α-Tocopherol and Trolox as Effective Natural Additives for Polyurethane Foams: A DFT and Experimental Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Computational Results
2.1.1. Optimized Geometries
2.1.2. Antioxidant Capability and Scavenging Mechanisms
2.1.3. Quantum Theory of Atoms in Molecules (QTAIM)
2.2. Experimental Results
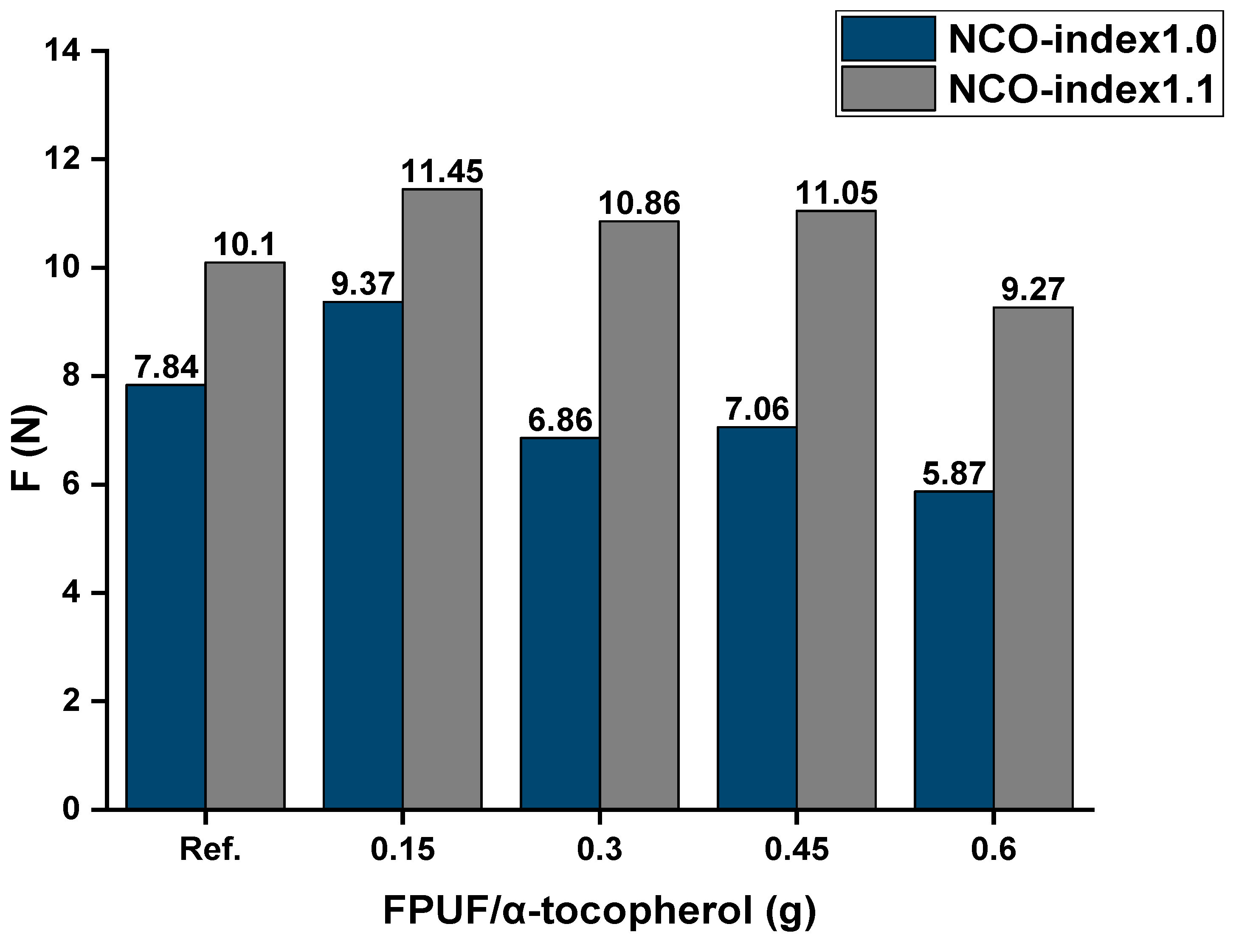
Mechanical Tests
3. Computational Details
3.1. Methods
3.2. Antioxidant Mechanism
4. Experimental Details
4.1. Preparation of Flexible Polyurethane Foam and α-Tocopherol as Additives (FPUF/α-Tocopherol)
4.2. Mechanical Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.U.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Cieślik, E.; Topolska, K. The sources of natural antioxidants. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–17. [Google Scholar]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, L.; Zhang, Y.; Sun, C.; Huang, Z. Enhancing the antioxidant potential of ESIPT-based naringenin flavonoids based on excited state hydrogen bond dynamics: A theoretical study. J. Photochem. Photobiol. B Biol. 2024, 258, 112996. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, L.; Zhang, Y.; Sun, C. Relationship between antioxidant activity and ESIPT process based on flavonoid derivatives: A comprehensive analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 327, 125370. [Google Scholar] [CrossRef]
- Lau, C.H.; Gan, S.; Lau, H.L.N.; Lee, L.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K. Insights into the effectiveness of synthetic and natural additives in improving biodiesel oxidation stability. Sustain. Energy Technol. Assess. 2022, 52, 102296. [Google Scholar] [CrossRef]
- Setiadi, D.H.; Chass, G.A.; Torday, L.L.; Varro, A.; Papp, J.G. Vitamin E models. Shortened sidechain models of α, β, γ and δ tocopherol and tocotrienol—A density functional study. J. Mol. Struct. THEOCHEM 2003, 637, 11–26. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F.C. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Tomassi, G.; Silano, V. An assessment of the safety of tocopherols as food additives. Food Chem. Toxicol. 1986, 24, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of tocopherol-rich extract (E 306), α-tocopherol (E 307), γ-tocopherol (E 308) and δ-tocopherol (E 309) as food additives. EFSA J. 2015, 13, 4247. [Google Scholar]
