[2+2]-Photocycloadditions of 1,4-Naphthoquinone Under Batch and Continuous-Flow Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Photocycloadditions Under Batch Conditions
2.1.1. Optimization Studies
Solvent Optimization
Wavelength Optimization
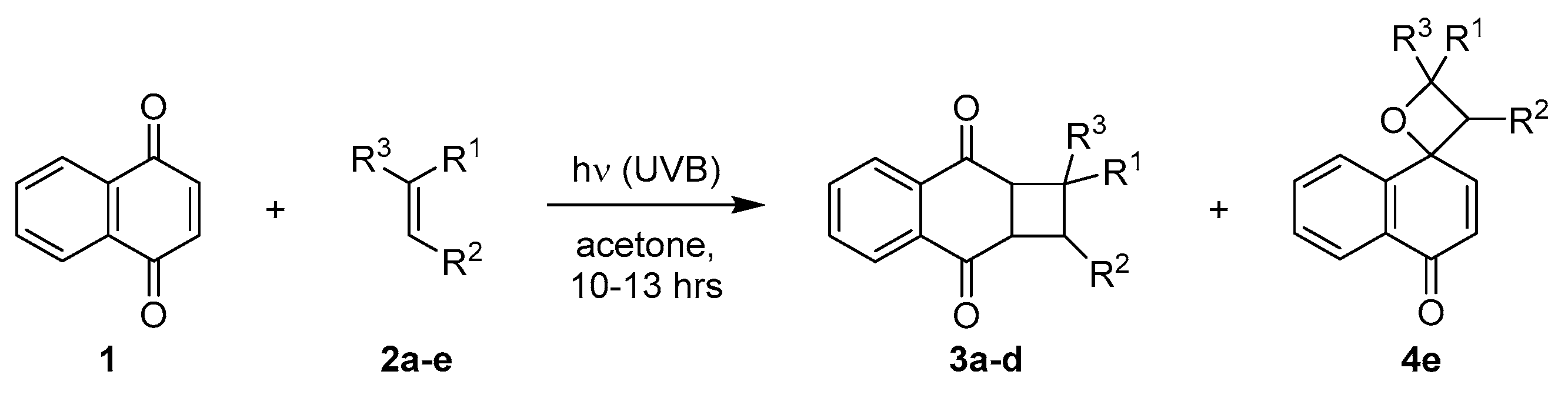
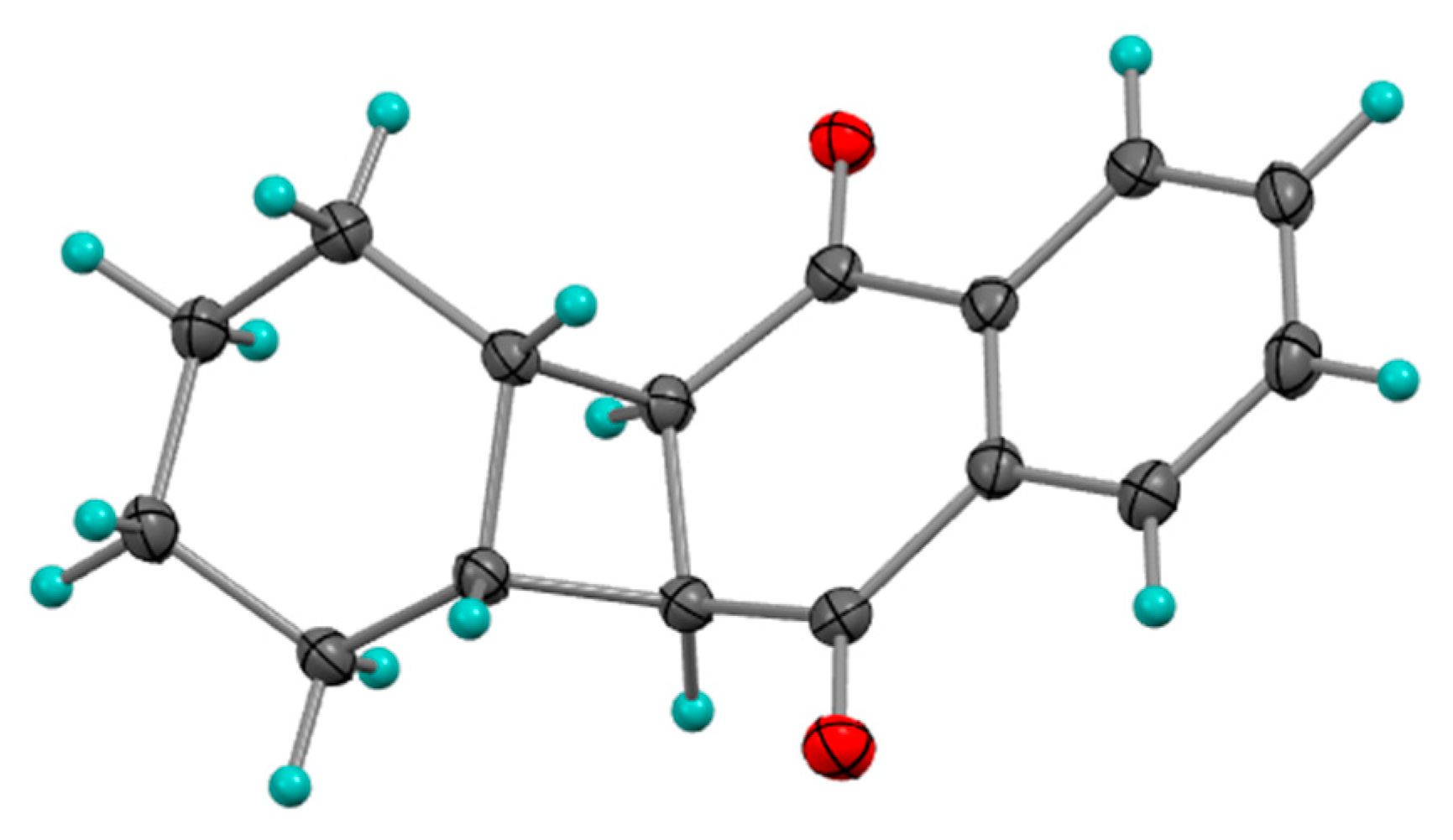
2.1.2. Photocycloadditions with Other Alkenes
Photocycloaddition with Styrene
Photocycloaddition with 1,1-Diphenyl Ethylene
Photocycloaddition with Cyclopentene
Photocycloaddition with Cyclohexene
Photocycloaddition with Trans-Stilbene
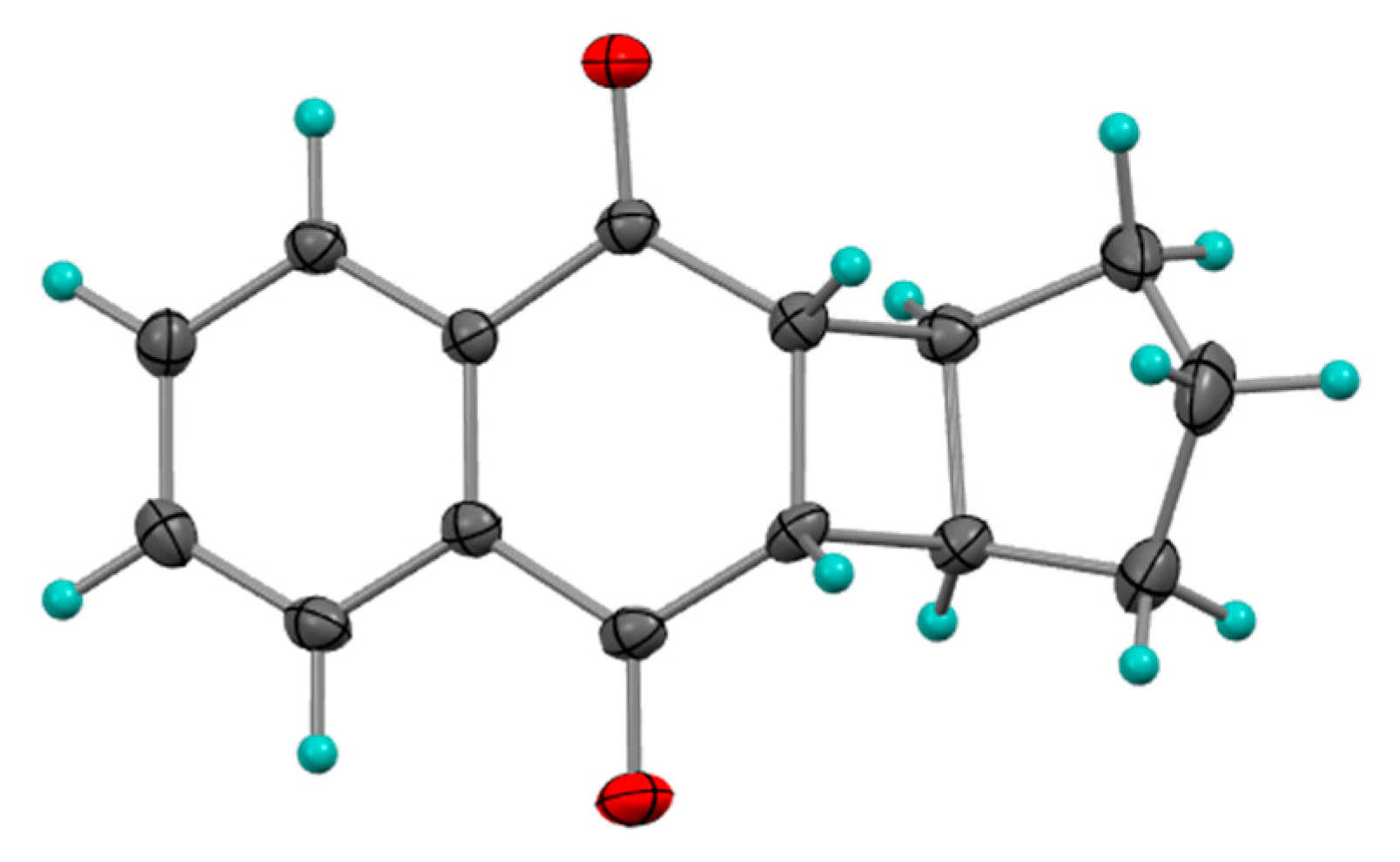
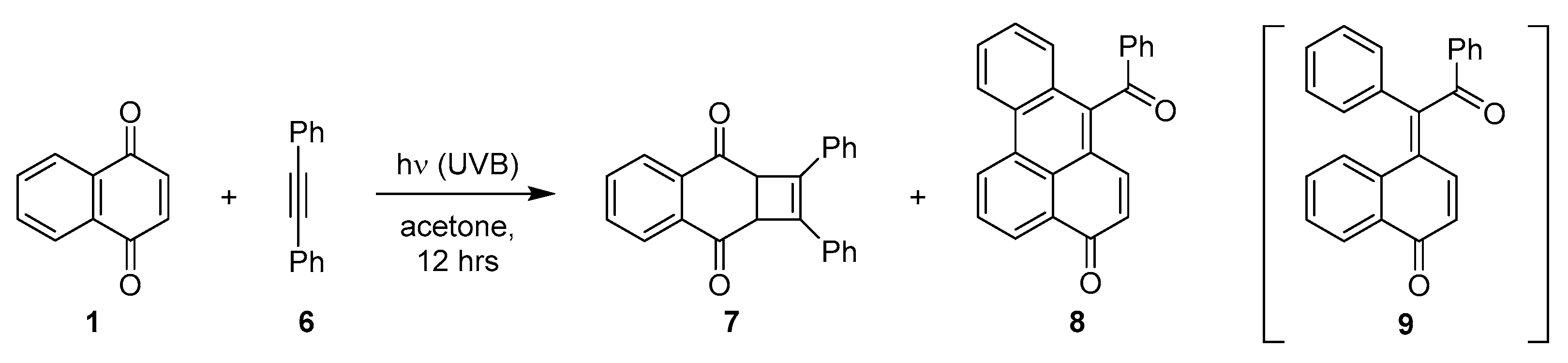
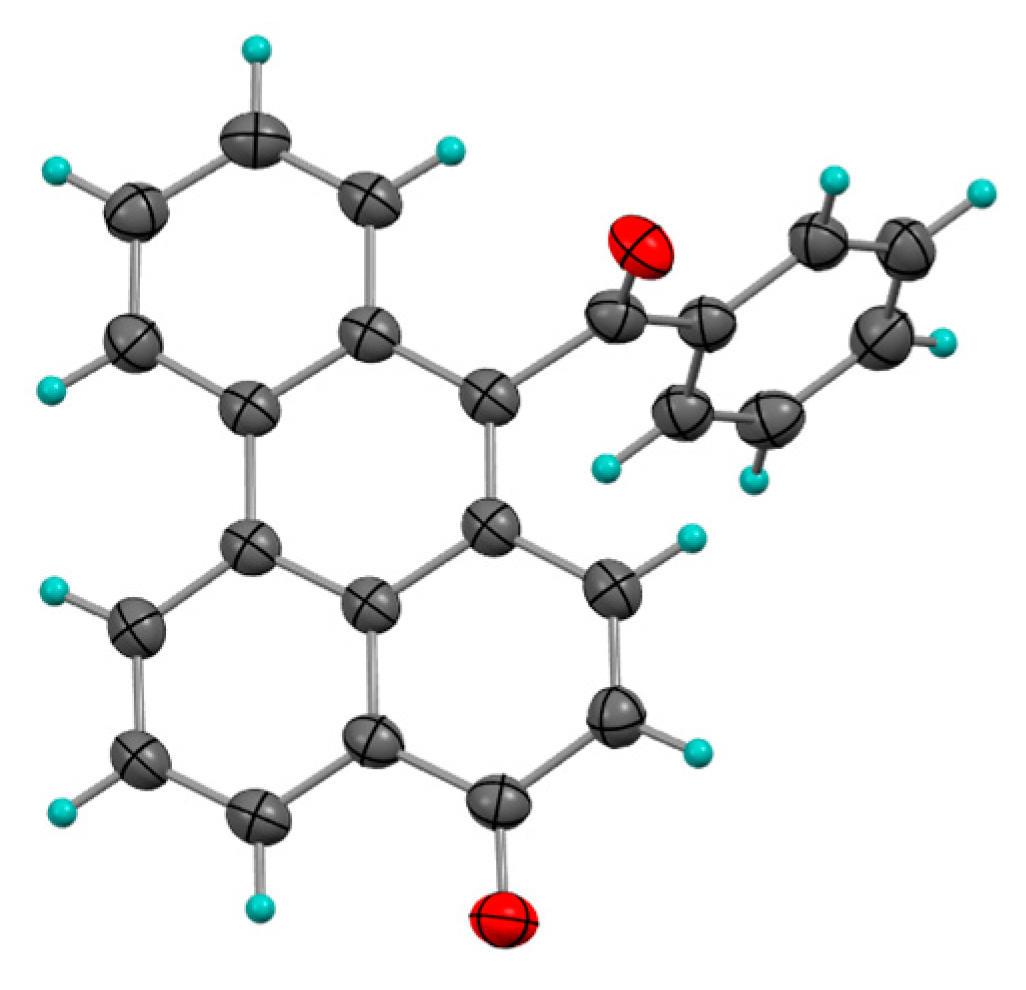
2.1.3. Photocycloadditions with Diphenylacetylene
2.2. Photocycloadditions Under Continuous-Flow Conditions
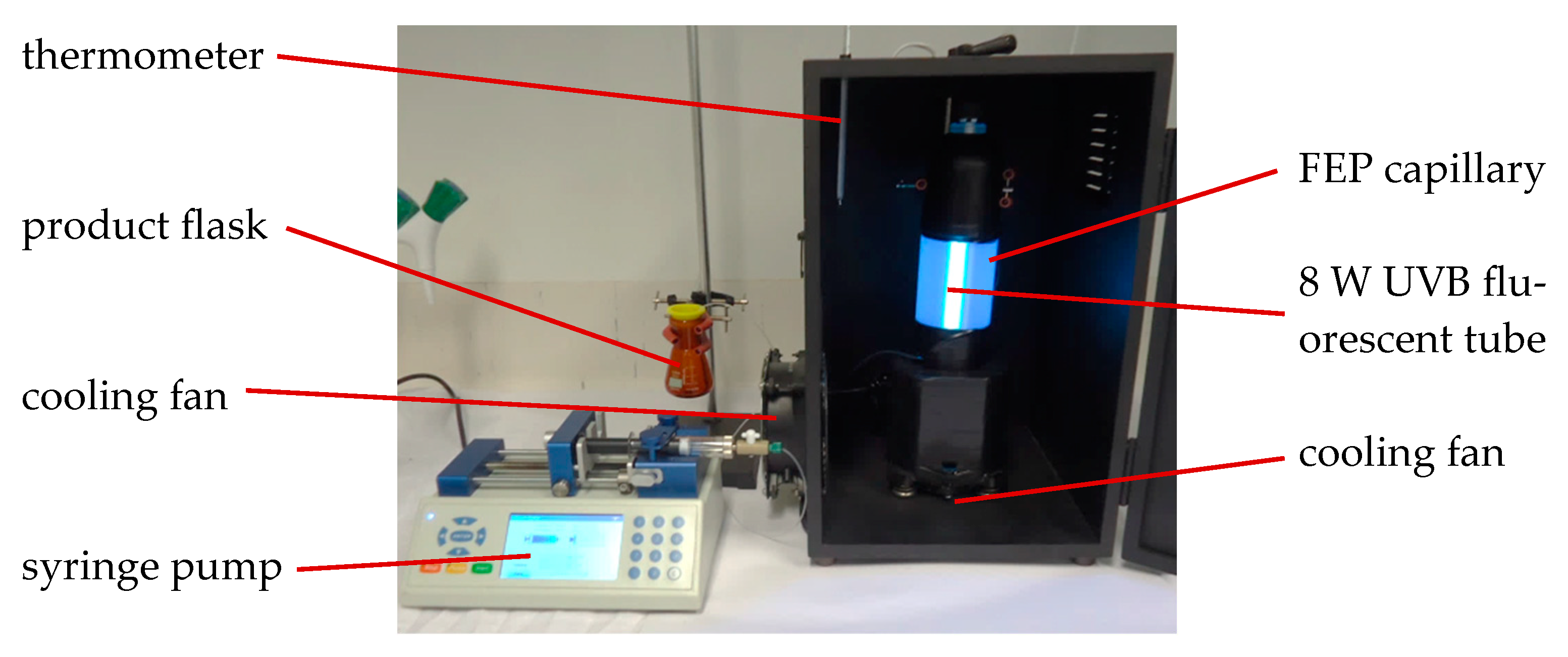
2.2.1. In-House Capillary Reactor
2.2.2. Residence Time and Flow Rate Study
2.2.3. Photocycloadditions with Other Alkenes
2.2.4. Photocycloadditions with Diphenylacetylene
3. Materials and Methods
3.1. General Information
3.2. Photocycloadditions
- General procedure for UVB photocycloadditions under batch conditions
- General procedure for UVB photocycloadditions under continuous-flow conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jha, R.K.; Kumar, S. Direct Functionalization of para-Quinones: A Historical Review and New Perspectives. Eur. J. Org. Chem. 2024, 27, e202400535. [Google Scholar] [CrossRef]
- Mone, N.S.; Bhagwat, S.A.; Sharma, D.; Chaskar, M.; Patil, R.H.; Zamboni, P.; Nawani, N.N.; Satpute, S.K. Naphthoquinones and Their Derivatives: Emerging Trends in Combating Microbial Pathogens. Coatings 2021, 11, 434. [Google Scholar] [CrossRef]
