The Amination of Waste Newsprint Paper with Various Aminating Agents (Ammonia Water, Ethylenediamine, and Diethylenetriamine) to Improve the Sorption Efficiency of Anionic Dyes
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Tested Sorbents (FTIR Analysis, Specific Surface Area, and Elementary Analysis)
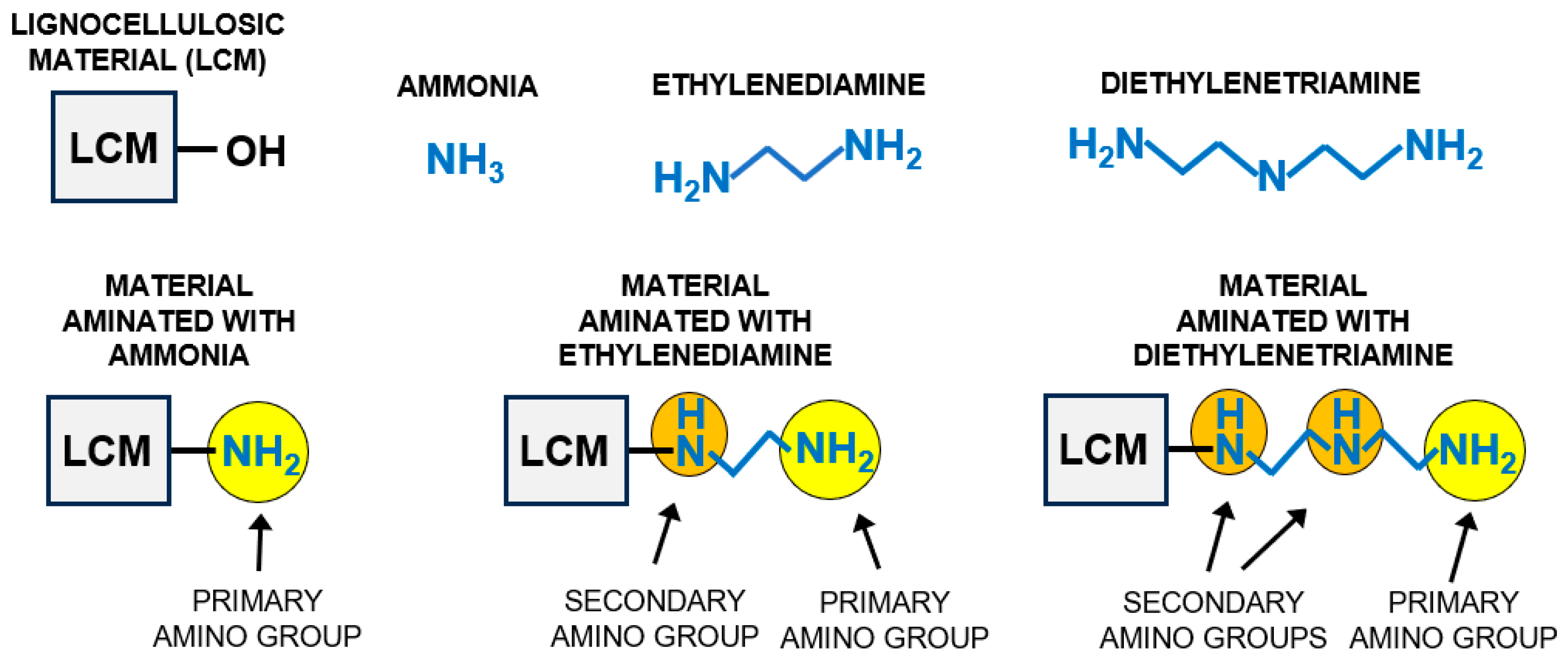
- Unmodified newsprint paper (N);
- Newsprint paper pre-activated with epichlorohydrin (NE);
- Newsprint paper pre-activated with epichlorohydrin and next aminated with ammonia water (NEN1);
- Newsprint paper pre-activated with epichlorohydrin and next aminated with ethylenediamine (NEN2);
- Newsprint paper pre-activated with epichlorohydrin and next aminated with diethylenetriamine (NEN3).
2.1.1. FTIR Analysis
2.1.2. Elemental Analysis
2.1.3. Analysis of Specific Surface Area and Porosity
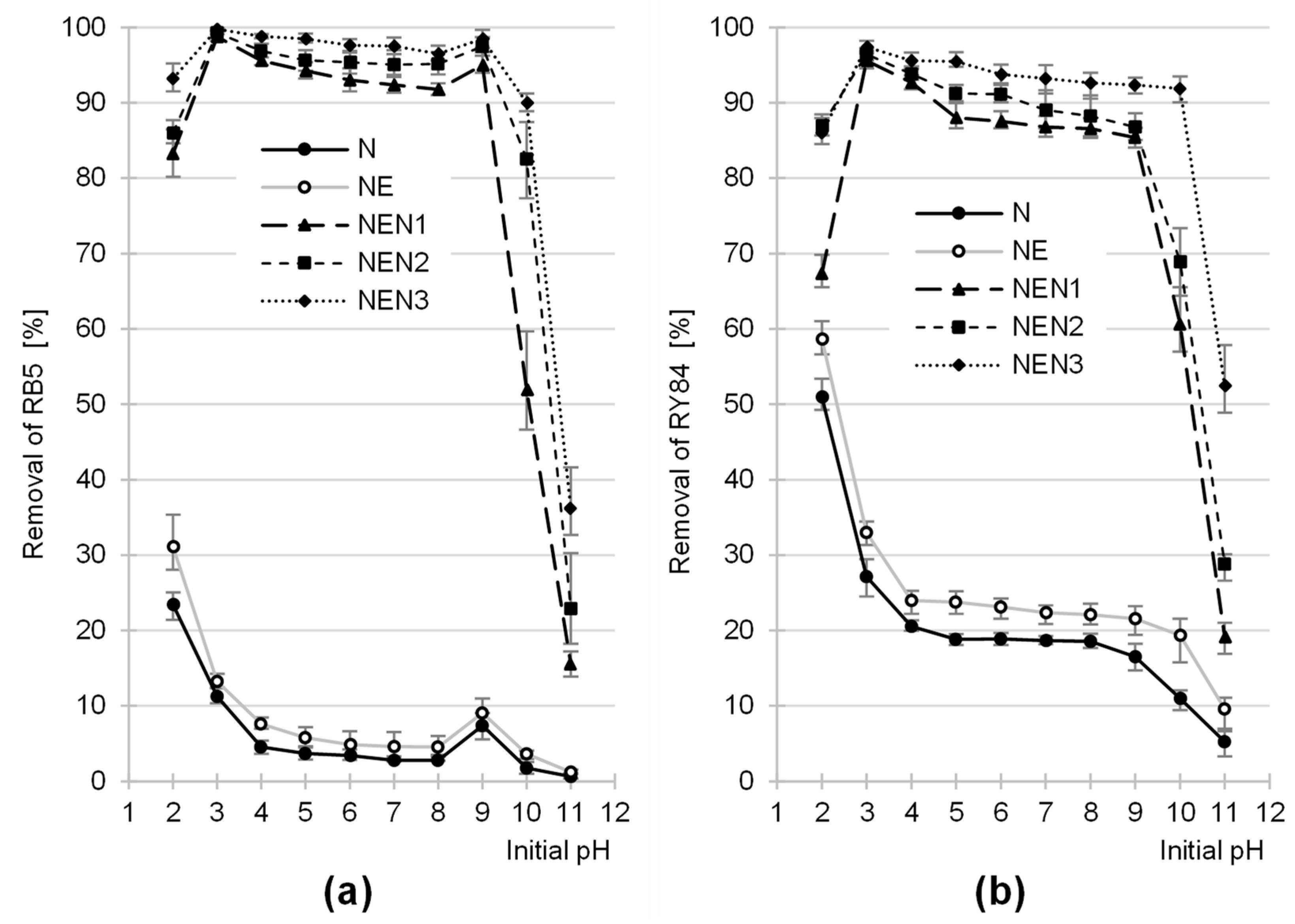
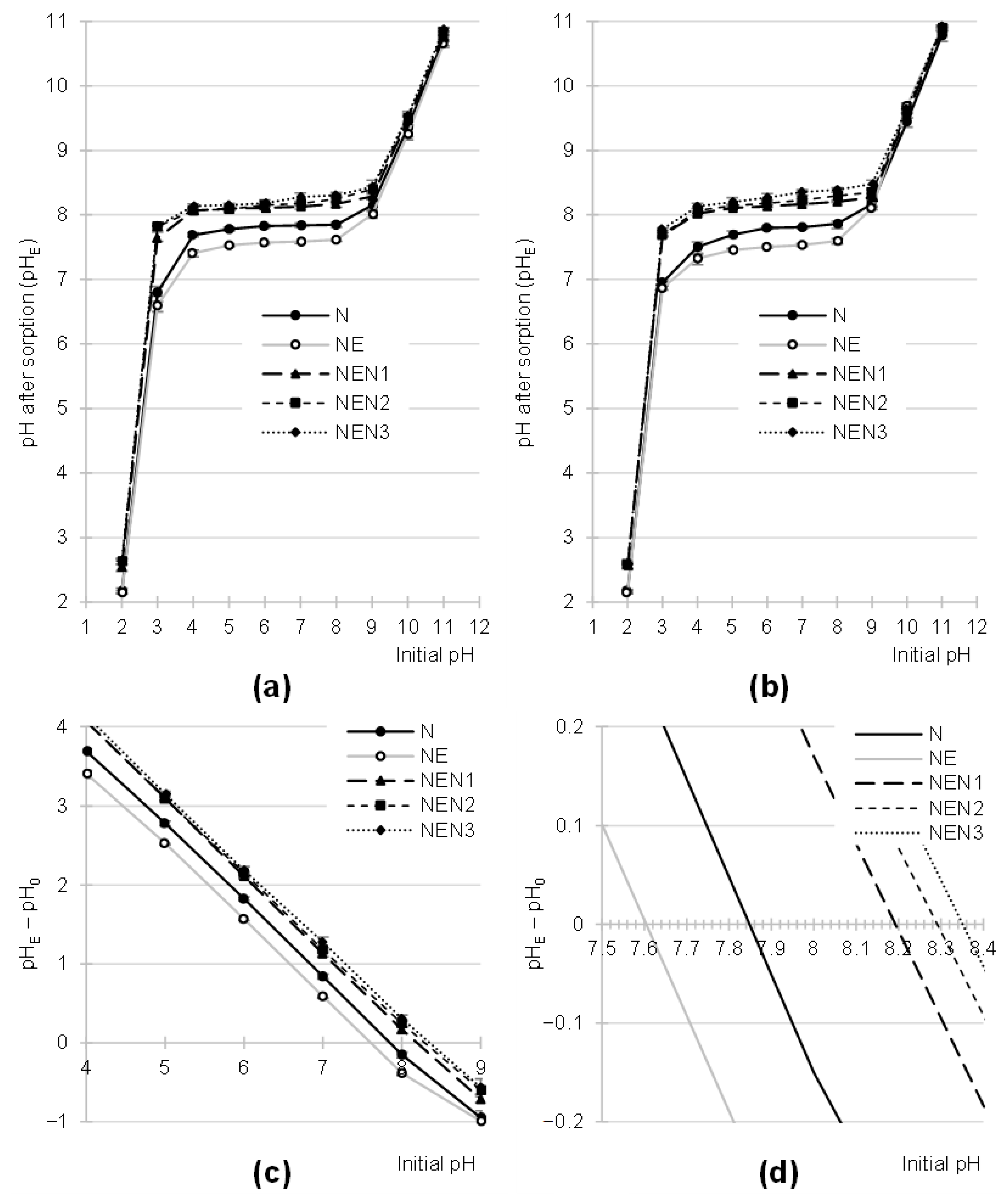
