Investigation of the Binary Nitrides YN, LaN and LuN by Solid-State NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. NMR of Spin-1: 14N
2.2. 14N Chemical Shift Relation to Crystal Structure
2.3. NMR of Cations
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podgornik, B.; Kosec, T.; Kocijan, A.; Donik, Č. Tribological behaviour and lubrication performance of hexagonal boron nitride (h-BN) as a replacement for graphite in aluminium forming. Tribol. Int. 2015, 81, 267–275. [Google Scholar] [CrossRef]
- Dridi, Z.; Bouhafs, B.; Ruterana, P. Pressure dependence of energy band gaps for AlxGa1−xN, InxGa1−xN und InxAl1−xN. New J. Phys. 2002, 4, 94. [Google Scholar] [CrossRef]
- Polster, T.; Hoffmann, M. Aluminum nitride based 3D, piezoelectric, tactile sensor. Procedia Chem. 2009, 1, 144–147. [Google Scholar] [CrossRef]
- Lima, L.; Diniz, J.; Doi, I.; Godoy Fo, J. Titanium nitride as electrode for MOS technology and Schottky diode: Alternative extraction method of titanium nitride work function. Microelectron. Eng. 2012, 92, 86–90. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Liu, L.; Manasa, P.; Kang, L.; Ran, F. Vanadium nitride for aqueous supercapacitors: A topic review. J. Mater. Chem. A 2020, 8, 8218–8233. [Google Scholar] [CrossRef]
- Soto, G.; Moreno-Armenta, M.; Reyes-Serrato, A. First principles study on the formation of yttrium nitride in cubic and hexagonal phases. Comp. Mater. Sci. 2008, 42, 8–13. [Google Scholar] [CrossRef]
- Ravi, C.; Sahu, H.; Valsakumar, M.; van de Walle, A. Cluster expansion Monte Carlo study of phase stability of vanadium nitrides. Phys. Rev. B 2010, 81, 104111. [Google Scholar] [CrossRef]
- Porte, L. Stoichiometric ScN and nitrogen deficient scandium nitride layers studied by photoelectron spectroscopy. J. Phys. C. Solid State Phys. 1985, 18, 6701–6709. [Google Scholar] [CrossRef]
- Čapková, P.; Smrčok, V.; Šlma, V.; Valvoda, V.; Chalupa, P.; Ettmayer, B. Thermal Vibrations in Substoichiometric Vanadium Nitrides. Dynamical Deformation Effect. Phys. Stat. Sol. 1987, 143, 471–476. [Google Scholar] [CrossRef]
- Guemmaz, M.; Mosser, A.; Ahuja, R.; Parlebas, J. Theoretical and experimental investigations on elastic properties of substoichiometric titanium nitrides: Influence of lattice vacancies. Int. J. Inorg. Mater. 2001, 3, 1319–1321. [Google Scholar] [CrossRef]
- Deng, Z.; Kioupakis, E. Semiconducting character of LaN: Magnitude of the bandgap and origin of the electrical conductivity. AIP Adv. 2021, 11, 065312. [Google Scholar] [CrossRef]
- De La Cruz, W.; Díaz, J.; Mancera, L.; Takeuchi, N.; Soto, G. Yttrium nitride thin films grown by reactive laser ablation. J. Phys. Chem. Solids 2003, 64, 2273–2279. [Google Scholar] [CrossRef]
- Larijani, M.M.; Kiani, M.; Tanhayi, M.; Majdabadi, A. Characterization of ion beam sputtered ZrN coatings prepared at different substrate temperatures. Cryst. Res. Technol. 2011, 46, 351–356. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yagi, T. Systematic study of formation and crystal structure of 3d-transition metal nitrides synthesized in a supercritical nitrogen fluid under 10 GPa and 1800 K using diamond anvil cell and YAG laser heating. J. Alloy. Compd. 2005, 403, 131–142. [Google Scholar] [CrossRef]
- Dumas, J.-B.A. Recherches de Chimie organique. Ann. Chim. Phys. 1833, 53, 164–181. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Motojima, S.; Baba, K.; Kitatani, K.; Takahashi, Y.; Sugiyama, K. Single crystal growth of titanium nitride by chemical vapour deposition and measurement of the linear growth rate on a (100) plane. J. Cryst. Growth 1976, 32, 141–148. [Google Scholar] [CrossRef]