- Poljšak, B.; Gazdag, Z.; Pesti, M.; Filipič, M.; Fujs, S.; Farkas, N.; Plesničar, S.; Raspor, P. Role of the vitamin E model compound Trolox in the prevention of Cr (VI)-induced cellular damage. Toxicol. Environ. Chem. 2006, 88, 141–157. [Google Scholar] [CrossRef]
- Boulebd, H. Comparative study of the radical scavenging behavior of ascorbic acid, BHT, BHA and Trolox: Experimental and theoretical study. J. Mol. Struct. 2020, 1201, 127210. [Google Scholar] [CrossRef]
- Ross, L.; Barclay, C.; Artz, J.D.; Mowat, J.J. Partitioning and antioxidant action of the water-soluble antioxidant, Trolox, between the aqueous and lipid phases of phosphatidylcholine membranes: 14C tracer and product studies. Biochim. Biophys. Acta (BBA)-Biomembr. 1995, 1237, 77–85. [Google Scholar] [CrossRef]
- Marcos, B.; Sárraga, C.; Castellari, M.; Kappen, F.; Schennink, G.; Arnau, J. Development of biodegradable films with antioxidant properties based on polyesters containing α-tocopherol and olive leaf extract for food packaging applications. Food Packag. Shelf Life 2014, 1, 140–150. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Issenhuth, S.; Burdick, D. The antioxidant role of vitamin E in polymers V. Separation of stereoisomers and characterisation of other oxidation products of dl-α-tocopherol formed in polyolefins during melt processing. Polym. Degrad. Stab. 2001, 73, 491–503. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer additives. Phys. Sci. Rev. 2017, 2, 20160130. [Google Scholar]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956. [Google Scholar] [CrossRef]
- Malewska, E.; Pulit-Prociak, J.; Zielina, M.; Matyjasik, W.; Hodacka, G.; Prociak, T.; Banach, M.; Kurańska, M.; Prociak, A. New thermal insulating polyurethane biofoams based on cherry seed oil. Clean Technol. Environ. Policy 2024, 26, 1–19. [Google Scholar] [CrossRef]