2.2. Effect of pH on the Efficiency of Dye Sorption
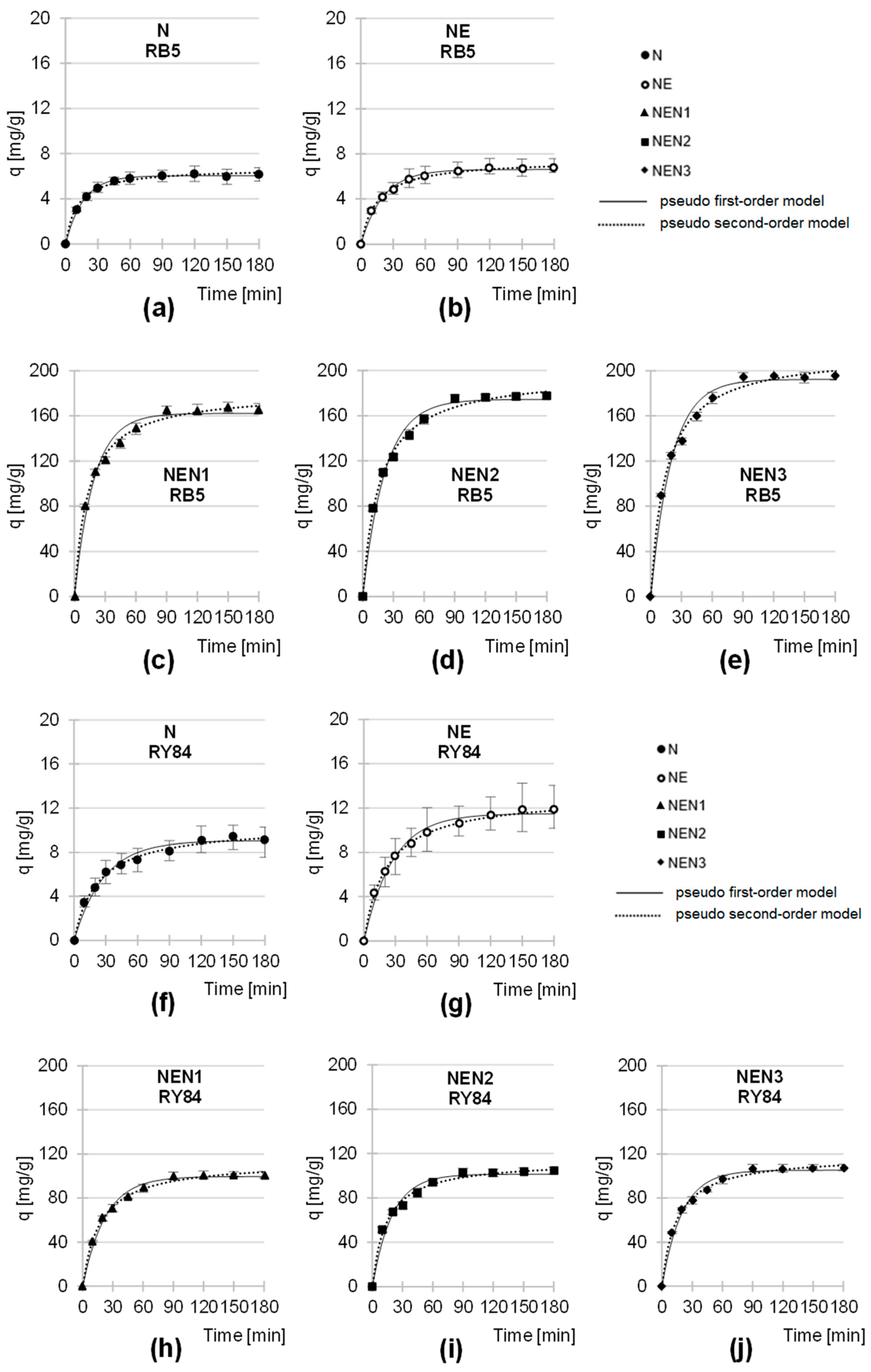
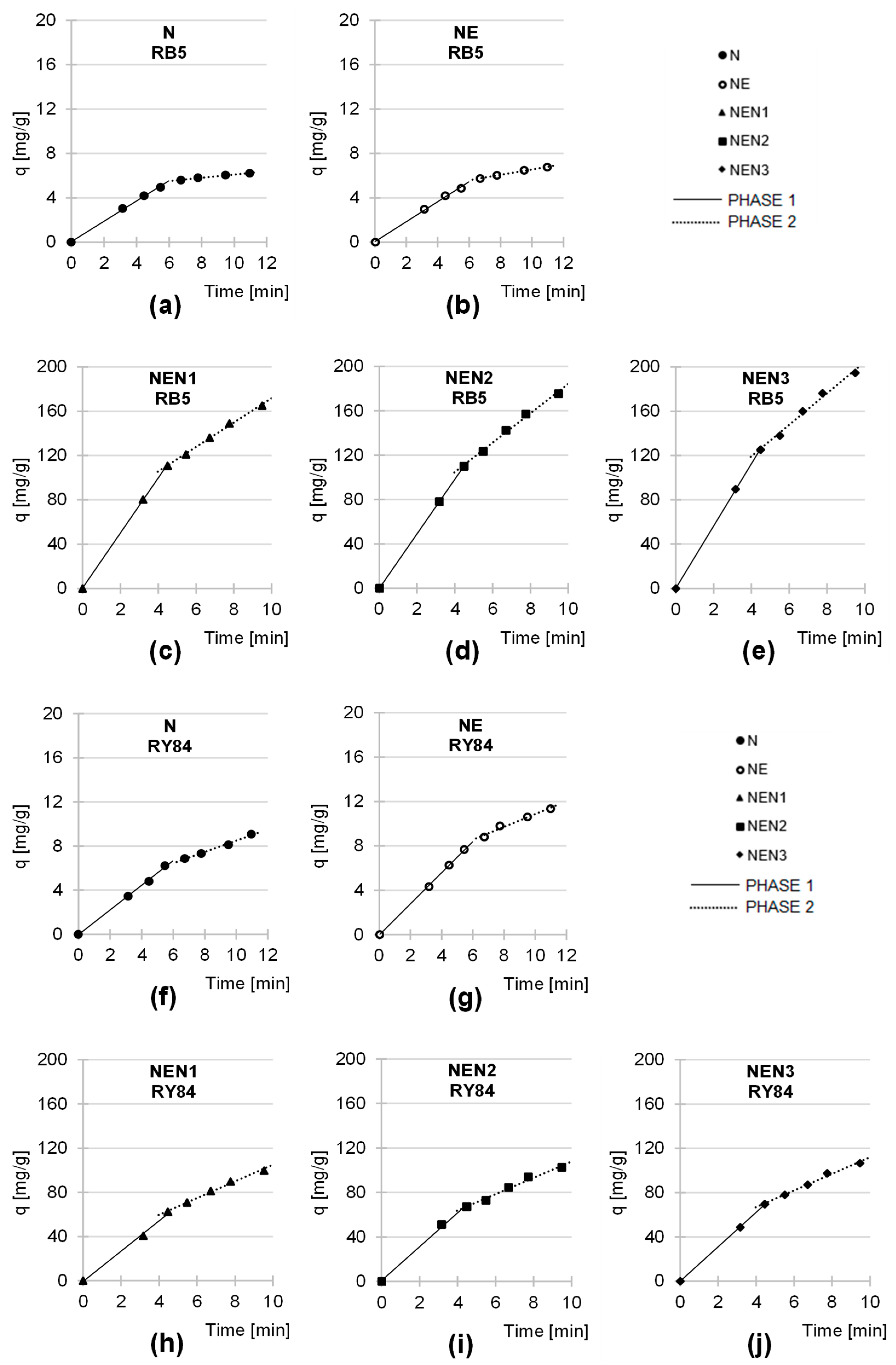
2.3. Kinetics of RB5 and RY84 Sorption onto Tested Sorbents
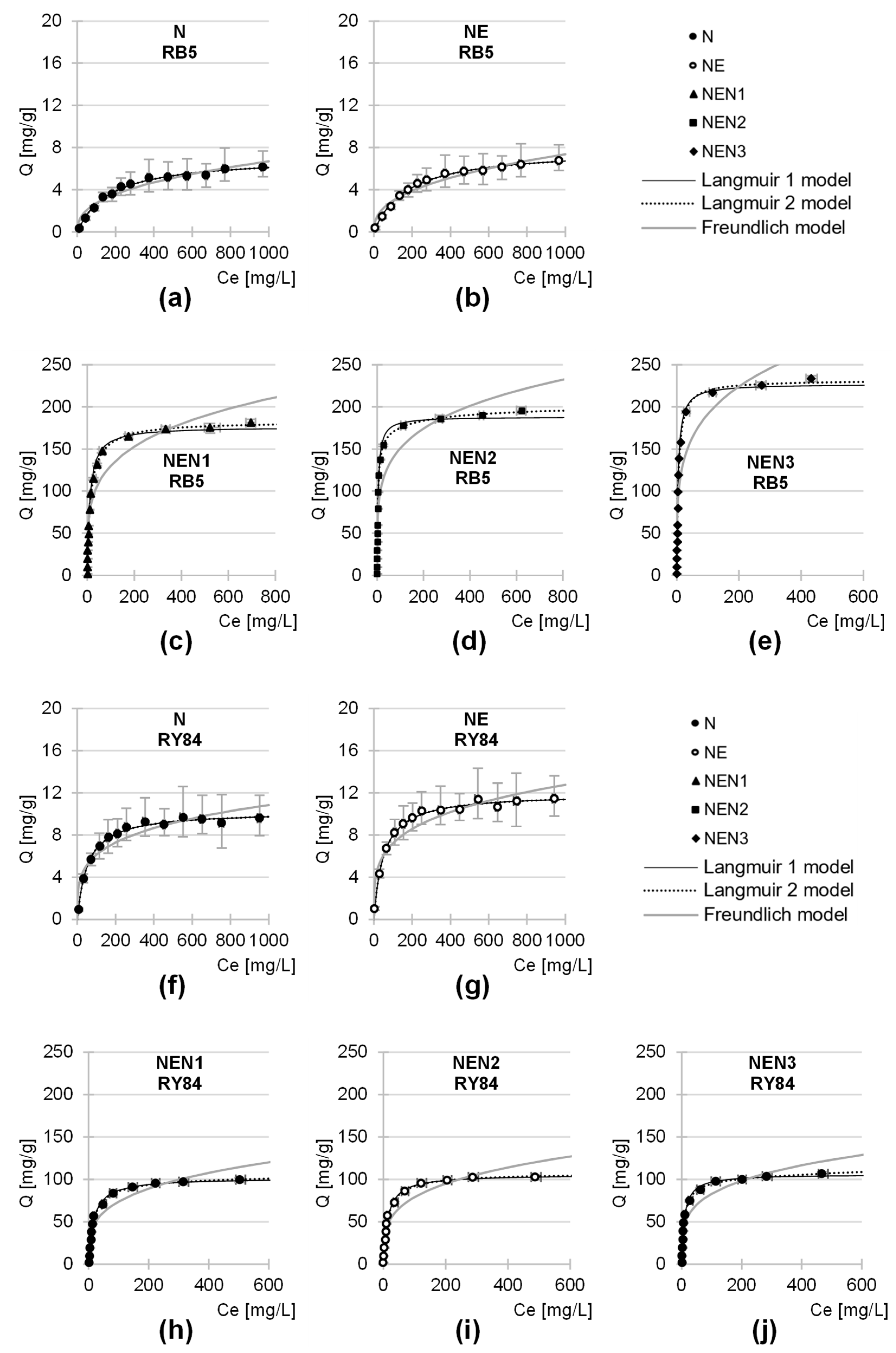
2.4. Maximum Sorption Capacity of the Tested Sorbents
3. Materials
3.1. Waste Newsprint Paper
3.2. Dyes
3.3. Chemical Reagents
3.4. Laboratory Equipment
- HI 110 pH meter (HANNA Instruments, Olsztyn, Poland)—for measuring and correcting the pH value of solutions;
- Water bath shaker type 357 (Elpin-Plus, Lubawa, Poland)—for modifying the sorbent with epichlorohydrin or aminating agents;
- Laboratory shaker SK-71 (JEIO TECH, Daejeon, Republic of Korea)—for dye sorption studies;
- Multi-Channel Stirrer MS-53M (JEIO TECH, Daejeon, Republic of Korea)—for dye sorption studies;
- UV-3100 PC spectrophotometer (VWR Spectrophotometer, VWR International LLC., Mississauga, ON, Canada)—for analyzing dye concentration in solutions;
