Improved Sensitivity of a Taste Sensor Composed of Trimellitic Acids for Sweetness
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Lipid and Modifier Content in Sweetness Sensor Membrane on Reference Potential and Relative Value
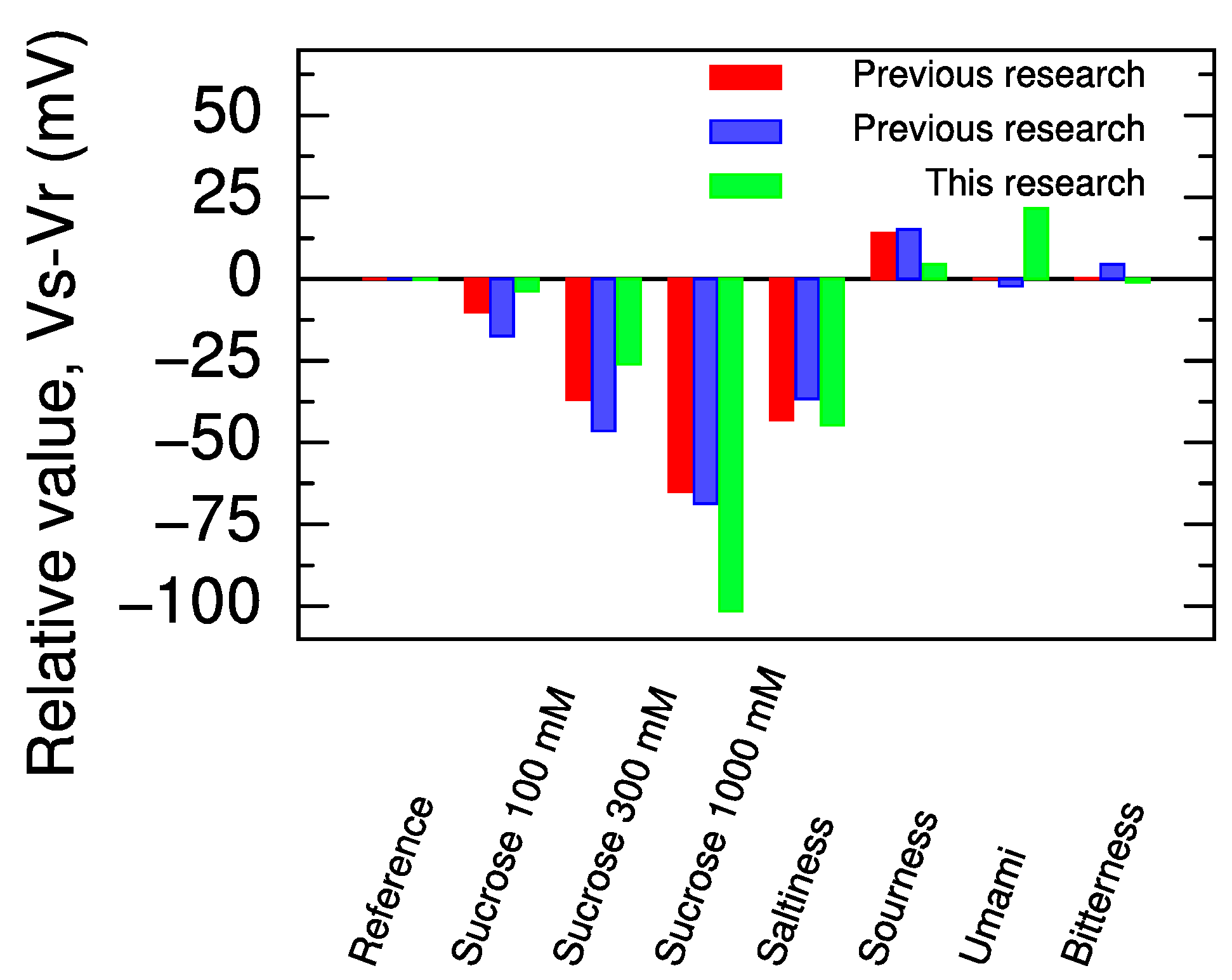
2.2. Response to Five Basic Taste Samples
3. Materials and Methods
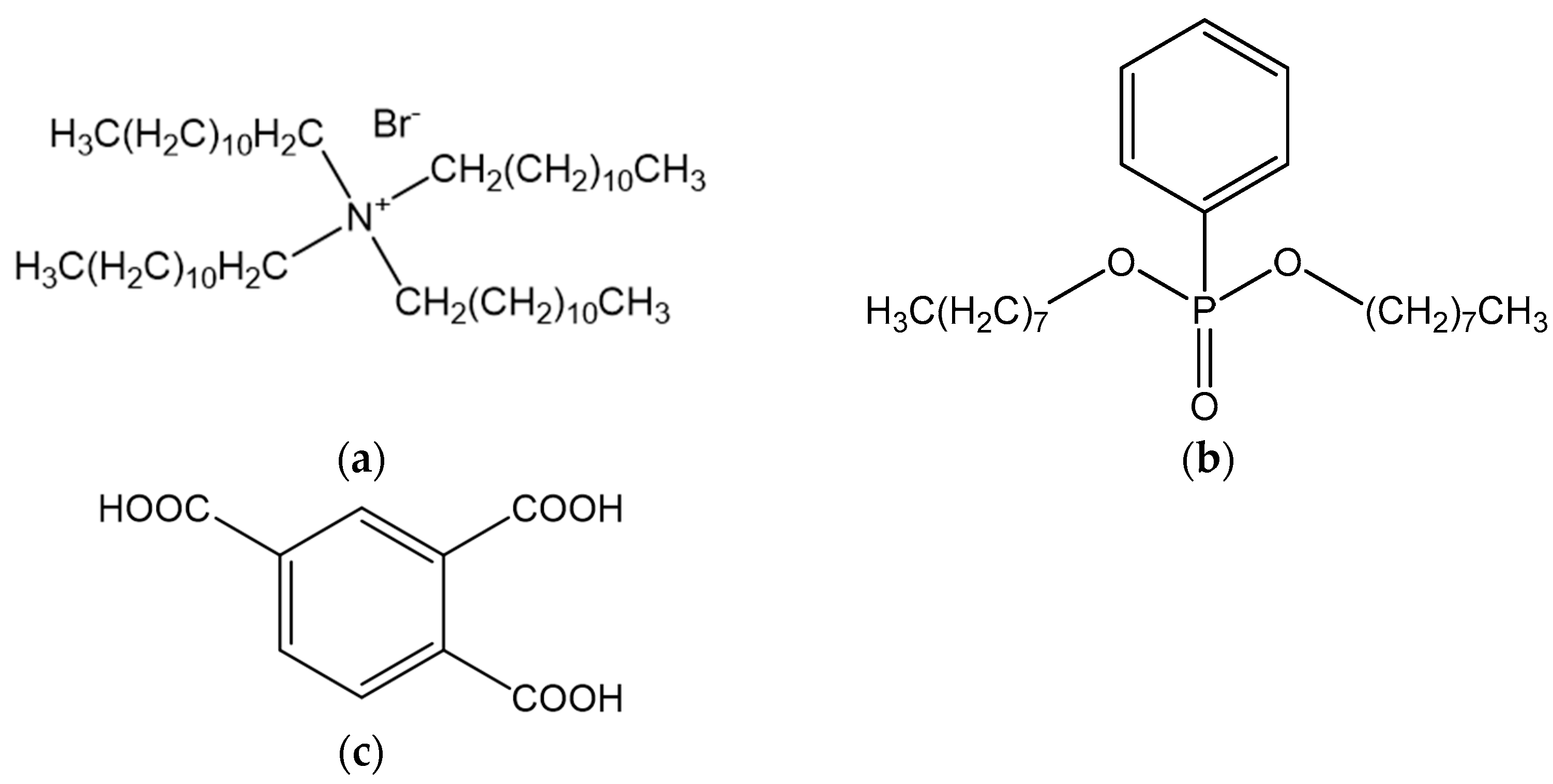
3.1. Reagent
3.2. Solution Preparation
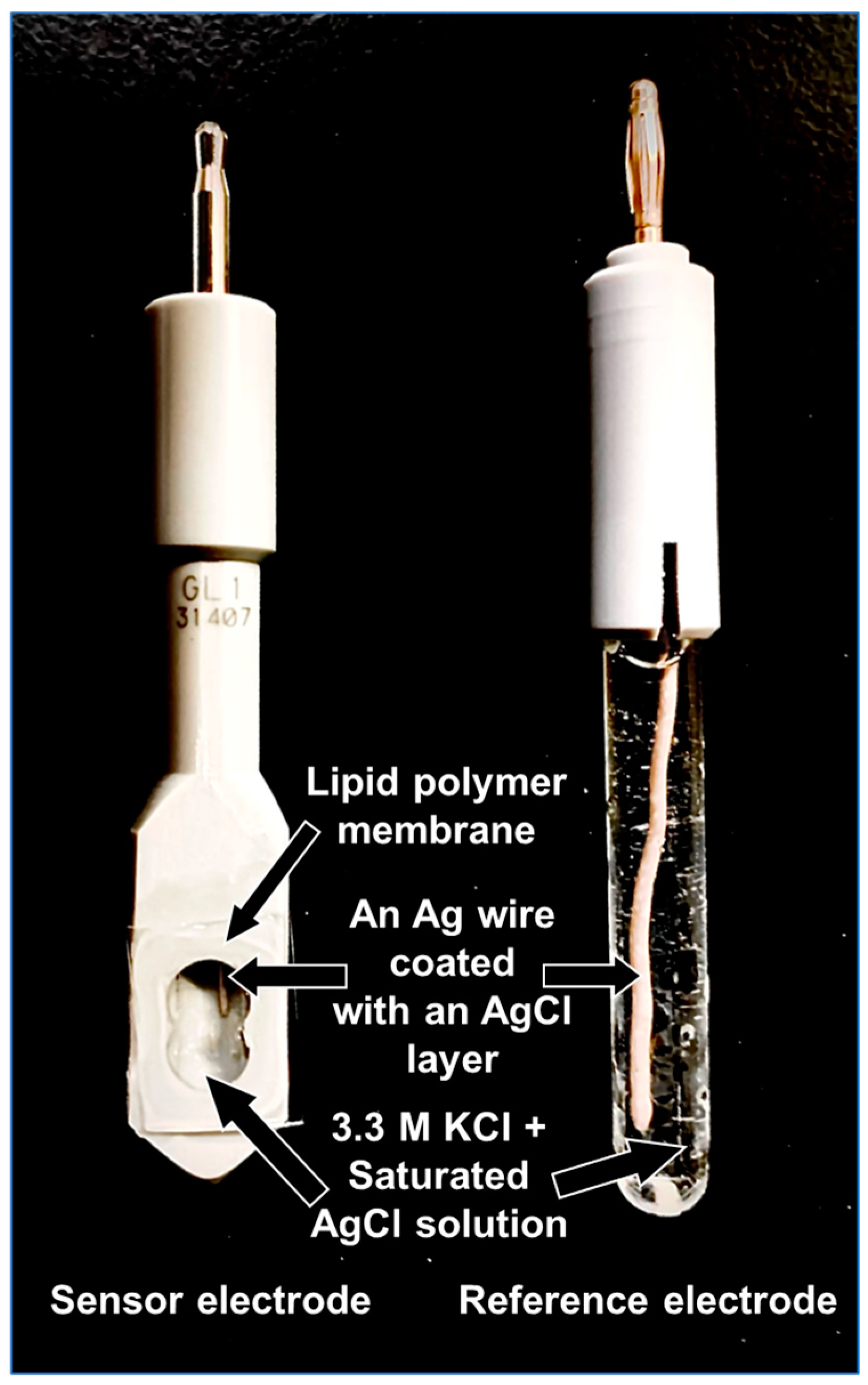
3.3. Formation of Lipid/Polymer Membrane and Fabrication of Sensor Electrodes
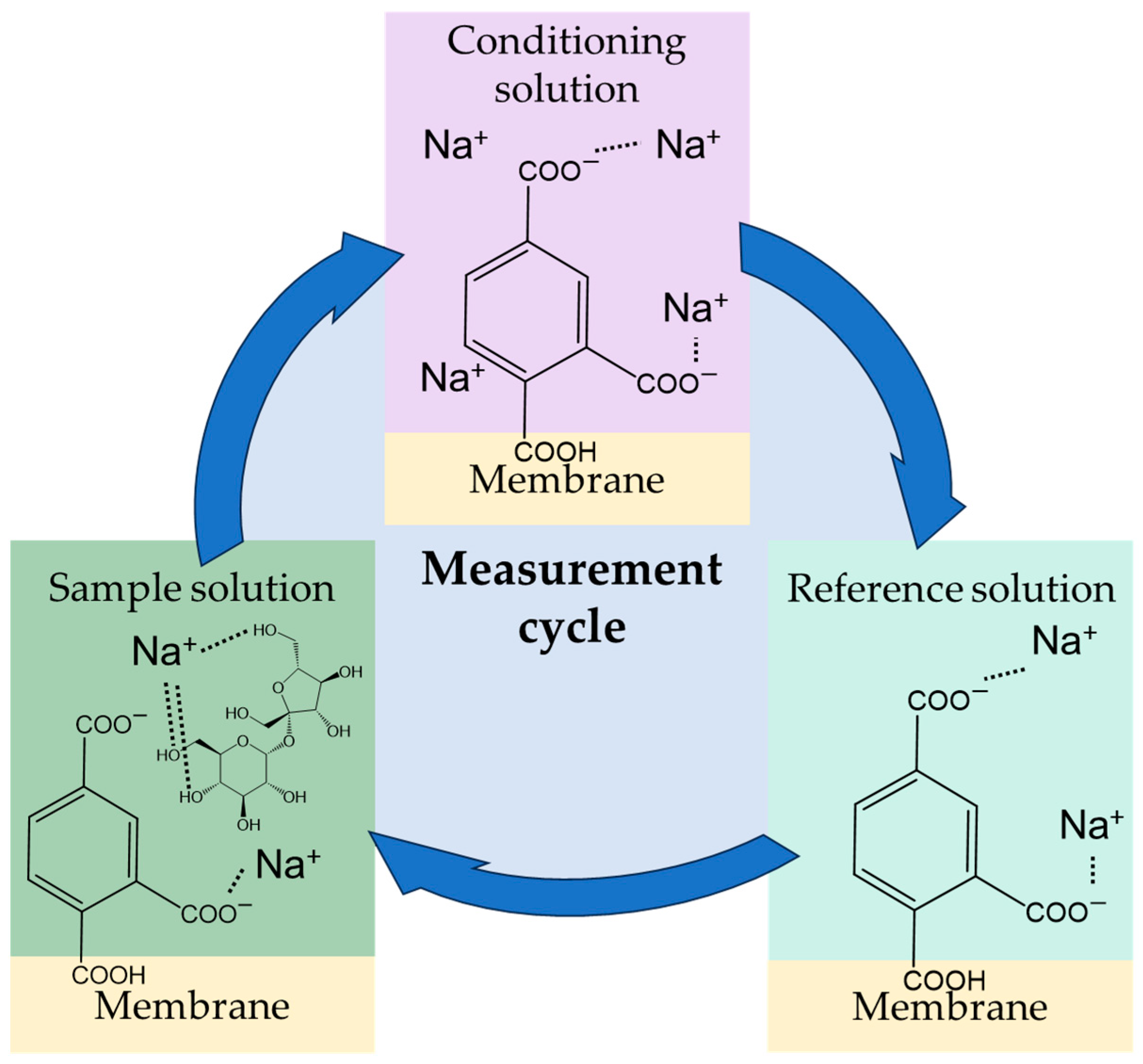
3.4. Measurement Procedure and Response Mechanism of Taste Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Beahm, D.R.; Ionov, S.; Sarpeshkar, R. Measuring and modeling energy and power consumption in living microbial cells with a synthetic ATP reporter. BMC Biol. 2021, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.P.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E. Transdisciplinary perspectives on sweetness. Chemosens. Percept. 2008, 1, 48. [Google Scholar] [CrossRef]
