Metal–Ligand Cooperation in Dihydrogen Activation by a Cationic Metallogermylene: Enhanced Activity from Tungsten to Molybdenum
Abstract
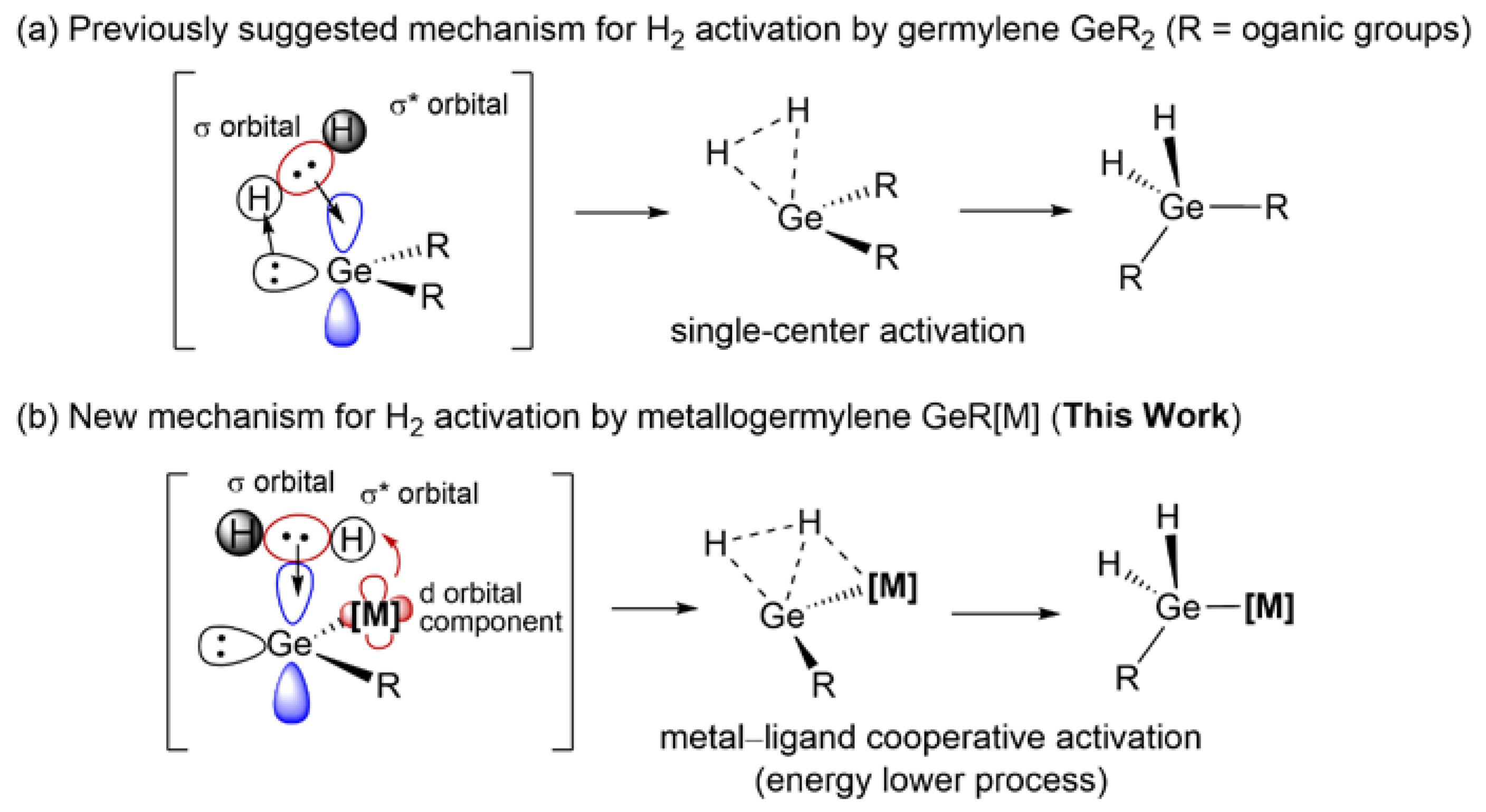
1. Introduction
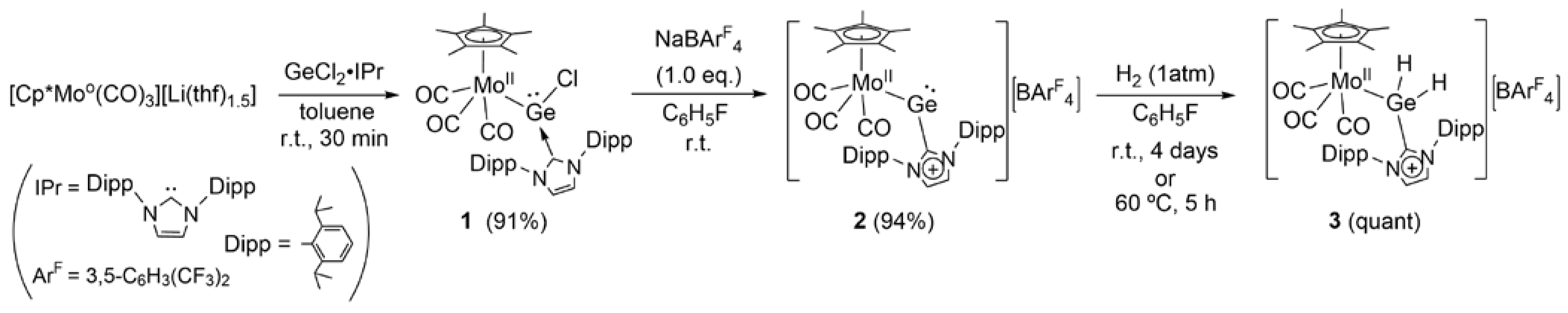
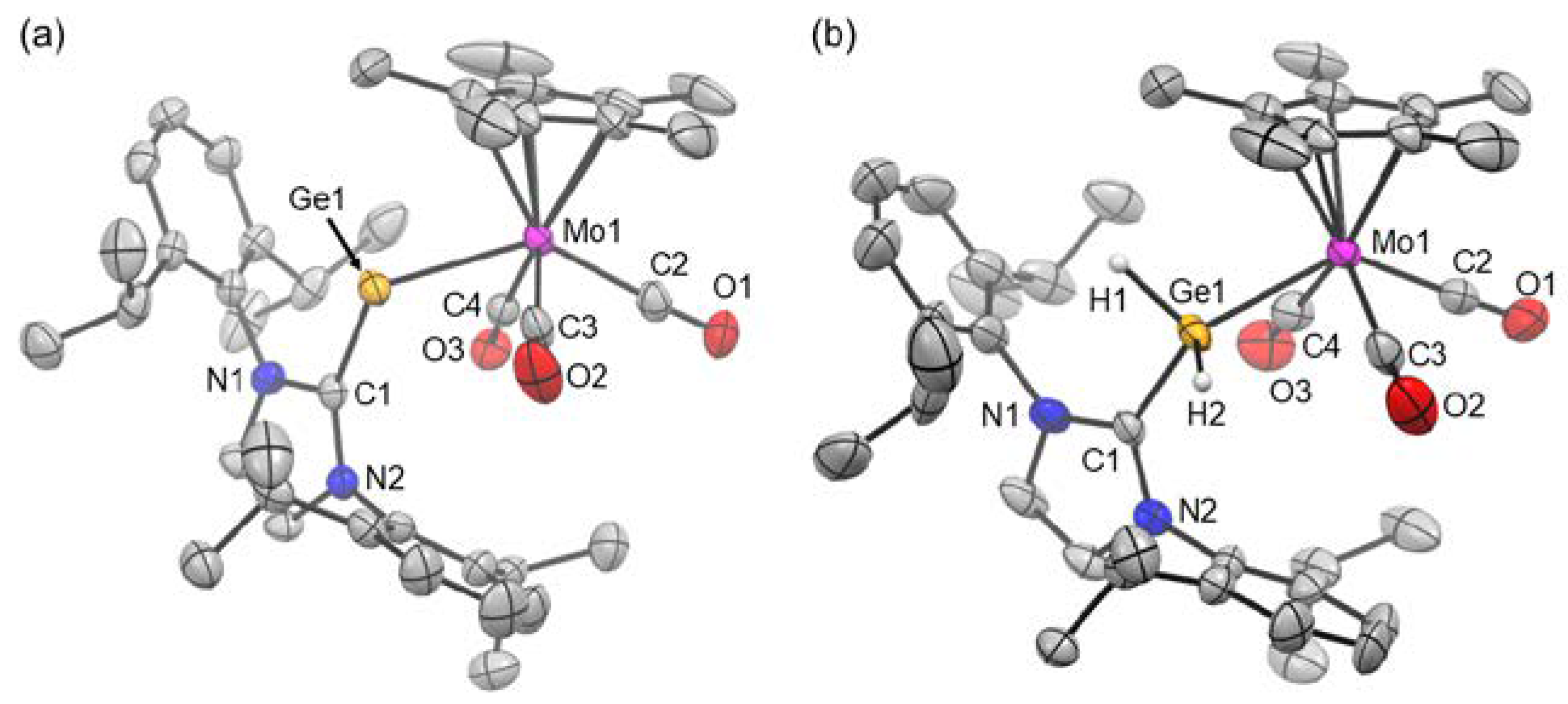
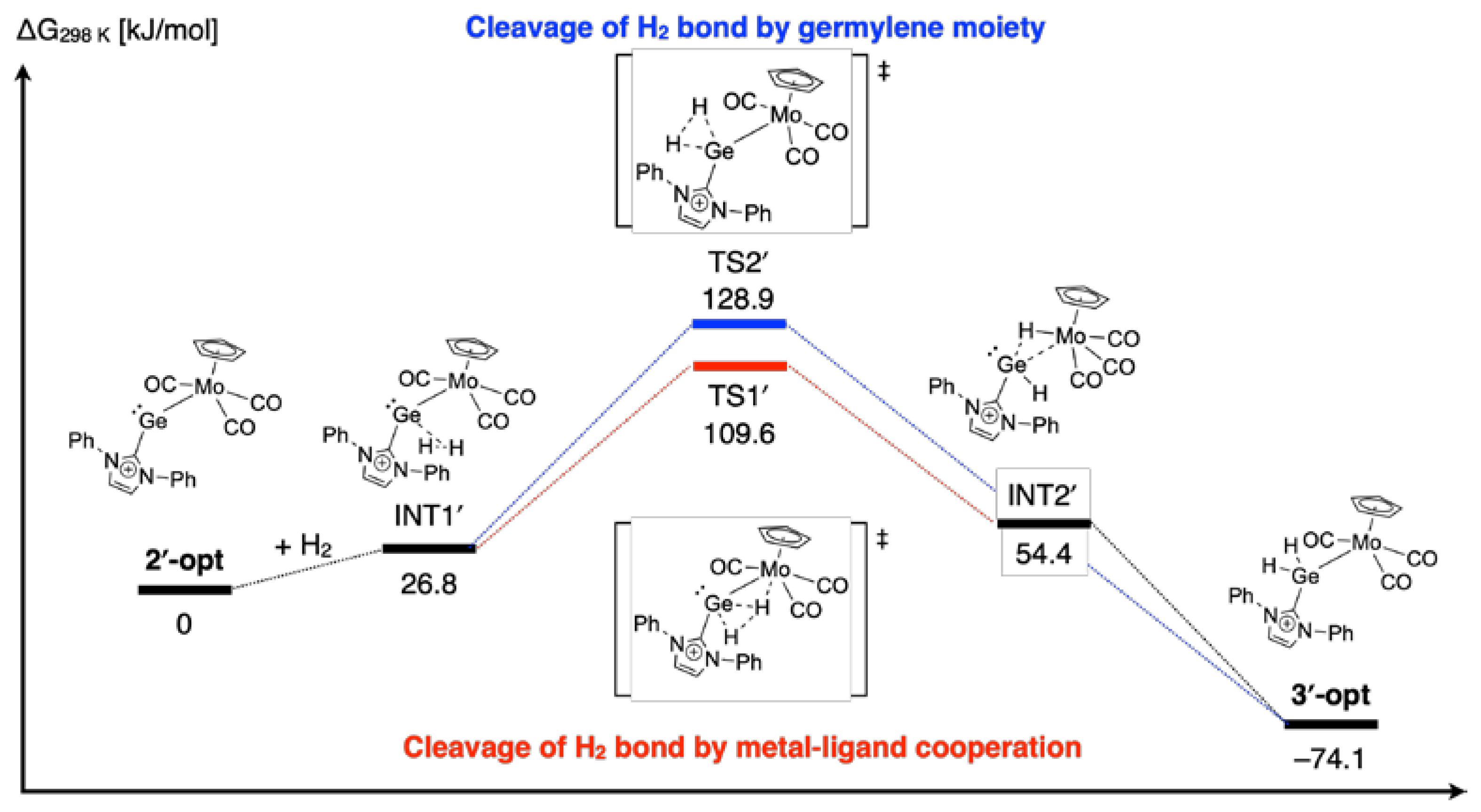
2. Results and Discussion
3. Experimental Section
3.1. General Procedures
3.2. Solvents, Reagents, and Precursors
3.3. Spectroscopic Measurements
3.4. X-Ray Crystallographic Analysis of 2 and 3
3.5. Computation Details
3.6. Synthesis of the Molybdenum Complexes (Figures S1–S18)
3.6.1. Modified Synthesis of [Cp*Mo(CO)3][Li(thf)1.5]
3.6.2. Synthesis of Cp*Mo(CO)3GeCl(IPr) (1)
3.6.3. Synthesis of [Cp*Mo(CO)3Ge(IPr)][BArF4] (2)
3.6.4. Reaction of 2 with Dihydrogen: Formation of [Cp*(CO)3MoGeH2(IPr)][BArF4] (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubas, G.J. Molecular hydrogen complexes: Coordination of a σ-bond to transition metals. Acc. Chem. Res. 1988, 21, 120–128. [Google Scholar] [CrossRef]
- Crabtree, R.H. Dihydrogen complexes: Some structural and chemical studies. Acc. Chem. Res. 1990, 23, 95–101. [Google Scholar] [CrossRef]