- FT/IR-4700LE FT-IR spectrometer with single reflection ATR attachment (JASCO International, Tokyo, Japan)—for the generation of FTIR spectra of sorbents;
- FLASH 2000 Analyzer (Thermo Scientific, Waltham, MA, USA)—for elemental analysis and measurement of carbon and nitrogen contents.
- ASAP 2020 (Micromeritics, Norcross, GA, USA)—for measuring the porosity and surface area of sorbents.
4. Methods
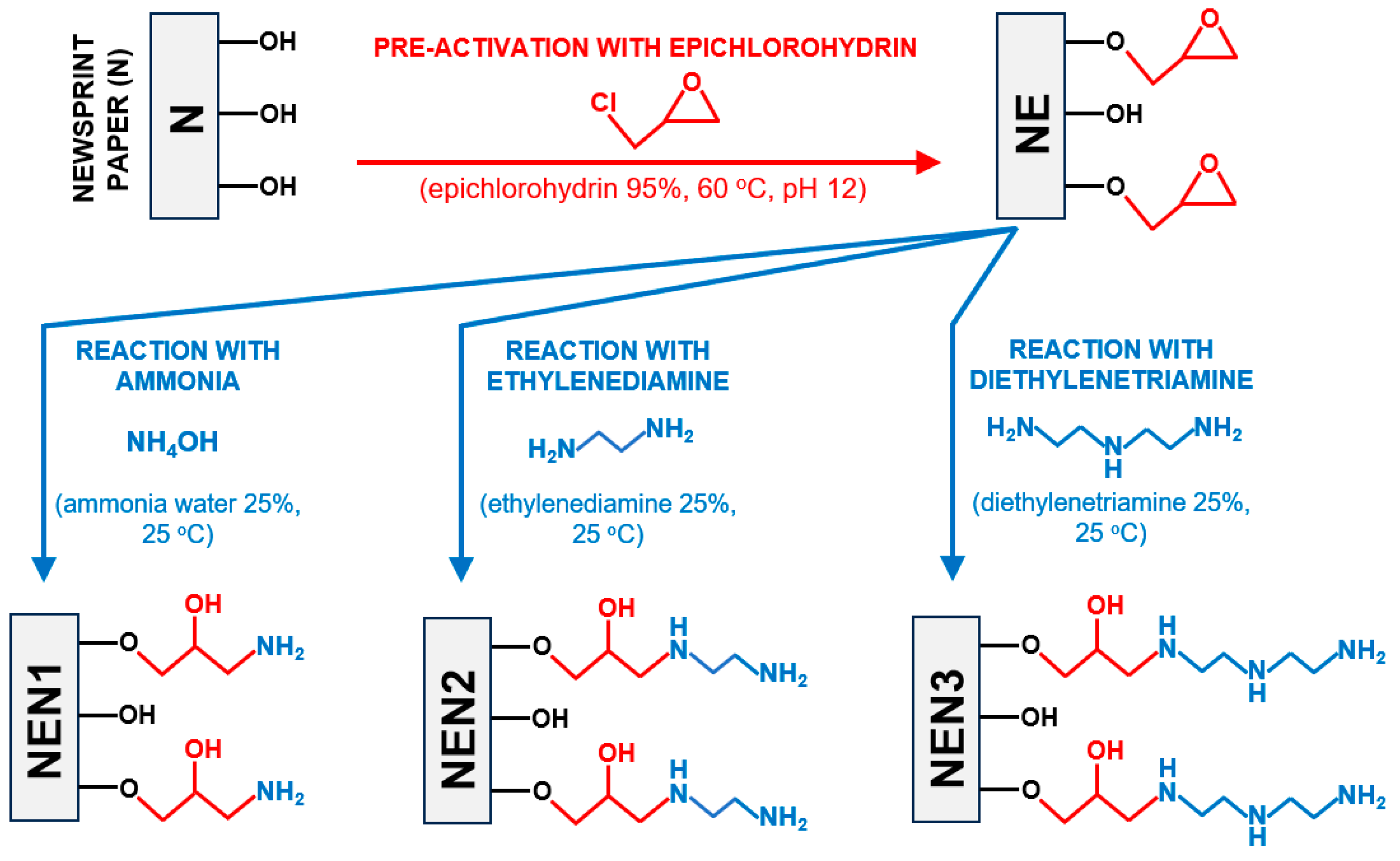
4.1. Preparation of a Sorbent Based on Waste Newsprint Paper (N)
4.2. Preparation of Newsprint Paper Modified with Epichlorohydrin (NE)
4.3. Preparation of Aminated Newsprint Paper (NEN1, NEN2, NEN3)
4.4. Studies on the Influence of pH on the Efficiency of Dye Sorption
4.5. Studies on the Kinetics of Dye Sorption
4.6. Studies on the Maximum Sorption Capacity of the Sorbents
- The sorption properties of each of the 5 sorbents tested (N, NE, NEN1, NEN2, and NEN3) were analyzed using 2 dyes (RB5 and RY84).
