Cyclobrachycoumarin from Gerbera piloselloides Inhibits Colorectal Cancer In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. Seven Active Constituents Were Isolated and Identified from G. piloselloides
2.2. Cyclobrachycoumarin Inhibited the Proliferation of CRC Cell Lines
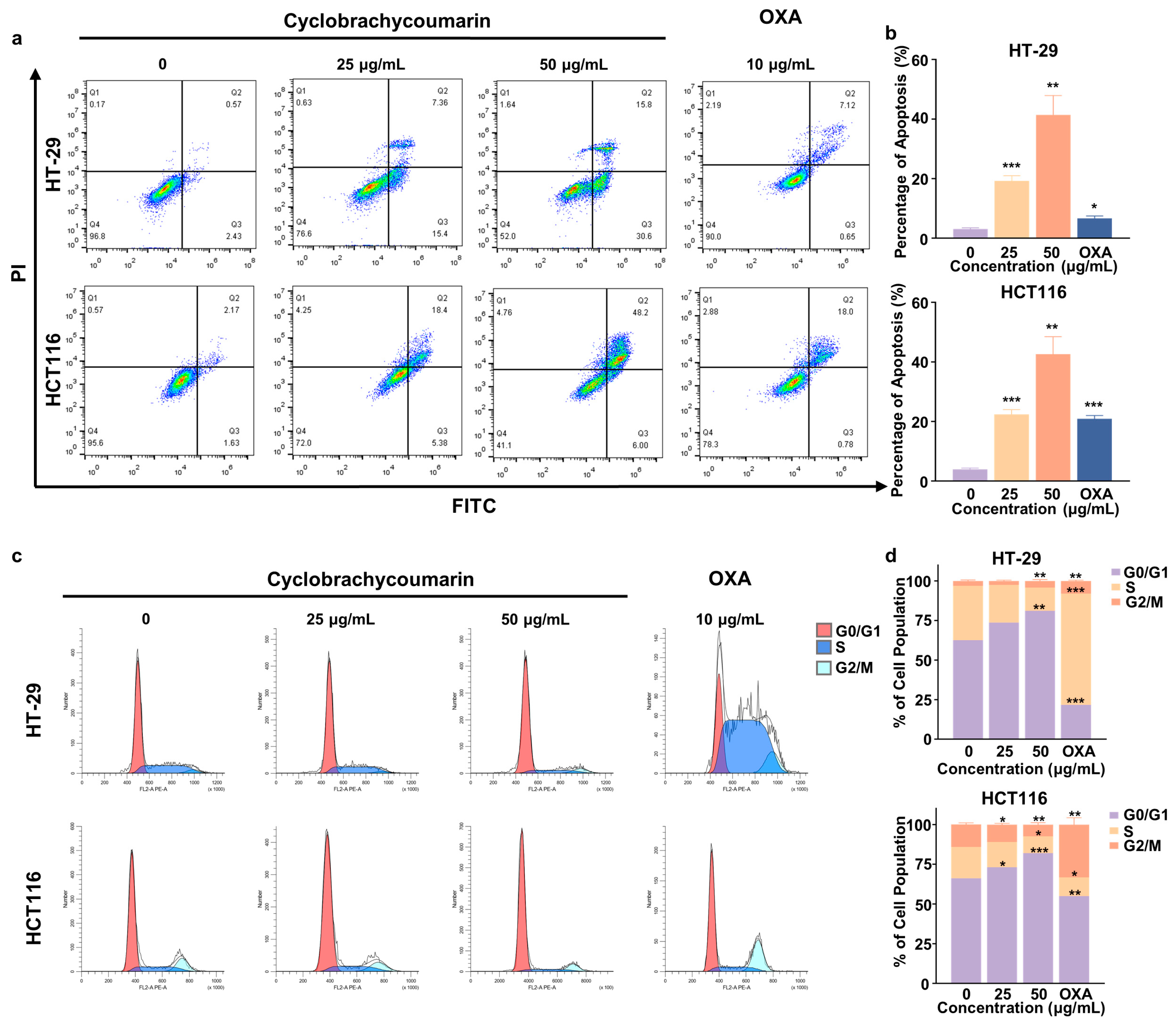
2.3. Cyclobrachycoumarin Induced Cell Cycle Arrest and Apoptosis in CRC Cell Lines
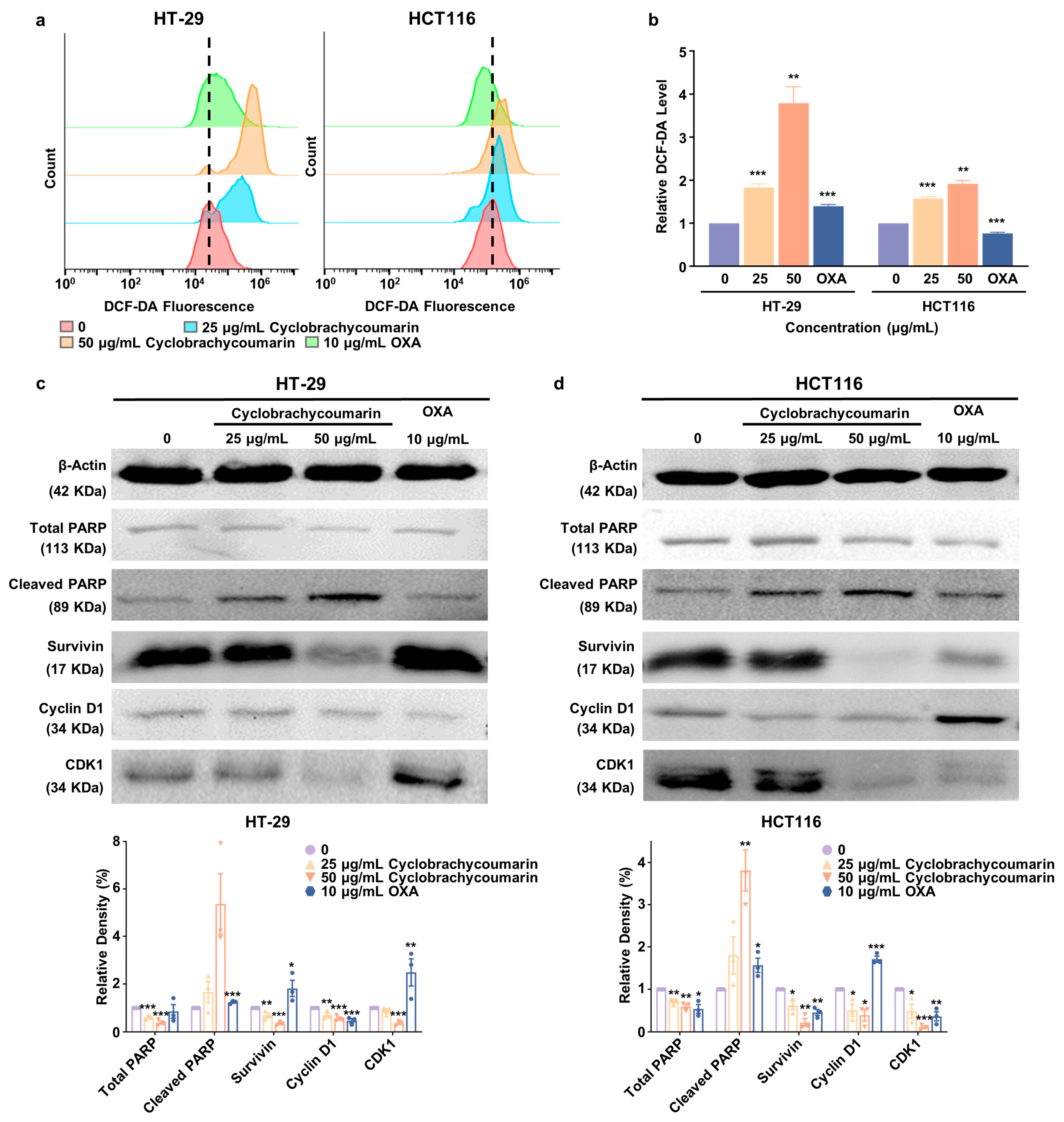
2.4. Cyclobrachycoumarin Inhibited CRC Cell Proliferation Through the Generation of ROS and the Modulation of Apoptosis and Cell Cycle-Related Pathways
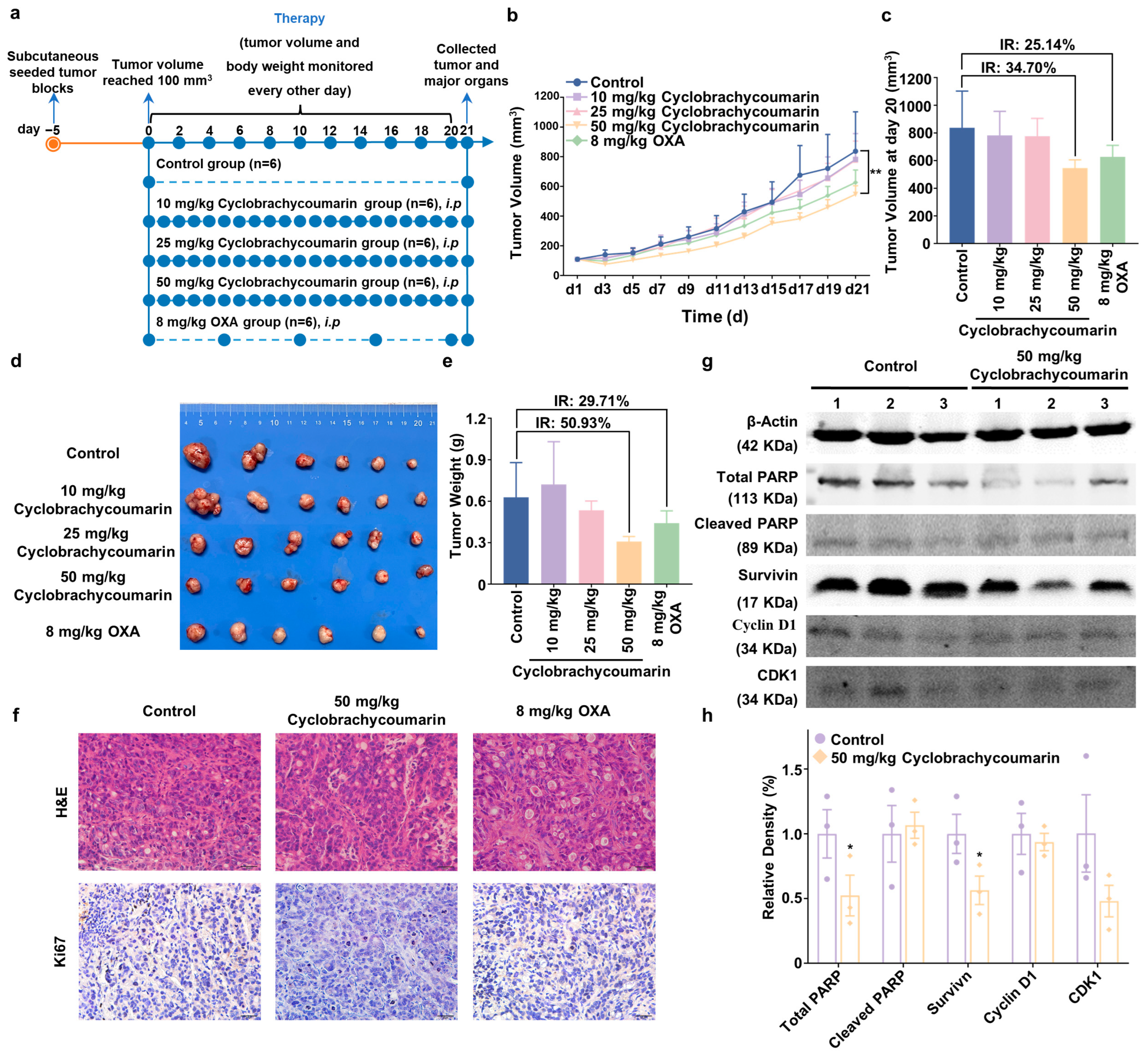
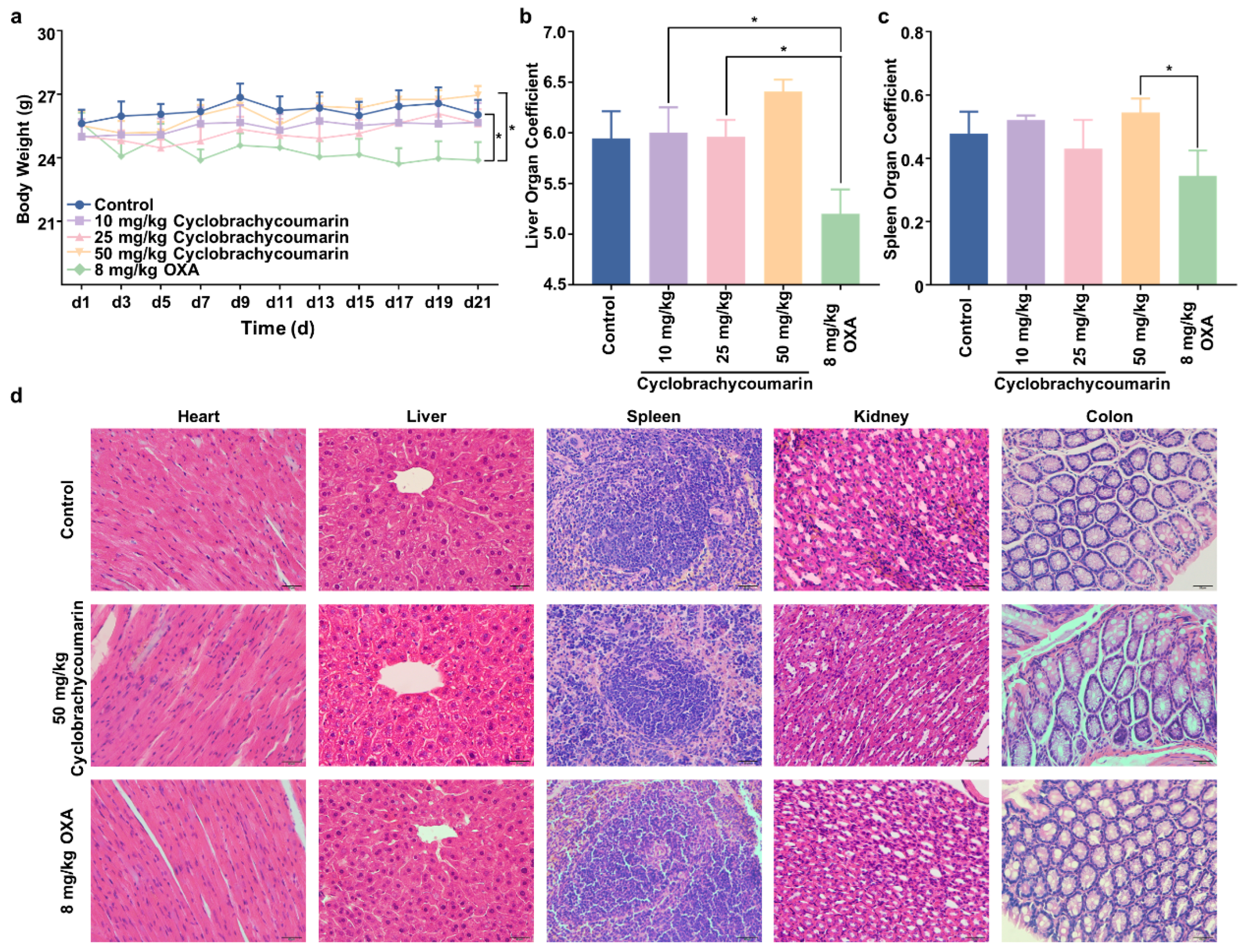
2.5. Cyclobrachycoumarin Suppressed Tumor Growth in Subcutaneous Xenograft Mice Models
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Cell Lines and Cell Culture
4.4. Cell Viability Assay
4.5. Colony Formation Assay
4.6. EdU Incorporation Assay
4.7. Cell Apoptosis Analysis
4.8. Cell Cycle Analysis
4.9. ROS Accumulation Assay
4.10. Western Blot Analysis
4.11. Xenograft Transplantation Assay
4.12. H&E Staining Assay
4.13. Immunohistochemistry (IHC) Assay
