Adsorption and Removal of 2,4,6-Trinitrotoluene by a Glycoluril-Derived Molecular-Clip-Based Supramolecular Organic Framework
Abstract
1. Introduction
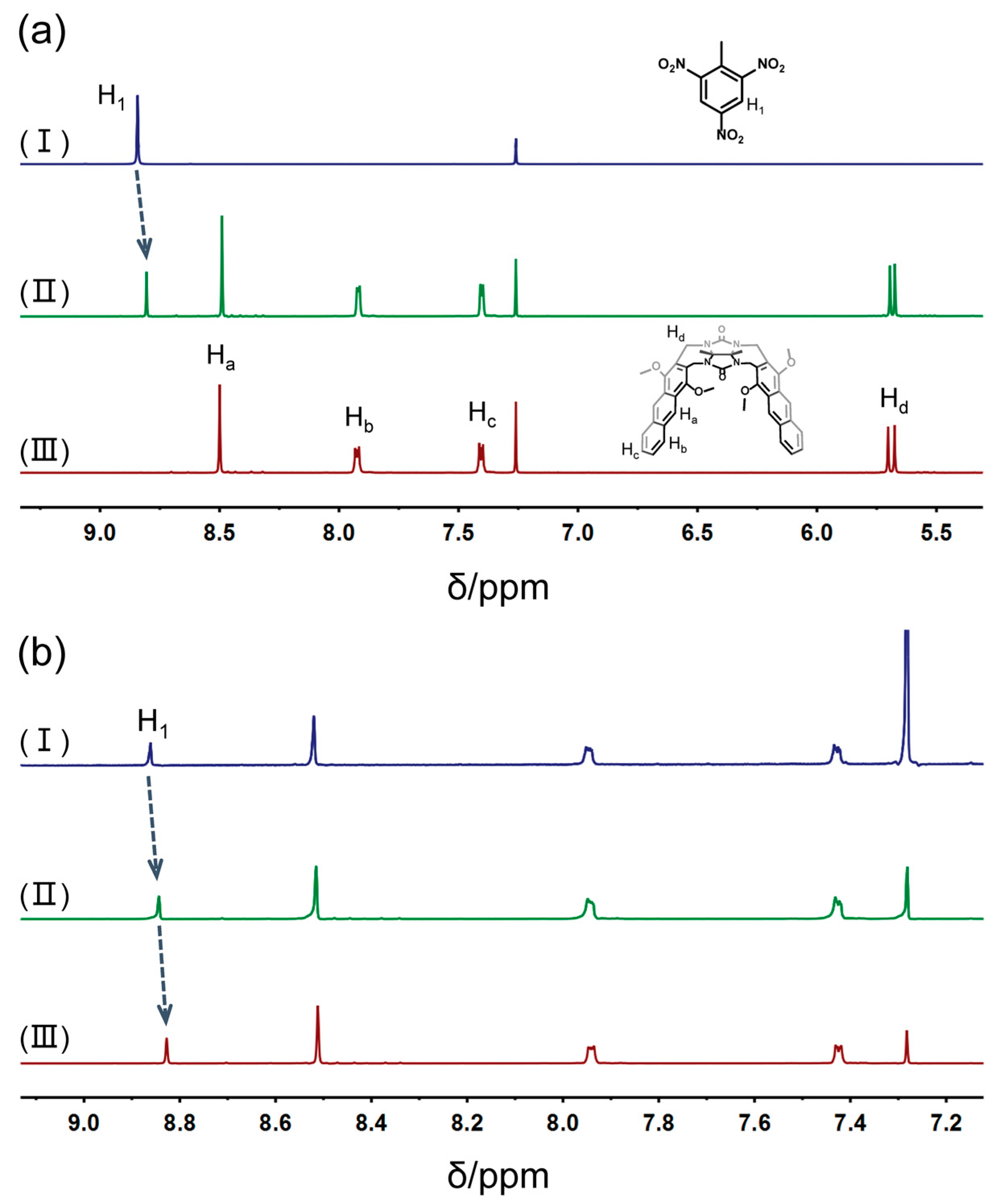
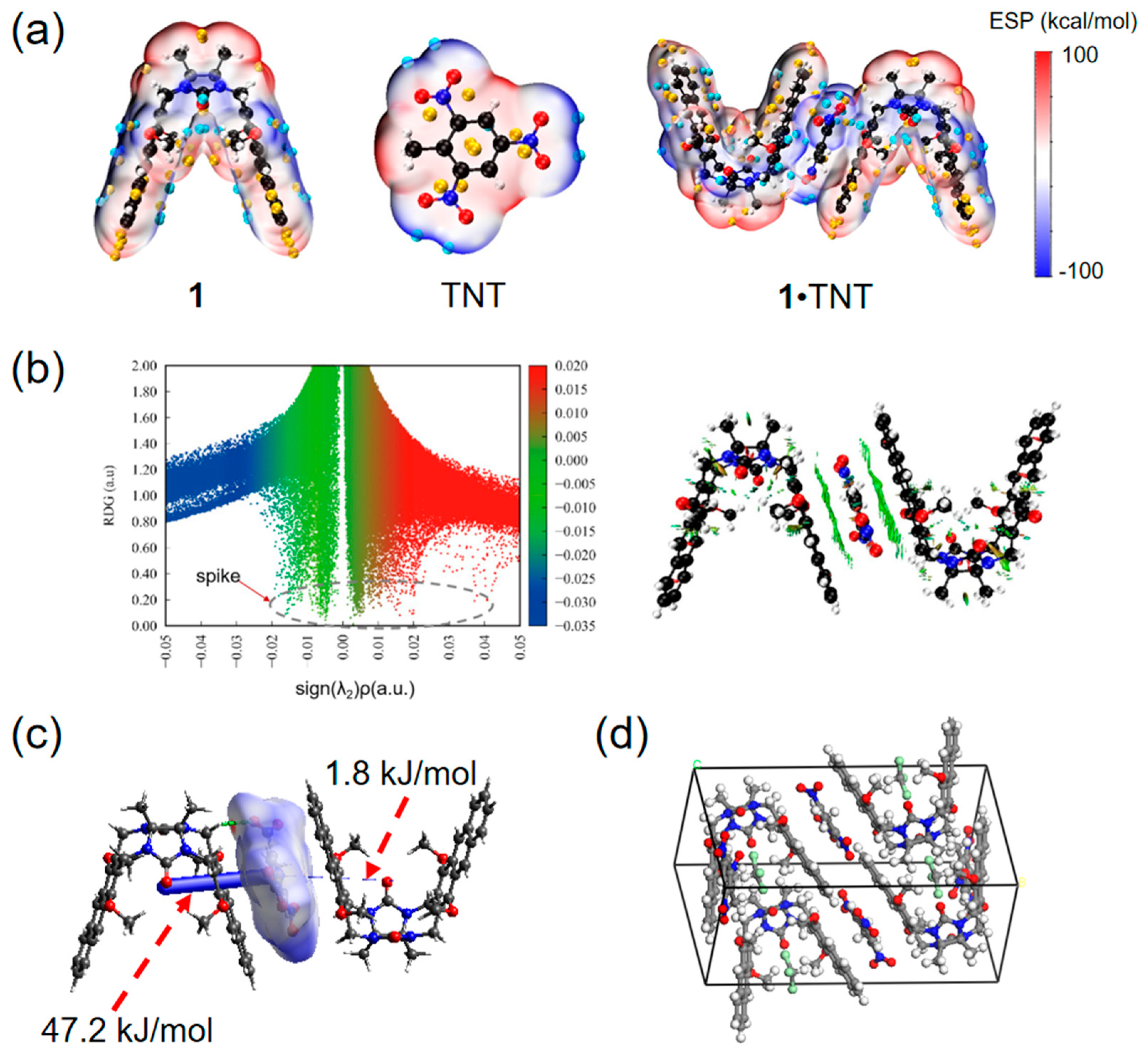
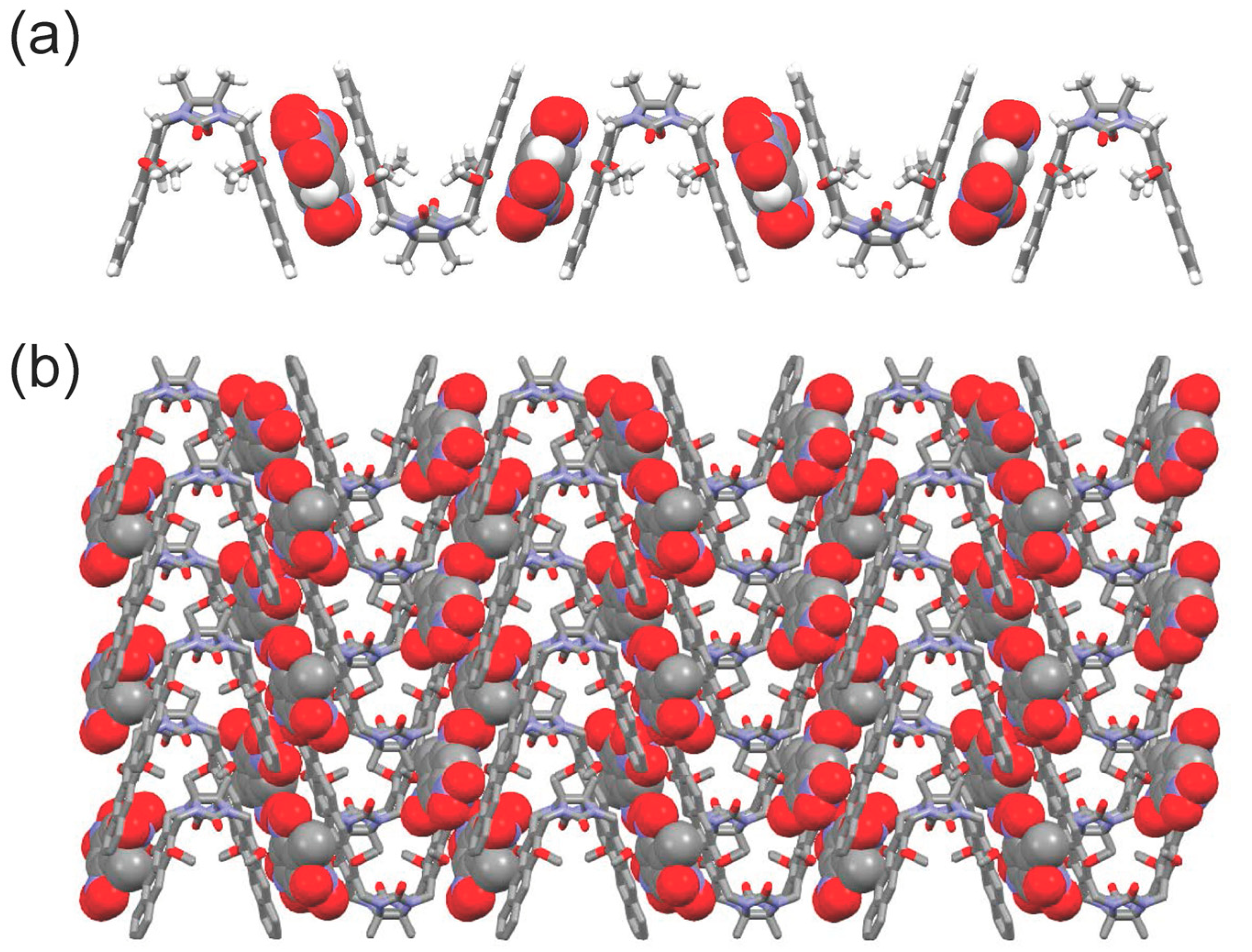
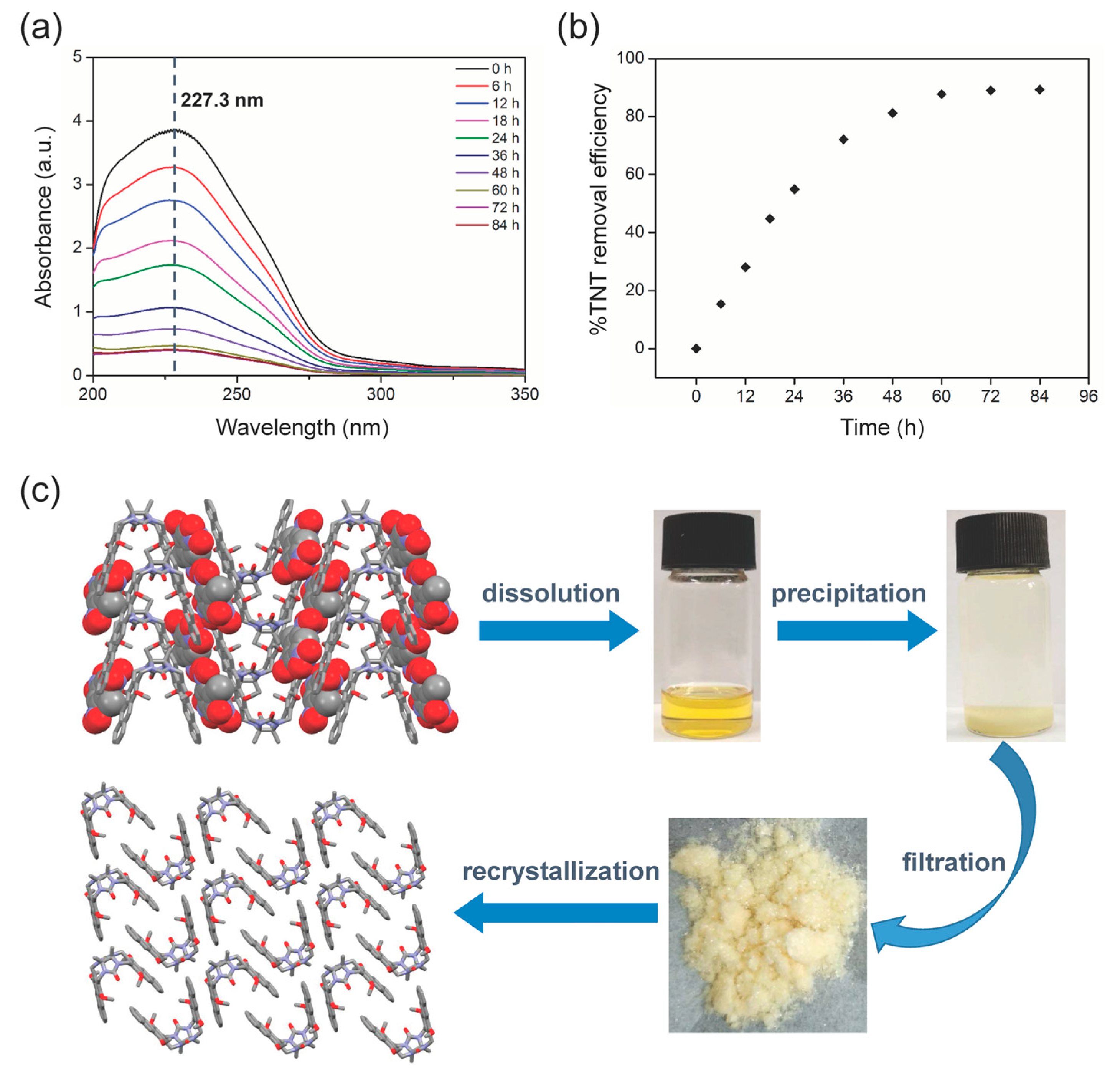
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Association Constant and Stoichiometry Determination for the Complexation Between Molecular Clip 1 and TNT
3.3. Calculation Methods
3.3.1. Molecular Surface Electrostatic Potential
3.3.2. Intermolecular Binding Energy
3.3.3. Optimize the Crystal Structures
3.4. Single Crystals Preparation Method of 1•TNT
3.5. TNT Adsorption and Removal Experiments
3.6. Clip-SOF Regeneration Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Wang, Q.; Feng, X.; Wang, B.; Yang, L. Explosives in the Cage: Metal–Organic Frameworks for High-Energy Materials Sensing and Desensitization. Adv. Mater. 2017, 29, 1701898. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, H.; Qu, Y.; Lü, C. Detection of TNT Based on Conjugated Polymer Encapsulated in Mesoporous Silica Nanoparticles through FRET. Chem. Commun. 2012, 48, 4633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, C.; Zheng, F.; Li, X.; Sun, C.-L.; Chen, W. Nitrogen and Sulfur Co-Doped Graphene Nanoribbons: A Novel Metal-Free Catalyst for High Performance Electrochemical Detection of 2, 4, 6-Trinitrotoluene (TNT). Carbon 2018, 126, 328–337. [Google Scholar] [CrossRef]
- Ariyarathna, T.; Vlahos, P.; Tobias, C.; Smith, R. Sorption Kinetics of TNT and RDX in Anaerobic Freshwater and Marine Sediments: Batch Studies. Enviro. Toxic Chem. 2016, 35, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Yang, L.; Ma, Y.; Chang, G. A Recyclable Indole-Based Polymer for Trinitrotoluene Adsorption via the Synergistic Effect of Dipole–π and donor–Acceptor Interactions. Polym. Chem. 2019, 10, 4632–4636. [Google Scholar] [CrossRef]