- All dye solutions were prepared with deionized water.
- pH values of the solutions were corrected by adding small amounts of 1 M HCl or 1 M NaOH under continuous pH measurement.
- The portions of sorbents added to the flasks or beakers were prepared on a precision balance with an accuracy of 0.001 g.
- The sorbent dose was 5.00 g/L in all test series.
- The selected mixing parameters on a multistage magnetic stirrer (200 rpm, magnetic stirrer with a Teflon coating of size 50 × 8 mm) ensured a uniform distribution of the sorbent throughout the entire volume of the solution during the process.
- The concentrations of the dyes remaining in the solution were determined spectrophotometrically using a UV-VIS spectrophotometer with a cuvette with an optical path length of 10 mm.
- The calibration curves plotted for the RB5 and RY84 dyes, entered into the spectrophotometer, enabled determinations of solutions with concentrations of 0–50 mg/L. Solutions with higher concentrations were diluted with deionized water.
- For each sorbent tested, an additional series of experiments were carried out without the dye. The aqueous solutions obtained in this way were used to calibrate the spectrophotometer before the dye concentration was analyzed. In this way, measurement errors due to possible color impurities released into the solutions by the sorbents tested could be eliminated.
- All analytical series were carried out in triplicate.
- A constant temperature of 25 °C was maintained in the laboratory during analyses.
4.7. FTIR Analysis of the Sorbents
4.8. Measurement of the Specific Surface Area and Porosity of the Sorbents
4.9. Calculation Methods
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doley, S.; Borauh, R.R.; Konwar, M. Study on Awareness of Consumers about Dye Related Problems. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1171–1176. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Paluch, D.; Bazan-Wozniak, A.; Wolski, R.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds. Molecules 2023, 28, 7786. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Paluch, D.; Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methylene Blue and Methyl Red from Aqueous Solutions Using Activated Carbons Obtained by Chemical Activation of Caraway Seed. Molecules 2023, 28, 6306. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Kyzas, G.Z.; Avranas, A.; Lazaridis, N.K. Multi-Parametric Adsorption Effects of the Reactive Dye Removal with Commercial Activated Carbons. J. Mol. Liq. 2016, 213, 381–389. [Google Scholar] [CrossRef]
- Qiu, H.; Shen, F.; Yin, A.; Liu, J.; Wu, B.; Li, Y.; Xiao, Y.; Hai, J.; Xu, B. Biodegradation and Detoxification of Azo Dyes by Halophilic/Halotolerant Microflora Isolated from the Salt Fields of Tibet Autonomous Region China. Front. Microbiol. 2022, 13, 877151. [Google Scholar] [CrossRef]
- Chequer, F.M.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Cardoso, J.C.; Zanoni, M.V.B.; de Oliveira, D.P. Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar] [CrossRef]
- Ouettar, L.; Guechi, E.K.; Hamdaoui, O.; Fertikh, N.; Saoudi, F.; Alghyamah, A. Biosorption of Triphenyl Methane Dyes (Malachite Green and Crystal Violet) from Aqueous Media by Alfa (Stipa tenacissima L.) Leaf Powder. Molecules 2023, 28, 3313. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef]
- Various, A.; Likozar, B.; D Alsukaibi, A.K. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Liu, P.; Lyu, J.; Bai, P. One-Step Synthesis of Al-Doped UiO-66 Nanoparticle for Enhanced Removal of Organic Dyes from Wastewater. Molecules 2023, 28, 2182. [Google Scholar] [CrossRef] [PubMed]
- Keshmiri-Naqab, R.; Taghavijeloudar, M. Efficient Adsorption of Acid Orange 7 from Wastewater Using Novel Bio-Natural Granular Bentonite-Sawdust-Corncob (GBSC): Mixture Optimization, Adsorption Kinetic and Regeneration. Environ. Res. 2024, 262, 119966. [Google Scholar] [CrossRef] [PubMed]
- Şenol, Z.M.; El Messaoudi, N.; Ciğeroglu, Z.; Miyah, Y.; Arslanoğlu, H.; Bağlam, N.; Kazan-Kaya, E.S.; Kaur, P.; Georgin, J. Removal of Food Dyes Using Biological Materials via Adsorption: A Review. Food Chem. 2024, 450, 139398. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ba Mohammed, B.; Oukhrib, R.; Dehbi, A.; Lamhasni, T.; Brahmi, Y.; El-Kordy, A.; Franco, D.S.P.; Georgin, J.; Lima, E.C.; et al. Adsorption of Various Inorganic and Organic Pollutants by Natural and Synthetic Zeolites: A Critical Review. Arab. J. Chem. 2024, 17, 105474. [Google Scholar] [CrossRef]
- Yadav, B.S.; Dasgupta, S. Effect of Time, PH, and Temperature on Kinetics for Adsorption of Methyl Orange Dye into the Modified Nitrate Intercalated MgAl LDH Adsorbent. Inorg. Chem. Commun. 2022, 137, 109203. [Google Scholar] [CrossRef]
- Kuptajit, P.; Sano, N.; Nakagawa, K.; Suzuki, T. A Study on Pore Formation of High Surface Area Activated Carbon Prepared by Microwave-Induced Plasma with KOH (MiWP-KOH) Activation: Effect of Temperature-Elevation Rate. Chem. Eng. Process. Process Intensif. 2021, 167, 108511. [Google Scholar] [CrossRef]
- Amran, F.; Abbas, M.; Zaini, A.; Bahru, J. Effects of Chemical Activating Agents on Physical Properties of Activated Carbons—A Commentary. Water Pract. Technol. 2020, 15, 863–876. [Google Scholar] [CrossRef]
- Fagbohun, E.O.; Wang, Q.; Spessato, L.; Zheng, Y.; Li, W.; Olatoye, A.G.; Cui, Y. Physicochemical Regeneration of Industrial Spent Activated Carbons Using a Green Activating Agent and Their Adsorption for Methyl Orange. Surf. Interfaces 2022, 29, 101696. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Zhang, Y.; Su, Y.; Zhang, H. The Deactivation Mechanisms, Regeneration Methods and Devices of Activated Carbon in Applications. J. Clean. Prod. 2024, 476, 143751. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green Sorbents from Agricultural Wastes: A Review of Sustainable Adsorption Materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The Utilization of Leaf-Based Adsorbents for Dyes Removal: A Review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. The Use of Bilberry Leaves (Vaccinium myrtillus L.) as an Efficient Adsorbent for Cationic Dye Removal from Aqueous Solutions. Polymers 2022, 14, 978. [Google Scholar] [CrossRef] [PubMed]
- Mussa, Z.H.; Al-Ameer, L.R.; Al-Qaim, F.F.; Deyab, I.F.; Kamyab, H.; Chelliapan, S. Comprehensive Review on Adsorption of Methylene Blue Dye Using Leaf Waste as a Bio-Sorbent: Isotherm Adsorption, Kinetics, and Thermodynamics Studies. Environ. Monit. Assess. 2023, 195, 940. [Google Scholar] [CrossRef]
- Mutunga, M.F.; Wanyonyi, W.C.; Ongera, G. Utilization of Macadamia Seed Husks as a Low-Cost Sorbent for Removing Cationic Dye (Basic Blue 3 Dye) from Aqueous Solution. Environ. Chem. Ecotoxicol. 2020, 2, 194–200. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Brym, S.; Kopeć, L. Use of Aminated Hulls of Sunflower Seeds for the Removal of Anionic Dyes from Aqueous Solutions. Int. J. Environ. Sci. Technol. 2020, 17, 1211–1224. [Google Scholar] [CrossRef]
- Jóźwiak, T.J.; Filipkowska, U. The Use of Rapeseed Husks to Remove Acidic and Basic Dyes from Aquatic Solutions. Appl. Sci. 2024, 14, 1174. [Google Scholar] [CrossRef]
- Kowalkowska, A.; Jóźwiak, T. Utilization of Pumpkin (Cucurbita pepo) Seed Husks as a Low-Cost Sorbent for Removing Anionic and Cationic Dyes from Aqueous Solutions. Desalination Water Treat. 2019, 171, 397–407. [Google Scholar] [CrossRef]
- Somasekhara Reddy, M.C.; Nirmala, V.; Ashwini, C. Bengal Gram Seed Husk as an Adsorbent for the Removal of Dye from Aqueous Solutions—Batch Studies. Arab. J. Chem. 2017, 10, S2554–S2566. [Google Scholar] [CrossRef]
