1.55–1.38 (m, 4H). 13C NMR (126 MHz, DMSO-d6) δ 166.20, 154.54, 154.47, 148.05, 130.53, 128.55, 128.06, 122.99, 121.43, 116.78, 116.55, 47.85, 42.18, 26.49, 25.80, 24.50. HRMS [M + H]+ (C18H20NO3): Calculated: 298.1416; Found: 298.1426.
N-Butyl-2-(4-hydroxy-3-methylphenoxy)-5-methylbenzamide (15q): 50.5%; m.p. 124–126 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.22 (s, 1H), 8.09 (t, J = 5.7 Hz, 1H), 7.48 (d, J = 2.3 Hz, 1H), 7.19 (dd, J = 8.5, 2.4 Hz, 1H), 6.82–6.75 (m, 2H), 6.73–6.66 (m, 2H), 3.24 (q, J = 6.8 Hz, 2H), 2.29 (s, 3H), 2.11 (s, 3H), 1.47–1.41 (m, 2H), 1.32–1.24 (m, 2H), 0.86 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.49, 153.69, 152.33, 148.45, 132.39, 131.98, 130.74, 126.72, 125.83, 122.04, 118.12, 117.89, 115.69, 39.16, 31.67, 20.55, 20.03, 16.56, 14.17. HRMS [M + H]+ (C19H24NO3): Calculated: 314.1729; Found: 314.1751.
N-Cyclohexyl-5-fluoro-2-(4-hydroxyphenoxy)benzamide (15r): 42.3%; m.p. 178–180 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.36 (s, 1H), 8.09 (d, J = 7.9 Hz, 1H), 7.41 (dd, J = 8.9, 3.3 Hz, 1H), 7.26 (ddd, J = 9.1, 7.9, 3.3 Hz, 1H), 6.93–6.88 (m, 2H), 6.86 (dd, J = 9.1, 4.5 Hz, 1H), 6.81–6.75 (m, 2H), 3.78–3.67 (m, 1H), 1.80–1.72 (m, 2H), 1.68–1.63 (m, 2H), 1.54 (dt, J = 8.8, 3.8 Hz, 1H), 1.34–1.19 (m, 4H), 1.18–1.08 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 163.42, 158.66, 156.75, 154.37, 151.67, 151.65, 148.89, 129.26, 129.20, 120.61, 120.39, 120.32, 118.56, 118.37, 116.69, 116.58, 116.39, 48.38, 32.55, 25.65, 24.81. HRMS [M + H]+ (C19H21FNO3): Calculated: 330.1427; Found: 330.1493.