- Shallenberger, R.S.; Acree, T.E. Molecular theory of sweet taste. Nature 1967, 216, 480. [Google Scholar] [CrossRef] [PubMed]
- Acree, T.E.; Shallenberger, R.S.; Ebeling, S. Thirty years of the AH-B theory. Dev. Food Sci. 1998, 40, 1–13. [Google Scholar]
- Eggers, S.C.; Acree, T.E.; Shallenberger, R.S. Sweetness chemoreception theory and sweetness transduction. Food Chem. 2000, 68, 45. [Google Scholar] [CrossRef]
- Kier, L.B. A molecular theory of sweet taste. J. Pharm. Sci. 1972, 61, 1394–1397. [Google Scholar] [CrossRef]
- Xu, H.; Staszewski, L.; Tang, H.; Adler, E.; Zoller, M.; Li, X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 14258–14263. [Google Scholar] [CrossRef]
- Chéron, J.B.; Golebiowski, J.; Antonczak, S.; Fiorucci, S. The anatomy of mammalian sweet taste receptors. Proteins 2017, 85, 332–341. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, X.; Fang, Y.; Sun, R.; Liu, B. The molecular theory of sweet taste: Revisit, update, and beyond. J. Med. Chem. 2024, 67, 3232. [Google Scholar] [CrossRef]
- Ardnaree, K.; Wongwilai, W.; Lapanantnopphakhun, S.; Grudpan, K. A simple lab on chip for quantitation of sugar content in a syrup. J. Flow Inject. Anal. 2013, 30, 114. [Google Scholar]