- Suleman, S.; Khan, S.M.; Gull, N.; Aleem, W.; Shafiq, M.; Jamil, T. A comprehensive short review on polyurethane foam. Int. J. Innov. Sci. Res 2014, 12, 165–169. [Google Scholar]
- Rohman, R.; Nath, R.; Kar, R. Revisiting the hydrogen atom transfer reactions through a simple and accurate theoretical model: Role of hydrogen bond energy in polyphenolic antioxidants. Comput. Theor. Chem. 2023, 1223, 114097. [Google Scholar] [CrossRef]
- Thbayh, D.K.; Reizer, E.; Kahaly, M.U.; Viskolcz, B.; Fiser, B. Antioxidant potential of santowhite as synthetic and ascorbic acid as natural polymer additives. Polymers 2022, 14, 3518. [Google Scholar] [CrossRef] [PubMed]
- Thbayh, D.K.; Fiser, B. Computational study of synthetic and natural polymer additives—Antioxidant potential of BHA, TBHQ, BHT, and curcumin. Polym. Degrad. Stab. 2022, 201, 109979. [Google Scholar] [CrossRef]
- Spiegel, M. Current Trends in Computational Quantum Chemistry Studies on Antioxidant Radical Scavenging Activity. J. Chem. Inf. Model. 2022, 62, 2639–2658. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Deng, G.; Chen, D.-F.; Liang, Q.; Guo, R.; Fu, Z.-M. Theoretical studies on the antioxidant activity of pinobanksin and its ester derivatives: Effects of the chain length and solvent. Food Chem. 2018, 240, 323–329. [Google Scholar] [CrossRef]
- Molski, M. Theoretical study on the radical scavenging activity of gallic acid. Heliyon 2023, 9, e12806. [Google Scholar] [CrossRef] [PubMed]
- Wayner, D.; Lusztyk, E.; Ingold, K.; Mulder, P. Application of Photoacoustic Calorimetry to the Measurement of the OH Bond Strength in Vitamin E (alpha-and delta-Tocopherol) and Related Phenolic Antioxidants (1). J. Org. Chem. 1996, 61, 6430–6433. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Klein, E.; Lukeš, V.; Ilčin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Cabiddu, S.; Fattuoni, C. Bond dissociation energies of O− H bonds in substituted phenols from equilibration studies. J. Org. Chem. 1996, 61, 9259–9263. [Google Scholar] [CrossRef]