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, R.; Ahopelto, K.; Hagström, J.; Böckelman, C.; Haglund, C.; Isoniemi, H. High TKTL1 expression as a sign of poor prognosis in colorectal cancer with synchronous rather than metachronous liver metastases. Cancer Biol. Ther. 2020, 21, 826–831. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Cantor, D.I.; Cheruku, H.R.; Westacott, J.; Shin, J.S.; Mohamedali, A.; Ahn, S.B. Proteomic investigations into resistance in colorectal cancer. Expert. Rev. Proteom. 2020, 17, 49–65. [Google Scholar] [CrossRef]
- Kim, J.H. Chemotherapy for colorectal cancer in the elderly. World J. Gastroenterol. 2015, 21, 5158–5166. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, Y.; Wang, J.; Yu, C.; Shen, W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front. Immunol. 2021, 11, 609705. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wong, Y.K.; Liao, F. What has traditional Chinese medicine delivered for modern medicine? Expert. Rev. Mol. Med. 2018, 20, e4. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fu, C.; Lu, Y.; Sun, J.; Liu, T.; Wang, Y.; Wang, A.; Huang, Y.; Li, Y. Integration of metabolomics and transcriptomics to reveal the mechanism of Gerberae piloselloidis herba in alleviating bronchial asthma. J. Ethnopharmacol. 