- Yamashita, Y.; Arakawa, M.; Funatsu, K. Internal quality analysis of fruit using near-infrared spectra. J. Comput. Aided Chem. 2011, 12, 37. [Google Scholar] [CrossRef][Green Version]
- Kamma, K.; Kaneko, H.; Funatsu, K. Development of a novel spectra analysis method to construct accurate NIR models. J. Comput. Aided Chem. 2014, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Nakasato, K.; Ono, T.; Ishiguro, T.; Takamatsu, M.; Tsukamoto, C.; Mikami, M. Rapid quantitative analysis of the major components in soymilk using fourier transform infrared spectroscopy (FT-IR). Food Sci. Technol. Res. 2004, 10, 137. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative research on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Taste sensing system (electronic tongues) for pharmaceutical applications. Int. J. Pharm. 2011, 417, 256. [Google Scholar] [CrossRef]
- Anand, V.; Kataria, M.; Kukkar, V.; Saharan, V.; Choudhury, P.K. The latest trends in the taste assessment of pharmaceuticals. Drug Discov. Today 2007, 12, 257–265. [Google Scholar] [CrossRef]
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic tongues—A review. IEEE Sens. J. 2013, 13, 3001. [Google Scholar] [CrossRef]
- Toko, K. Research and development of taste sensors as a novel analytical tool. Proc. Jpn. Acad. Ser. B 2023, 99, 173–189. [Google Scholar] [CrossRef]
- Toko, K.; Onodera, T.; Tahara, Y. Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences; Bagchi, D., Bagchi, M., Moriyama, H., Shahidi, F., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; p. 270. [Google Scholar]
- Ishiwaki, T. Biochemical Sensors: Mimicking Gustatory and Olfactory Senses; Toko, K., Ed.; Pan Stanford Publishing: Singapore, 2013; p. 83. [Google Scholar]
- Savage, N. Technology: The taste of things to come. Nature 2012, 486, 18. [Google Scholar] [CrossRef] [PubMed]
- Citterio, D.; Suzuki, K. Smart taste sensors. Anal. Chem. 2008, 80, 396. [Google Scholar]
- Winquist, F. Voltammetric electronic tongues–basic principles and applications. Microchim. Acta 2008, 163, 3. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Yamashita, M.; Kato, M.; Suzuki, T.; Omori, M.; Chen, R. Evaluation of the taste of tea with different degrees of fermentation using a taste sensing system. Sens. Mater. 2011, 23, 501. [Google Scholar]
- Cui, S.; Wang, J.; Geng, L.; Wei, Z.; Tian, X. Determination of ginseng with different ages using a taste-sensing system. Sens. Mater. 2013, 25, 241. [Google Scholar]
- Harada, T.; Uchida, T.; Yoshida, M.; Kobayashi, Y.; Narazaki, R.; Ohwaki, T. A new method for evaluating the bitterness of medicines in development using a taste sensor and a disintegration testing apparatus. Chem. Pharm. Bull. 2010, 58, 1009. [Google Scholar] [CrossRef]
- Toyota, K.; Cui, H.; Abe, K.; Habara, M.; Toko, K.; Ikezaki, H. Sweetness sensor with lipid/polymer membranes: Sweet-responsive substances. Sens. Mater. 2011, 23, 465. [Google Scholar]
- Yasuura, M.; Tahara, Y.; Ikezaki, H.; Toko, K. Development of a sweetness sensor for aspartame, a positively charged high-potency sweetener. Sensors 2014, 14, 7359. [Google Scholar] [CrossRef]