- Wright, J.S.; Carpenter, D.J.; McKay, D.J.; Ingold, K. Theoretical calculation of substituent effects on the O− H bond strength of phenolic antioxidants related to vitamin E. J. Am. Chem. Soc. 1997, 119, 4245–4252. [Google Scholar] [CrossRef]
- Baj, A.; Cedrowski, J.; Olchowik-Grabarek, E.; Ratkiewicz, A.; Witkowski, S. Synthesis, DFT calculations, and in vitro antioxidant study on novel carba-analogs of vitamin E. Antioxidants 2019, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, P.; Ardalan, T.; Momen Heravi, M. A DFT Study on Antioxidant Activity of Trolox and Substituted Trolox and Their Radicals. Appl. Chem. 2013, 8, 45–50. [Google Scholar]
- Zuquan, J. Carbon—Hydrogen bond dissociation energies and stretching frequencies. J. Mol. Struct. THEOCHEM 1985, 123, 443–455. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Zhao, X.; Li, Y. Effects of nitro-and amino-group on the antioxidant activity of genistein: A theoretical study. Food Chem. 2019, 275, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Tsioptsias, C.; Gkouzouma, G.; Leontiadis, K.; Tsivintzelis, I. Effect of an Antioxidant and a Compatibilizer on the Mechanical Properties of Virgin and Thermally Aged Polypropylene Drawn Fibers. Textiles 2022, 2, 499–510. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision d. 01; Gaussian Inc.: Wallingford, CT, USA, 2009; Volume 201. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- de Souza, G.L.; Peterson, K.A. Benchmarking antioxidant-related properties for gallic acid through the use of DFT, MP2, CCSD, and CCSD (T) approaches. J. Phys. Chem. A 2021, 125, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Chiodo, S.G.; Russo, N.; Toscano, M. Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J. Chem. Theory Comput. 2011, 7, 4218–4233. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, W.; Wang, D.; Bian, X.; Zhang, H.; Shi, P. Reaction mechanism of ferulic acid scavenging OH and NO2 radicals: A theoretical study. Struct. Chem. 2022, 33, 641–647. [Google Scholar] [CrossRef]
- Pandithavidana, D.R.; Jayawardana, S.B. Comparative study of antioxidant potential of selected dietary vitamins; computational insights. Molecules 2019, 24, 1646. [Google Scholar] [CrossRef]