- Marinović, V.; Ristić, M.; Dostanić, M. Dynamic Adsorption of Trinitrotoluene on Granular Activated Carbon. J. Hazard. Mater. 2005, 117, 121–128. [Google Scholar] [CrossRef]
- Yao, Y.; Xue, M.; Chen, J.; Zhang, M.; Huang, F. An Amphiphilic Pillar[5]Arene: Synthesis, Controllable Self-Assembly in Water, and Application in Calcein Release and TNT Adsorption. J. Am. Chem. Soc. 2012, 134, 15712–15715. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Y.; Lv, F.; Chu, P.K.; Shang, J. Removal of Organic Materials from TNT Red Water by Bamboo Charcoal Adsorption. Chem. Eng. J. 2012, 193–194, 39–49. [Google Scholar] [CrossRef]
- Zhong, C.; He, P.; Yuan, R.; Sun, Y.; Deng, H.; Zhang, T.; Liang, S.; Kang, B.; Chang, G.; Xu, Y. Hydrophobic Porous Polymer Containing Isoxazoline and Siloxane Groups for 2,4,6-Trinitrotoluene (TNT) Adsorption via Synergistic Effect of Lewis Acid-Base, Dipole-π, and π-π Interactions. J. Non-Cryst. Solids 2023, 602, 122079. [Google Scholar] [CrossRef]
- Ayoub, K.; Nélieu, S.; Van Hullebusch, E.D.; Maia-Grondard, A.; Cassir, M.; Bermond, A. TNT Oxidation by Fenton Reaction: Reagent Ratio Effect on Kinetics and Early Stage Degradation Pathways. Chem. Eng. J. 2011, 173, 309–317. [Google Scholar] [CrossRef]
- Matta, R.; Hanna, K.; Kone, T.; Chiron, S. Oxidation of 2,4,6-Trinitrotoluene in the Presence of Different Iron-Bearing Minerals at Neutral pH. Chem. Eng. J. 2008, 144, 453–458. [Google Scholar] [CrossRef]
- Casey, M.C.; Cliffel, D.E. Surface Adsorption and Electrochemical Reduction of 2,4,6-Trinitrotoluene on Vanadium Dioxide. Anal. Chem. 2015, 87, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, Y.; Shan, X.; Chen, Z. Degradation of 2,4,6-Trinitrotoluene (TNT) from Explosive Wastewater Using Nanoscale Zero-Valent Iron. Chem. Eng. J. 2010, 158, 566–570. [Google Scholar] [CrossRef]