- Jessop, P.G.; Morris, R.H. Reactions of transition metal dihydrogen complexes. Coord. Chem. Rev. 1992, 121, 155–284. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous Catalysis for Sustainable Energy: Hydrogen and Methanol Economies, Fuels from Biomass, and Related Topics. Chem. Rev. 2022, 122, 385–441. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Han, Z.; Chai, Y.; Wu, G.; Li, L. Catalytic hydrogen storage in liquid hydrogen carriers. EES Catal. 2023, 1, 459–494. [Google Scholar] [CrossRef]
- Kubas, G.J. Catalytic processes involving dihydrogen complexes and other sigma-bond complexes. Catal. Lett. 2005, 104, 79–101. [Google Scholar] [CrossRef]
- Crabtree, R.H. Dihydrogen complexation. Chem. Rev. 2016, 116, 8750–8769. [Google Scholar] [CrossRef] [PubMed]
- Gutsulyak, D.V.; Piers, W.E.; Borau-Garcia, J.; Parvez, M. Activation of Water, Ammonia, and Other Small Molecules by PCcarbeneP Nickel Pincer Complexes. J. Am. Chem. Soc. 2013, 135, 11776–11779. [Google Scholar] [CrossRef]
- Brown, R.M.; Garcia, J.B.; Valjus, J.; Roberts, C.J.; Tuononen, H.M.; Parvez, M.; Roesler, R. Ammonia Activation by a Nickel NCN-Pincer Complex featuring a Non-Innocent N-Heterocyclic Carbene: Ammine and Amido Complexes in Equilibrium. Angew. Chem. Int. Ed. 2015, 54, 6274–6277. [Google Scholar] [CrossRef] [PubMed]
- Dauth, A.; Gellrich, U.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. The Ferraquinone–Ferrahydroquinone Couple: Combining Quinonic and Metal-Based Reactivity. J. Am. Chem. Soc. 2017, 139, 2799–2807. [Google Scholar] [CrossRef]