- Ahmadi, E.S.; Tajbakhsh, A.; Iranshahy, M.; Asili, J.; Kretschmer, N.; Shakeri, A.; Sahebkar, A. Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity. Mini-Rev. Med. Chem. 2020, 20, 2019–2035. [Google Scholar] [CrossRef] [PubMed]
- López, L.I.; Leyva, E.; García de la Cruz, R.F. Naphthoquinones: More than natural pigments. Rev. Mex. Cienc. Farm. 2011, 42, 6–17. [Google Scholar]
- Jang, J.; Lee, G.; Cho, E.J. Visible light induced reactions of quinones. Bull. Korean Chem. Soc. 2024, 45. in print. [Google Scholar] [CrossRef]
- de Lucas, N.C.; Ferreira, A.B.B.; Netto-Ferreira, J.C. Fotoquímica de Naftoquinonas. Rev. Virtual Quim. 2015, 7, 403–463. [Google Scholar] [CrossRef]
- Maruyama, K.; Osuka, A. Recent advances in the photochemistry of quinones. In The Chemistry of Quinonoid Compounds; Patai, S., Rappaport, Z., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 1988; Volume 2, Part 1, Chapter 13; pp. 759–878. [Google Scholar] [CrossRef]
- Gilbert, A. 1,4-Quinone Cycloaddition Reactions with Alkenes, Alkynes, and Related Compounds. In CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W.M., Lenci, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; Chapter 87; pp. 1–12. [Google Scholar] [CrossRef]
- Creed, D. 1,4-Quinone cycloaddition reactions with alkene, alkynes and related compounds. In CRC Handbook of Organic Photochemistry and Photobiology; Horspool, W.M., Song, P.-S., Eds.; CRC Press: Boca Raton, FL, USA, 1995; Chapter 59; pp. 737–747. [Google Scholar]
- Maejima, S.; Yamaguchi, E.; Itoh, A. Visible-Light-Induced Regioselective Functionalization of α-Olefin: Development of One-Pot Photo-Synthesis of C3-Substituted Dihydrobenzofurans. Org. Lett. 2023, 25, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, F.; Zhang, X.; Gao, Y.; Su, W. Visible-Light-Induced Paternò-Büchi Reaction of Anthraquinones for the Synthesis of Spirocyclic Oxetanes. Asian J. Org. Chem. 2023, 12, e202300069. [Google Scholar] [CrossRef]