- Yasuura, M.; Okazaki, H.; Tahara, Y.; Ikezaki, H.; Toko, K. Development of sweetness sensor with selectivity to negatively charged high-potency sweeteners. Sens. Actuators B 2014, 201, 329. [Google Scholar] [CrossRef]
- Ye, Z.; Ai, T.; Wu, X.; Onodera, T.; Ikezaki, H.; Toko, K. Elucidation of response mechanism of a potentiometric sweetness sensor with a lipid/polymer membrane for uncharged sweeteners. Chemosens 2022, 10, 166. [Google Scholar] [CrossRef]
- Angyal, S.J. Complex formation between sugars and metal ions. Pure Appl. Chem. 1973, 35, 131. [Google Scholar] [CrossRef]
- Träuble, H.; Teubner, M.; Woolley, P.; Eibl, H. Electrostatic interactions at charged lipid membranes. I. Effects of pH and univalent cations on membrane structure. Biophys. Chem. 1976, 4, 319. [Google Scholar] [CrossRef] [PubMed]
- Payens, T.A.J. Ionized monolayers. Philips Res. Rep. 1955, 10, 425. [Google Scholar]
- Kamo, N.; Oikawa, M.; Kobatake, Y. Effective fixed charge density governing membrane phenomena. V. A reduced expression of permselectivity. J. Phys. Chem. 1973, 77, 92. [Google Scholar] [CrossRef] [PubMed]
- Kamo, N.; Kobatake, Y. Interpretation of asymmetric membrane potential. J. Colloid Interface Sci. 1974, 46, 85. [Google Scholar] [CrossRef]