- Wang, Q.; Manzano, R.A.; Tinnermann, H.; Sung, S.; Leforestier, B.; Krämer, T.; Young, R.D. Access to and Reactivity of Fe0, Fe−I, FeI, and FeII PCcarbeneP Pincer Complexes. Angew. Chem. Int. Ed. 2021, 60, 18168–18177. [Google Scholar] [CrossRef]
- Alig, L.; Fritz, M.; Schneider, S. First-row transition metal (de) hydrogenation catalysis based on functional pincer ligands. Chem. Rev. 2019, 119, 2681–2751. [Google Scholar] [CrossRef]
- Kubas, G.J. Metal Dihydrogen and σ-Bond Complexes. In Modern Inorganic Chemistry; Fackler, J.P., Jr., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; pp. 1–472. [Google Scholar]
- Karunananda, M.K.; Mankad, N.P. Cooperative strategies for catalytic hydrogenation of unsaturated hydrocarbons. ACS Catal. 2017, 7, 6110–6119. [Google Scholar] [CrossRef]
- Higashi, T.; Kusumoto, S.; Nozaki, K. Cleavage of Si–H, B–H, and C–H Bonds by Metal–Ligand Cooperation. Chem. Rev. 2019, 119, 10393–10402. [Google Scholar] [CrossRef]
- Elsby, M.R.; Baker, R.T. Strategies and mechanisms of metal-ligand cooperativity in first-row transition metal complex catalysts. Chem. Soc. Rev. 2020, 49, 8933–8987. [Google Scholar] [CrossRef] [PubMed]