- Tan, S.-B.; Huang, C.; Chen, X.; Wu, Y.; Zhou, M.; Zhang, C.; Zhang, Y. Small molecular inhibitors of miR-1 identified from photocycloadducts of acetylenes with 2-methoxy-1,4-naphthalenequinone. Bioorg. Med. Chem. 2013, 21, 6124–6131. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, C.; Zhang, W.; Wu, Y.; Chen, X.; Zhang, C.-Y.; Zhang, Y. A universal activator of micro RNAs identified from photoreaction products. Chem. Comm. 2012, 48, 6432–6434. [Google Scholar] [CrossRef] [PubMed]
- Bochet, C.G. On the Sustainability of Photochemical Reactions. Chimia 2019, 73, 720–723. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M.; Albini, A. Visible Light Photocatalysis. A Green Choice? Curr. Org. Chem. 2013, 17, 2366–2373. [Google Scholar] [CrossRef]
- Alfano, A.I.; García-Lacuna, J.; Griffiths, O.M.; Ley, S.V.; Baumann, M. Continuous Flow Synthesis enabling Reaction Discovery. Chem. Sci. 2024, 15, 4618–4630. [Google Scholar] [CrossRef] [PubMed]
- Alfano, A.I.; Pelliccia, S.; Rossino, G.; Chianese, O.; Summa, V.; Collina, S.; Brindisi, M. Continuous-Flow Technology for Chemical Rearrangements: A Powerful Tool to Generate Pharmaceutically Relevant Compounds. ACS Med. Chem. Lett. 2023, 14, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, G.; Sharley, J.S.; Baxendale, I.R. A Comprehensive Review of Flow Chemistry Techniques Tailored to the Flavours and Fragrances Industries. Beilstein J. Org. Chem. 2021, 17, 1181–1312. [Google Scholar] [CrossRef]