- Dominguez, M.; Mendoza, J.; Figueroa, K. Adsorption of Methylene Blue Dye Using Common Walnut Shell (Juglans regia) like Biosorbent: Implications for Wastewater Treatment. Green Chem. Lett. Rev. 2024, 17, 2362257. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Ramírez-Montoya, L.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A.; Ramírez-López, E.M. Sorption Mechanism of Anionic Dyes on Pecan Nut Shells (Carya illinoinensis) Using Batch and Continuous Systems. Ind. Crops Prod. 2013, 48, 89–97. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Bouslamti, R.; Nahali, L.; Mejbar, F.; Lairini, S. The Fast-Efficient Adsorption Process of the Toxic Dye onto Shells Powders of Walnut and Peanut: Experiments, Equilibrium, Thermodynamic, and Regeneration Studies. Chem. Afr. 2022, 5, 375–393. [Google Scholar] [CrossRef]
- Nhung, N.T.H.; Quynh, B.T.P.; Thao, P.T.T.; Bich, H.N.; Giang, B.L. Pretreated Fruit Peels as Adsorbents for Removal of Dyes from Water. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 159. [Google Scholar]
- Tolkou, A.K.; Tsoutsa, E.K.; Kyzas, G.Z.; Katsoyiannis, I.A. Sustainable Use of Low-Cost Adsorbents Prepared from Waste Fruit Peels for the Removal of Selected Reactive and Basic Dyes Found in Wastewaters. Environ. Sci. Pollut. Res. Int. 2024, 31, 14662–14689. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, T.; Filipkowska, U.; Zajko, P. Use of Citrus Fruit Peels (Grapefruit, Mandarin, Orange, and Lemon) as Sorbents for the Removal of Basic Violet 10 and Basic Red 46 from Aqueous Solutions. Desalination Water Treat. 2019, 163, 385–397. [Google Scholar] [CrossRef]
- Bouhadjra, K.; Lemlikchi, W.; Ferhati, A.; Mignard, S. Enhancing Removal Efficiency of Anionic Dye (Cibacron Blue) Using Waste Potato Peels Powder. Sci. Rep. 2021, 11, 2090. [Google Scholar] [CrossRef]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; El Maguana, Y. Efficiency of Sawdust as Low-Cost Adsorbent for Dyes Removal. J. Chem. 2020, 2020, 8813420. [Google Scholar] [CrossRef]
- Pimentel, C.H.; Freire, M.S.; Gómez-Díaz, D.; González-Álvarez, J. Removal of Wood Dyes from Aqueous Solutions by Sorption on Untreated Pine (Pinus radiata) Sawdust. Cellulose 2023, 30, 4587–4608. [Google Scholar] [CrossRef]
- Zhou, G.; Li, S.; Meng, Q.; Niu, C.; Zhang, X.; Wang, Q. A New Type of Highly Efficient Fir Sawdust-Based Super Adsorbent: Remove Cationic Dyes from Wastewater. Surf. Interfaces 2023, 36, 102637. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Barral, M.T.; Al-Degs, Y.; Paradelo, R. Competitive Removal of Textile Dyes from Solution by Pine Bark-Compost in Batch and Fixed Bed Column Experiments. Environ. Technol. Innov. 2022, 27, 102421. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an Anionic Dye (Congo Red) from Aqueous Solutions by Pine Bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Zamouche, M.; Hamdaoui, O. A Use of Cedar Cone for the Removal of a Cationic Dye from Aqueous Solutions by Sorption. Energy Procedia 2012, 18, 1047–1058. [Google Scholar] [CrossRef]
- Debnath, S.; Ballav, N.; Maity, A.; Pillay, K. Competitive Adsorption of Ternary Dye Mixture Using Pine Cone Powder Modified with β-Cyclodextrin. J. Mol. Liq. 2017, 225, 679–688. [Google Scholar] [CrossRef]
- Mishra, Y.; Sowmya, V.; Shanthakumar, S. Adsorption Studies of Basic Dyes onto Teak (Tectona grandis) Leaf Powder. J. Urban Environ. Eng. 2015, 9, 102–108. [Google Scholar] [CrossRef]
- Gul, S.; Gul, A.; Gul, H.; Khattak, R.; Ismail, M.; Khan, S.U.; Khan, M.S.; Aouissi, H.A.; Krauklis, A. Removal of Brilliant Green Dye from Water Using Ficus Benghalensis Tree Leaves as an Efficient Biosorbent. Materials 2023, 16, 521. [Google Scholar] [CrossRef]
- Gul, S.; Gul, S.; Jamila, N.; Iqbal, F.; Gul, H.; Shahd, T.A. Dye Removal from Waste Water by Java Plum Tree Leaves as an Effective Biosorbent. 2024. Available online: https://www.researchsquare.com/article/rs-3961875/v1 (accessed on 3 December 2024).
- de Oliveira, D.M.; de Bomfim, A.S.C.; Benini, K.C.C.d.C.; Cioffi, M.O.H.; Voorwald, H.J.C.; Rodrigue, D. Waste Paper as a Valuable Resource: An Overview of Recent Trends in the Polymeric Composites Field. Polymers 2023, 15, 426. [Google Scholar] [CrossRef]
- Abushammala, H.; Masood, M.A.; Ghulam, S.T.; Mao, J. On the Conversion of Paper Waste and Rejects into High-Value Materials and Energy. Sustainability 2023, 15, 6915. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Bednarowicz, A.; Zielińska, D.; Wiśniewska-Wrona, M. The Use of Various Types of Waste Paper for the Removal of Anionic and Cationic Dyes from Aqueous Solutions. Molecules 2024, 29, 2809. [Google Scholar] [CrossRef]
- Mniej Papieru Dla Gazet to Wpływ Wysokich Cen Energii i Spowolnienia Gospodarczego—Press.Pl—Najnowsze Informacje z Branży Medialnej, Marketingowej, Reklamowej i Public Relations. Available online: https://www.press.pl/tresc/77563,mniej-papieru-dla-gazet-to-wplyw-wysokich-cen-energii-i-spowolnienia-gospodarczego (accessed on 16 April 2024).
- Moawed, E.A.; El-Hagrasy, M.A.; Farhat, A.A.M. Application of Magnetic Isothiouronium Polyurethane Sorbent for the Removal of Acidic and Basic Dyes from Wastewater. J. Clean. Prod. 2017, 157, 232–242. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Brym, S.; Zyśk, M. The Use of Aminated Cotton Fibers as an Unconventional Sorbent to Remove Anionic Dyes from Aqueous Solutions. Cellulose 2020, 27, 3957–3969. [Google Scholar] [CrossRef]
- Józwiak, T.; Filipkowska, U.; Kowalkowska, A.; Struk-Sokoowska, J.; Werbowy, D. The Influence of Amination of Sorbent Based on Buckwheat (Fagopyrum esculentum) Husks on the Sorption Effectiveness of Reactive Black 5 Dye. J. Environ. Chem. Eng. 2021, 9, 105092. [Google Scholar] [CrossRef]
- Paczyńska, K.; Jóźwiak, T.; Filipkowska, U. The Effect of Modifying Canadian Goldenrod (Solidago canadensis) Biomass with Ammonia and Epichlorohydrin on the Sorption Efficiency of Anionic Dyes from Water Solutions. Materials 2023, 16, 4586. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, T.; Filipkowska, U. Aminated Rapeseed Husks (Brassica napus) as an Effective Sorbent for Removing Anionic Dyes from Aqueous Solutions. Molecules 2024, 29, 843. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, T.; Filipkowska, U.; Walczak, P. The Use of Aminated Wheat Straw for Reactive Black 5 Dye Removal from Aqueous Solutions as a Potential Method of Biomass Valorization. Energies 2022, 15, 6257. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Pavithra, R.; Gunasekaran, S.; Sailatha, E.; Kamatchi, S. Investigations on Paper Making Raw Materials and Determination of Paper Quality by FTIR-UATR and UV-Vis DRS Spectroscopy. Int. J. Curr. Res. Acad. Rev. 2015, 3, 42–59. [Google Scholar]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform—Materials Analysis; IntechOpen: London, UK, 2012. [Google Scholar]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Asri, S.E.A.M.; Ismail, A.F. Physicochemical Properties of “Green” Nanocrystalline Cellulose Isolated from Recycled Newspaper. RSC Adv. 2015, 5, 29842–29849. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical Functional Groups of Extractives, Cellulose and Lignin Extracted from Native Leucaena Leucocephala Bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Lebiocka, M.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Gułkowski, S. Mature Landfill Leachate as a Medium for Hydrodynamic Cavitation of Brewery Spent Grain. Energies 2021, 14, 1150. [Google Scholar] [CrossRef]
- Kim, Y.; Caumon, M.C.; Barres, O.; Sall, A.; Cauzid, J. Identification and Composition of Carbonate Minerals of the Calcite Structure by Raman and Infrared Spectroscopies Using Portable Devices. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 261, 119980. [Google Scholar] [CrossRef] [PubMed]