- Santos, J.L.; Kauffmann, A.C.; da Silva, S.C.; Silva, V.C.; de Souza, G.L. Probing structural properties and antioxidant activity mechanisms for eleocarpanthraquinone. J. Mol. Model. 2020, 26, 233. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.; Joubert, L. On the influence of density functional approximations on some local Bader’s atoms-in-molecules properties. J. Phys. Chem. A 2011, 115, 5505–5515. [Google Scholar] [CrossRef]
- Fradera, X.; Austen, M.A.; Bader, R.F. The Lewis model and beyond. J. Phys. Chem. A 1999, 103, 304–314. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll (Version 15.05. 18); TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Thbayh, D.K.; Rágyanszki, A.; Fiser, B. Antioxidnat potential of butylated hydroxytoludine (BHT)—A theoretical study. Mater. Sci. Eng. 2021, 46, 63–69. [Google Scholar]
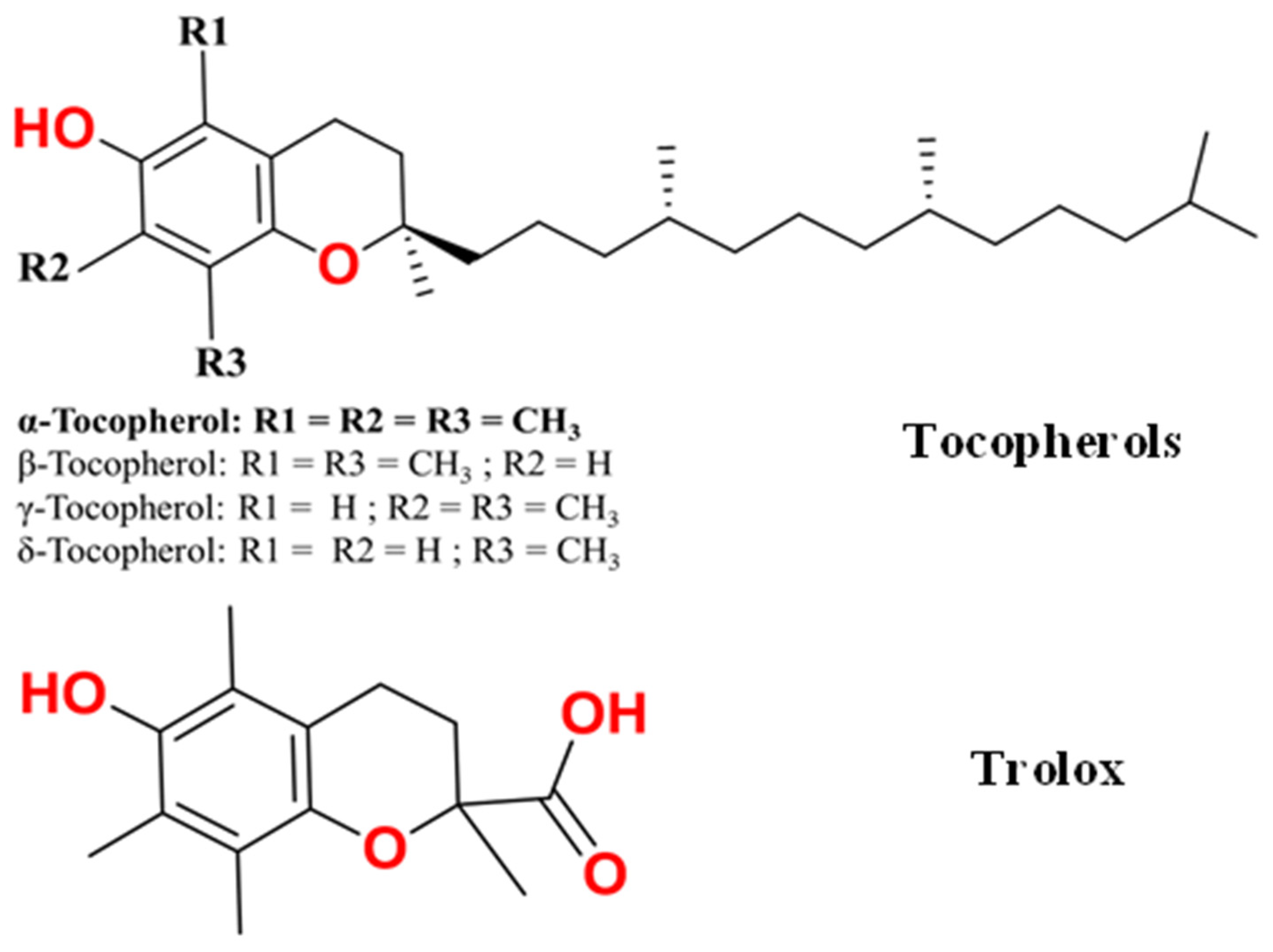
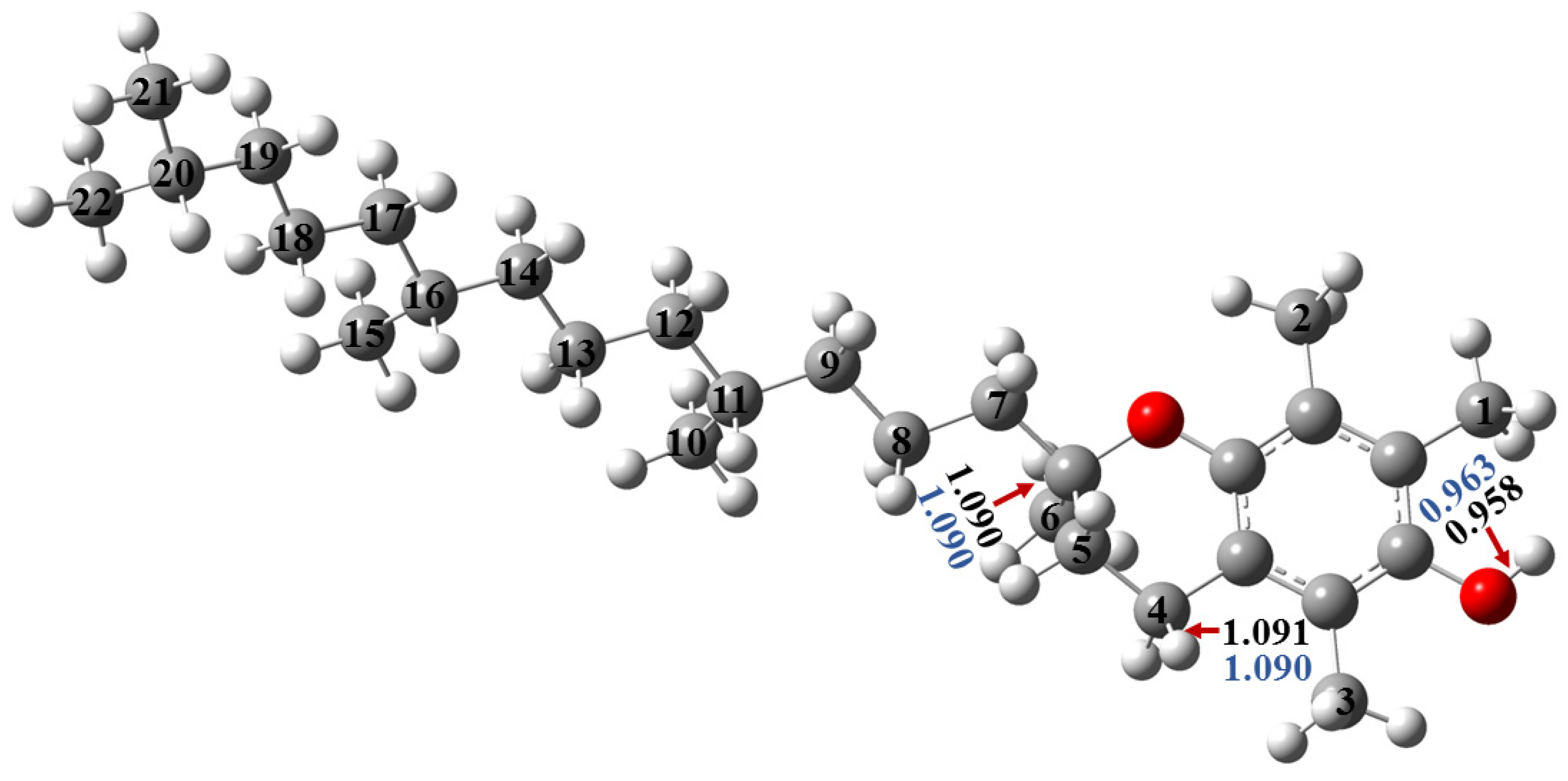
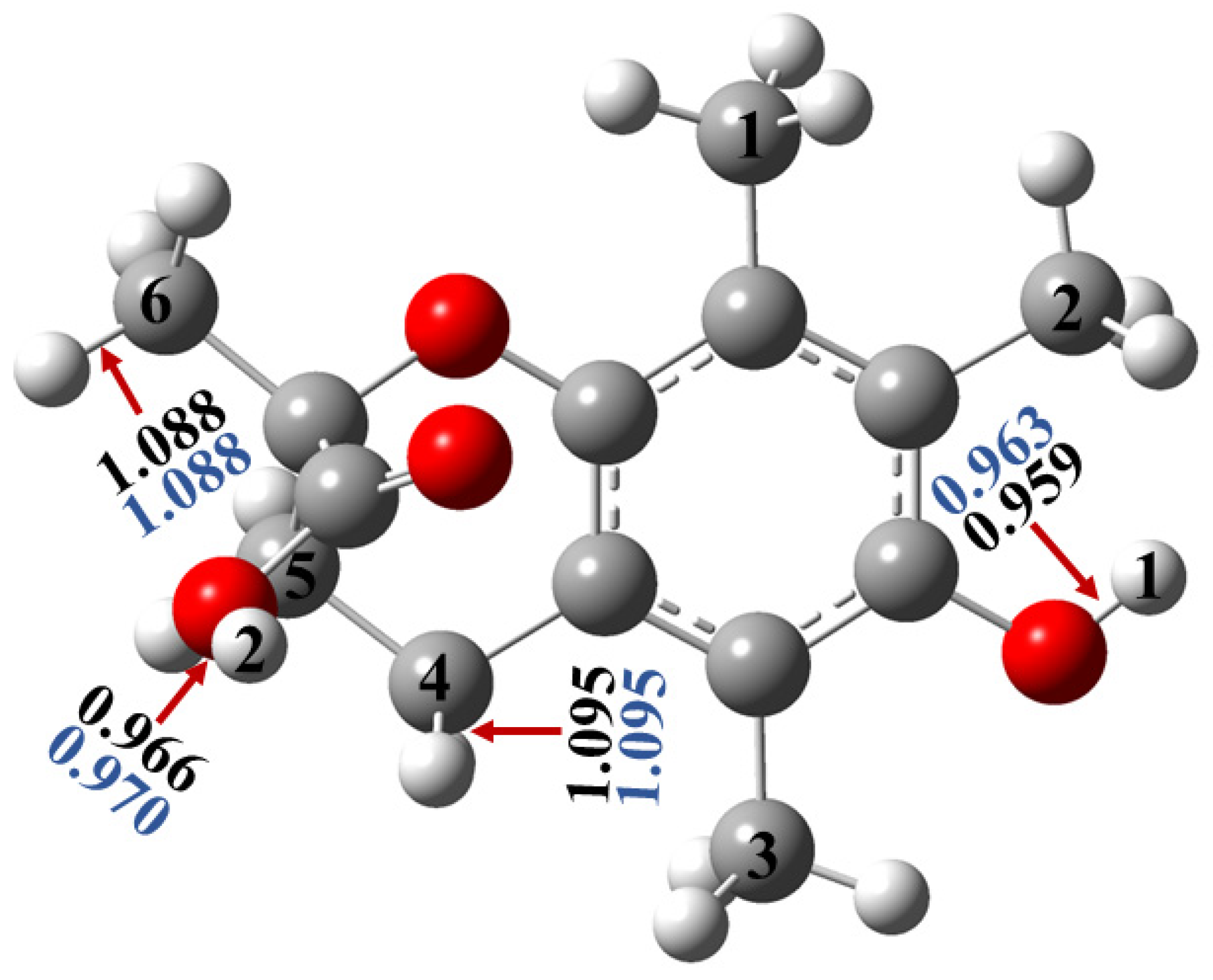
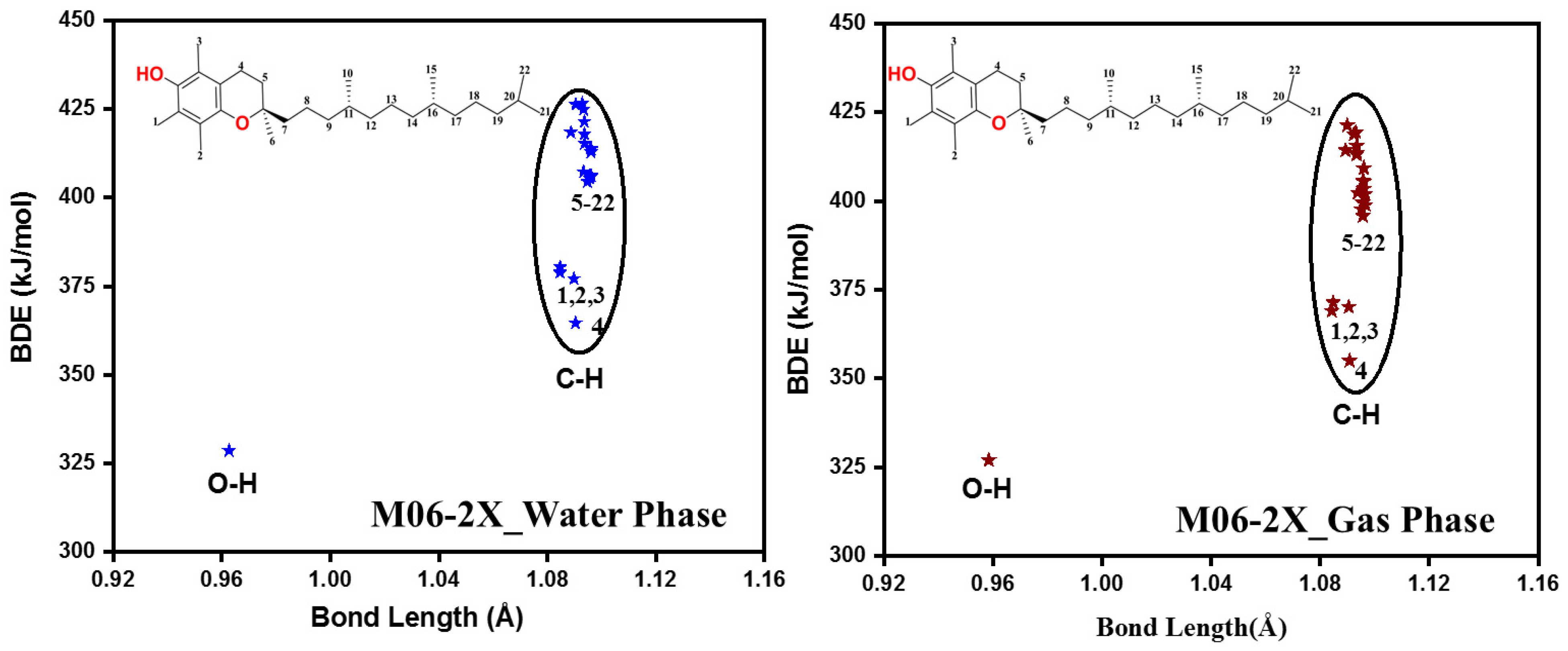
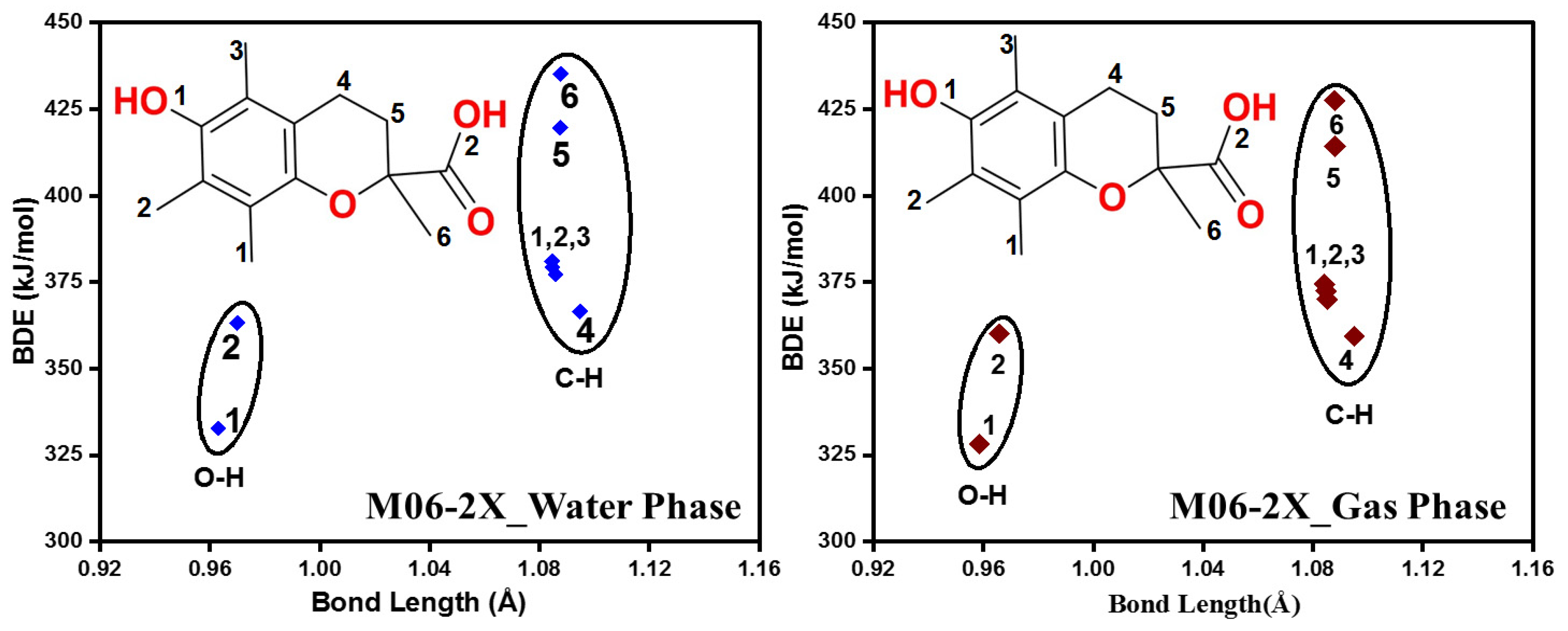
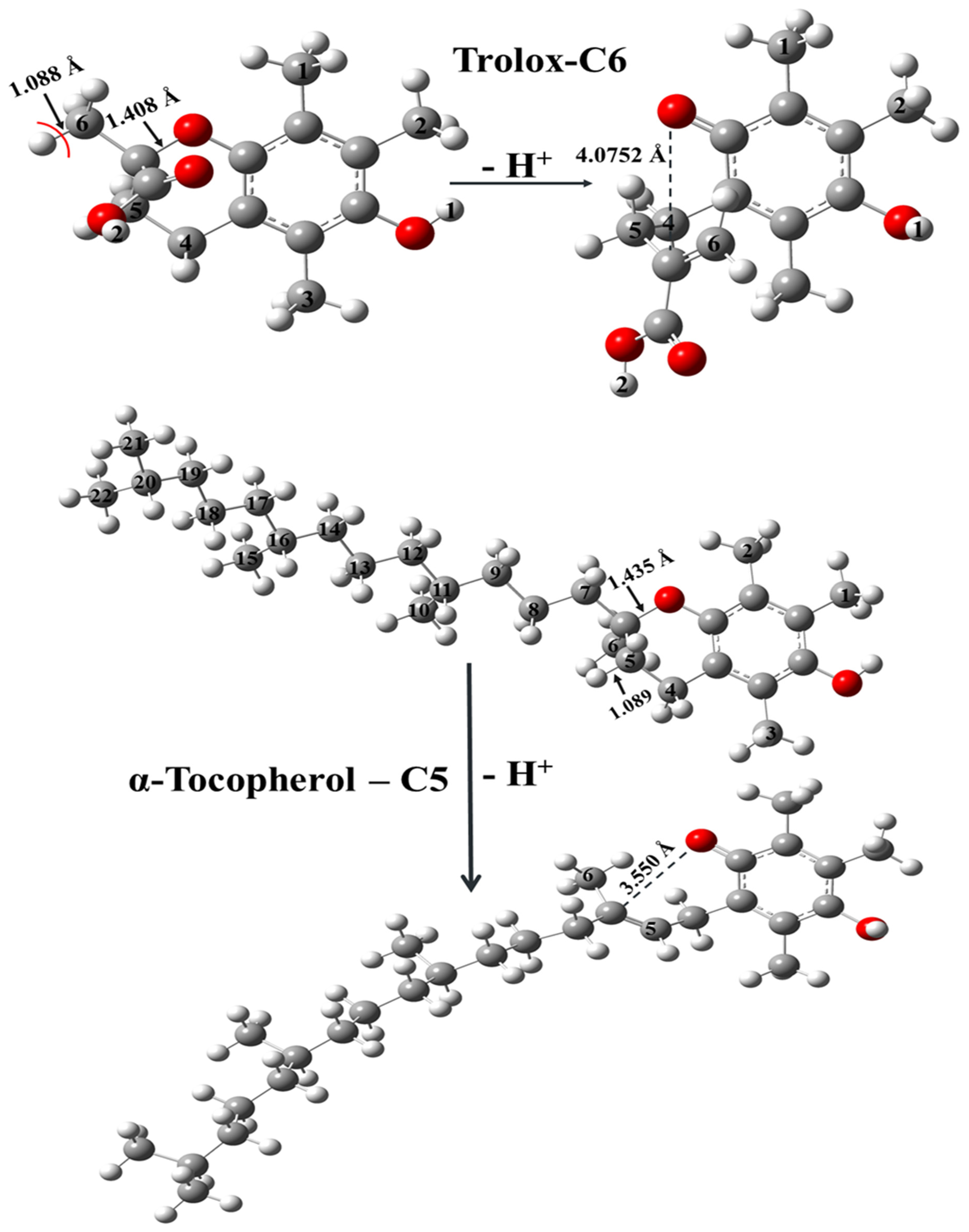
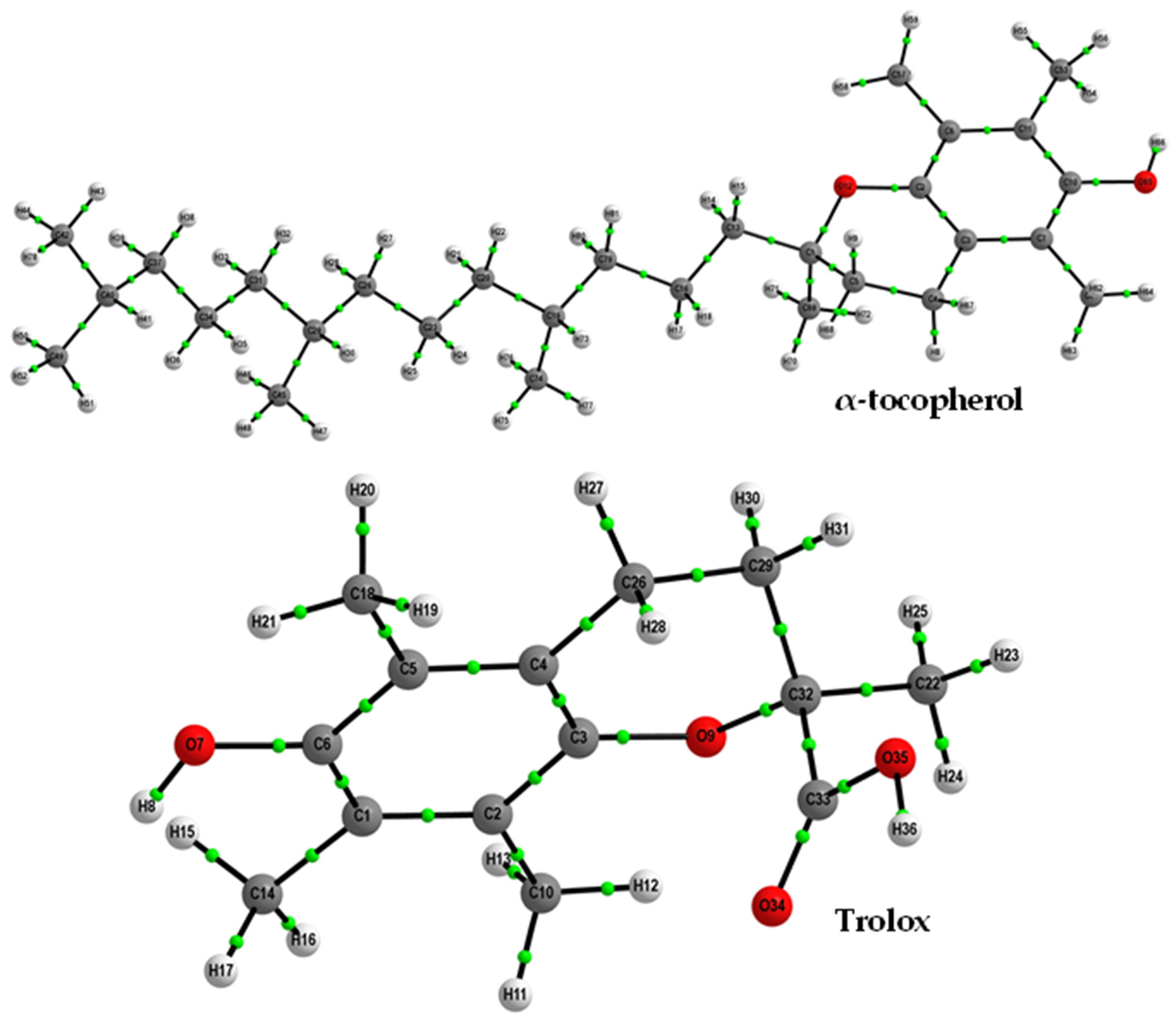
| Compound | B.L. | BDE | IP | PDE | IP + PDE | PA | ETE | PA + ETE |
|---|---|---|---|---|---|---|---|---|
| α-Tocopherol | 662.7 | |||||||
| O-H | 0.958 | 327.0 | 972.9 | 1635.6 | 1461.4 | 174.2 | 1635.6 | |
| C1-H | 1.085 | 371.4 | 1020.6 | 1683.3 | 1564.5 | 118.0 | 1682.5 | |
| C2-H | 1.084 | 369.1 | 1019.8 | 1682.5 | 1583.4 | 99.1 | 1682.5 | |
| C3-H | 1.090 | 370.2 | 1018.6 | 1681.3 | 1585.5 | 95.8 | 1681.3 | |
| C4-H | 1.091 | 355.0 | 1006.1 | 1668.8 | 1569.1 | 99.6 | 1668.8 | |
| C5-H * | 1.089 | 414.3 | - * | - * | - * | - * | - * | |
| C6-H | 1.090 | 421.3 | 1069.7 | 1732.4 | 1674.3 | 58.1 | 1732.4 | |
| C7-H | 1.093 | 413.6 | 1059.3 | 1722.0 | 1675.2 | 49.5 | 1724.7 | |
| C8-H | 1.094 | 402.4 | 1049.9 | 1712.6 | 1668.0 | 46.3 | 1714.3 | |
| C9-H | 1.096 | 405.6 | 1054.5 | 1717.2 | 1685.7 | 31.5 | 1717.2 | |
| C10-H | 1.093 | 413.2 | 1062.6 | 1725.3 | 1686.8 | 38.4 | 1725.3 | |
| C11-H | 1.097 | 398.9 | 1047.3 | 1710.0 | 1673.0 | 36.5 | 1709.5 | |
