The Distinct Biological Effects of 6-Hydroxy-L-Nicotine in Representative Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
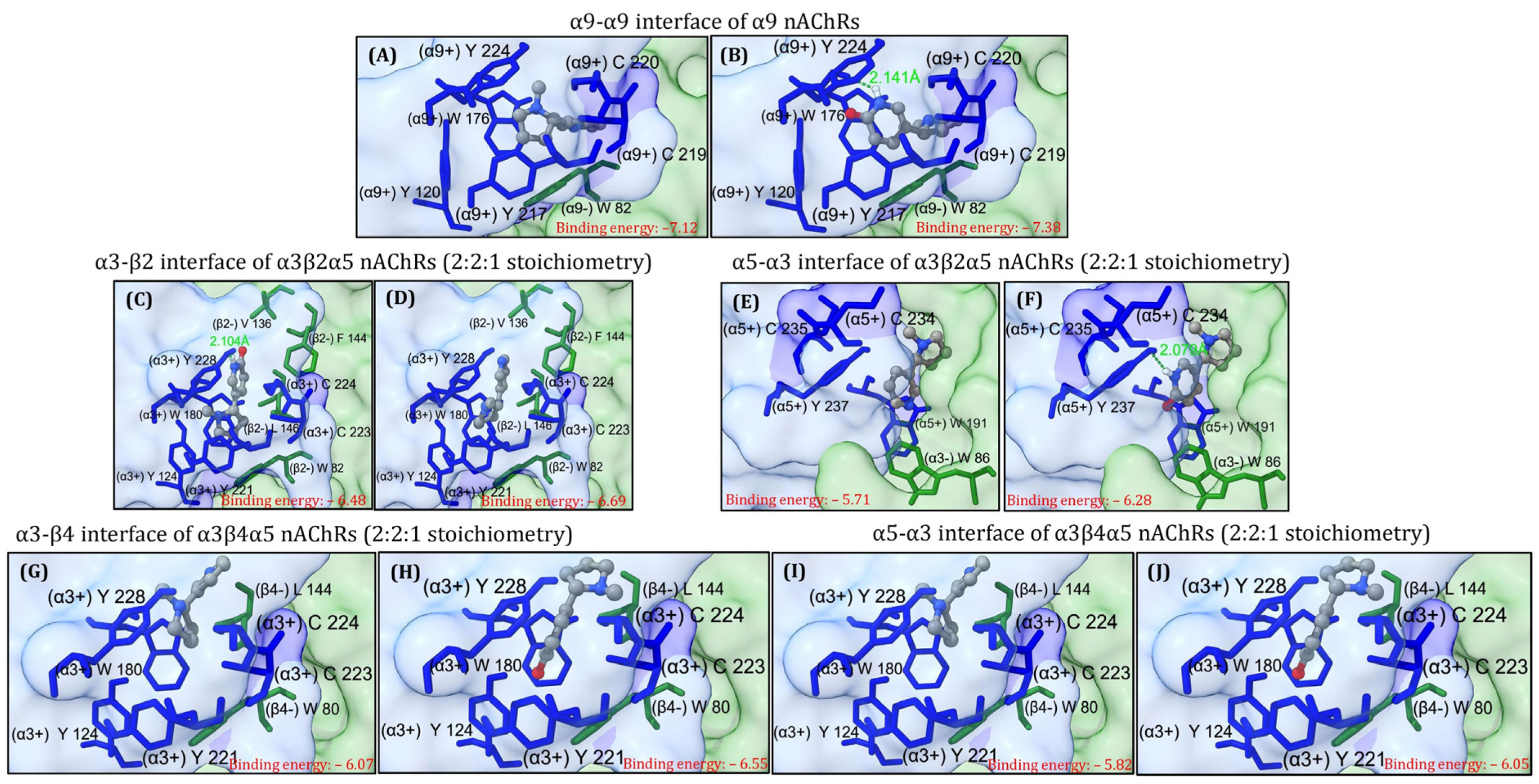
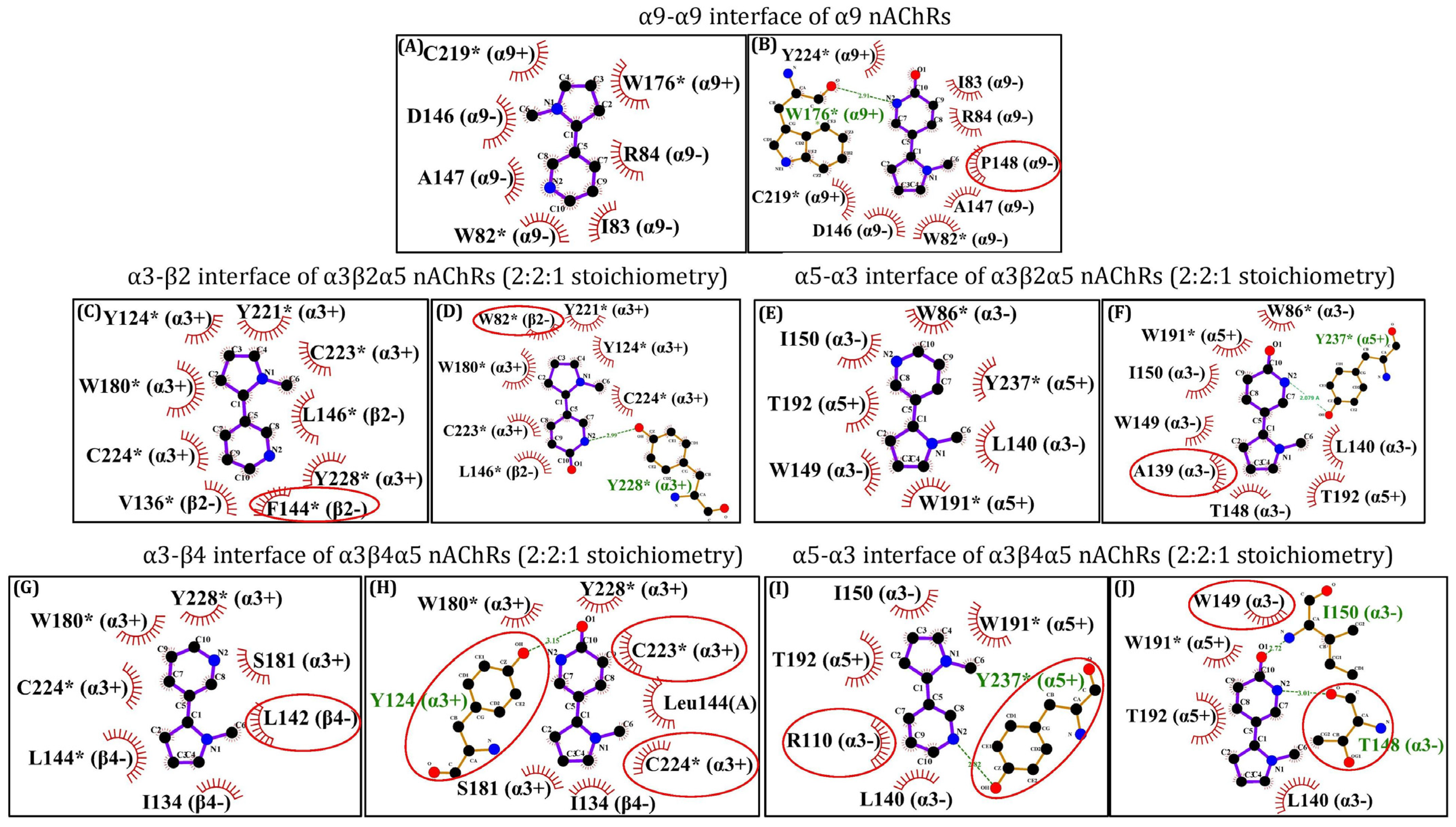
2.1. α9,α3β2α5 and α3β4α5 nAChR Structures Prediction and In Silico Molecular Docking of 6HLN and NIC
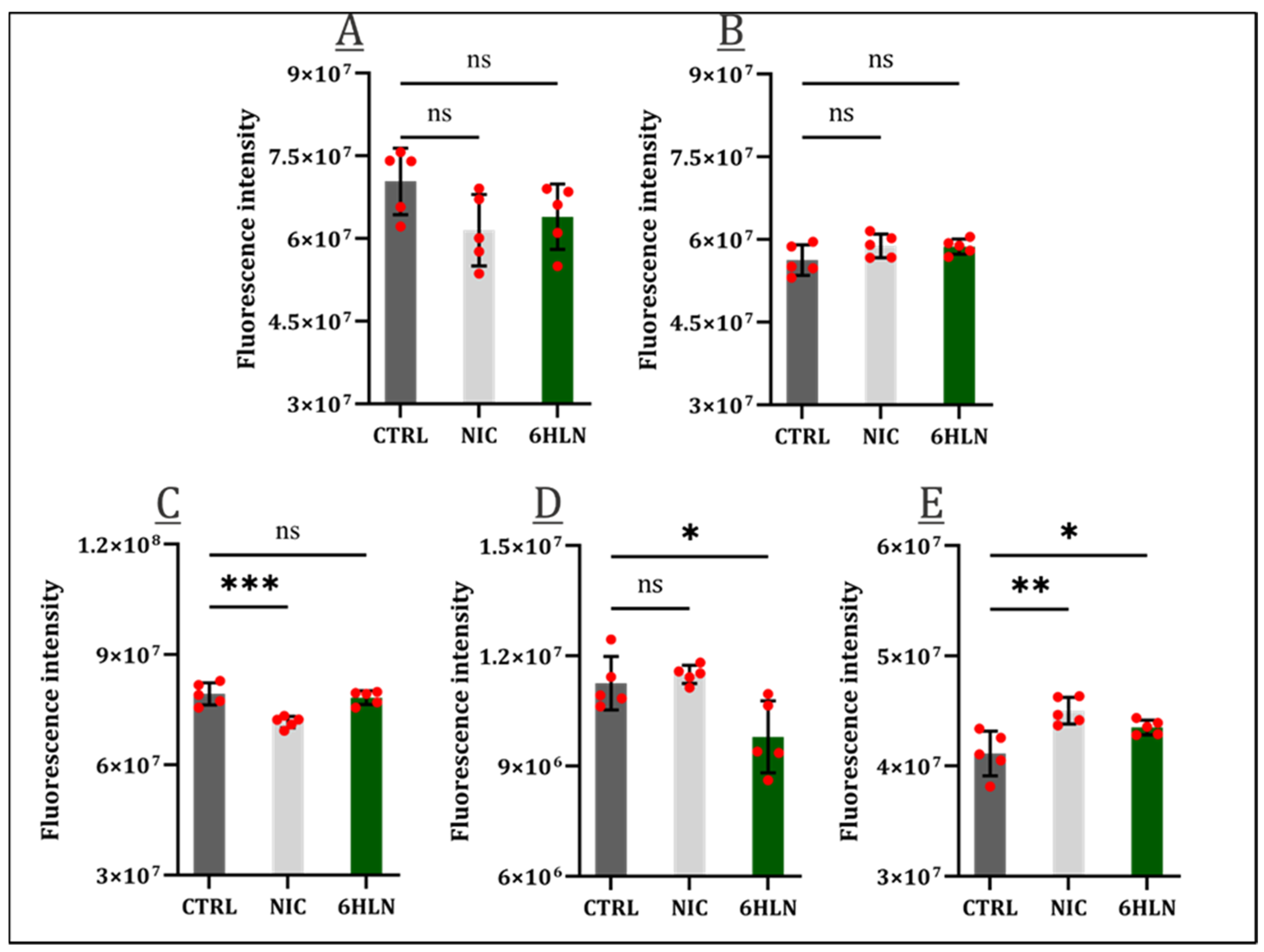
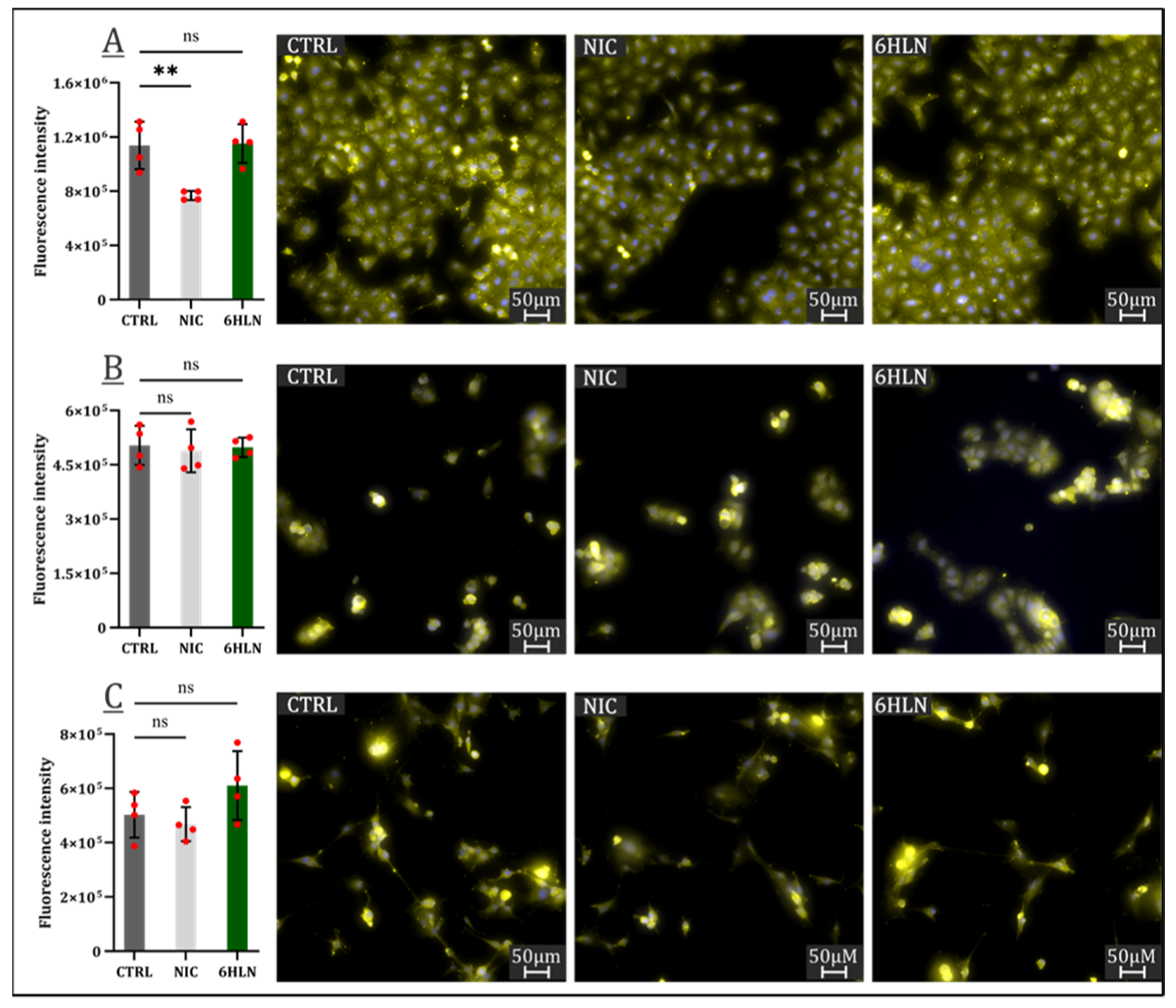
2.2. Influence of 6HLN and NIC on Proliferative Behavior
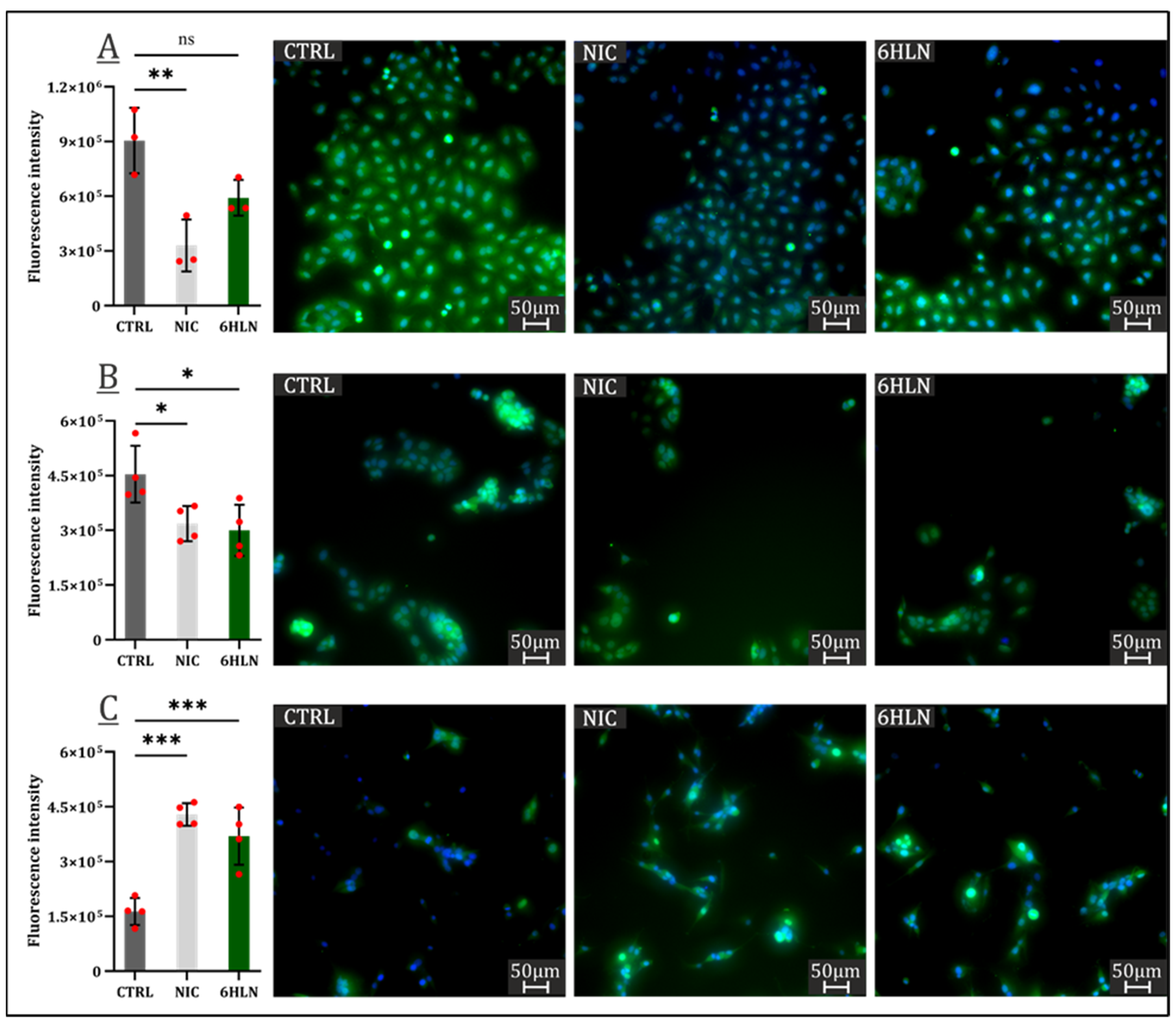
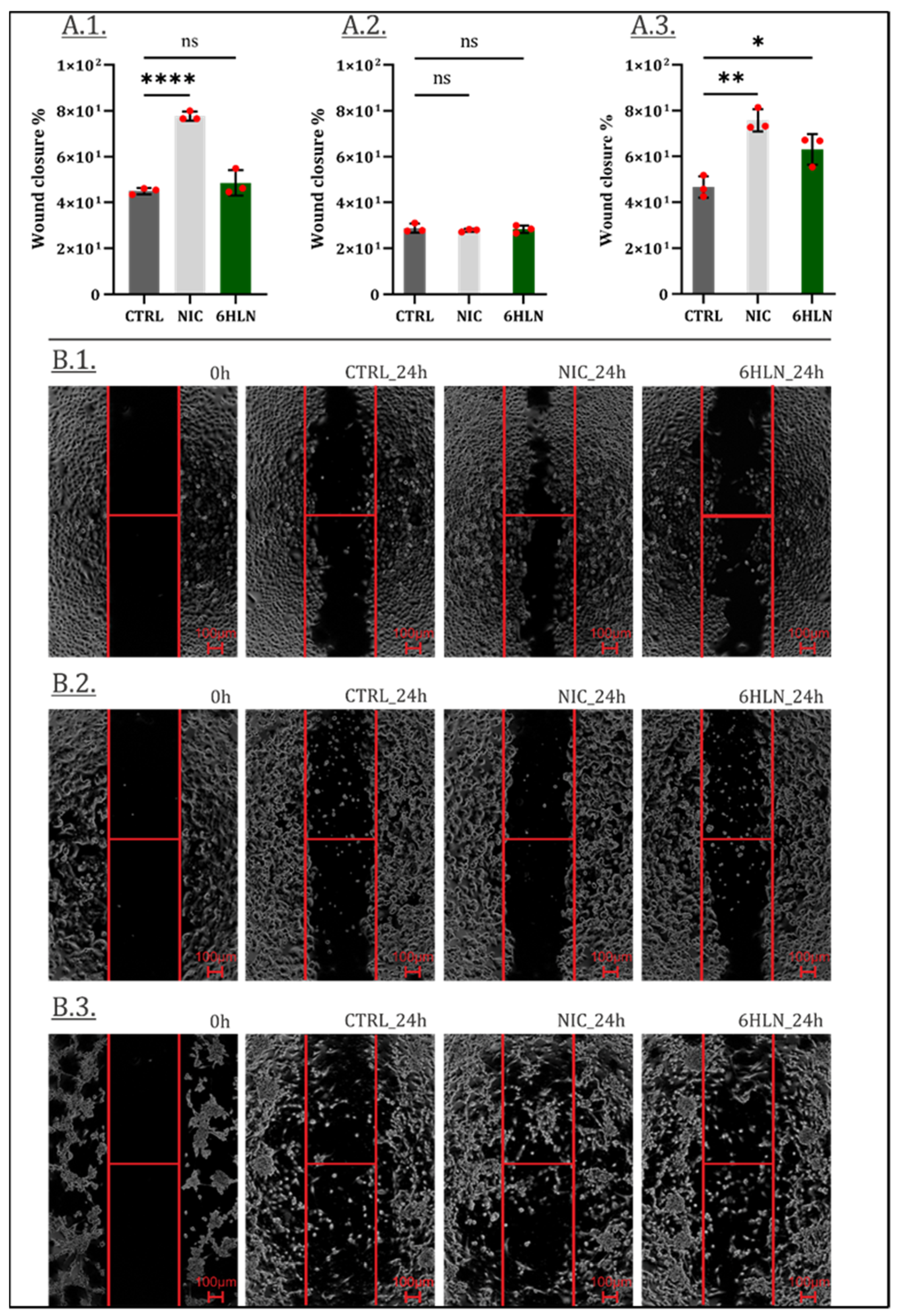
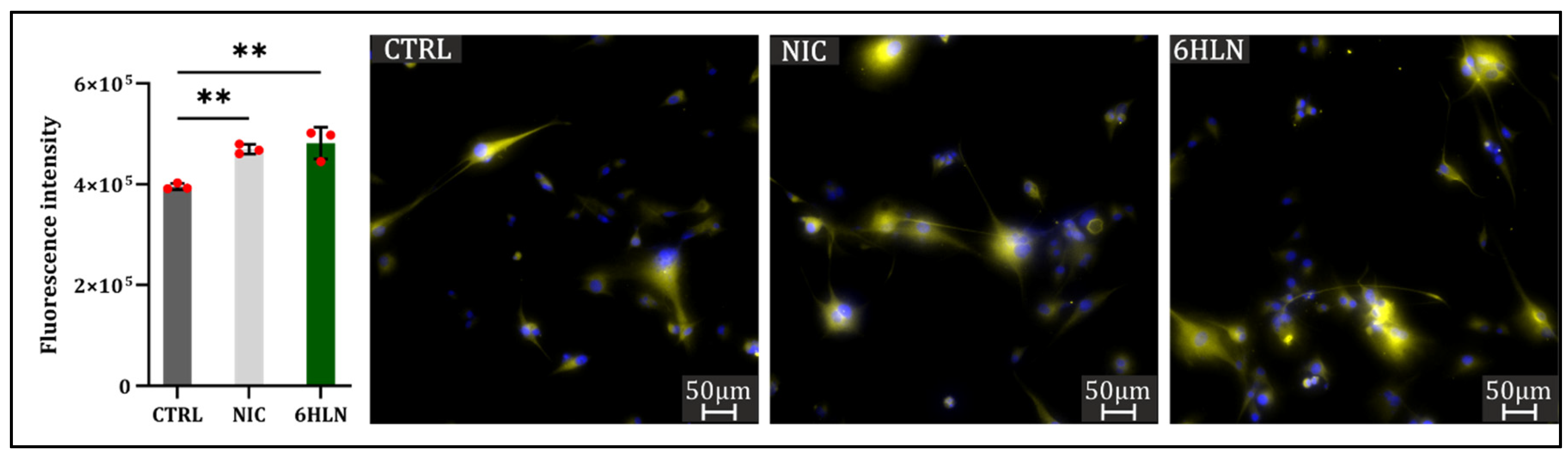
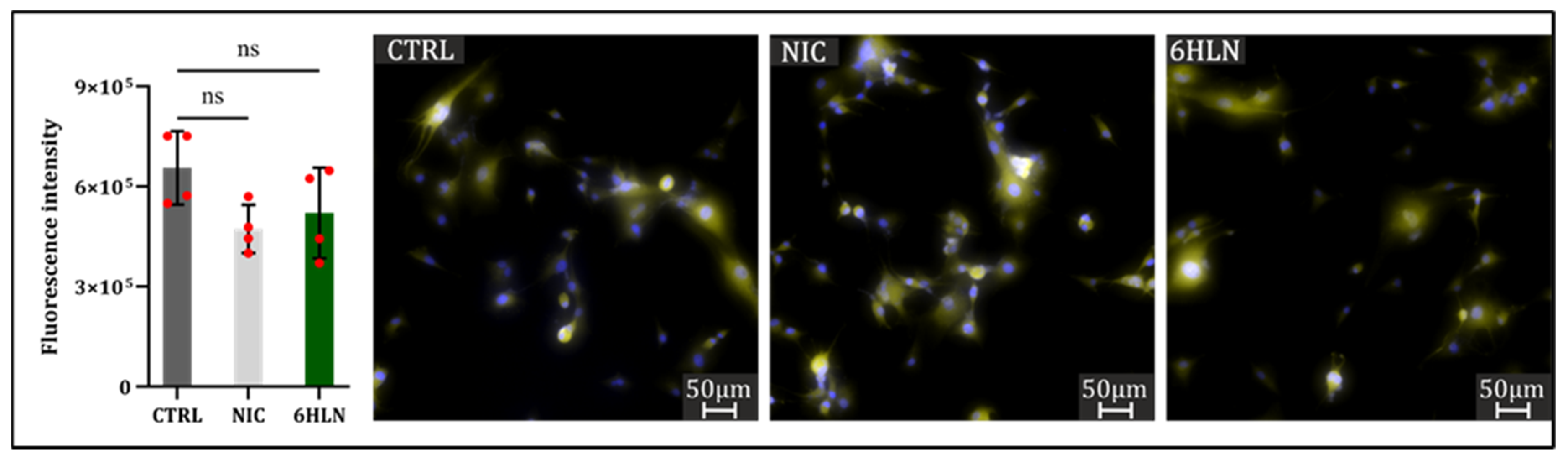
2.3. Influence of 6HLN and NIC on Migratory Behavior
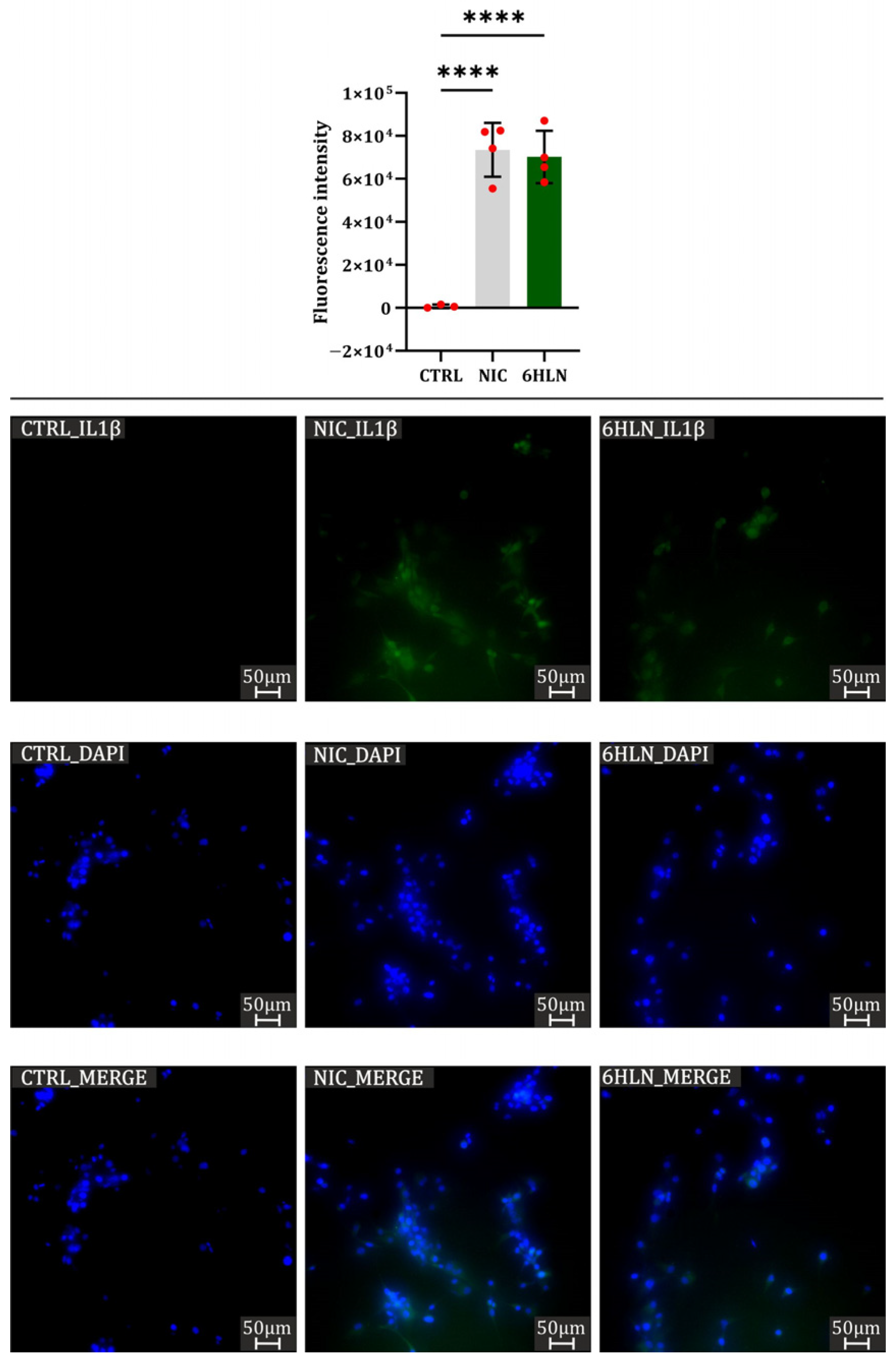
2.4. Influence of 6HLN and NIC on Pro-Inflammatory Cytokines
3. Materials and Methods
3.1. Cell Cultures
3.2. Cell Viability
3.3. Wound Healing Assay
3.4. Immunocytofluorescence
3.5. AlphaFold2 Multimer Structure Prediction of α9, α3β2α5 and α3β4α5 nAChRs and Molecular Docking Simulations
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.-H.; Hu, L.-P.; Wang, X.; Li, J.; Zhang, Z.-G. Neurotransmitters: Emerging Targets in Cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M.; Chakravarthy, H.; Sharma, S.; Shree, S.; Bhat, A.R.; Pradeepkiran, J.A.; Devanathan, V. Neurotransmitter Systems in the Etiology of Major Neurological Disorders: Emerging Insights and Therapeutic Implications. Ageing Res. Rev. 2023, 89, 101994. [Google Scholar] [CrossRef]