- Louati, S.; Baklouti, S.; Samet, B. Geopolymers Based on Phosphoric Acid and Illito-Kaolinitic Clay. Adv. Mater. Sci. Eng. 2016, 2016, 2359759. [Google Scholar] [CrossRef]
- Srivastava, K.; Rathore, A.K.; Srivastava, D. Studies on the Structural Changes during Curing of Epoxy and Its Blend with CTBN. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bach, Q.V.; Vu, C.M.; Vu, H.T.; Vu, H.B.; Van Nguyen, T.; Chang, S.W.; Nguyen, D.D.; Thi, T.A.D.; Doan, V.N. Significant Enhancement of Fracture Toughness and Mechanical Properties of Epoxy Resin Using CTBN-Grafted Epoxidized Linseed Oil. J. Appl. Polym. Sci. 2020, 137, 48276. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Lu, Y.; Rong, M.Z.; Zhang, M.Q. A Thermally Remendable and Reprocessable Crosslinked Methyl Methacrylate Polymer Based on Oxygen Insensitive Dynamic Reversible C–ON Bonds. RSC Adv. 2016, 6, 6350–6357. [Google Scholar] [CrossRef]
- Singh, B.P.; Choudhary, V.; Teotia, S.; Gupta, T.K.; Singh, V.N.; Dhakate, S.R.; Mathur, R.B. Solvent Free, Efficient, Industrially Viable, Fast Dispersion Process Based Amine Modified MWCNT Reinforced Epoxy Composites of Superior Mechanical Properties. Adv. Mater. Lett. 2015, 6, 104–113. [Google Scholar] [CrossRef]
- Filipkowska, U.; Jόźwiak, T.; Szymczyk, P.; Kuczajowska-Zadrożna, M. The Use of Active Carbon Immobilised on Chitosan Beads for RB5 and BV10 Dye Removal from Aqueous Solutions. Prog. Chem. Appl. Chitin Its Deriv. 2017, 22, 14–26. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Rodziewicz, J.; Mielcarek, A.; Owczarkowska, D. Application of Compost as a Cheap Sorbent for Dyes Removal from Aqueous Solutions. Rocz. Ochr. Srodowiska 2013, 15, 2398–2411. [Google Scholar]
- Jóźwiak, T.; Filipkowska, U.; Bakuła, T.; Bralewska-Piotrowicz, B.; Karczmarczyk, K.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Szyryńska, N.; Lewczuk, B. The Use of Chitin from the Molts of Mealworm (Tenebrio molitor) for the Removal of Anionic and Cationic Dyes from Aqueous Solutions. Materials 2023, 16, 545. [Google Scholar] [CrossRef]
- Szymczyk, P.; Filipkowska, U.; Jóźwiak, T.; Kuczajowska-Zadrożna, M. Use of Sawdust Immobilised on Chitosan for Disposal of Dyes from Water Solutions. Prog. Chem. Appl. Chitin Its Deriv. 2017, 22, 207–219. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Kuczajowska-Zadrożna, M.; Mielcarek, A. Removal of Dyes from Aqueous Solutions by Glauconite Immobilised on Chitosan Hydrogel Beads. Prog. Chem. Appl. Chitin Its Deriv. 2017, 22, 54–65. [Google Scholar] [CrossRef]
- Güzel, F.; Sayʇili, H.; Akkaya Sayʇili, G.; Koyuncu, F. New Low-Cost Nanoporous Carbonaceous Adsorbent Developed from Carob (Ceratonia siliqua) Processing Industry Waste for the Adsorption of Anionic Textile Dye: Characterization, Equilibrium and Kinetic Modeling. J. Mol. Liq. 2015, 206, 244–255. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-Bed Adsorption of Reactive Azo Dye onto Granular Activated Carbon Prepared from Waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, B.F.; de Araujo, C.M.B.; do Nascimento, A.C.; da Costa, G.R.B.; Gomes, B.F.M.L.; da Silva, M.P.; da Silva Santos, R.K.; da Motta Sobrinho, M.A. Adsorption of Reactive Black 5 and Basic Blue 12 Using Biochar from Gasification Residues: Batch Tests and Fixed-Bed Breakthrough Predictions for Wastewater Treatment. Bioresour. Technol. Rep. 2021, 15, 100767. [Google Scholar] [CrossRef]
- Ip, A.W.M.; Barford, J.P.; McKay, G. A Comparative Study on the Kinetics and Mechanisms of Removal of Reactive Black 5 by Adsorption onto Activated Carbons and Bone Char. Chem. Eng. J. 2010, 157, 434–442. [Google Scholar] [CrossRef]
- Nabil, G.M.; El-Mallah, N.M.; Mahmoud, M.E. Enhanced Decolorization of Reactive Black 5 Dye by Active Carbon Sorbent-Immobilized-Cationic Surfactant (AC-CS). J. Ind. Eng. Chem. 2014, 20, 994–1002. [Google Scholar] [CrossRef]
- Eren, Z.; Acar, F.N. Adsorption of Reactive Black 5 from an Aqueous Solution: Equilibrium and Kinetic Studies. Desalination 2006, 194, 1–10. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Azadeh, E.; Abdulkhani, A. Removal of Acid Orange 7 and Remazol Black 5 Reactive Dyes from Aqueous Solutions Using a Novel Biosorbent. Mater. Sci. Eng. C 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.S. Removal of Anionic Dyes (Reactive Black 5 and Congo Red) from Aqueous Solutions Using Banana Peel Powder as an Adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef]
- Mook, W.T.; Aroua, M.K.; Szlachta, M. Palm Shell-Based Activated Carbon for Removing Reactive Black 5 Dye: Equilibrium and Kinetics Studies. Bioresources 2016, 11, 1432–1447. [Google Scholar] [CrossRef]
- Heibati, B.; Rodriguez-Couto, S.; Amrane, A.; Rafatullah, M.; Hawari, A.; Al-Ghouti, M.A. Uptake of Reactive Black 5 by Pumice and Walnut Activated Carbon: Chemistry and Adsorption Mechanisms. J. Ind. Eng. Chem. 2014, 20, 2939–2947. [Google Scholar] [CrossRef]
- Ucun, H. Equilibrium, Thermodynamic and Kinetics of Reactive Black 5 Biosorption on Loquat (Eriobotrya Japonica) Seed. Sci. Res. Essays 2011, 6, 4113–4124. [Google Scholar] [CrossRef]
- Uçar, D.; Armağan, B. The Removal of Reactive Black 5 from Aqueous Solutions by Cotton Seed Shell. Water Environ. Res. 2012, 84, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Felista, M.M.; Wanyonyi, W.C.; Ongera, G. Adsorption of Anionic Dye (Reactive Black 5) Using Macadamia Seed Husks: Kinetics and Equilibrium Studies. Sci. Afr. 2020, 7, e00283. [Google Scholar] [CrossRef]
- Osma, J.F.; Saravia, V.; Toca-Herrera, J.L.; Couto, S.R. Sunflower Seed Shells: A Novel and Effective Low-Cost Adsorbent for the Removal of the Diazo Dye Reactive Black 5 from Aqueous Solutions. J. Hazard. Mater. 2007, 147, 900–905. [Google Scholar] [CrossRef]
- Józwiak, T.; Filipkowska, U.; Bugajska, P.; Kalkowski, T. The Use of Coconut Shells for the Removal of Dyes from Aqueous Solutions. J. Ecol. Eng. 2018, 19, 129–135. [Google Scholar] [CrossRef]
- Indhu, S.; Muthukumaran, K. Removal and Recovery of Reactive Yellow 84 Dye from Wastewater and Regeneration of Functionalised Borassus Flabellifer Activated Carbon. J. Environ. Chem. Eng. 2018, 6, 3111–3121. [Google Scholar] [CrossRef]
- Şahin, E. Interpretation of Sorption Kinetics for Mixtures of Reactive Dyes on Wool. Turk. J. Chem. 2005, 29, 617–625. [Google Scholar]
- 2024 Export Prices—Letsrecycle.Com. Available online: https://www.letsrecycle.com/prices/waste-paper/export-prices/2024-export-prices/ (accessed on 3 December 2024).
- About Newsprint Paper|Newsprint Primer|PaperIndex Academy|PaperIndex. Available online: https://www.paperindex.com/academy/paper-grades/newsprint-primer (accessed on 16 April 2024).
- Epichlorohydrin Price Index—Businessanalytiq. Available online: https://businessanalytiq.com/procurementanalytics/index/epichlorohydrin-price-index/#google_vignette (accessed on 3 December 2024).
- Ammonia Price Index—Businessanalytiq. Available online: https://businessanalytiq.com/procurementanalytics/index/ammonia-price-index/ (accessed on 3 December 2024).
- Electricity Rates for Every State—EnergyBot. Available online: https://www.energybot.com/electricity-rates/ (accessed on 3 December 2024).
- Electricity Prices Around the World|GlobalPetrolPrices.Com. Available online: https://www.globalpetrolprices.com/electricity_prices/ (accessed on 3 December 2024).
- Activated Carbon Prices—Businessanalytiq. Available online: https://businessanalytiq.com/procurementanalytics/index/activated-carbon-prices/ (accessed on 3 December 2024).