2024, 325, 117852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, J.; Hu, Y.; Li, L.; Yu, M.; Huang, Y.; Zhang, T.; Shang, H.; Zou, Z. (+)/(−)-Gerbeloid A, a pair of unprecedented coumarin-based polycyclic meroterpenoid enantiomers from Gerbera piloselloides: Structural elucidation, semi-synthesis, and lipid-lowering activity. Acta Pharm. Sin. B 2024, 14, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, J.; Cheng, X.; Wang, R.; Qu, H.; Jiang, H.; Bai, Y.; Cao, W. 8-methoxysmyrindiol from Gerbera piloselloides (L.) Cass. and its vasodilation effects on isolated rat mesenteric arteries. Fitoterapia. 2019, 138, 104299. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, Y.; Li, J.B.; Nohara, T. Two novel dicoumaro-p-menthanes from Gerbera piloselloides (L.) CASS. Chem. Pharm. Bull. 2004, 52, 1362–1364. [Google Scholar] [CrossRef]
- He, F.; Wang, M.; Gao, M.; Zhao, M.; Bai, Y.; Zhao, C. Chemical composition and biological activities of Gerbera anandria. Molecules 2014, 19, 4046–4057. [Google Scholar] [CrossRef]
- Wang, J.; Petrova, V.; Wu, S.B.; Zhu, M.; Kennelly, E.J.; Long, C. Antioxidants from Gerbera piloselloides: An ethnomedicinal plant from southwestern China. Nat. Prod. Res. 2014, 28, 2072–2075. [Google Scholar] [CrossRef]
- Li, T.; Ma, X.; Fedotov, D.; Kjaerulff, L.; Frydenvang, K.; Coriani, S.; Hansen, P.R.; Kongstad, K.T.; Staerk, D. Structure Elucidation of Prenyl- and Geranyl-Substituted Coumarins in Gerbera piloselloides by NMR Spectroscopy, Electronic Circular Dichroism Calculations, and Single Crystal X-ray Crystallography. Molecules 2020, 25, 1706. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, H.; Li, J.; Yu, M.; Wu, J.; Zhang, H.; Zhang, T.; Zou, Z. Bioactive constituents from Gerbera piloselloides with anti-inflammatory and antiproliferative activities. Fitoterapia 2022, 161, 105258. [Google Scholar] [CrossRef]
- CZdero, F.; Bohlmann, R.M.; King, H. Robinson. Further 5-methyl coumarins and other constituents from the subtribe mutisiinae. Phytochemistry 1986, 25, 509–516. [Google Scholar] [CrossRef]
- Lei, L.; Xue, Y.B.; Liu, Z.; Peng, S.S.; He, Y.; Zhang, Y.; Fang, R.; Wang, J.P.; Luo, Z.W.; Yao, G.M.; et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 2015, 5, 13544. [Google Scholar] [CrossRef] [PubMed]
- Luz, R.F.; Vieira, I.J.C.; Braz-Filho, R.; Moreira, V.F. 13C-NMR Data from Coumarins from Moraceae Family. Am. J. Anal. Chem. 2015, 6, 851–866. [Google Scholar] [CrossRef]
- Miyazawa, M.; Tsukamoto, T.; Anzai, J.; Ishikawa, Y. Insecticidal effect of phthalides and furanocoumarins from Angelica acutiloba against Drosophila melanogaster. J. Agric. Food Chem. 2004, 52, 4401–4405. [Google Scholar] [CrossRef]