- Lolas, G.; Bianchi, A.; Syrigos, K.N. Tumour-Induced Neoneurogenesis and Perineural Tumour Growth: A Mathematical Approach. Sci. Rep. 2016, 6, 20684. [Google Scholar] [CrossRef]
- Li, Z.J.; Cho, C.H. Neurotransmitters, More than Meets the Eye–Neurotransmitters and Their Perspectives in Cancer Development and Therapy. Eur. J. Pharmacol. 2011, 667, 17–22. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, R.D.; Albores-García, D.; Cervantes-Villagrana, A.R.; García-Acevez, S.J. Tumor-Induced Neurogenesis and Immune Evasion as Targets of Innovative Anti-Cancer Therapies. Signal Transduct. Target. Ther. 2020, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Borniger, J.C.; D’Silva, N.J.; Deneen, B.; Dirks, P.B.; Fattahi, F.; Frenette, P.S.; Garzia, L.; Gutmann, D.H.; Hanahan, D.; et al. Roadmap for the Emerging Field of Cancer Neuroscience. Cell 2020, 181, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Venkatesh, H.S.; Amit, M.; Batchelor, T.; Demir, I.E.; Deneen, B.; Gutmann, D.H.; Hervey-Jumper, S.; Kuner, T.; Mabbott, D.; et al. Cancer Neuroscience: State of the Field, Emerging Directions. Cell 2023, 186, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Świt, P.; Herian, M.; Gołembiowska, K. Improvement of Analytical Results Quality in Neuroscience—Good Methodology Practice in the Acetylcholine Determination. Microchem. J. 2021, 168, 106404. [Google Scholar] [CrossRef]
- Dang, N.; Meng, X.; Song, H. Nicotinic Acetylcholine Receptors and Cancer. Biomed. Rep. 2016, 4, 515–518. [Google Scholar] [CrossRef]
