Stokes–Einstein Relation in Different Models of Water
Abstract
1. Introduction
2. Materials and Methods
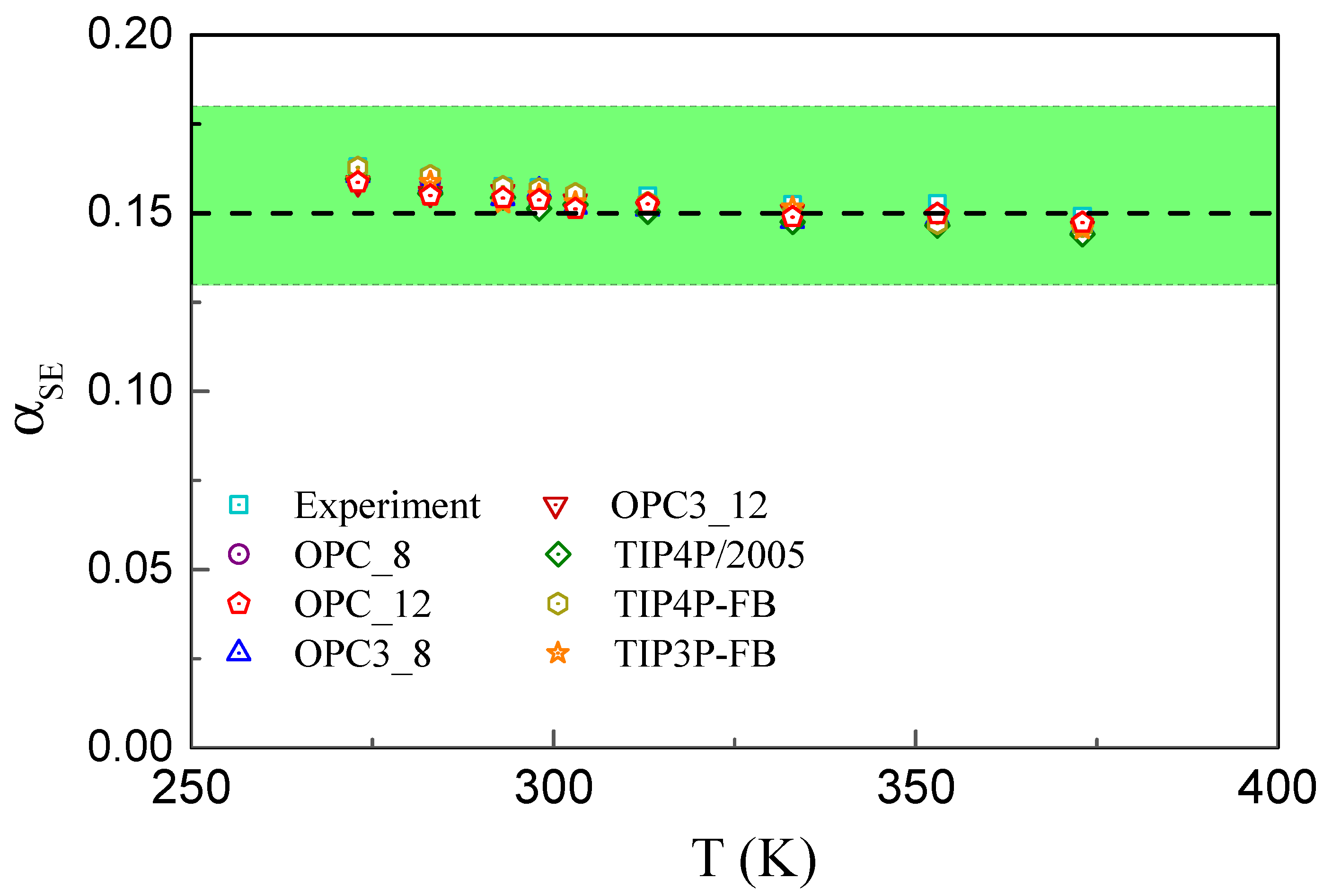
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SE | Stokes–Einstein |
| TIP | Transferable Intermolecular Potential |
| OPC | Optimal Point Charge |
References
- Landau, L.D.; Lifshitz, E.M. Fluid Mechanics; Butterworth-Heinemann: Oxford, UK, 1987. [Google Scholar]
- Balucani, U.; Zoppi, M. Dynamics of the Liquid State; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Costigliola, L.; Heyes, D.M.; Schrøder, T.B.; Dyre, J.C. Revisiting the Stokes-Einstein relation without a hydrodynamic diameter. J. Chem. Phys. 2019, 150, 21101. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, Y. Kinetic Theory of Liquids; Dover: New York, NY, USA, 1955. [Google Scholar]
- Zwanzig, R. On the relation between self-diffusion and viscosity of liquids. J. Chem. Phys. 1983, 79, 4507–4508. [Google Scholar] [CrossRef]
- Balucani, U.; Vallauri, R.; Gaskell, T. Generalized Stokes-Einstein Relation. Berichte Bunsenges. Phys. Chem. 1990, 94, 261–264. [Google Scholar] [CrossRef]
- Khrapak, S. Elementary vibrational model for transport properties of dense fluids. Phys. Rep. 2024, 1050, 1. [Google Scholar] [CrossRef]
- Khrapak, S. Stokes–Einstein relation in simple fluids revisited. Mol. Phys. 2019, 118, e1643045. [Google Scholar] [CrossRef]
- Khrapak, S.A.; Khrapak, A.G. Excess entropy and Stokes-Einstein relation in simple fluids. Phys. Rev. E 2021, 104, 44110. [Google Scholar] [CrossRef]
- Khrapak, S.; Kryuchkov, N.P.; Mistryukova, L.A.; Yurchenko, S.O. From soft- to hard-sphere fluids: Crossover evidenced by high-frequency elastic moduli. Phys. Rev. E 2021, 103, 52117. [Google Scholar] [CrossRef]
- Daligault, J. Liquid-State Properties of a One-Component Plasma. Phys. Rev. Lett. 2006, 96, 65003. [Google Scholar] [CrossRef]
- Daligault, J.; Rasmussen, K.; Baalrud, S.D. Determination of the shear viscosity of the one-component plasma. Phys. Rev. E 2014, 90, 33105. [Google Scholar] [CrossRef] [PubMed]
- Khrapak, S.A. Self-Diffusion in Simple Liquids as a Random Walk Process. Molecules 2021, 26, 7499. [Google Scholar] [CrossRef]