- Son, H.-S.; Lee, S.-J.; Cho, I.-H.; Zoh, K.-D. Kinetics and Mechanism of TNT Degradation in TiO2 Photocatalysis. Chemosphere 2004, 57, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Yang, T.-H.; Shim, W.-G.; Kwon, T.-O.; Moon, I.-S. Equilibria and Dynamics of Liquid-Phase Trinitrotoluene Adsorption on Granular Activated Carbon: Effect of Temperature and pH. J. Hazard. Mater. 2007, 141, 185–192. [Google Scholar] [CrossRef]
- Chatterjee, S.; Paital, A.R. Functionalized Cubic Mesoporous Silica as a Non-Chemodosimetric Fluorescence Probe and Adsorbent for Selective Detection and Removal of Bisulfite Anions along with Toxic Metal Ions. Adv. Funct. Mater. 2018, 28, 1704726. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Yang, L.; Chang, G. An Indole-Based Smart Aerogel for Simultaneous Visual Detection and Removal of Trinitrotoluene in Water via Synergistic Effect of Dipole-π and donor-Acceptor Interactions. Chem. Eng. J. 2020, 384, 123358. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, X.; Li, Y.; Du, R.; Zhang, Z.; An, F.; Gao, B. Selective Detection of TNT Using Molecularly Imprinted Polymer Microsphere. Desalination Water Treat. 2015, 55, 278–283. [Google Scholar] [CrossRef]
- An, F.; Gao, B.; Feng, X. Adsorption of 2,4,6-Trinitrotoluene on a Novel Adsorption Material PEI/SiO2. J. Hazard. Mater. 2009, 166, 757–761. [Google Scholar] [CrossRef]
- An, F.; Gao, B.; Feng, X. Adsorption Performance and Mechanism of 2,4,6-Trinitrotoluene on a Novel Adsorption Material Polyvinylbenzyl Acid/SiO2. Appl. Surf. Sci. 2009, 255, 5031–5035. [Google Scholar] [CrossRef]
- Bharti; Khurana, I.; Shaw, A.K.; Saxena, A.; Khurana, J.M.; Rai, P.K. Removal of Trinitrotoluene with Nano Zerovalent Iron Impregnated Graphene Oxide. Water Air Soil Pollut 2018, 229, 17. [Google Scholar] [CrossRef]