- Fukuyama, T.; Kasakado, T.; Hyodo, M.; Ryu, I. Improved Efficiency of Photo-induced Synthetic Reactions Enabled by Advanced Photo Flow Technologies. Photochem. Photobiol. Sci. 2022, 21, 761–775. [Google Scholar] [CrossRef]
- Politano, F.; Oksdath-Mansilla, G. Light on the Horizon: Current Research and Future Perspectives in Flow Photochemistry. Org. Process Res. Dev. 2018, 22, 1045–1062. [Google Scholar] [CrossRef]
- Rehm, T.H. Flow Photochemistry as a Tool in Organic Synthesis. Chem. Eur. J. 2020, 26, 16952–16974. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Hoffmann, N.; Shvydkiv, O. From ‘Lab & Light on a Chip’ to Parallel Microflow Photochemistry. Austr. J. Chem. 2014, 67, 337–342. [Google Scholar] [CrossRef]
- Sender, M.; Ziegenbalg, D. Light Sources for Photochemical Processes—Estimation of Technological Potentials. Chem. Ing. Tech. 2017, 89, 1159–1173. [Google Scholar] [CrossRef]
- Khan, H.; Rajesh, V.M.; Ravva, M.K.; Sen, S. Optimization of Blue LED Photo-Flow Synthesis in Continuous Flow Reactors Using Design of Experiments (DoE): Efficient Synthesis of Diverse Diaryl Ketones. Chem. Eng. J. 2024, 501, 157657. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Mumtaz, S.; Hunter, R.L.; Wall, D.; Belluau, V.; Robertson, M.J.; Oelgemöller, M. Continuous-Flow Photochemical Transformations of 1,4-Naphthoquinones and Phthalimides in a Concentrating Solar Trough Reactor. Austr. J. Chem. 2020, 73, 1149–1157, Erratum in Austr. J. Chem. 2020, 73, 1301. [Google Scholar] [CrossRef]
- Maruyama, K.; Otsuki, T.; Takuwa, A.; Kako, S. Photochemical Reaction of 1, 4-Naphthoquinone with Olefins. Bull. Inst. Chem. Res. Kyoto Uni. 1972, 50, 344–347. [Google Scholar]
- Dekker, J.; van Vuuren, P.J.; Venter, D.P. Photodimerization. I. The syn and anti-Photodimers of 1,4-Naphthoquinone. J. Org. Chem. 1968, 33, 464–466. [Google Scholar] [CrossRef]