- Nowicka, A.; Jóźwiak, T.; Zieliński, M. The Possibility of Using Waste from Dye Sorption for Methane Production. Energies 2024, 17, 4756. [Google Scholar] [CrossRef]
- Chen, H.; Han, Q.; Venditti, R.A.; Jameel, H. Enzymatic Hydrolysis of Pretreated Newspaper Having High Lignin Content for Bioethanol Production. Bioresources 2015, 10, 4077–4098. [Google Scholar] [CrossRef]
- Ioelovich, M. Waste Paper as Promising Feedstock for Production of Biofuel. J. Sci. Res. Rep. 2014, 3, 905–916. [Google Scholar] [CrossRef]

| Tested Sorbent | Nitrogen Content [%] | Carbon Content [%] | Hydrogen Content [%] | Approximate Amount of Nitrogen Introduced During Amination [%] (Based on Average Values) |
|---|---|---|---|---|
| N | 0.64 ± 0.07 | 46.08 ± 0.16 | 6.14 ± 0.10 | - |
| NE | 0.68 ± 0.12 | 45.12 ± 0.18 | 6.10 ± 0.09 | - |
| NEN1 | 1.34 ± 0.03 | 45.88 ± 0.28 | 6.34 ± 0.09 | 0.70 |
| NEN2 | 1.94 ± 0.09 | 45.41 ± 0.17 | 6.39 ± 0.05 | 1.30 |
| NEN3 | 2.70 ± 0.18 | 46.01 ± 0.10 | 6.36 ± 0.10 | 2.03 |
| Tested Sorbent | BET Surface Area [m2/g] | Pore Volume [cm3/g] | Pore Size (Average) [nm] |
|---|---|---|---|
| N | 0.488 ± 0.0331 | 0.00212 | 25.0 |
| NE | 0.389 ± 0.0331 | 0.00495 | 37.9 |
| NEN1 | 0.499 ± 0.0331 | 0.00327 | 20.8 |
| NEN2 | 0.528 ± 0.0472 | 0.00311 | 24.6 |
| NEN3 | 0.377 ± 0.0350 | 0.00378 | 23.7 |
| Tested Sorbent | Dye | Dye Conc. | Pseudo-First-Order Model | Pseudo-Second-Order Model | Exp. Data | Equil. Time | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe,cal. | R2 | k2 | qe,cal. | R2 | qe,exp. | ||||
| [mg/L] | [1/min] | [mg/g] | - | [g/mg × min] | [mg/g] | - | [mg/g] | [min] | ||
| N | RB5 | 1000 | 0.0610 | 6.06 | 0.9954 | 0.0134 | 6.70 | 0.9971 | 6.22 | 120 |
| RY84 | 1000 | 0.0357 | 9.06 | 0.9784 | 0.0043 | 10.42 | 0.9943 | 9.42 | 150 | |
| NE | RB5 | 1000 | 0.0485 | 6.62 | 0.9915 | 0.0087 | 7.49 | 0.9978 | 6.76 | 120 |
| RY84 | 1000 | 0.0368 | 11.49 | 0.9879 | 0.0036 | 13.17 | 0.9971 | 11.86 | 150 | |
| NEN1 | RB5 | 1000 | 0.0532 | 162.24 | 0.9802 | 0.0004 | 181.50 | 0.9957 | 161.25 | 90 |
| RY84 | 1000 | 0.0447 | 99.60 | 0.9915 | 0.0005 | 113.56 | 0.9961 | 100.41 | 90 | |
| NEN2 | RB5 | 1000 | 0.0460 | 174.40 | 0.9854 | 0.0003 | 197.73 | 0.9960 | 176.17 | 90 |
| RY84 | 1000 | 0.0511 | 101.51 | 0.9757 | 0.0006 | 113.72 | 0.9936 | 102.63 | 90 | |
| NEN3 | RB5 | 1000 | 0.0489 | 192.22 | 0.9841 | 0.0003 | 216.51 | 0.9949 | 195.31 | 90 |
| RY84 | 1000 | 0.0501 | 105.35 | 0.9862 | 0.0006 | 118.56 | 0.9960 | 106.36 | 90 | |
| Tested Sorbent | Dye | Dye Conc. | Phase 1 | Phase 2 | ||||
|---|---|---|---|---|---|---|---|---|
| kd1 | Duration | R2 | kd2 | Duration | R2 | |||
| [mg/L] | [mg/(g × min0.5)] | [min] | - | [mg/(g × min0.5)] | [min] | - | ||
| N | RB5 | 1000 | 0.903 | 30 | 0.9981 | 0.145 | 90 | 0.9817 |
| RY84 | 1000 | 1.117 | 30 | 0.9978 | 0.482 | 120 | 0.9907 | |
| NE | RB5 | 1000 | 0.899 | 30 | 0.9974 | 0.242 | 90 | 0.9961 |
| RY84 | 1000 | 1.403 | 30 | 0.9998 | 0.534 | 120 | 0.9777 | |
| NEN1 | RB5 | 1000 | 24.587 | 20 | 0.9996 | 11.050 | 70 | 0.9964 |
| RY84 | 1000 | 13.733 | 20 | 0.9964 | 7.192 | 70 | 0.9891 | |
| NEN2 | RB5 | 1000 | 24.871 | 20 | 0.9999 | 13.259 | 70 | 0.9933 |
| RY84 | 1000 | 15.254 | 20 | 0.9965 | 7.264 | 70 | 0.9853 | |
| NEN3 | RB5 | 1000 | 28.075 | 20 | 0.9999 | 14.258 | 70 | 0.9898 |
| RY84 | 1000 | 15.547 | 20 | 0.9999 | 7.380 | 70 | 0.9845 | |
| Tested SorBent | Dye | Langmuir 1 Model | Langmuir 2 Model | Freundlich Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qmax | Kc | R2 | Qmax | b1 | K1 | b2 | K2 | R2 | k | n | R2 | ||
| [mg/g] | [L/mg] | - | mg/g | mg/g | L/mg | mg/g | L/mg | - | - | - | - | ||
| N | RB5 | 7.12 | 0.0060 | 0.9913 | 7.12 | 3.57 | 0.0060 | 3.55 | 0.0060 | 0.9913 | 0.458 | 0.388 | 0.9422 |
| RY84 | 10.24 | 0.0190 | 0.9931 | 10.24 | 5.77 | 0.0190 | 4.47 | 0.0190 | 0.9931 | 1.967 | 0.247 | 0.8753 | |
| NE | RB5 | 7.89 | 0.0057 | 0.9960 | 7.89 | 4.02 | 0.0057 | 3.87 | 0.0057 | 0.9960 | 0.484 | 0.394 | 0.9498 |
| RY84 | 11.95 | 0.0201 | 0.9945 | 11.94 | 5.93 | 0.0201 | 6.01 | 0.0201 | 0.9945 | 2.323 | 0.247 | 0.8810 | |
| NEN1 | RB5 | 176.46 | 0.0905 | 0.9841 | 182.78 | 147.05 | 0.0477 | 35.73 | 1.3001 | 0.9967 | 44.462 | 0.233 | 0.9107 |
| RY84 | 101.64 | 0.0603 | 0.9955 | 106.58 | 95.77 | 0.0664 | 10.81 | 0.0360 | 0.9960 | 21.067 | 0.272 | 0.9089 | |
| NEN2 | RB5 | 188.23 | 0.2355 | 0.9930 | 202.70 | 169.85 | 0.0550 | 32.85 | 0.2932 | 0.9961 | 58.159 | 0.207 | 0.8589 |
| RY84 | 105.71 | 0.0720 | 0.9966 | 108.15 | 99.84 | 0.0783 | 8.31 | 0.0075 | 0.9968 | 23.333 | 0.265 | 0.8988 | |
| NEN3 | RB5 | 227.08 | 0.2334 | 0.9946 | 231.50 | 186.48 | 0.1460 | 45.02 | 1.3704 | 0.9984 | 67.530 | 0.226 | 0.8715 |
| RY84 | 105.94 | 0.1076 | 0.9929 | 133.31 | 100.79 | 0.1179 | 32.52 | 0.0007 | 0.9940 | 26.953 | 0.244 | 0.8963 | |
| Sorbent | Qmax [mg/g] | pH of Sorption | Time of Sorption [min] | Source |
|---|---|---|---|---|
| Newsprint paper aminated with diethylenetriamine (pre-activated with epichlorohydrin) (NEN3) | 231.5 | 3 | 90 | This work |
| Newsprint paper aminated with ethylenediamine (pre-activated with epichlorohydrin) (NEN2) | 202.7 | 3 | 90 | This work |
| Activated carbon Filtrasorb 400 (commercial) | 198.0 | 5.2 | 720 | [79] |
| Newsprint paper aminated with ammonia (pre-activated with epichlorohydrin) (NEN1) | 182.8 | 3 | 90 | This work |
| Rapeseed hulls—aminated with ammonia (pre-activated with epichlorohydrin) | 135.8 | 3 | 120 | [55] |
| Activated carbon (powder) | 125.8 | 2 | 240 | [71] |
| Wheat straw aminated with ammonia (pre-activated with epichlorohydrin) | 91.0 | 3 | 210 | [56] |
| Buckwheat hulls aminated with ammonia (pre-activated with epichlorohydrin) | 85.2 | 3 | 300 | [53] |
| Goldenrot biomass aminated with ammonia (pre-activated with epichlorohydrin) | 71.3 | 3 | 120 | [54] |
| Activated carbon modified with SPC | 69.9 | 2 | <60 | [80] |