| C12-H | 1.096 | 409.2 | 1057.6 | 1720.4 | 1686.5 | 28.6 | 1715.1 | |
| C13-H | 1.096 | 395.8 | 1046.2 | 1709.0 | 1691.7 | 15.2 | 1706.9 | |
| C14-H | 1.096 | 405.5 | 1053.3 | 1716.0 | 1700.8 | 20.8 | 1721.7 | |
| C15-H | 1.093 | 415.6 | 1064.0 | 1726.7 | 1694.0 | 31.5 | 1725.5 | |
| C16-H | 1.097 | 402.0 | 1050.4 | 1713.1 | 1683.8 | 29.4 | 1713.2 | |
| C17-H | 1.096 | 403.4 | 1051.8 | 1714.5 | 1693.7 | 20.2 | 1713.8 | |
| C18-H | 1.096 | 399.6 | 1047.9 | 1710.6 | 1696.7 | 13.8 | 1710.5 | |
| C19-H | 1.096 | 409.3 | 1053.6 | 1716.3 | 1700.3 | 15.9 | 1716.2 | |
| C20-H | 1.095 | 397.7 | 1046.1 | 1708.8 | 1697.7 | 11.2 | 1708.9 | |
| C21-H | 1.092 | 418.8 | 1067.1 | 1729.8 | 1713.9 | 15.9 | 1729.9 | |
| C22-H | 1.093 | 419.4 | 1068.3 | 1731.0 | 1707.0 | 25.6 | 1732.7 |
| Compound | B.L | BDE | IP | PDE | IP + PDE | PA | ETE | PA + ETE |
|---|---|---|---|---|---|---|---|---|
| α-Tocopherol | 423.6 | |||||||
| O-H | 0.963 | 328.6 | 63.7 | 487.3 | 174.1 | 312.5 | 486.5 | |
| C1-H | 1.085 | 380.4 | 110.6 | 534.2 | 310.8 | 229.0 | 539.8 | |
| C2-H | 1.085 | 378.9 | 114.6 | 538.2 | 319.7 | 218.5 | 538.2 | |
| C3-H | 1.090 | 377.1 | 113.5 | 537.1 | 319.8 | 216.8 | 536.7 | |
| C4-H | 1.090 | 364.6 | 100.6 | 524.2 | 323.8 | 197.8 | 521.6 | |
| C5-H | 1.089 | 418.5 | 149.5 | 573.1 | 383.6 | 191.7 | 575.3 | |
| C6-H | 1.090 | 426.3 | 162.2 | 585.9 | 380.7 | 205.1 | 585.9 | |
| C7-H | 1.094 | 415.3 | 151.0 | 574.6 | 397.3 | 177.6 | 574.9 | |
| C8-H | 1.093 | 407.3 | 140.6 | 564.3 | 406.0 | 160.6 | 566.6 | |
| C9-H | 1.096 | 413.9 | 149.4 | 573.1 | 405.9 | 167.5 | 573.4 | |
| C10-H | 1.094 | 421.4 | 157.4 | 581.0 | 390.6 | 187.9 | 578.5 | |
| C11-H | 1.096 | 406.3 | 142.2 | 565.8 | 411.7 | 148.4 | 560.1 | |
| C12-H | 1.096 | 412.9 | 148.7 | 572.3 | 407.3 | 165.0 | 572.3 | |
| C13-H | 1.095 | 405.5 | 141.4 | 565.0 | 409.7 | 155.3 | 565.0 | |
| C14-H | 1.096 | 413.1 | 149.0 | 572.6 | 407.7 | 164.9 | 572.6 | |
| C15-H | 1.094 | 418.0 | 156.7 | 580.3 | 393.8 | 183.8 | 577.5 | |
| C16-H | 1.096 | 406.1 | 142.0 | 565.7 | 412.3 | 153.2 | 565.5 | |
| C17-H | 1.096 | 413.0 | 148.8 | 572.4 | 404.5 | 168.0 | 572.5 | |