- Wachter, P. Physical Properties of Some Stoichiometric Rare Earth Nitride Single Crystals. Adv. Mater. Phys. Chem. 2015, 5, 96–131. [Google Scholar] [CrossRef]
- Ye, T.-N.; Park, S.-W.; Lu, Y.; Li, J.; Sasase, M.; Kitano, M.; Tada, T.; Hosono, H. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 2020, 583, 391–395. [Google Scholar] [CrossRef]
- Kim, N.; Stebbins, J.F. Vacancy and Cation Distribution in Yttria-Doped Ceria: An 89Y and 17O MAS NMR Study. Chem. Mater. 2007, 19, 5742–5747. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Hung, I.; Mentink-Vigier, F.; Wang, X.; Qi, G.; Wang, X.; Gan, Z.; Xu, J.; Deng, F. Mapping the oxygen structure of γ-Al2O3 by high-field solid-state NMR spectroscopy. Nat. Comm. 2020, 11, 3620. [Google Scholar] [CrossRef] [PubMed]
- Eckert, H.; Deo, G.; Wachs, I.; Hirt, A. Solid state 51V NMR structural studies of vanadium(V) oxide catalysts supported on TiO2(anatase) and TiO2(rutile). The influence of surface impurities on the vanadium(V) coordination. Colloids Surf. 1990, 45, 347–359. [Google Scholar] [CrossRef]
- Bräuniger, T.; Kempgens, P.; Harris, R.K.; Howes, A.P.; Liddell, K.; Thompson, D.P. A combined 14N/27Al nuclear magnetic resonance and powder X-ray diffraction study of impurity phases in β-sialon ceramics. Solid State Nucl. Magn. Reson. 2003, 23, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G.; Hoffbauer, W.; Jansen, M. A comprehensive NMR study of cubic and hexagonal boron nitride. Solid State Nucl. Magn. Reson. 1998, 12, 1–7. [Google Scholar] [CrossRef]
- Bräuniger, T.; Müller, T.; Pampel, A.; Abicht, H.-P. Study of Oxygen–Nitrogen Replacement in BaTiO3 by 14N Solid-State Nuclear Magnetic Resonance. Chem. Mater. 2005, 17, 4114–4117. [Google Scholar] [CrossRef]
- Kempgens, P.; Britton, J. Powder-XRD and 14N magic angle-spinning solid-state NMR spectroscopy of some metal nitrides. Magn. Reson. Chem. 2016, 54, 371–376. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Moudrakovski, I.L.; Hartmann, C.; Indris, S.; Bräuniger, T. Local Electronic Structure in AlN Studied by Single-Crystal 27Al and 14N NMR and DFT Calculations. Molecules 2020, 25, 469. [Google Scholar] [CrossRef]
- Steinadler, J.; Eisenburger, L.; Bräuniger, T. Characterization of the Binary Nitrides VN and ScN by Solid-State NMR Spectroscopy. Z. Anorg. Allg. Chem. 2022, 648, e202200201. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Luo, H. Superconducting and normal-state properties of vanadium nitride. Phys. Rev. B 1984, 29, 6198–6202. [Google Scholar] [CrossRef]
- Gupta, S.; Moatti, A.; Bhaumik, A.; Sachan, R.; Narayan, J. Room-temperature ferromagnetism in epitaxial titanium nitride thin films. Acta Mater. 2019, 166, 221–230. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Meinhold, R.H.; McGavin, D.G.; Ripmeester, J.A.; Moudrakovski, I.L. Titanium carbide, nitride and carbonitrides: A 13C, 14N, 15N and 47,49Ti solid-state nuclear magnetic resonance study. Solid State Nucl. Magn. Reson. 1995, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Reif, F. Quadrupole Effects in Nuclear Magnetic Resonance Studies of Solids. Solid State Phys. 1957, 5, 321–438. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Bräuniger, T. Quantifying the quadrupolar interaction by 45Sc-NMR spectroscopy of single crystals. Solid State Nucl. Magn. Reson. 2022, 117, 101775. [Google Scholar] [CrossRef]
- Bräuniger, T. High-Precision Determination of NMR Interaction Parameters by Measurement of Single Crystals: A Review of Classical and Advanced Methods. Molecules 2024, 29, 4148. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Year-2017 nuclear quadrupole moments. Mol. Phys. 2018, 116, 1328–1338. [Google Scholar] [CrossRef]