| Activated carbon—powdered | 58.8 | - | - | [81] |
| Sunflower seed shells aminated with ammonia (pre-activated with epichlorohydrin) | 51.0 | 3 | 240 | [26] |
| Activated carbon from bamboo | 39.0 | 2 | 60 | [77] |
| Activated carbon from Carob tree | 36.9 | 2 | 120 | [76] |
| Biochar from gasification residues | 35.7 | - | 90 | [78] |
| Rape stalks (waste) | 32.8 | 2.5 | 30 | [82] |
| Banana peel (powder) | 26.9 | 3 | 60 | [83] |
| Rapeseed hulls aminated with ammonia (without activation) | 26.3 | 3 | 150 | [55] |
| Activated carbon from palm shell | 25.1 | 2 | 300 | [84] |
| Wood (walnut) activated carbon | 19.3 | 5 | 400 | [85] |
| Wheat straw aminated with ammonia (without activation) | 17.5 | 3 | 210 | [56] |
| Wheat straw | 15.7 | 7 | 195 | [56] |
| Rapeseed husks | 15.2 | 3 | 180 | [55] |
| Beech sawdust | 13.9 | 3 | 1440 | [74] |
| Seed scales of Eriobotrya japonica | 13.8 | 3 | 150 | [86] |
| Cotton seed husks | 12.9 | 2 | 30 | [87] |
| Goldenrot biomass aminated with ammonia (without activation) | 10.6 | 3 | 150 | [54] |
| Newsprint paper activated with epichlorohydrin (NE) | 7.9 | 3 | 120 | This work |
| Buckwheat hulls aminated with ammonia (without activation) | 7.4 | 3 | 300 | [53] |
| Newsprint paper (N) | 7.1 | 3 | 120 | This work |
| Buckwheat hulls | 4.4 | 3 | 300 | [53] |
| Sunflower seed hulls | 2.9 | 3 | 210 | [26] |
| Cotton fibers | 2.7 | 3 | 240 | [52] |
| Goldenrot biomass | 2.3 | 3 | 150 | [54] |
| Macadamia seed husks | 1.2 | 3 | 510 | [88] |
| Sunflower biomass | 1.1 | 2 | 210 | [89] |
| Pumpkin seed husks | 1.0 | 3 | 60 | [28] |
| Coconut shells | 0.8 | 2 | 60 | [90] |
| Sorbent | Qmax [mg/g] | pH of Sorption | Time of Sorption [min] | Source |
|---|---|---|---|---|
| Newsprint paper aminated with diethylenetriamine (pre-activated with epichlorohydrin) (NEN3) | 133.3 | 3 | 90 | This work |
| Rapeseed husks aminated with ammonia (activated with epichlorohydrin) | 114.2 | 3 | 120 | [55] |
| Newsprint paper aminated with ethylenediamine (pre-activated with epichlorohydrin) (NEN2) | 108.2 | 3 | 90 | This work |
| Newsprint paper aminated with ammonia (pre-activated with epichlorohydrin) (NEN1) | 106.6 | 3 | 90 | This work |
| Sunflower seed hulls aminated with ammonia (pre-activated with epichlorohydrin) | 63.3 | 3 | 240 | [26] |
| Goldenrot biomass aminated with ammonia (pre-activated with epichlorohydrin) | 59.3 | 3 | 120 | [54] |
| Cotton fibers aminated with ammonia (pre-activated with epichlorohydrin) | 43.3 | 2 | 240 | [52] |
| Activated carbon from the Borassus flabellifer plant | 40.0 | - | - | [91] |
| Rapeseed husks aminated with ammonia (without activation) | 19.7 | 3 | 150 | [55] |
| Cotton fibers | 15.9 | 2 | 240 | [52] |
| Rapeseed husks | 13.7 | 3 | 180 | [55] |
| Newsprint paper activated with epichlorohydrin (NE) | 11.9 | 3 | 150 | This work |
| Wool | 11.0 | 7 | 180 | [92] |
| Newsprint paper (N) | 10.2 | 3 | 150 | This work |
| Sunflower seed hulls aminated with ammonia (without activation) | 9.9 | 3 | 180 | [26] |
| Sunflower seed hulls | 4.2 | 3 | 90 | [26] |
| Goldenrot biomass | 2.3 | 3 | 180 | [54] |
| Compost | 2.2 | 3 | 180 | [72] |
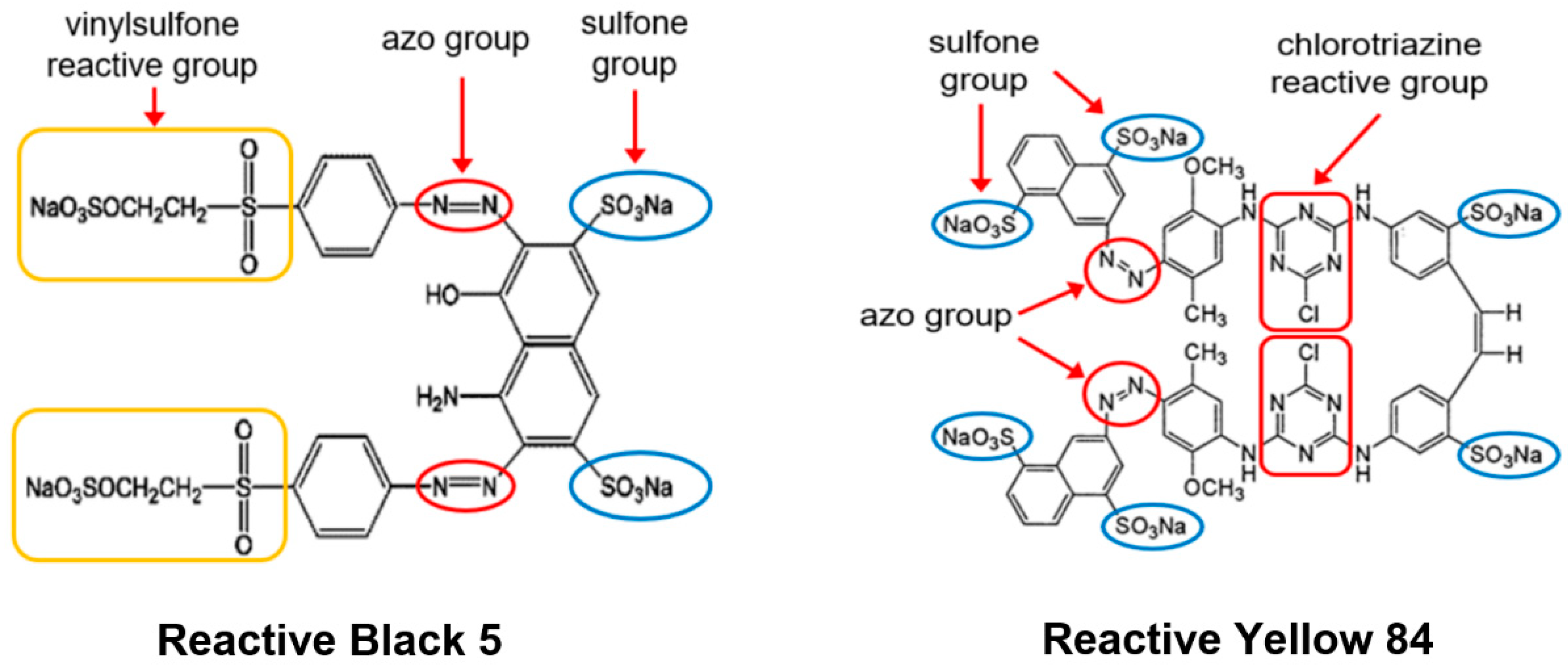
| Dymolecules-29-06024e Name | Reactive Black 5 (RB5) | Reactive Yellow 84 (RY84) |
|---|---|---|
| Chemical formula | C26H21N5Na4O19S6 | C56H38Cl2N14Na6O20S6 |
| Molecular weight | 991.0 g/mol | 1628.0 g/mol |
| Dye type | anionic (reactive) | anionic (reactive) |
| Dye class | double azo dye | double azo dye |
| Type of reactive groups | vinylsulfone | chlorotriazine |
| λmax | 600 nm | 356 nm |
| Uses | dyeing of cotton, viscose, wool | dyeing of polyester, cotton, synthetic silk |
| Other trade names | Begazol Black B; Remazol Black B | Active Yellow HE-4R; Lamafix Yellow HER. |
| Purity of the commercial product | dye content 70% | dye content 97% |
| Name | Structural Formula (Simplified) | Molecular Weight [g/mol] | Purity | Application in This Study |
|---|---|---|---|---|
| Hydrochloric acid | 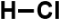 | 36.5 | 37% | diluted, for the correction of the pH of solutions |
| Sodium hydroxide (microgranules) | 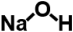 | 40.0 | 99.9% | in the solution form, for the correction of the pH of solutions |
| Epichlorohydrin | 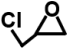 | 92.5 | >99% | for the modification/activation of sorbent |
| Ammonia (ammonia water) | 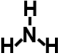 | 17.0 | 25.0% (water solution) | for the amination of sorbent |
| Ethylenediamine | 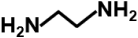 | 60.1 | >99% | for the amination of sorbent |
| Diethylenetriamine |  | 103.2 | >99% | for the amination of sorbent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak, T. The Amination of Waste Newsprint Paper with Various Aminating Agents (Ammonia Water, Ethylenediamine, and Diethylenetriamine) to Improve the Sorption Efficiency of Anionic Dyes. Molecules 2024, 29, 6024. https://doi.org/10.3390/molecules29246024
Jóźwiak T. The Amination of Waste Newsprint Paper with Various Aminating Agents (Ammonia Water, Ethylenediamine, and Diethylenetriamine) to Improve the Sorption Efficiency of Anionic Dyes. Molecules. 2024; 29(24):6024. https://doi.org/10.3390/molecules29246024
Chicago/Turabian StyleJóźwiak, Tomasz. 2024. "The Amination of Waste Newsprint Paper with Various Aminating Agents (Ammonia Water, Ethylenediamine, and Diethylenetriamine) to Improve the Sorption Efficiency of Anionic Dyes" Molecules 29, no. 24: 6024. https://doi.org/10.3390/molecules29246024
APA StyleJóźwiak, T. (2024). The Amination of Waste Newsprint Paper with Various Aminating Agents (Ammonia Water, Ethylenediamine, and Diethylenetriamine) to Improve the Sorption Efficiency of Anionic Dyes. Molecules, 29(24), 6024. https://doi.org/10.3390/molecules29246024