- Heyes, D.M.; Brańka, A.C. Physical properties of soft repulsive particle fluids. Phys. Chem. Chem. Phys. 2007, 9, 5570. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, N.; Ishii, Y. Explicit expression for the Stokes-Einstein relation for pure Lennard-Jones liquids. Phys. Rev. E 2015, 91, 12111. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, N.; Miyamoto, S.; Ishii, Y. Breakdown of the Stokes-Einstein relation in pure Lennard-Jones fluids: From gas to liquid via supercritical states. Phys. Rev. E 2017, 95, 52122. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, N.; Uchiyama, H.; Ishii, Y. The Stokes-Einstein relation for simple fluids: From hard-sphere to Lennard-Jones via WCA potentials. J. Chem. Phys. 2018, 149, 214501. [Google Scholar] [CrossRef] [PubMed]
- Khrapak, S.; Khrapak, A. Freezing density scaling of transport coefficients in the Weeks-Chandler-Andersen fluid. J. Chem. Phys. 2024, 160, 134504. [Google Scholar] [CrossRef]
- Pieprzyk, S.; Bannerman, M.N.; Brańka, A.C.; Chudak, M.; Heyes, D.M. Thermodynamic and dynamical properties of the hard sphere system revisited by molecular dynamics simulation. Phys. Chem. Chem. Phys. 2019, 21, 6886–6899. [Google Scholar] [CrossRef]
- Li, Q.; Sun, T.; Zhang, Y.; Xian, J.W.; Vocadlo, L. Atomic transport properties of liquid iron at conditions of planetary cores. J. Chem. Phys. 2021, 155, 194505. [Google Scholar] [CrossRef]
- Ranieri, U.; Klotz, S.; Gaal, R.; Koza, M.M.; Bove, L.E. Diffusion in dense supercritical methane from quasi-elastic neutron scattering measurements. Nat. Commun. 2021, 12, 1958. [Google Scholar] [CrossRef]
- Khrapak, S. Diffusion, viscosity, and Stokes-Einstein relation in dense supercritical methane. J. Mol. Liq. 2022, 354, 118840. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, C.; Li, Q.; Lin, Y.; Liu, L. Atomic transport properties of silicon melt at high temperature. J. Cryst. Growth 2022, 590, 126701. [Google Scholar] [CrossRef]
- Gomez, S.; Rovigatti, L. Diffusion, viscosity, and linear rheology of valence-limited disordered fluids. J. Chem. Phys. 2024, 160, 184901. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, N.; Kondo, Y.; Shintani, K.; Murakami, T.; Nobuta, T.; Ishii, Y. The Stokes-Einstein Relation for Non-spherical Molecular Liquids. Chem. Lett. 2020, 49, 379–382. [Google Scholar] [CrossRef]
- Abascal, J.L.F.; Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Danilov, I.V.; Tsiok, O.B. Mysteries of Water and Other Anomalous Liquids: “Slow” Sound and Relaxing Compressibility and Heat Capacity (Brief Review). JETP Lett. 2023, 117, 840–856. [Google Scholar] [CrossRef]
- Khrapak, S.A. System Size Dependence of the Diffusion Coefficients in MD Simulations: A Simple Correction Formula for Pure Dense Fluids. J. Phys. Chem. B 2024, 128, 287. [Google Scholar] [CrossRef]