- Schuller, H.M. Is Cancer Triggered by Altered Signalling of Nicotinic Acetylcholine Receptors? Nat. Rev. Cancer 2009, 9, 195–205. [Google Scholar] [CrossRef]
- Zhao, Y. The Oncogenic Functions of Nicotinic Acetylcholine Receptors. J. Oncol. 2016, 2016, 9650481. [Google Scholar] [CrossRef] [PubMed]
- Afrashteh Nour, M.; Hajiasgharzadeh, K.; Kheradmand, F.; Asadzadeh, Z.; Bolandi, N.; Baradaran, B. Nicotinic Acetylcholine Receptors in Chemotherapeutic Drugs Resistance: An Emerging Targeting Candidate. Life Sci. 2021, 278, 119557. [Google Scholar] [CrossRef] [PubMed]
- Arunrungvichian, K.; Vajragupta, O.; Hayakawa, Y.; Pongrakhananon, V. Targeting Alpha7 Nicotinic Acetylcholine Receptors in Lung Cancer: Insights, Challenges, and Therapeutic Strategies. ACS Pharmacol. Transl. Sci. 2024, 7, 28–41. [Google Scholar] [CrossRef]
- Pucci, S.; Zoli, M.; Clementi, F.; Gotti, C. A9-Containing Nicotinic Receptors in Cancer. Front. Cell. Neurosci. 2022, 15, 805123. [Google Scholar] [CrossRef]
- Bordas, A.; Cedillo, J.L.; Arnalich, F.; Esteban-Rodriguez, I.; Guerra-Pastrián, L.; de Castro, J.; Martín-Sánchez, C.; Atienza, G.; Fernández-Capitan, C.; Rios, J.J.; et al. Expression Patterns for Nicotinic Acetylcholine Receptor Subunit Genes in Smoking-Related Lung Cancers. Oncotarget 2017, 8, 67878–67890. [Google Scholar] [CrossRef] [PubMed]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Petre, B.A.; Hritcu, L. Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease. Antioxidants 2020, 9, 768. [Google Scholar] [CrossRef]