- Tian, J.; Chen, L.; Zhang, D.-W.; Liu, Y.; Li, Z.-T. Supramolecular Organic Frameworks: Engineering Periodicity in Water Through Host-Guest Chemistry. Chem. Commun. 2016, 52, 6351–6362. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-T.; Yu, S.-B.; Liu, Y.; Tian, J.; Zhang, D.-W. Supramolecular Organic Frameworks: Exploring Water-Soluble, Regular Nanopores for Biomedical Applications. Acc. Chem. Res. 2022, 55, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Yoon, M.; Lim, S.; Park, S.M.; Seo, G.; Kim, K. Highly Selective Carbon Dioxide Sorption in an Organic Molecular Porous Material. J. Am. Chem. Soc. 2010, 132, 12200–12202. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ma, S.; Thallapally, P.K.; Fowler, D.; McGrail, B.P.; Atwood, J.L. Cucurbit[7]Uril: An Amorphous Molecular Material for Highly Selective Carbon Dioxide Uptake. Chem. Commun. 2011, 47, 7626. [Google Scholar] [CrossRef]
- Hasell, T.; Schmidtmann, M.; Cooper, A.I. Molecular Doping of Porous Organic Cages. J. Am. Chem. Soc. 2011, 133, 14920–14923. [Google Scholar] [CrossRef]
- Hasell, T.; Chong, S.Y.; Jelfs, K.E.; Adams, D.J.; Cooper, A.I. Porous Organic Cage Nanocrystals by Solution Mixing. J. Am. Chem. Soc. 2012, 134, 588–598. [Google Scholar] [CrossRef]
- Sozzani, P.; Bracco, S.; Comotti, A.; Ferretti, L.; Simonutti, R. Methane and Carbon Dioxide Storage in a Porous van Der Waals Crystal. Angew. Chem. Int. Ed. 2005, 44, 1816–1820. [Google Scholar] [CrossRef]
- He, Y.; Xiang, S.; Chen, B. A Microporous Hydrogen-Bonded Organic Framework for Highly Selective C2H2/C2H4 Separation at Ambient Temperature. J. Am. Chem. Soc. 2011, 133, 14570–14573. [Google Scholar] [CrossRef]
- Mastalerz, M.; Oppel, I.M. Rational Construction of an Extrinsic Porous Molecular Crystal with an Extraordinary High Specific Surface Area. Angew. Chem. Int. Ed. 2012, 51, 5252–5255. [Google Scholar] [CrossRef]