- Bunce, N.J.; Ridley, J.E.; Zerner, M.C. On the Excited States of p-Quinones and an Interpretation of the Photocycloaddition of p-Quinones to Alkenes. Theoret. Chim. Acta 1977, 45, 283–300. [Google Scholar] [CrossRef]
- Bunce, N.J.; Hadley, M. On the Mechanism of Oxetane Formation in the Photocycloaddition of p-Benzoquinone to Alkenes. Canad. J. Chem. 1975, 53, 3240–3246. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Leshina, T.; Polyakov, N. The Mechanism of Photoreduction of Quinones by Alcohols from Proton CIDNP Data in high and low Magnetic Fields. J. Phys. Chem. 1990, 94, 4379–4382. [Google Scholar] [CrossRef]
- Albini, A. Norrish’ Type I and II Reactions and their Role in the Building of Photochemical Science. Photochem. Photobiol. Sci. 2021, 20, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T. Photo and Radiation Chemistry of Quinones. Proc. Indian Natl. Sci. Acad. USA 2000, 66, 239–266. [Google Scholar]
- Wernerova, M.; Hudlicky, T. On the Practical Limits of Determining Isolated Product Yields and Ratios of Stereoisomers: Reflections, Analysis, and Redemption. Synlett 2010, 2010, 2701–2707. [Google Scholar] [CrossRef]
- Bryce-Smith, D.; Evans, E.H.; Gilbert, A.; McNeill, H.S. Photoaddition of Ethenes to 1,4-Naphthoquinone: Factors Influencing the Site of Reaction. J. Chem. Soc. Perkin Trans.1 1992, 485–489. [Google Scholar] [CrossRef]
- Dragojlovic, V. Conformational analysis of cycloalkanes. ChemTexts 2015, 1, 14. [Google Scholar] [CrossRef]
- Cotton, F.A.; Frenz, B.A. Conformations of cyclobutane. Tetrahedron 1974, 30, 1587–1594. [Google Scholar] [CrossRef]