- Toko, K.; Hara, D.; Tahara, Y.; Yasuura, M.; Ikezaki, H. Relationship between the amount of bitter substances adsorbed onto lipid/polymer membrane and the electric response of taste sensors. Sensors 2014, 14, 16274. [Google Scholar] [CrossRef]
- Mikhelson, K.N. Ion-selective electrode in PVC matrix. Sens. Actuators B 1994, 18–19, 31. [Google Scholar] [CrossRef]
- Watanabe, M.; Toko, K.; Sato, K.; Kina, K.; Takahashi, Y.; Iiyama, S. Charged impurities of plasticizer used for ion-selective electrode and taste sensor. Sens. Mater. 1998, 10, 103. [Google Scholar]
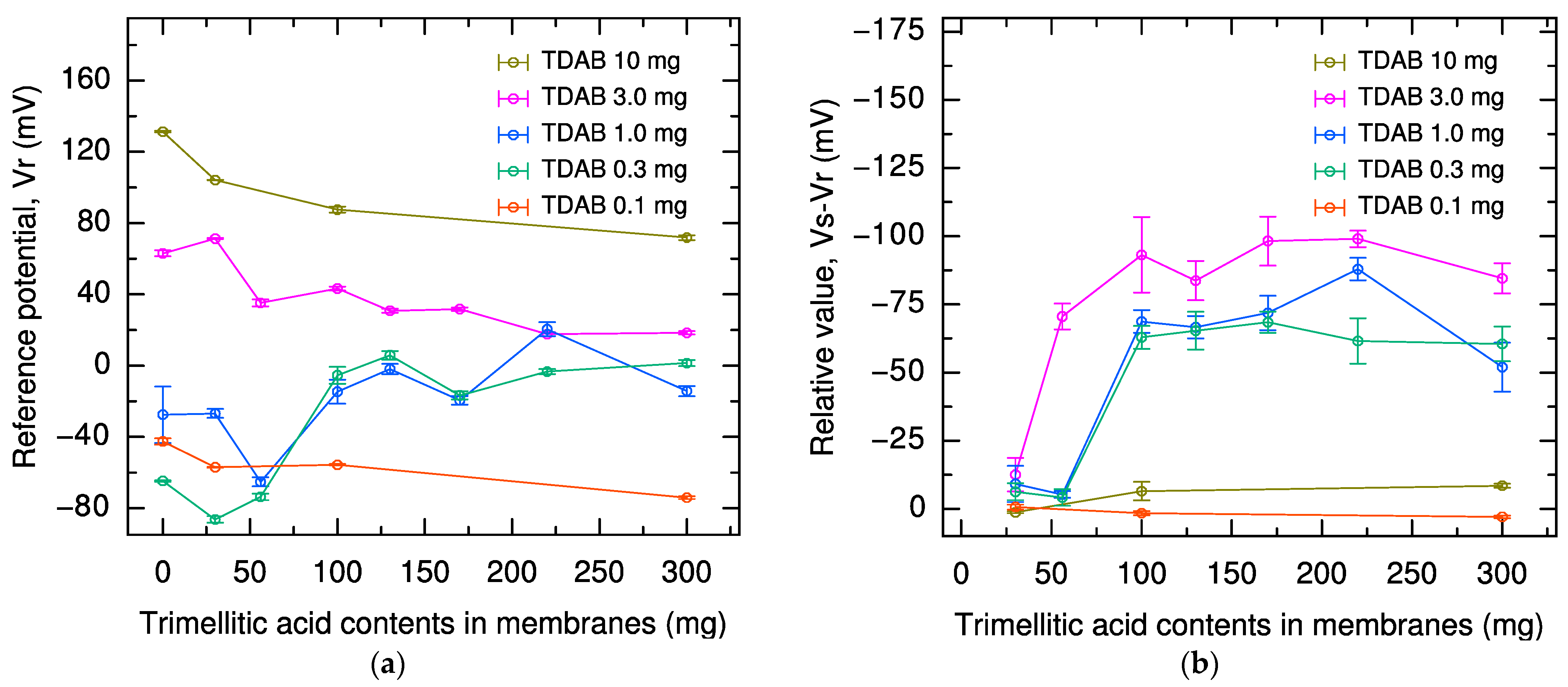
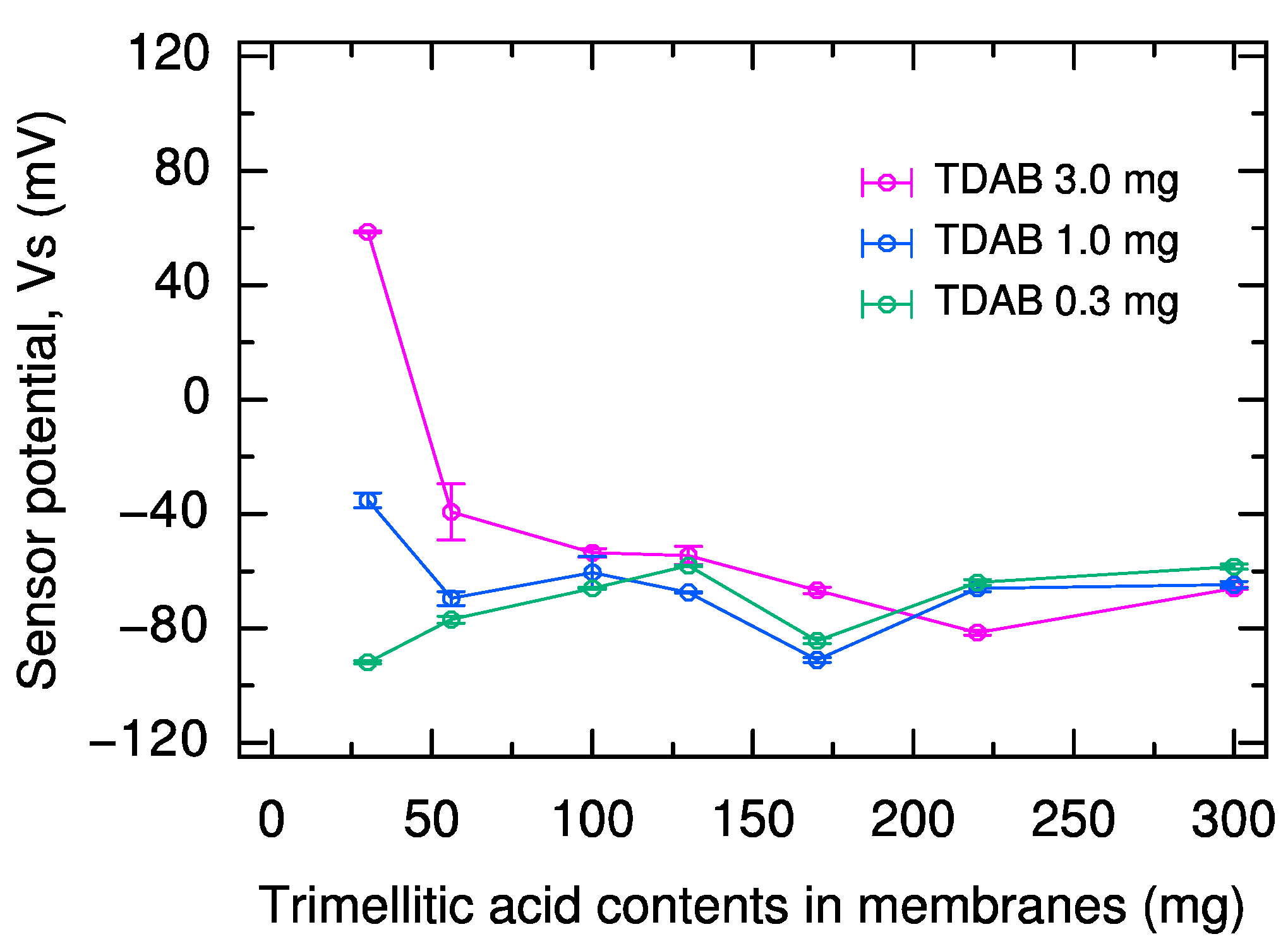
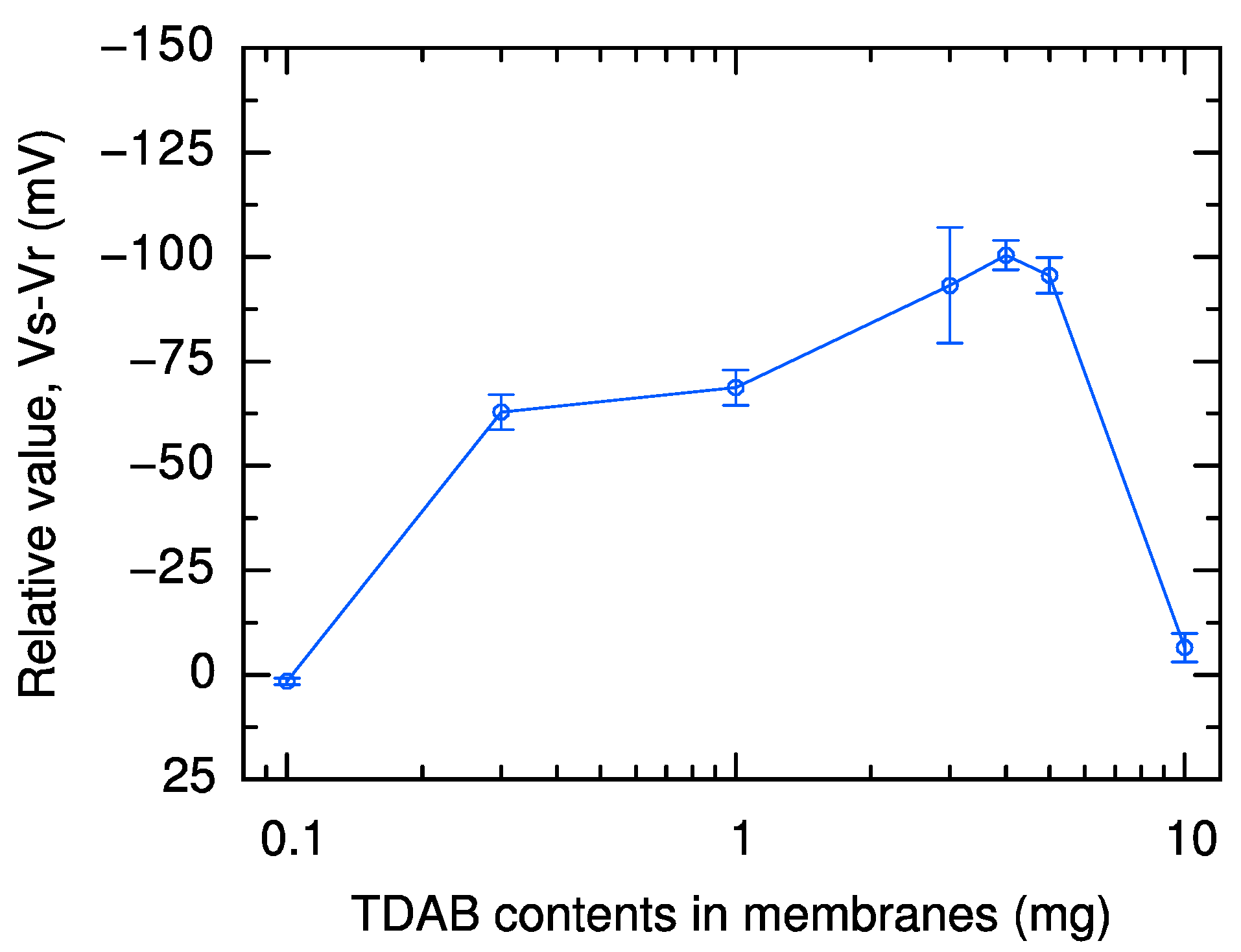
| Reference Potential | Relative Value | Vs |
|---|---|---|
| Positive shift with increasing TDAB content | Increasing up to 3 mg TDAB, or up to 100 mg trimellitic acid | Only minor fluctuations above 100 mg trimellitic acid |
| Sample | Composition | Concentration |
|---|---|---|
| Reference | KCl and Tartaric acid | 30 mM and 0.3 mM, respectively |