- Hritcu, L.; Ionita, R.; Motei, D.E.; Babii, C.; Stefan, M.; Mihasan, M. Nicotine versus 6-Hydroxy-L-Nicotine against Chlorisondamine Induced Memory Impairment and Oxidative Stress in the Rat Hippocampus. Biomed. Pharmacother. 2017, 86, 102–108. [Google Scholar] [CrossRef]
- Bele, T.; Turk, T.; Križaj, I. Nicotinic Acetylcholine Receptors in Cancer: Limitations and Prospects. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166875. [Google Scholar] [CrossRef]
- Kolodziej, M.A.; Gött, H.; Kopischke, B.; Bender, M.K.F.; Weigand, M.A.; Di Fazio, P.; Schwarm, F.P.; Uhle, F. Antiproliferative Effect of GTS-21 in Glioblastoma Cells. Oncol. Lett. 2021, 22, 759. [Google Scholar] [CrossRef]
- Pucci, S.; Fasoli, F.; Moretti, M.; Benfante, R.; Di Lascio, S.; Viani, P.; Daga, A.; Gordon, T.J.; McIntosh, M.; Zoli, M.; et al. Choline and Nicotine Increase Glioblastoma Cell Proliferation by Binding and Activating A7- and A9- Containing Nicotinic Receptors. Pharmacol. Res. 2021, 163, 105336. [Google Scholar] [CrossRef]
- Mucchietto, V.; Fasoli, F.; Pucci, S.; Moretti, M.; Benfante, R.; Maroli, A.; Di Lascio, S.; Bolchi, C.; Pallavicini, M.; Dowell, C.; et al. A9- and A7-Containing Receptors Mediate the pro-Proliferative Effects of Nicotine in the A549 Adenocarcinoma Cell Line. Br. J. Pharmacol. 2018, 175, 1957–1972. [Google Scholar] [CrossRef] [PubMed]
- Murayama, M.A.; Takada, E.; Takai, K.; Arimitsu, N.; Shimizu, J.; Suzuki, T.; Suzuki, N. Nicotine Treatment Regulates PD-L1 and PD-L2 Expression via Inhibition of Akt Pathway in HER2-Type Breast Cancer Cells. PLoS ONE 2022, 17, e0260838. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.M.; Roh, S.-H.; Gharpure, A.; Morales-Perez, C.L.; Teng, J.; Hibbs, R.E. Structural Principles of Distinct Assemblies of the Human A4β2 Nicotinic Receptor. Nature 2018, 557, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Zhang, J.; Chen, Y.; Ding, Y. Nicotine Promotes Chronic Obstructive Pulmonary Disease via Inducing Pyroptosis Activation in Bronchial Epithelial Cells. Mol. Med. Rep. 2022, 25, 92. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/MTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Degan, S.E.; Gelman, I.H. Emerging Roles for AKT Isoform Preference in Cancer Progression Pathways. Mol. Cancer Res. 2021, 19, 1251–1257. [Google Scholar] [CrossRef]
- Hinz, N.; Jücker, M. Distinct Functions of AKT Isoforms in Breast Cancer: A Comprehensive Review. Cell Commun. Signal. 2019, 17, 154. [Google Scholar] [CrossRef]
- Joy, A.; Kapoor, M.; Georges, J.; Butler, L.; Chang, Y.; Li, C.; Crouch, A.; Smirnov, I.; Nakada, M.; Hepler, J.; et al. The Role of AKT Isoforms in Glioblastoma: AKT3 Delays Tumor Progression. J. Neuro-Oncol. 2016, 130, 43–52. [Google Scholar] [CrossRef]
- Tu, S.-H.; Lin, Y.-C.; Huang, C.-C.; Yang, P.-S.; Chang, H.-W.; Chang, C.-H.; Wu, C.-H.; Chen, L.-C.; Ho, Y.-S. Protein Phosphatase Mg2+/Mn2+ Dependent 1F Promotes Smoking-Induced Breast Cancer by Inactivating Phosphorylated-P53-Induced Signals. Oncotarget 2016, 7, 77516–77531. [Google Scholar] [CrossRef]
