β,β-Difluoro Peroxides as Fluorinated C2-Building Blocks for the Construction of Functionalized Indolizines
Abstract
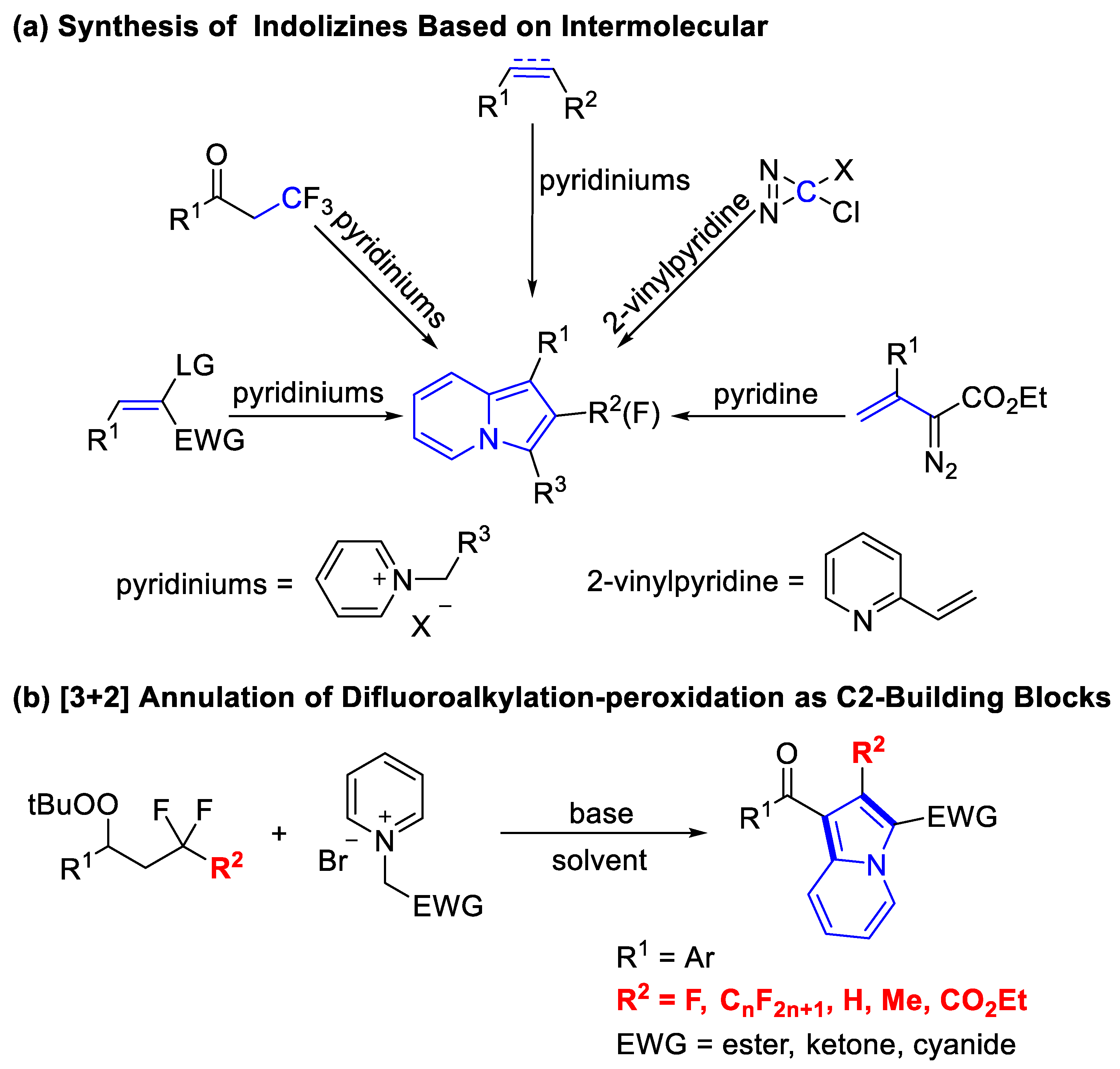
1. Introduction
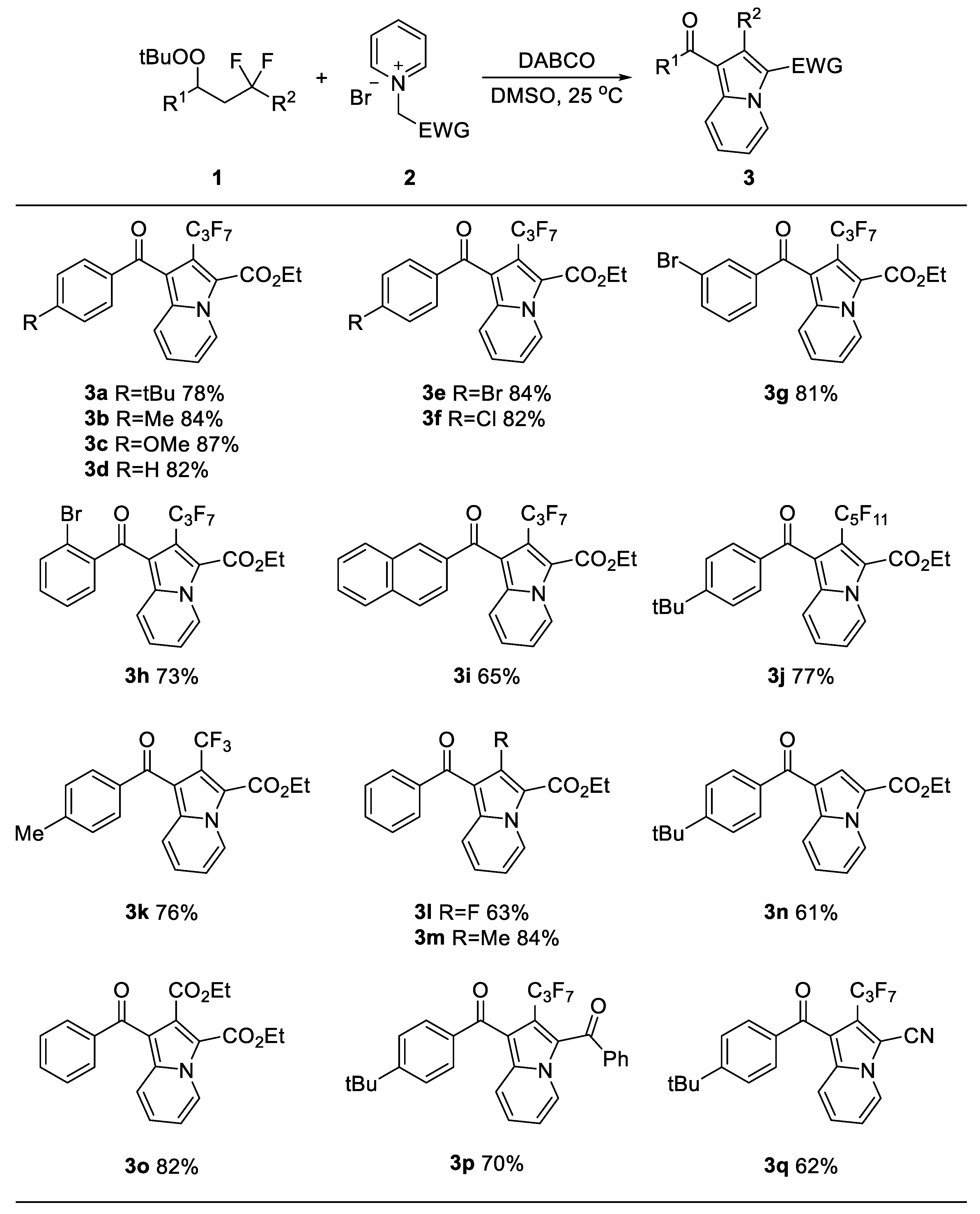
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedures for Synthesis of β,β-Difluoro Peroxides 1
3.3. General Procedures for Synthesis of 1,2,3-Trifunctionalized Indolizines 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, B.M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef] [PubMed]
- Prchalová, E.; Stepánek, O.; Smrcek, S.; Kotora, M. Medicinal Applications of Perfluoroalkylated Chain-containing Compounds. Future Med. Chem. 2014, 6, 1201–1229. [Google Scholar] [CrossRef]
- Han, J.; Remete, A.M.; Dobson, L.S.; Kiss, L.; Izawa, K.; Moriwaki, H.; Soloshonok, V.A.; O’Hagan, D. Next Generation Organofluorine Containing Blockbuster Drugs. J. Fluor. Chem. 2020, 239, 109639. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Kirk, K.L. Fluorination in Medicinal Chemistry: Methods, Strategies, and Recent Developments. Org. Process Res. Dev. 2008, 12, 305–321. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Tu, H.-Y.; Wang, F.; Huo, L.; Li, Y.; Zhu, S.; Zhao, X.; Li, H.; Qing, F.-L.; Chu, L. Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins through Nickel-Catalyzed Cross-Electrophile Coupling. J. Am. Chem. Soc. 2020, 142, 9604–9611. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.-L.; Xu, X.-H.; Qing, F.-L. Oxidative Hydro-, Bromo-, and Chloroheptafluoroisopropylation of Unactivated Alkenes with Heptafluoroisopropyl Silver. Org. Lett. 2019, 21, 9532–9535. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Hu, J. The Unique Fluorine Effects in Organic Reactions: Recent Facts and Insights into Fluoroalkylations. Chem. Soc. Rev. 2016, 45, 5441–5454. [Google Scholar] [CrossRef]