- Yeh, I.C.; Hummer, G. System-Size Dependence of Diffusion Coefficients and Viscosities from Molecular Dynamics Simulations with Periodic Boundary Conditions. J. Phys. Chem. B 2004, 108, 15873–15879. [Google Scholar] [CrossRef]
- Busch, J.; Paschek, D. OrthoBoXY: A Simple Way to Compute True Self-Diffusion Coefficients from MD Simulations with Periodic Boundary Conditions without Prior Knowledge of the Viscosity. J. Phys. Chem. B 2023, 127, 7983–7987. [Google Scholar] [CrossRef]
- Abascal, J.L.F.; Sanz, E.; Fernandez, R.G.; Vega, C. A potential model for the study of ices and amorphous water: TIP4P/Ice. J. Chem. Phys. 2005, 122, 234511. [Google Scholar] [CrossRef]
- Baran, L.; Rzysko, W.; MacDowell, L.G. Self-diffusion and shear viscosity for the TIP4P/Ice water model. J. Chem. Phys. 2023, 158, 64503. [Google Scholar] [CrossRef]
- Khrapak, S.A.; Khrapak, A.G. Stokes–Einstein relation without hydrodynamic diameter in the TIP4P/Ice water model. J. Chem. Phys. 2023, 158, 206101. [Google Scholar] [CrossRef]
- Khrapak, S.A.; Khrapak, A.G. Quasiuniversal behavior of shear relaxation times in simple fluids. Phys. Rev. E 2024, 110, 54101. [Google Scholar] [CrossRef]
- Dueby, S.; Dubey, V.; Daschakraborty, S. Decoupling of Translational Diffusion from the Viscosity of Supercooled Water: Role of Translational Jump Diffusion. J. Phys. Chem. B 2019, 123, 7178–7189. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Erimban, S.; Indra, S.; Daschakraborty, S. Understanding the Origin of the Breakdown of the Stokes–Einstein Relation in Supercooled Water at Different Temperature–Pressure Conditions. J. Phys. Chem. B 2019, 123, 10089–10099. [Google Scholar] [CrossRef]
- Dubey, V.; Dueby, S.; Daschakraborty, S. Breakdown of the Stokes–Einstein relation in supercooled water: The jump-diffusion perspective. Phys. Chem. Chem. Phys. 2021, 23, 19964–19986. [Google Scholar] [CrossRef]
- Ando, T. Shear viscosity of OPC and OPC3 water models. J. Chem. Phys. 2023, 159, 101102. [Google Scholar] [CrossRef]
- Izadi, S.; Anandakrishnan, R.; Onufriev, A. Building Water Models: A Different Approach. J. Phys. Chem. Lett. 2014, 5, 3863–3871. [Google Scholar] [CrossRef]
- Izadi, S.; Onufriev, A. Accuracy limit of rigid 3-point water models. J. Chem. Phys. 2016, 145, 74501. [Google Scholar] [CrossRef]
- Wang, L.P.; Martinez, T.; Pande, V. Building Force Fields: An Automatic, Systematic, and Reproducible Approach. J. Phys. Chem. Lett. 2014, 5, 1885–1891. [Google Scholar] [CrossRef]
- Holz, M.; Heil, S.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology Database. Available online: https://webbook.nist.gov/chemistry/fluid (accessed on 17 October 2024).