| C18-H | 1.096 | 406.0 | 142.1 | 565.7 | 409.9 | 155.5 | 565.4 | |
| C19-H | 1.096 | 413.7 | 149.5 | 573.2 | 409.0 | 164.3 | 573.2 | |
| C20-H | 1.095 | 404.5 | 140.3 | 564.0 | 410.2 | 153.8 | 564.0 | |
| C21-H | 1.093 | 426.5 | 162.4 | 586.1 | 397.3 | 188.8 | 586.1 | |
| C22-H | 1.093 | 424.9 | 160.8 | 584.4 | 394.8 | 189.4 | 584.2 |
| Compound | B.L | BDE | IP | PDE | IP + PDE | PA | ETE | PA + ETE |
|---|---|---|---|---|---|---|---|---|
| Trolox | 677.2 | |||||||
| O1-H | 0.959 | 328.2 | 961.9 | 1639.1 | 1460.5 | 178.8 | 1639.3 | |
| O2-H | 0.966 | 360.2 | 991.4 | 1668.6 | 1406.0 | 262.4 | 1668.4 | |
| C1-H | 1.084 | 374.5 | 1007.4 | 1684.6 | 1588.0 | 97.6 | 1685.6 | |
| C2-H | 1.085 | 372.5 | 1006.5 | 1683.7 | 1564.1 | 119.5 | 1683.6 | |
| C3-H | 1.085 | 370.1 | 1003.9 | 1681.2 | 1584.6 | 96.5 | 1681.2 | |
| C4-H | 1.095 | 359.4 | 993.3 | 1670.5 | 1566.7 | 103.8 | 1670.5 | |
| C5-H | 1.088 | 414.3 | 1048.2 | 1725.4 | 1625.2 | 100.2 | 1725.4 | |
| C6-H* | 1.088 | 427.5 | - * | - * | - * | - * | - * |
| Compound | B.L. | BDE | IP | PDE | IP + PDE | PA | ETE | PA + ETE |
|---|---|---|---|---|---|---|---|---|
| Trolox | 436.2 | |||||||
| O1-H | 0.963 | 332.8 | 56.2 | 492.3 | 171.8 | 320.6 | 492.3 | |
| O2-H | 0.970 | 363.2 | 86.6 | 522.8 | 102.1 | 420.6 | 522.8 | |
| C1-H | 1.085 | 379.4 | 103.6 | 539.8 | 315.8 | 222.9 | 538.8 | |
| C2-H | 1.085 | 381.1 | 104.4 | 540.5 | 309.9 | 230.7 | 540.6 | |
| C3-H | 1.086 | 377.2 | 100.6 | 536.8 | 315.8 | 221.0 | 536.8 | |
| C4-H | 1.095 | 366.6 | 90.0 | 526.2 | 318.0 | 208.1 | 526.2 | |
| C5-H | 1.088 | 419.7 | 143.7 | 579.8 | 363.8 | 215.4 | 579.2 | |
| C6-H | 1.088 | 435.2 | 158.6 | 594.8 | 346.1 | 248.6 | 594.7 |
| NCO Index = 1.0 | ||
|---|---|---|
| Percentage of α-Tocopherol (% wt) | F (N) | h (mm) |
| 0 | 7.84 | 34.33 |
| 0.25 | 9.37 | 34.14 |
| 0.5 | 6.86 | 34.66 |
| 0.75 | 7.06 | 35.69 |
| 1 | 5.87 | 34.67 |
| NCO Index = 1.1 | ||
| 0 | 10.10 | 34.55 |
| 0.25 | 11.45 | 35.70 |
| 0.5 | 11.05 | 36.21 |
| 0.75 | 10.86 | 35.21 |
| 1 | 9.27 | 36.63 |
| Polyol Premix Ongropur FFP-303 | Ratio pphb | ||
|---|---|---|---|
| Wanol F3160 | 100 | ||
| Water | 3.6 | ||