- Zhao, X.; Tu, H.-Y.; Guo, L.; Zhu, S.; Qing, F.-L.; Chu, L. Intermolecular Selective Carboacylation of Alkenes via Nickel-catalyzed Reductive Radical Relay. Nat. Commun. 2018, 9, 3488. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-L.; Liu, J.-B.; Xu, X.-H.; Qing, F.-L. Synthesis of Pentafluoroethyl Ethers by Silver-Mediated Oxidative Pentafluoroethylation of Alcohols and Phenols. J. Org. Chem. 2017, 82, 3702–3709. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Hu, M.; Hu, J. Good Partnership between Sulfur and Fluorine: Sulfur-Based Fluorination and Fluoroalkylation Reagents for Organic Synthesis. Chem. Rev. 2015, 115, 765–825. [Google Scholar] [CrossRef]
- Nagib, D.A.; MacMillan, D.W.C. Trifluoromethylation of Arenes and Heteroarenes by Means of Photoredox Catalysis. Nature 2011, 480, 224–228. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Dixon, J.A.; O’Hara, F.; Funder, E.D.; Dixon, D.D.; Rodriguez, R.A.; Baxter, R.D.; Herle, B.; Sach, N.; Collins, M.R.; et al. Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles. Nature 2012, 492, 95–99. [Google Scholar] [CrossRef]
- Barata-Vallejo, S.; Cooke, M.V.; Postigo, A. Radical Fluoroalkylation Reactions. ACS Catal. 2018, 8, 7287–7307. [Google Scholar] [CrossRef]
- Cheng, B.; Ge, D.; Wang, X.; Chu, X. Perfluoroalkyl Halides as Fluorine-Containing Building Blocks for the Synthesis of Fluoroalkylated Heterocycles. Chin. J. Org. Chem. 2021, 41, 1925–1938. [Google Scholar] [CrossRef]
- Meador, W.E.; Autry, S.A.; Bessetti, R.N.; Gayton, J.N.; Flynt, A.S.; Hammer, N.I.; Delcamp, J.H. Water-Soluble NIR Absorbing and Emitting Indolizine Cyanine and Indolizine Squaraine Dyes for Biological Imaging. J. Org. Chem. 2020, 85, 4089–4095. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, B.B.; da Silva, T.S.; Correia, J.T.M.; Coelho, F. Brønsted-acid-catalyzed selective Friedel-Crafts monoalkylation of Isatins with Indolizines in Water. Org. Biomol. Chem. 2020, 18, 7330–7335. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.T.M.; List, B.; Coelho, F. Catalytic Asymmetric Conjugate Addition of Indolizines to α,β-Unsaturated Ketones. Angew. Chem. Int. Ed. 2017, 56, 7967–7970. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pu, X.; Niu, D.; Fu, Z.; Zhang, X. Copper-Catalyzed Asymmetric Propargylation of Indolizines. Org. Lett. 2019, 21, 8553–8557. [Google Scholar] [CrossRef]
- Gundersen, L.-L.; Negussie, A.H.; Rise, F.; Østby, O.B. Antimycobacterial Activity of 1-Substituted Indolizines. Arch. Pharm. 2003, 336, 191–195. [Google Scholar] [CrossRef]
- Hazra, A.; Mondal, S.; Maity, A.; Naskar, S.; Saha, P.; Paira, R.; Sahu, K.B.; Paira, P.; Ghosh, S.; Sinha, C.; et al. Amberlite-IRA-402 (OH) ion Exchange Resin Mediated Synthesis of Indolizines, Pyrrolo [1,2-a] Quinolines and Isoquinolines: Antibacterial and Antifungal Evaluation of the Products. Eur. J. Med. Chem. 2011, 46, 2132–2140. [Google Scholar] [CrossRef]
- Bloch, W.M.; Derwent-Smith, S.M.; Issa, F.; Morris, J.C.; Rendina, L.M.; Sumby, C.J. Fused Pyrazino[2,3-b]indolizine and Indolizino[2,3-b]quinoxaline Derivatives; Synthesis, Structures, and Properties. Tetrahedron 2011, 67, 9368–9375. [Google Scholar] [CrossRef]
- Dawood, K.M.; Abdel-Gawad, H.; Ellithey, M.; Mohamed, H.A.; Hegazi, B. Synthesis, Anticonvulsant, and Anti-inflammatory Activities of Some New Benzofuran-based Heterocycles. Arch. Pharm. 2006, 339, 133–140. [Google Scholar] [CrossRef]
- Østby, O.B.; Dalhus, B.; Gundersen, L.-L.; Rise, F.; Bast, A.; Haenen, G.R.M.M. Synthesis of 1-Substituted 7-Cyano-2,3-diphenylindolizines and Evaluation of Antioxidant Properties. Eur. J. Org. Chem. 2000, 2000, 3763–3770. [Google Scholar] [CrossRef]