- Oz, M.; King, J.R.; Yang, K.-H.S.; Khushaish, S.; Tchugunova, Y.; Khajah, M.A.; Luqmani, Y.A.; Kabbani, N. A7 Nicotinic Acetylcholine Receptor Interaction with G Proteins in Breast Cancer Cell Proliferation, Motility, and Calcium Signaling. PLoS ONE 2023, 18, e0289098. [Google Scholar] [CrossRef]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine Induces Cell Proliferation, Invasion and Epithelial-Mesenchymal Transition in a Variety of Human Cancer Cell Lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Jameson, M.J.; Broaddus, W.C.; Lin, P.S.; Chung, T.D. Nicotine Enhances Proliferation, Migration, and Radioresistance of Human Malignant Glioma Cells through EGFR Activation. Brain Tumor Pathol. 2013, 30, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Z.; Zhou, C.; Liu, L.; Huang, C. Epithelial-Mesenchymal Transition: The History, Regulatory Mechanism, and Cancer Therapeutic Opportunities. MedComm 2022, 3, e144. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling Mechanisms of the Epithelial-Mesenchymal Transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- McConnell, D.; Herbert, J.; Miller, D.; Litofsky, N. Repetitive Nicotine Exposure on Efficacy of Temozolomide and Radiotherapy on Cultured Glioblastoma Cell Lines. Med. Res. Arch. 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Karimi, N.; Kheiri, H.; Zarrinpour, V.; Forghanifard, M.M. Bioinformatic Analysis of MMP Family Members in GBM. Inform. Med. Unlocked 2023, 39, 101240. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. The Relationship between Nonsteroidal Anti-Inflammatory Drugs and Cancer Incidence: An Umbrella Review. Heliyon 2024, 10, e23203. [Google Scholar] [CrossRef]