- Luo, X.-Z.; Jia, X.-J.; Deng, J.-H.; Zhong, J.-L.; Liu, H.-J.; Wang, K.-J.; Zhong, D.-C. A Microporous Hydrogen-Bonded Organic Framework: Exceptional Stability and Highly Selective Adsorption of Gas and Liquid. J. Am. Chem. Soc. 2013, 135, 11684–11687. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Hamada, T.; Hisaki, I.; Miyata, M.; Tohnai, N. Dynamically Deformable Cube-like Hydrogen-Bonding Networks in Water-Responsive Diamondoid Porous Organic Salts. Angew. Chem. Int. Ed. 2013, 52, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, Y.; Guang, J.; Weng, L.; Zhao, J.C.-G.; Xiang, S.; Chen, B. A Homochiral Microporous Hydrogen-Bonded Organic Framework for Highly Enantioselective Separation of Secondary Alcohols. J. Am. Chem. Soc. 2014, 136, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.S.; Banerjee, D.; Zhang, C.; Thallapally, P.K.; Atwood, J.L. Selective CO2 Adsorption in a Supramolecular Organic Framework. Angew. Chem. Int. Ed. 2016, 55, 4523–4526. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Du, J.; Wang, J.; Chen, Z. Supramolecular Organic Frameworks of a Schiff Base Showing Selective Guest Adsorption. J. Appl. Crystallogr. 2015, 48, 909–912. [Google Scholar] [CrossRef]
- Lü, J.; Perez-Krap, C.; Suyetin, M.; Alsmail, N.H.; Yan, Y.; Yang, S.; Lewis, W.; Bichoutskaia, E.; Tang, C.C.; Blake, A.J.; et al. A Robust Binary Supramolecular Organic Framework (SOF) with High CO2 Adsorption and Selectivity. J. Am. Chem. Soc. 2014, 136, 12828–12831. [Google Scholar] [CrossRef]
- Yu, S.; Qi, Q.; Yang, B.; Wang, H.; Zhang, D.; Liu, Y.; Li, Z. Enhancing Hydrogen Generation Through Nanoconfinement of Sensitizers and Catalysts in a Homogeneous Supramolecular Organic Framework. Small 2018, 14, 1801037. [Google Scholar] [CrossRef]
- Yan, M.; Liu, X.-B.; Gao, Z.-Z.; Wu, Y.-P.; Hou, J.-L.; Wang, H.; Zhang, D.-W.; Liu, Y.; Li, Z.-T. A Pore-Expanded Supramolecular Organic Framework and Its Enrichment of Photosensitizers and Catalysts for Visible-Light-Induced Hydrogen Production. Org. Chem. Front. 2019, 6, 1698–1704. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Y.; Liu, H.; Yu, S.; Niu, K.-K.; Xing, L.-B. Construction of a Novel Pyrene-based Two-Dimensional Supramolecular Organic Framework and the Selective Regulation of Reactive Oxygen Species for Photocatalysis. J. Mater. Chem. A 2024, 12, 4752–4760. [Google Scholar] [CrossRef]