- Sun, D.; Hubig, S.M.; Kochi, J.K. Oxetanes from [2+2] Cycloaddition of Stilbenes to Quinone via Photoinduced Electron Transfer. J. Org. Chem. 1999, 64, 2250–2258. [Google Scholar] [CrossRef]
- Covell, C.; Gilbert, A.; Richter, C. Sunlight-induced Regio-and Stereo-specific (2π+2π) Cycloaddition of Arylethenes to 2-Substituted-1, 4-naphthoquinones. J. Chem. Res. Synop. 1998, 316–317. [Google Scholar] [CrossRef]
- Waldeck, D.H. Photoisomerization Dynamics of Stilbenes. Chem. Rev. 1991, 91, 415–436. [Google Scholar] [CrossRef]
- Moore, W.M.; Morgan, D.D.; Stermitz, F.R. The Photochemical Conversion of Stilbene to Phenanthrene. The Nature of the Intermediate. J. Am. Chem. Soc. 1963, 85, 829–830. [Google Scholar] [CrossRef]
- Farid, S.; Kothe, W.; Pfundt, G. Competitive Photoadditions of Acetylenes to the C=C and C=O Bonds of p-Quinones. Tetrahedron Lett. 1968, 9, 4147–4150. [Google Scholar] [CrossRef]
- Bosch, E.; Hubig, S.; Kochi, J. Paterno-Büchi Coupling of (Diaryl) Acetylenes and Quinone via Photoinduced Electron Transfer. J. Am. Chem. Soc. 1998, 120, 386–395. [Google Scholar] [CrossRef]
- Pappas, S.; Portnoy, N.A. Substituent Effects on the Photoaddition of Diphenylacetylene to 1, 4-Naphthoquinones. J. Org. Chem. 1968, 33, 2200–2203. [Google Scholar] [CrossRef]
- Mumtaz, S.; Robertson, M.J.; Oelgemöller, M. Continuous Flow Photochemical and Thermal Multi-step Synthesis of Bioactive 3-Arylmethylene-2,3-dihydro-1H-isoindolin-1-ones. Molecules 2019, 24, 4527. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sabio, J.C.; Hartman, R.L. When Solids Stop Flow Chemistry in Commercial Tubing. J. Flow Chem. 2015, 5, 166–171. [Google Scholar] [CrossRef]