- Sadowski, B.; Klajn, J.; Gryko, D.T. Recent Advances in the Synthesis of Indolizines and Their π-Expanded Analogues. Org. Biomol. Chem. 2016, 14, 7804–7828. [Google Scholar] [CrossRef]
- Yadagiri, D.; Rivas, M.; Gevorgyan, V. Denitrogenative Transformations of Pyridotriazoles and Related Compounds: Synthesis of N-Containing Heterocyclic Compounds and Beyond. J. Org. Chem. 2020, 85, 11030–11046. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Liu, Z.; Bi, W.; Shen, J.; Li, G. Efficient Solvent-Free Synthesis of Indolizines Using CuBr Catalyst from Pyridine, Acetophenone, and Electron-Deficient Alkenes. Molecules 2024, 29, 2061. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xu, Y.-H.; Han, J.; Deng, H.-M.; Shao, M.; Chen, J.; Zhang, H.; Cao, W.-G. Facile One-pot Tandem Synthesis of Perfluoroalkylated Indolizines under Metal-Free Mild Conditions. Tetrahedron 2017, 73, 938–944. [Google Scholar] [CrossRef]
- Shen, B.-X.; Li, B.; Wang, B.-Q. Rh(III)-Catalyzed Oxidative Annulation Leading to Substituted Indolizines by Cleavage of C(sp2)-H/C(sp3)-H Bonds. Org. Lett. 2016, 18, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Brioche, J.; Meyer, C.; Cossy, J. Synthesis of 2-Aminoindolizines by 1,3-Dipolar Cycloaddition of Pyridinium Ylides with Electron-Deficient Ynamides. Org. Lett. 2015, 17, 2800–2803. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Qandalee, M.; Behboodi, V.; Gorji, A.N.; Pasha, G.F. Chemoselective Synthesis of Novel Aminoindolizines Using Aminopyridines, Acetylenic Diesters and α-Halo Ketones. Chin. Chem. Lett. 2016, 27, 361–364. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Feng, J.-J.; Zhu, Y.-L.; Gu, N.; Kan, Y.-H. Copper Acetate Monohydrate: A Cheap but Efficient Oxidant for Synthesizing Multi-Substituted Indolizines from Pyridinium Ylides and Electron Deficient Alkenes. RSC Adv. 2012, 2, 8637–8644. [Google Scholar] [CrossRef]