- Yin, C.; Yan, Z.-A.; Yan, R.; Xu, C.; Ding, B.; Ji, Y.; Ma, X. A 3D Phosphorescent Supramolecular Organic Framework in Aqueous Solution. Adv. Funct. Mater. 2024, 34, 2316008. [Google Scholar] [CrossRef]
- Tedesco, C.; Erra, L.; Brunelli, M.; Cipolletti, V.; Gaeta, C.; Fitch, A.N.; Atwood, J.L.; Neri, P. Methane Adsorption in a Supramolecular Organic Zeolite. Chem. A Eur. J 2010, 16, 2371–2374. [Google Scholar] [CrossRef] [PubMed]
- Tsue, H.; Ono, K.; Tokita, S.; Ishibashi, K.; Matsui, K.; Takahashi, H.; Miyata, K.; Takahashi, D.; Tamura, R. Spontaneous and Selective CO2 Sorption under Ambient Conditions in Seemingly Nonporous Molecular Crystal of Azacalix[5]Arene Pentamethyl Ether. Org. Lett. 2011, 13, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Zhou, Y.; Li, Q.-L.; Yang, Y.-W. Enzyme-Responsive Supramolecular Nanovalves Crafted by Mesoporous Silica Nanoparticles and Choline-Sulfonatocalix[4]Arene [2]Pseudorotaxanes for Controlled Cargo Release. Chem. Commun. 2013, 49, 9033. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Mouchaham, G.; Shkurenko, A.; Hoang, P.; Moosa, B.; Bhatt, P.M.; Adil, K.; Salama, K.N.; Eddaoudi, M.; Khashab, N.M. Trianglamine-Based Supramolecular Organic Framework with Permanent Intrinsic Porosity and Tunable Selectivity. J. Am. Chem. Soc. 2018, 140, 14571–14575. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, H.; Tao, Y.; Zhang, S.X.; Wang, B.; Yang, Y. Pillar[5]arene-Based Supramolecular Organic Frameworks for Highly Selective CO2 -Capture at Ambient Conditions. Adv. Mater. 2014, 26, 7027–7031. [Google Scholar] [CrossRef]