- Cussans, N.J.; Huckerby, T.N. Carbon-13 NMR spectroscopy of heterocyclic compounds—IV: A 20 MHz study of chemical shifts and carbon-proton coupling constants in a series of hydroxy, methoxy and glucosyl coumarins. Tetrahedron 1975, 31, 2719–2726. [Google Scholar] [CrossRef]
- Rocha, D.D.; Dantas, I.N.; Albuquerque, M.R.; Montenegro, R.C.; Pessoa, C.; de Moraes, M.O.; Pessoa, O.D.; Silveira, E.R.; Costa-Lotufo, L.V. Studies on the cytotoxicity of miscellaneous compounds from Eupatorium betonicaeforme (D.C.) Baker (Asteraceae). Chem. Biodivers. 2007, 4, 2835–2844. [Google Scholar] [CrossRef]
- Zhou, K.; Lu, D.; You, J.; Liu, T.; Sun, J.; Lu, Y.; Pan, J.; Li, Y.; Liu, C. Integrated plasma pharmacochemistry and network pharmacology to explore the mechanism of Gerberae Piloselloidis Herba in treatment of allergic asthma. J. Ethnopharmacol. 2022, 298, 115624. [Google Scholar] [CrossRef]
- Du, H.; Huang, Y.; Hou, X.; Quan, X.; Jiang, J.; Wei, X.; Liu, Y.; Li, H.; Wang, P.; Zhan, M.; et al. Two novel camptothecin derivatives inhibit colorectal cancer proliferation via induction of cell cycle arrest and apoptosis in vitro and in vivo. Eur. J. Pharm. Sci. 2018, 123, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Bergmann, A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008, 18, 467–473. [Google Scholar] [CrossRef]