- Milstein, D. Metal–ligand cooperation by aromatization–dearomatization as a tool in single bond activation. Philos. Trans. R. Soc. A 2015, 373, 20140189. [Google Scholar] [CrossRef]
- Van Der Vlugt, J.I. Cooperative catalysis with first-row late transition metals. Eur. J. Inorg. Chem. 2012, 2012, 363–375. [Google Scholar] [CrossRef]
- Grützmacher, H. Cooperating ligands in catalysis. Angew. Chem. Int. Ed. 2008, 47, 1814–1818. [Google Scholar] [CrossRef]
- Hadlington, T.J. Heavier tetrylene- and tetrylyne-transition metal chemistry: It’s no carbon copy. Chem. Soc. Rev. 2024, 53, 9738–9831. [Google Scholar] [CrossRef] [PubMed]
- Somerville, R.J.; Campos, J. Cooperativity in Transition Metal Tetrylene Complexes. Eur. J. Inorg. Chem. 2021, 2021, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.; Gaurav, K.; Khan, S. Applications of low-valent compounds with heavy group-14 elements. Chem. Soc. Rev. 2024, 53, 6150–6243. [Google Scholar] [CrossRef] [PubMed]
- Jutzi, P.; Leue, C. (Supermesityl)chlorogermylene (Supermesityl = Mes* = 2,4,6-tBu3C6H2): Synthesis and Derivatization to (Supermesityl)ferriogermylenes. Organometallics 1994, 13, 2898–2899. [Google Scholar] [CrossRef]
- Hashimoto, H.; Nagata, K. Transition-metal Complexes with Triple Bonds to Si, Ge, Sn, and Pb and Relevant Complexes. Chem. Lett. 2021, 50, 778–787. [Google Scholar] [CrossRef]