- Shi, B.; Shangguan, L.; Wang, H.; Zhu, H.; Xing, H.; Liu, P.; Liu, Y.; Liu, J.; Huang, F. Pillar[5]Arene-Based Molecular Recognition Induced Crystal-to-Crystal Transformation and Its Application in Adsorption of Adiponitrile in Water. ACS Mater. Lett. 2019, 1, 111–115. [Google Scholar] [CrossRef]
- Hardouin–Lerouge, M.; Hudhomme, P.; Sallé, M. Molecular Clips and Tweezers Hosting Neutral Guests. Chem. Soc. Rev. 2011, 40, 30–43. [Google Scholar] [CrossRef]
- Klärner, F.-G.; Kahlert, B. Molecular Tweezers and Clips as Synthetic Receptors. Molecular Recognition and Dynamics in Receptor−Substrate Complexes. Acc. Chem. Res. 2003, 36, 919–932. [Google Scholar] [CrossRef]
- Schrader, T.; Bitan, G.; Klärner, F.-G. Molecular Tweezers for Lysine and Arginine—Powerful Inhibitors of Pathologic Protein Aggregation. Chem. Commun. 2016, 52, 11318–11334. [Google Scholar] [CrossRef]
- Jono, K.; Suzuki, A.; Akita, M.; Albrecht, K.; Yamamoto, K.; Yoshizawa, M. A Polyaromatic Molecular Clip That Enables the Binding of Planar, Tubular, and Dendritic Compounds. Angew. Chem. Int. Ed. 2017, 56, 3570–3574. [Google Scholar] [CrossRef]
- Harmata, M. Chiral Molecular Tweezers. Acc. Chem. Res. 2004, 37, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Xiang, J.; Cao, L.; Wu, A.; Isaacs, L. Dimeric Packing of Molecular Clips Induced by Interactions between π-Systems. CrystEngComm 2015, 17, 2486–2495. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Mukherjee, P.S. Self-Assembled Discrete Molecules for Sensing Nitroaromatics. Chem. A Eur. J. 2015, 21, 6656–6666. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Shi, B.; Jiao, T.; Ju, H.; Liu, P.; Huang, F. Cocrystallization with a Clip-Type Molecule Catcher: A New Method to Determine Structures of Liquid Molecules. Org. Chem. Front. 2020, 7, 742–746. [Google Scholar] [CrossRef]
- Deposition Numbers 1960287 (for 1) and 2219664 (for 1•TNT) Contain the Supplementary Crystallographic Data for This Paper. These Data Are Provided Free of Charge by the Joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures Service.