- Bakshi, D.; Singh, A. Transition-Metal-Free Synthesis of Nitrogen Containing Heterocycles with Fully Substituted N-fused Pyrrole Rings. Asian J. Org. Chem. 2016, 5, 70–73. [Google Scholar] [CrossRef]
- Shu, S.; Wang, L.; Lv, L.; Li, Z. Copper-Catalyzed [3+2] Annulation of Pyridinium Ylides with α-CF3 Ketones: Synthesis of Functionalized 2-Fluoroindolizines. Asian J. Org. Chem. 2023, 12, e202300075. [Google Scholar] [CrossRef]
- Bonneau, R.; Ramoshin, Y.N.; Liu, M.T.H.; MacPherson, S.E. Synthesis of 3-Substituted Indolizines from the Reaction of Chlorocarbenes with 2-Vinylpyridine. J. Chem. Soc. Chem. Commun. 1994, 4, 509–510. [Google Scholar] [CrossRef]
- Barluenga, J.; Lonzi, G.; Riesgo, L.; LóPez, L.A.; TomáS, M. Pyridine Activation via Copper(I)-Catalyzed Annulation toward Indolizines. J. Am. Chem. Soc. 2010, 132, 13200–13202. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.A.; Murphy, M. The Synthesis of Indolizines: The Reaction of .alpha.-halo Pyridinium Salts with .beta.-Dicarbonyl Species. J. Org. Chem. 1987, 52, 2206–2208. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Lou, C.; Li, Z. DABCO-Mediated [4+1] Cycloaddition of β,β-Dihalo Peroxides with Sodium Azide toward Isoxazoles. Asian J. Org. Chem. 2020, 9, 1018–1021. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, L.; Li, Z. β-Perfluoroalkyl Peroxides as Fluorinated C3-Building Blocks for the Construction of Benzo[4,5]imidazo[1,2-a]pyridines. J. Org. Chem. 2022, 87, 1564–1573. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Lv, L.; Li, Z. Regioselective Synthesis of Emission Color-Tunable Pyrazolo[1,5-a]pyrimidines with β,β-Difluoro Peroxides as 1,3-Bis-Electrophiles. Adv. Synth. Catal. 2021, 363, 3233–3239. [Google Scholar] [CrossRef]
- The pKa Data Can Be Obtained Free of Charge. Available online: https://organicchemistrydata.org/hansreich/resources/pka/#pka_-water_compilation_evans (accessed on 1 November 2024).