- Queen, J.D.; Phung, A.C.; Caputo, C.A.; Fettinger, J.C.; Power, P.P. Metathetical Exchange between Metal–Metal Triple Bonds. J. Am. Chem. Soc. 2020, 142, 2233–2237. [Google Scholar] [CrossRef] [PubMed]
- Jutzi, P.; Leszczyńska, K.; Mix, A.; Neumann, B.; Rummel, B.; Schoeller, W.; Stammler, H.-G. Synthesis and Characterization of the Ferrio-Substituted Silicon(II)Compound Me5C5(CO)2FeSiC5Me. Organometallics 2010, 29, 4759–4761. [Google Scholar] [CrossRef]
- Filippou, A.C.; Baars, B.; Chernov, O.; Lebedev, Y.N.; Schnakenburg, G. Silicon–Oxygen Double Bonds: A Stable Silanone with a Trigonal-Planar Coordinated Silicon Center. Angew. Chem. Int. Ed. 2014, 53, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Watanabe, T.; Tobita, H. Catonic Metallogermylene and Dicationic Dimetallodigermenes: Synthesis by Chloride Abstraction from N-Heterocyclic Carbene-Stabilized Chlorometallogermylenes. J. Am. Chem. Soc. 2014, 136, 14341–14344. [Google Scholar] [CrossRef] [PubMed]
- Inomata, K.; Watanabe, T.; Miyazaki, Y.; Tobita, H. Insertion of a Cationic Metallogermylene into E–H Bonds (E = H, B, Si). J. Am. Chem. Soc. 2015, 137, 11935–11937. [Google Scholar] [CrossRef]
- Based on a Survey of the Cambridge Structural Database (CSD), Version 5.45; Cambridge Crystallographic Data Centre: Cambridge, UK, December 2024.