- Jacob, C.R.; Visscher, L.; Thierfelder, C.; Schwerdtfeger, P. Nuclear quadrupole moment of 139La from relativistic electronic structure calculations of the electric field gradients in LaF, LaCl, LaBr, and LaI. J. Chem. Phys. 2007, 127, 204303. [Google Scholar] [CrossRef]
- Dey, W.; Ebersold, P.; Leisi, H.J.; Scheck, F.; Walter, H.K.; Zehnder, A. Nuclear spectroscopic ground-state quadrupole moments from muonic atoms. The quadrupole moment of 175Lu. Nucl. Phys. A 1979, 326, 418–444. [Google Scholar] [CrossRef]
- Benndorf, C.; de Oliveira Junior, M.; Bradtmüller, H.; Stegemann, F.; Pöttgen, R.; Eckert, H. Rare-earth solid-state NMR spectroscopy of intermetallic compounds: The case of the 175Lu isotope. Solid State Nucl. Magn. Reson. 2019, 101, 63–67. [Google Scholar] [CrossRef] [PubMed]
- d’Espinose de Lacaillerie, J.-B.; Fretigny, C.; Massiot, D. MAS NMR spectra of quadrupolar nuclei in disordered solids: The Czjzek model. J. Magn. Reson. 2008, 192, 244–251. [Google Scholar] [CrossRef]
- O’Dell, L.A. Direct detection of nitrogen-14 in solid-state NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 295–318. [Google Scholar] [CrossRef]
- Bak, M.; Rasmussen, J.T.; Nielsen, N.C. SIMPSON: A general simulation program for solid-state NMR spectroscopy. J. Magn. Reson. 2011, 213, 366–400. [Google Scholar] [CrossRef]
- Grimmer, A.-R.; Radeglia, R. Correlation between the isotropic 29Si chemical shifts. Chem. Phys. Lett. 1984, 106, 262–265. [Google Scholar] [CrossRef]
- Dupree, R.; Howes, A.P.; Kohn, S.C. Natural abundance solid state 43Ca NMR. Chem. Phys. Lett. 1997, 276, 399–404. [Google Scholar] [CrossRef]
- Stebbins, J.F. Cation sites in mixed-alkali oxide glasses: Correlations of NMR chemical shift data with site size and bond distance. Solid State Ionics 1998, 112, 137–141. [Google Scholar] [CrossRef]
- Zeman, O.E.O.; Steinadler, J.; Hochleitner, R.; Bräuniger, T. Determination of the Full 207Pb Chemical Shift Tensor of Anglesite, PbSO4, and Correlation of the Isotropic Shift to Lead–Oxygen Distance in Natural Minerals. Crystals 2019, 9, 43. [Google Scholar] [CrossRef]
- Steinadler, J.; Zeman, O.E.O.; Bräuniger, T. Correlation of the Isotropic NMR Chemical Shift with Oxygen Coordination Distances in Periodic Solids. Oxygen 2022, 2, 327–336. [Google Scholar] [CrossRef]
- Eichhorn, K.; Kirfel, A.; Grochowski, J.; Serda, P. Accurate structure analysis with synchrotron radiation. An application to borazone, cubic BN. Acta Cryst. 1991, B47, 843–848. [Google Scholar] [CrossRef]
- Budak, E.; Bozkurt, C. Synthesis of hexagonal boron nitride with the presence of representative metals. Physics B 2010, 405, 4702–4705. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Jin, S.F.; Li, D.D.; Yang, J.W.; Hu, W.J.; Wang, W.J. Debye temperature of wurtzite AlN determined by X-ray powder diffraction. Powder Diffr. 2014, 29, 352–355. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Ching, W.Y. Electronic, optical, and structural properties of some wurtzite crystals. Phys. Rev. B 1993, 48, 4335–4351. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Černý, R.; Krukowski, S. Rietveld refinement for indium nitride in the 105–295 K range. Powder Diffr. 2003, 18, 114–121. [Google Scholar] [CrossRef]
- Inamura, S.; Nobugai, K.; Kanamaru, F. The preparation of NaCl-type Ti1−xAlxN solid solution. J. Solid State Chem. 1987, 68, 124–127. [Google Scholar] [CrossRef]
- Christensen, A.N. A Neutron Diffraction Investigation on Single Crystals of Titanium Carbide, Titanium Nitride, and Zirconium Nitride. Acta Chem. Scand. A 1975, 29, 563–564. [Google Scholar] [CrossRef]
- Spear, K.E.; Leitnaker, J.M. Equilibrium investigations of carbon-rich V(C,N) solutions. High Temp. Sci. 1969, 1, 401–411. [Google Scholar]