- Cowieson, N.P.; Aragao, D.; Clift, M.; Ericsson, D.J.; Gee, C.; Harrop, S.J.; Mudie, N.; Panjikar, S.; Price, J.R.; Riboldi-Tunnicliffe, A.; et al. MX1: A Bending-magnet Crystallography Beamline Serving both Chemical and Macromolecular Crystallography Communities at the Australian Synchrotron. J. Synchrotron Rad. 2015, 22, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.; Kothe, W.; Pfundt, G. NMR-study of Cyclobutene Derivatives. Tetrahedron Lett. 1968, 9, 4151–4154. [Google Scholar] [CrossRef]
- Donnelly, K.; Baumann, M. Scalability of Photochemical Reactions in Continuous Flow Mode. J. Flow Chem. 2021, 11, 223–241. [Google Scholar] [CrossRef]
- Hunter, R.; Josland, S.; Moore, J.; Guthrie, D.; Robertson, M.J.; Oelgemöller, M. Rapid Photochemical Reaction Studies under Continuous-flow Conditions in the Vapourtec UV-150 Reactor—A Technical Note. Curr. Org. Chem. 2018, 22, 2501–2508. [Google Scholar] [CrossRef]

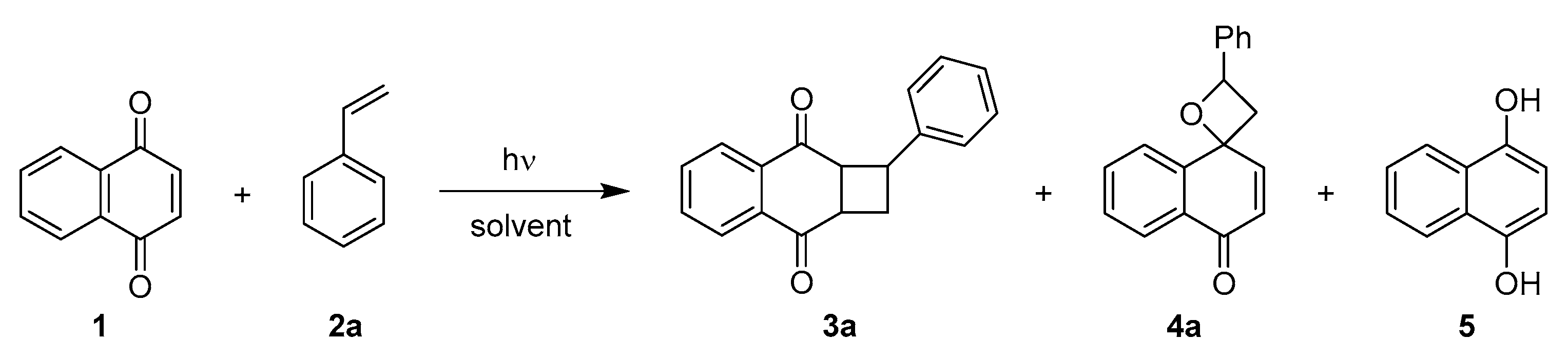

| Entry | Solvent | Conversion (%) 1 | Composition 1 | Yield of 3a (%) 2 | |
|---|---|---|---|---|---|
| 3a (%) | 4a (%) | ||||
| 1 | acetonitrile | 88 | 93 | 7 | 51 |
| 2 | trifluorotoluene | 87 | 94 | 6 | 58 |
| 3 | chloroform | 100 | 78 | 22 | 55 |
| 4 | acetone | 96 | 100 | 0 | 69 |
| 5 | methanol | 100 | photoreduction to 5 | n.d. 3 | |
| Entry | Irradiation Conditions | Solvent | Conversion (%) 1 | Composition 1 | |
|---|---|---|---|---|---|
| 3a (%) | 4a (%) | ||||
| 1 | 400–700 nm, 2 Pyrex | acetone | 73 | 63 | 37 |
| 2 | 419 ± 25 nm, Pyrex | acetone | 89 | 82 | 18 |
| 3 | 419 ± 25 nm, Pyrex | trifluorotoluene | 74 | 81 | 19 |
| 4 | 419 ± 25 nm, Pyrex | acetonitrile | 69 | 90 | 10 |
| 5 | 350 ± 25 nm, Pyrex | acetone | 93 | 95 | 5 |
| 6 | 300 ± 25 nm, Pyrex | acetone | 96 | 100 | 0 |
| 7 | 300 ± 25 nm, Quartz | acetone | 96 | 100 | 0 |