- Sidiropoulos, A.; Jones, C.; Stasch, A.; Klen, S.; Frening, G. N-Heterocyclic Carbene Stabilized Digermanium(0). Angew. Chem. Int. Ed. 2009, 48, 9701–9704. [Google Scholar] [CrossRef]
- Yakelis, N.A.; Bergman, R.G. Safe Preparation and Purification of Sodium Tetrakis[(3,5-trifluoromethyl)phenyl]borate (NaBArF24): Reliable and Sensitive Analysis of Water in Solutions of Fluorinated Tetraarylborates. Organometallics 2005, 24, 3579–3581. [Google Scholar] [CrossRef] [PubMed]
- Lindsell, W.E.; McCullough, K.J.; Plancq, S. Binuclear complexes of rhodium or copper with pentamethylcyclopentadienyltricarbonyltungsten. Crystal structure of (η5-C5Me5)(CO)W(μ-CO)2Rh(PPh3)2. J. Organomet. Chem. 1995, 491, 275–281. [Google Scholar] [CrossRef]
- Higashi, T. NUMBAS; Rigaku Corporation: Tokyo, Japan, 1995. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Determination; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXS-16/6, Program for Crystal Structure Determination; University of Göttingen: Göttingen, Germany, 2016. [Google Scholar]
- Wakita, K. Yadokari-XG, Software for Crystal Structure Analyses; University of Tokyo: Tokyo, Japan, 2001. [Google Scholar]
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of software (Yadokari-XG 2009) for crystal structure analyses. Nippon. Kessho Gakkaishi 2009, 51, 218–224. (In Japanese) [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Hay, P.J.; Wadt, W.R.J. temporary Σ and Π anions of the chloroethylenes and chlorofluoroethylenes. Chem. Phys. 1985, 82, 270–283. [Google Scholar]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, R.; Nagata, K.; Nakamura, R.; Watanabe, T.; Hashimoto, H. Metal–Ligand Cooperation in Dihydrogen Activation by a Cationic Metallogermylene: Enhanced Activity from Tungsten to Molybdenum. Molecules 2024, 29, 5974. https://doi.org/10.3390/molecules29245974
Matsumoto R, Nagata K, Nakamura R, Watanabe T, Hashimoto H. Metal–Ligand Cooperation in Dihydrogen Activation by a Cationic Metallogermylene: Enhanced Activity from Tungsten to Molybdenum. Molecules. 2024; 29(24):5974. https://doi.org/10.3390/molecules29245974
Chicago/Turabian StyleMatsumoto, Rikiya, Koichi Nagata, Ryo Nakamura, Takahito Watanabe, and Hisako Hashimoto. 2024. "Metal–Ligand Cooperation in Dihydrogen Activation by a Cationic Metallogermylene: Enhanced Activity from Tungsten to Molybdenum" Molecules 29, no. 24: 5974. https://doi.org/10.3390/molecules29245974
APA StyleMatsumoto, R., Nagata, K., Nakamura, R., Watanabe, T., & Hashimoto, H. (2024). Metal–Ligand Cooperation in Dihydrogen Activation by a Cationic Metallogermylene: Enhanced Activity from Tungsten to Molybdenum. Molecules, 29(24), 5974. https://doi.org/10.3390/molecules29245974