| Alcupol F3231 | 1 | ||
| Tegostab B4113 | 0.5 | ||
| Tegoamin DEOA 85 | 0.5 | ||
| DABCO 33LV | 0.15 | ||
| Jaffcat ZF-22 | 0.1 | ||
| NCO Index | Polyol Premix Ongropur FFP-303 (%m/m) | Isocyanate Ongronat TR4040 (%m/m) | |
| 1.0 | 63.61% | 36.39% | |
| 1.1 | 59.98% | 40.02% | |
| NCO-Index-1.0 | |||
|---|---|---|---|
| Percentage of α-Tocopherol (% wt) | Weight of α-Tocopherol [g] | Polyol Premix | Isocyanate |
| Ongropur FFP-303 [g] | Ongronat TR4040 [g] | ||
| 0 | 0 | 38.16 | 21.83 |
| 0.25 | 0.15 | 38.16 | 21.83 |
| 0.5 | 0.3 | 38.16 | 21.83 |
| 0.75 | 0.45 | 38.16 | 21.83 |
| 1 | 0.6 | 38.16 | 21.83 |
| NCO-Index-1.1 | |||
| Percentage of α-Tocopherol (% wt) | Weight of α-Tocopherol [g] | Polyol Premix | Isocyanate |
| Ongropur FFP-303 [g] | Ongronat TR4040 [g] | ||
| 0 | 0 | 35.98 | 24.01 |
| 0.25 | 0.15 | 35.98 | 24.01 |
| 0.5 | 0.3 | 35.98 | 24.01 |
| 0.75 | 0.45 | 35.98 | 24.01 |
| 1 | 0.6 | 35.98 | 24.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thbayh, D.K.; Mentes, D.; Boros, Z.R.; Palusiak, M.; Farkas, L.; Viskolcz, B.; Fiser, B. α-Tocopherol and Trolox as Effective Natural Additives for Polyurethane Foams: A DFT and Experimental Study. Molecules 2024, 29, 6037. https://doi.org/10.3390/molecules29246037
Thbayh DK, Mentes D, Boros ZR, Palusiak M, Farkas L, Viskolcz B, Fiser B. α-Tocopherol and Trolox as Effective Natural Additives for Polyurethane Foams: A DFT and Experimental Study. Molecules. 2024; 29(24):6037. https://doi.org/10.3390/molecules29246037
Chicago/Turabian StyleThbayh, Dalal K., Dóra Mentes, Zsanett R. Boros, Marcin Palusiak, László Farkas, Béla Viskolcz, and Béla Fiser. 2024. "α-Tocopherol and Trolox as Effective Natural Additives for Polyurethane Foams: A DFT and Experimental Study" Molecules 29, no. 24: 6037. https://doi.org/10.3390/molecules29246037
APA StyleThbayh, D. K., Mentes, D., Boros, Z. R., Palusiak, M., Farkas, L., Viskolcz, B., & Fiser, B. (2024). α-Tocopherol and Trolox as Effective Natural Additives for Polyurethane Foams: A DFT and Experimental Study. Molecules, 29(24), 6037. https://doi.org/10.3390/molecules29246037