- Gong, Y.-N.; Jiang, L.; Lu, T.-B. A Highly Stable Dynamic Fluorescent Metal–Organic Framework for Selective Sensing of Nitroaromatic Explosives. Chem. Commun. 2013, 49, 11113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Zhao, Q.L.; Ye, Z.F. Organic Pollutants Removal from 2,4,6-Trinitrotoluene (TNT) Red Water Using LowCost Activated Coke. J. Environ. Sci. 2011, 23, 1962–1969. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and qu-antitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- He, X.; Chen, C.; Zhang, Z.; Yu, T.; Wen, L.; Cao, Y.; Liu, Y. Molecule Empowerment and Crystal Desensitization: A Multilevel Structure-Property Analysis toward Designing High-Energy Low-Sensitivity Layered Energetic Materials. ACS Appl. Mater. Interfaces 2024, 16, 47429–47442. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An Ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zeng, S.; He, X.; Wu, Y.; Liu, Y.; Wang, Y. Adsorption and Removal of 2,4,6-Trinitrotoluene by a Glycoluril-Derived Molecular-Clip-Based Supramolecular Organic Framework. Molecules 2024, 29, 5822. https://doi.org/10.3390/molecules29245822
Liu Y, Zeng S, He X, Wu Y, Liu Y, Wang Y. Adsorption and Removal of 2,4,6-Trinitrotoluene by a Glycoluril-Derived Molecular-Clip-Based Supramolecular Organic Framework. Molecules. 2024; 29(24):5822. https://doi.org/10.3390/molecules29245822
Chicago/Turabian StyleLiu, Yuezhou, Shu Zeng, Xiaokai He, Yang Wu, Yang Liu, and Yinglei Wang. 2024. "Adsorption and Removal of 2,4,6-Trinitrotoluene by a Glycoluril-Derived Molecular-Clip-Based Supramolecular Organic Framework" Molecules 29, no. 24: 5822. https://doi.org/10.3390/molecules29245822
APA StyleLiu, Y., Zeng, S., He, X., Wu, Y., Liu, Y., & Wang, Y. (2024). Adsorption and Removal of 2,4,6-Trinitrotoluene by a Glycoluril-Derived Molecular-Clip-Based Supramolecular Organic Framework. Molecules, 29(24), 5822. https://doi.org/10.3390/molecules29245822