| Sweetness | Sucrose | 100, 300, 1000 mM |
| Saltiness | KCl | 300 mM |
| Sourness | Tartaric acid | 3 mM |
| Umami | Monosodium L-glutamate (MSG) | 10 mM |
| Bitterness | Quinine hydrochloride | 0.1 mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, T.; Kumura, S.; Kimura, S.; Toko, K. Improved Sensitivity of a Taste Sensor Composed of Trimellitic Acids for Sweetness. Molecules 2024, 29, 5573. https://doi.org/10.3390/molecules29235573
Watanabe T, Kumura S, Kimura S, Toko K. Improved Sensitivity of a Taste Sensor Composed of Trimellitic Acids for Sweetness. Molecules. 2024; 29(23):5573. https://doi.org/10.3390/molecules29235573
Chicago/Turabian StyleWatanabe, Tatsukichi, Sojiro Kumura, Shunsuke Kimura, and Kiyoshi Toko. 2024. "Improved Sensitivity of a Taste Sensor Composed of Trimellitic Acids for Sweetness" Molecules 29, no. 23: 5573. https://doi.org/10.3390/molecules29235573
APA StyleWatanabe, T., Kumura, S., Kimura, S., & Toko, K. (2024). Improved Sensitivity of a Taste Sensor Composed of Trimellitic Acids for Sweetness. Molecules, 29(23), 5573. https://doi.org/10.3390/molecules29235573