- Wang, L.; Yeung, J.H.; Hu, T.; Lee, W.Y.; Lu, L.; Zhang, L.; Shen, J.; Chan, R.L.; Wu, W.K.; Cho, C.H. Dihydrotanshinone induces p53-independent but ROS-dependent apoptosis in colon cancer cells. Life Sci. 2013, 93, 344–351. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, L.; Niu, D.; Chen, M.; Zhang, W.; Liu, Y.; Xu, J.; Wang, D. Copper (II) complex of salicylate phenanthroline induces the apoptosis of colorectal cancer cells, including oxaliplatin-resistant cells. Oncol. Rep. 2023, 50, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Gao, H.; Yu, M.; Zhao, J.P.; He, B.X.; Wu, J.P.; Zhang, H.X.; Zhang, T.; Zou, Z.M. 1H-NMR-guided isolation of enantiomeric coumarin-monoterpenes with anti-inflammatory activity from Gerbera piloselloides. Phytochemistry 2022, 203, 113346. [Google Scholar] [CrossRef]
- Chen, S.L.; Gao, H.; Zhao, C.X.; Zhang, T.; Zou, Z.M. LC-MS coupled with diagnostic ion strategy facilitated the discovery of 5-methylcoumarin meroterpenoids from Gerbera piloselloides. Phytochemistry 2025, 229, 114296. [Google Scholar] [CrossRef]
- Bhattarai, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer Potential of Coumarin and its Derivatives. Mini Rev. Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Shelake, S.; Sankpal, U.T.; Eslin, D.; Bowman, W.P.; Simecka, J.W.; Raut, S.; Ray, A.; Basha, R. Clotam enhances anti-proliferative effect of vincristine in Ewing sarcoma cells. Apoptosis 2019, 24, 21–32. [Google Scholar] [CrossRef]