| 8 | 254 nm, Quartz | acetone | 91 | 100 | 0 |
| 9 | 254 nm, Quartz | acetonitrile | 65 | 100 | 0 |
| Entry | Alkene (2) | Time (h) | Conversion (%) 1 | Yield (%) 2 | ||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | ||||
| 1 | Ph | H | H | 10 | 96 | 69 (3a) |
| 2 | Ph | H | Ph | 12 | 78 | 42 (3b) |
| 3 | -(CH2)3- | H | 13 | 67 | 50 (3c) | |
| 4 | -(CH2)4- | H | 12 | 84 | 25 (3d) | |
| 5 | H | Ph | Ph | 13 | 40 | 27 (4e) |
| 6 3 | H | Ph | Ph | 6 | 93 | 78 (4e) |
| Entry | Alkene (2) | Conversion (%) 1 | Yield (%) 2 | ||
|---|---|---|---|---|---|
| R1 | R2 | R3 | |||
| 1 | Ph | H | H | 99 | 84 (3a) |
| 2 | Ph | H | Ph | 86 | 60 (3b) |
| 3 | -(CH2)3- | H | 88 | 73 (3c) | |
| 4 | -(CH2)4- | H | 78 | 55 (4d) | |
| 5 | H | Ph | Ph | 50 | 47 (4e) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, M.A.; Guo, Z.; Junk, P.C.; Oelgemöller, M. [2+2]-Photocycloadditions of 1,4-Naphthoquinone Under Batch and Continuous-Flow Conditions. Molecules 2024, 29, 5920. https://doi.org/10.3390/molecules29245920
Yaseen MA, Guo Z, Junk PC, Oelgemöller M. [2+2]-Photocycloadditions of 1,4-Naphthoquinone Under Batch and Continuous-Flow Conditions. Molecules. 2024; 29(24):5920. https://doi.org/10.3390/molecules29245920
Chicago/Turabian StyleYaseen, Madyan A., Zhifang Guo, Peter C. Junk, and Michael Oelgemöller. 2024. "[2+2]-Photocycloadditions of 1,4-Naphthoquinone Under Batch and Continuous-Flow Conditions" Molecules 29, no. 24: 5920. https://doi.org/10.3390/molecules29245920
APA StyleYaseen, M. A., Guo, Z., Junk, P. C., & Oelgemöller, M. (2024). [2+2]-Photocycloadditions of 1,4-Naphthoquinone Under Batch and Continuous-Flow Conditions. Molecules, 29(24), 5920. https://doi.org/10.3390/molecules29245920