- Lemmon, E.W.; Bell, I.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar] [CrossRef]
- Wagner, W.; Pruss, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Huber, M.L.; Perkins, R.A.; Laesecke, A.; Friend, D.G.; Sengers, J.V.; Assael, M.J.; Metaxa, I.N.; Vogel, E.; Mareš, R.; Miyagawa, K. New International Formulation for the Viscosity of H2O. J. Phys. Chem. Ref. Data 2009, 38, 101–125. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Fomin, Y.D.; Lyapin, A.G.; Ryzhov, V.N.; Trachenko, K. Two liquid states of matter: A dynamic line on a phase diagram. Phys. Rev. E 2012, 85, 31203. [Google Scholar] [CrossRef] [PubMed]
- Brazhkin, V.V.; Fomin, Y.D.; Lyapin, A.G.; Ryzhov, V.N.; Tsiok, E.N.; Trachenko, K. “Liquid-gas” transition in the supercritical region: Fundamental changes in the particle dynamics. Phys. Rev. Lett. 2013, 111, 145901. [Google Scholar] [CrossRef]
- Khrapak, S.A. Gas-liquid crossover in the Lennard-Jones system. J. Chem. Phys. 2022, 156, 116101. [Google Scholar] [CrossRef]
- Trachenko, K. Theory of Liquids: From Excitations to Thermodynamics; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar]
- Khrapak, S.A.; Khrapak, A.G. Minima of shear viscosity and thermal conductivity coefficients of classical fluids. Phys. Fluids 2022, 34, 27102. [Google Scholar] [CrossRef]
- Becker, S.R.; Poole, P.H.; Starr, F.W. Fractional Stokes-Einstein and Debye-Stokes-Einstein Relations in a Network-Forming Liquid. Phys. Rev. Lett. 2006, 97, 55901. [Google Scholar] [CrossRef]
- Xu, L.; Mallamace, F.; Yan, Z.; Starr, F.W.; Buldyrev, S.V.; Eugene Stanley, H. Appearance of a fractional Stokes-Einstein relation in water and a structural interpretation of its onset. Natl. Phys. 2009, 5, 565–569. [Google Scholar] [CrossRef]
- Harris, K.R. The fractional Stokes-Einstein equation: Application to Lennard-Jones, molecular, and ionic liquids. J. Chem. Phys. 2009, 131, 54503. [Google Scholar] [CrossRef]
- Dehaoui, A.; Issenmann, B.; Caupin, F. Viscosity of deeply supercooled water and its coupling to molecular diffusion. Proc. Nat. Acad. Sci. USA 2015, 112, 12020–12025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrapak, S.; Khrapak, A. Stokes–Einstein Relation in Different Models of Water. Molecules 2024, 29, 5587. https://doi.org/10.3390/molecules29235587
Khrapak S, Khrapak A. Stokes–Einstein Relation in Different Models of Water. Molecules. 2024; 29(23):5587. https://doi.org/10.3390/molecules29235587
Chicago/Turabian StyleKhrapak, Sergey, and Alexey Khrapak. 2024. "Stokes–Einstein Relation in Different Models of Water" Molecules 29, no. 23: 5587. https://doi.org/10.3390/molecules29235587
APA StyleKhrapak, S., & Khrapak, A. (2024). Stokes–Einstein Relation in Different Models of Water. Molecules, 29(23), 5587. https://doi.org/10.3390/molecules29235587