- Yang, L.-M.; Zhang, Y.-Y.; Deng, J.-T.; Ma, A.-J.; Zhang, X.-Z.; Zhang, S.-Y.; Peng, J.-B. Oxidative [3+2] Annulation of Pyridinium Salts with gem-Difluoroalkenes: Synthesis of 2-Fluoroindolizines. Asian J. Org. Chem. 2021, 10, 1679–1682. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Hu, D.; Song, J.; Ren, H. [3 + 2]-Annulation of gem-Difluoroalkenes and Pyridinium Ylides: Access to Functionalized 2-Fluoroindolizines. J. Org. Chem. 2021, 86, 4646–4660. [Google Scholar] [CrossRef]
- Shi, E.; Liu, J.; Liu, C.; Shao, Y.; Wang, H.; Lv, Y.; Ji, M.; Bao, X.; Wan, X. Difunctionalization of Styrenes with Perfluoroalkyl and tertButylperoxy Radicals: Room Temperature Synthesis of (1-(tertbutylperoxy)-2-perfluoroalkyl)-ethylbenzene. J. Org. Chem. 2016, 81, 5878–5885. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Ma, Y.; Li, Z. Cobalt-Catalyzed Three-Component Difluoroalkylation-Peroxidation of Alkenes. J. Org. Chem. 2019, 84, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lou, C.; Lv, L.; Li, Z. Photoinduced Fluoroalkylation-Peroxidation of Alkenes Enabled by Ligand-to-Iron Charge Transfer Mediated Decarboxylation. Chem. Commun. 2024, 60, 12389–12392. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lv, L.; Ma, Y.; Li, Z. Copper-Catalyzed Radical Difluoromethylation-Peroxidation of Alkenes: Synthesis of β-Difluoromethyl Peroxides. Asian J. Org. Chem. 2022, 11, e202200393. [Google Scholar] [CrossRef]


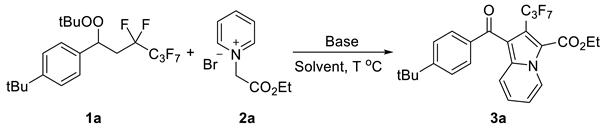 | ||||
| Entry | Base | Solvent | T (°C) | Yield (%) b |
| 1 | DABCO | MeCN | 25 | 54 |
| 2 | DIPEA | MeCN | 25 | N.R. |
| 3 | NEt3 | MeCN | 25 | trace |
| 4 | K3PO4 | MeCN | 25 | N.R. |
| 5 | CsCO3 | MeCN | 25 | trace |
| 6 | KOH | MeCN | 25 | N.R. |
| 7 | DABCO | EA | 25 | 11 |
| 8 | DABCO | DCM | 25 | 13 |
| 9 | DABCO | MeOH | 25 | trace |
| 10 | DABCO | DMF | 25 | 59 |
| 11 | DABCO | DMSO | 25 | 82 |
| 12 | DABCO | DMSO | 60 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, H.; Du, X.; Fang, S.; Yin, K.; Du, G.; Tian, Z.; Zhou, Z. β,β-Difluoro Peroxides as Fluorinated C2-Building Blocks for the Construction of Functionalized Indolizines. Molecules 2024, 29, 5927. https://doi.org/10.3390/molecules29245927
Ma Y, Zhang H, Du X, Fang S, Yin K, Du G, Tian Z, Zhou Z. β,β-Difluoro Peroxides as Fluorinated C2-Building Blocks for the Construction of Functionalized Indolizines. Molecules. 2024; 29(24):5927. https://doi.org/10.3390/molecules29245927
Chicago/Turabian StyleMa, Yangyang, Hua Zhang, Xiaoxiao Du, Shurui Fang, Kexin Yin, Gangfeng Du, Zhengshan Tian, and Zhonghao Zhou. 2024. "β,β-Difluoro Peroxides as Fluorinated C2-Building Blocks for the Construction of Functionalized Indolizines" Molecules 29, no. 24: 5927. https://doi.org/10.3390/molecules29245927
APA StyleMa, Y., Zhang, H., Du, X., Fang, S., Yin, K., Du, G., Tian, Z., & Zhou, Z. (2024). β,β-Difluoro Peroxides as Fluorinated C2-Building Blocks for the Construction of Functionalized Indolizines. Molecules, 29(24), 5927. https://doi.org/10.3390/molecules29245927