- Ozleyen, A.; Yilmaz, Y.B.; Donmez, S.; Atalay, H.N.; Antika, G.; Tumer, T.B. Looking at NSAIDs from a Historical Perspective and Their Current Status in Drug Repurposing for Cancer Treatment and Prevention. J. Cancer Res. Clin. Oncol. 2023, 149, 2095–2113. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, H.; Zou, M.; Yuan, Q.; Huang, Z.; Pan, X.; Zhang, W. Nicotine in Inflammatory Diseases: Anti-Inflammatory and Pro-Inflammatory Effects. Front. Immunol. 2022, 13, 826889. [Google Scholar] [CrossRef]
- Ke, W.; Zhang, L.; Dai, Y. The Role of IL-6 in Immunotherapy of Non-Small Cell Lung Cancer (NSCLC) with Immune-Related Adverse Events (IrAEs). Thorac. Cancer 2020, 11, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J. IL-6: The Link Between Inflammation, Immunity and Breast Cancer. Front. Oncol. 2022, 12, 903800. [Google Scholar] [CrossRef]
- Kitzberger, C.; Shehzad, K.; Morath, V.; Spellerberg, R.; Ranke, J.; Steiger, K.; Kälin, R.E.; Multhoff, G.; Eiber, M.; Schilling, F.; et al. Interleukin-6-Controlled, Mesenchymal Stem Cell-Based Sodium/Iodide Symporter Gene Therapy Improves Survival of Glioblastoma-Bearing Mice. Mol. Ther. Oncol. 2023, 30, 238–253. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Arranz, L.; del Mar Arriero, M.; Villatoro, A. Interleukin-1β as Emerging Therapeutic Target in Hematological Malignancies and Potentially in Their Complications. Blood Rev. 2017, 31, 306–317. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein Complex Prediction with AlphaFold-Multimer. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Miteva, M.A.; Guyon, F.; Tuff?ry, P. Frog2: Efficient 3D Conformation Ensemble Generator for Small Compounds. Nucleic Acids Res. 2010, 38, W622–W627. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J.; Huey, R.; Lindstrom, W.; Sanner, M.F.; et al. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An Environment for Comparative Protein Modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postu, P.A.; Boiangiu, R.S.; Mihasan, M.; Stache, A.B.; Tiron, A.; Hritcu, L. The Distinct Biological Effects of 6-Hydroxy-L-Nicotine in Representative Cancer Cell Lines. Molecules 2024, 29, 5593. https://doi.org/10.3390/molecules29235593
Postu PA, Boiangiu RS, Mihasan M, Stache AB, Tiron A, Hritcu L. The Distinct Biological Effects of 6-Hydroxy-L-Nicotine in Representative Cancer Cell Lines. Molecules. 2024; 29(23):5593. https://doi.org/10.3390/molecules29235593
Chicago/Turabian StylePostu, Paula Alexandra, Razvan Stefan Boiangiu, Marius Mihasan, Alexandru Bogdan Stache, Adrian Tiron, and Lucian Hritcu. 2024. "The Distinct Biological Effects of 6-Hydroxy-L-Nicotine in Representative Cancer Cell Lines" Molecules 29, no. 23: 5593. https://doi.org/10.3390/molecules29235593
APA StylePostu, P. A., Boiangiu, R. S., Mihasan, M., Stache, A. B., Tiron, A., & Hritcu, L. (2024). The Distinct Biological Effects of 6-Hydroxy-L-Nicotine in Representative Cancer Cell Lines. Molecules, 29(23), 5593. https://doi.org/10.3390/molecules29235593