- Blanc-Brude, O.P.; Yu, J.; Simosa, H.; Conte, M.S.; Sessa, W.C.; Altieri, D.C. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat. Med. 2002, 8, 987–994. [Google Scholar] [CrossRef]
- Mughal, M.J.; Bhadresha, K.; Kwok, H.F. CDK inhibitors from past to present: A new wave of cancer therapy. Semin. Cancer Biol. 2023, 88, 106–122. [Google Scholar] [CrossRef] [PubMed]

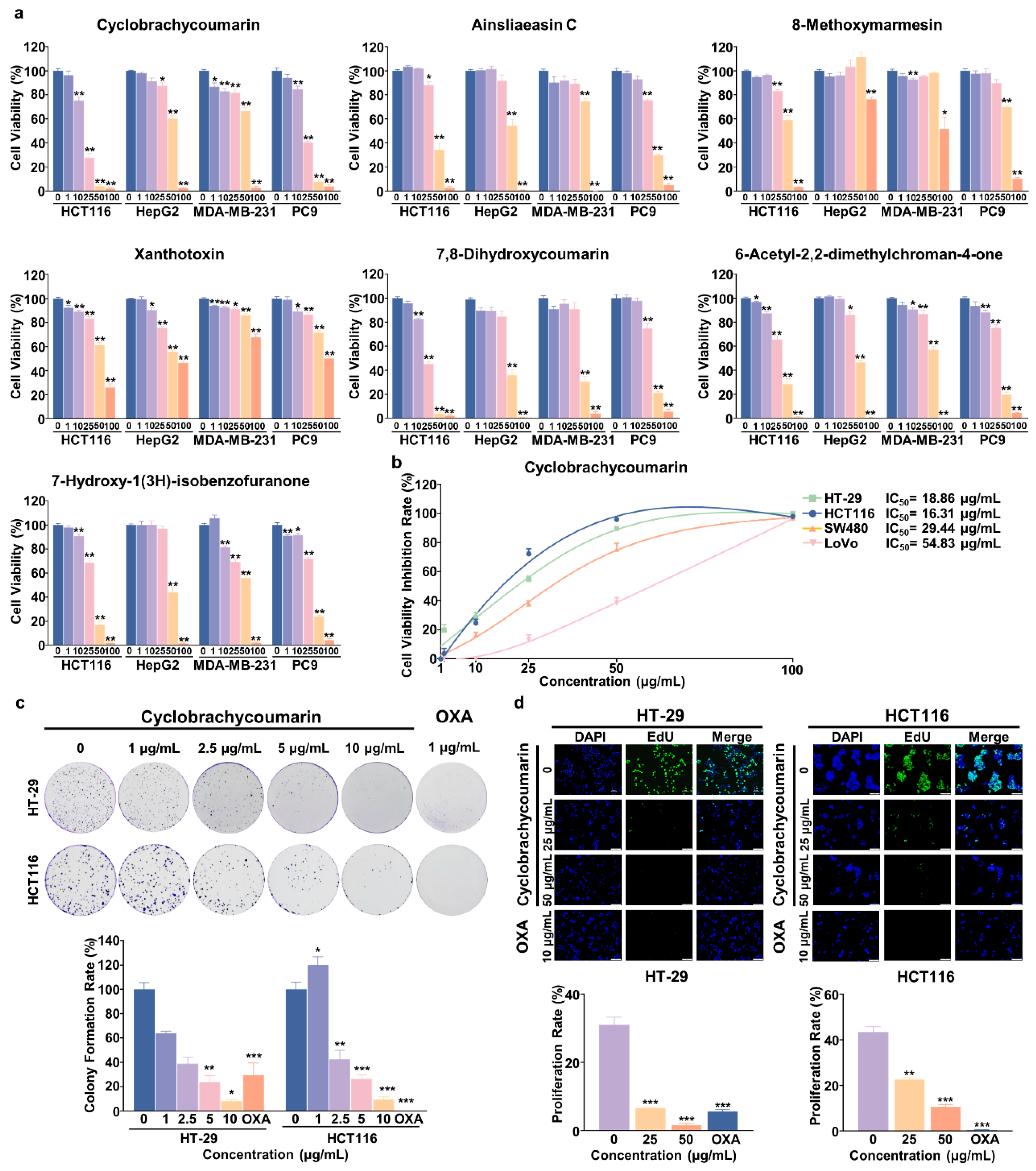
| IC50 (Mean ± SEM, μg/mL) of Seven Compounds on Different Cancer Cell Lines at 48 h | ||||
|---|---|---|---|---|
| Compounds | Colorectal Cancer | Liver Cancer | Breast Cancer | Lung Cancer |
| HCT116 | HepG2 | MDA-MB-231 | PC9 | |
| Cyclobrachycoumarin | 16.31 ± 1.34 | 53.76 ± 3.46 | 56.47 ± 5.81 | 20.82 ± 1.49 |
| Ainsliaeasin C | 42.23 ± 2.47 | 51.35 ± 3.15 | 57.03 ± 0.84 | 36.90 ± 1.73 |
| 8-Methoxymarmesin | 52.28 ± 4.49 | >100 | >100 | 60.72 ± 3.50 |
| Xanthotoxin | 60.51 ± 4.91 | 77.83 ± 8.62 | >100 | >100 |
| 7,8-Dihydroxycoumarin | 21.44 ± 1.39 | 41.58 ± 4.13 | 39.48 ± 3.18 | 34.48 ± 2.04 |
| 6-Acetyl-2,2-dimethylchroman-4-one | 32.12 ± 2.59 | 46.60 ± 2.38 | 52.22 ± 2.76 | 33.87 ± 2.60 |
| 7-Hydroxy-1(3H)-isobenzofuranone | 31.21 ± 1.98 | 47.61 ± 2.20 | 42.22 ± 7.02 | 33.95 ± 1.98 |
| Cell Viability IC50 (μg/mL) ± SEM | ||||
|---|---|---|---|---|
| Colorectal Cancer | ||||
| HT-29 | HCT116 | SW480 | LoVo | |
| Cyclobrachycoumarin | 18.86 ± 3.62 | 16.31 ± 1.34 | 29.44 ± 2.56 | 54.83 ± 4.59 |
| OXA | >25.00 | 7.58 ± 1.05 | 12.53 ± 2.1 | 8.85 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Ye, X.; Fang, Q.; Li, X.; Wang, H.; Sun, B.; Shu, X.; Hou, X.; Liu, Y. Cyclobrachycoumarin from Gerbera piloselloides Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules 2024, 29, 5678. https://doi.org/10.3390/molecules29235678
Fan L, Ye X, Fang Q, Li X, Wang H, Sun B, Shu X, Hou X, Liu Y. Cyclobrachycoumarin from Gerbera piloselloides Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules. 2024; 29(23):5678. https://doi.org/10.3390/molecules29235678
Chicago/Turabian StyleFan, Limei, Xiansheng Ye, Qian Fang, Xiaoxuan Li, Haiping Wang, Binlian Sun, Xiji Shu, Xiaoying Hou, and Yuchen Liu. 2024. "Cyclobrachycoumarin from Gerbera piloselloides Inhibits Colorectal Cancer In Vitro and In Vivo" Molecules 29, no. 23: 5678. https://doi.org/10.3390/molecules29235678
APA StyleFan, L., Ye, X., Fang, Q., Li, X., Wang, H., Sun, B., Shu, X., Hou, X., & Liu, Y. (2024). Cyclobrachycoumarin from Gerbera piloselloides Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules, 29(23), 5678. https://doi.org/10.3390/molecules29235678