- Klesnar, H.P.; Rogl, P. Phase relations in the ternary systems rare-earth metal(RE)-boron-nitrogen, where RE = Tb, Dy, Ho, Er, Tm, Lu, Sc and Y. High Temp. High Press. 1990, 22, 453–457. [Google Scholar]
- Schuster, J.C.; Bauer, J. The ternary systems Sc–Al–N and Y–Al–N. J. Less-Common Met. 1985, 109, 345–350. [Google Scholar] [CrossRef]
- Holleck, H.; Smailos, E. Mischnitride von Thorium mit Seltenen Erden. J. Nucl. Mater. 1980, 91, 237–239. [Google Scholar] [CrossRef]
- Vendl, A. Über die gegenseitige Mischbarkeit von LaN, CeN, NdN und GdN. J. Nucl. Mater. 1979, 79, 246–248. [Google Scholar] [CrossRef]
- Khitrin, A.K.; Fung, B.M. 14N nuclear magnetic resonance of polycrystalline solids with fast spinning at or very near the magic angle. J. Chem. Phys. 1999, 111, 8963–8969. [Google Scholar] [CrossRef]
- Healy, M.A.; Morris, A. Reference compounds for 14N nuclear magnetic resonance; the relative chemical shifts of aqueous nitrate ion and nitromethane. Spectrochim. Acta A 1975, 31, 1695–1697. [Google Scholar] [CrossRef]
- Samoson, A. Satellite transition high-resolution NMR of quadrupolar nuclei in powders. Chem. Phys. Lett. 1985, 119, 29–32. [Google Scholar] [CrossRef]
- Harazono, T.; Watanabe, T. Chemical Shift, Chemical Shift Anisotropy, and Spin-Lattice Relaxation Time in 89Y-MAS and-Static NMR of Yttrium Compounds. Bull. Chem. Soc. Jpn. 1997, 70, 2383–2388. [Google Scholar] [CrossRef]
- Florian, P.; Gervais, M.; Douy, A.; Massiot, D.; Coutures, J.-P. A Multi-nuclear Multiple-Field Nuclear Magnetic Resonance Study of the Y2O3–Al2O3 Phase Diagram. J. Phys. Chem. B 2001, 105, 379–391. [Google Scholar] [CrossRef]
- Kunwar, A.C.; Turner, G.L.; Oldfield, E. Solid-state spin-echo Fourier transform NMR of 39K and 67Zn salts at high field. J. Magn. Reson. 1986, 69, 124–127. [Google Scholar] [CrossRef]
- Koller, H.; Engelhardt, G.; Kentgens, A.P.M.; Sauer, J. 23Na NMR Spectroscopy of Solids: Interpretation of Quadrupole Interaction Parameters and Chemical Shifts. J. Phys. Chem. 1994, 98, 1544–1551. [Google Scholar] [CrossRef]
- Ebert, H.; Ködderitzsch, D. Minár, J. Calculating condensed matter properties using the KKR-Green’s function method—Recent developments and applications. Rep. Prog. Phys. 2011, 74, 096501. [Google Scholar] [CrossRef]
- Freysoldt, C.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Kresse, G.; Janotti, A.; Van de Walle, C.G. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86, 253–305. [Google Scholar] [CrossRef]
- Niklaus, R.; Minár, J.; Häusler, J.; Schick, W. First-principles and experimental characterization of the electronic properties of CaGaSiN3 and CaAlSiN3: The impact of chemical disorder. Phys. Chem. Chem. Phys. 2017, 19, 9292–9299. [Google Scholar] [CrossRef] [PubMed]
- Schnick, W.; Huppertz, H.; Lauterbach, R. High temperature syntheses of novel nitrido- and oxonitrido-silicates and sialons using rf furnaces. J. Mater. Chem. 1999, 9, 289–296. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Schütze, M. Feinreinigung von Gasen mit einem hochaktiven Kupferkatalysator. Angew. Chem. 1958, 70, 697–699. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS-Academic, Version 6; Coelho Software: Brisbane, Australia, 2016.
- Cheary, R.W.; Coelho, A. A Fundamental Parameters Approach to X-ray Line-Profile Fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A.A.; Cline, J.P. Fundamental Parameters Line Profile Fitting in Laboratory Diffractometers. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 1–25. [Google Scholar] [CrossRef] [PubMed]
- OriginPro, Version 2019b; OriginLab Corporation: Northhampton, MA, USA, 2019.
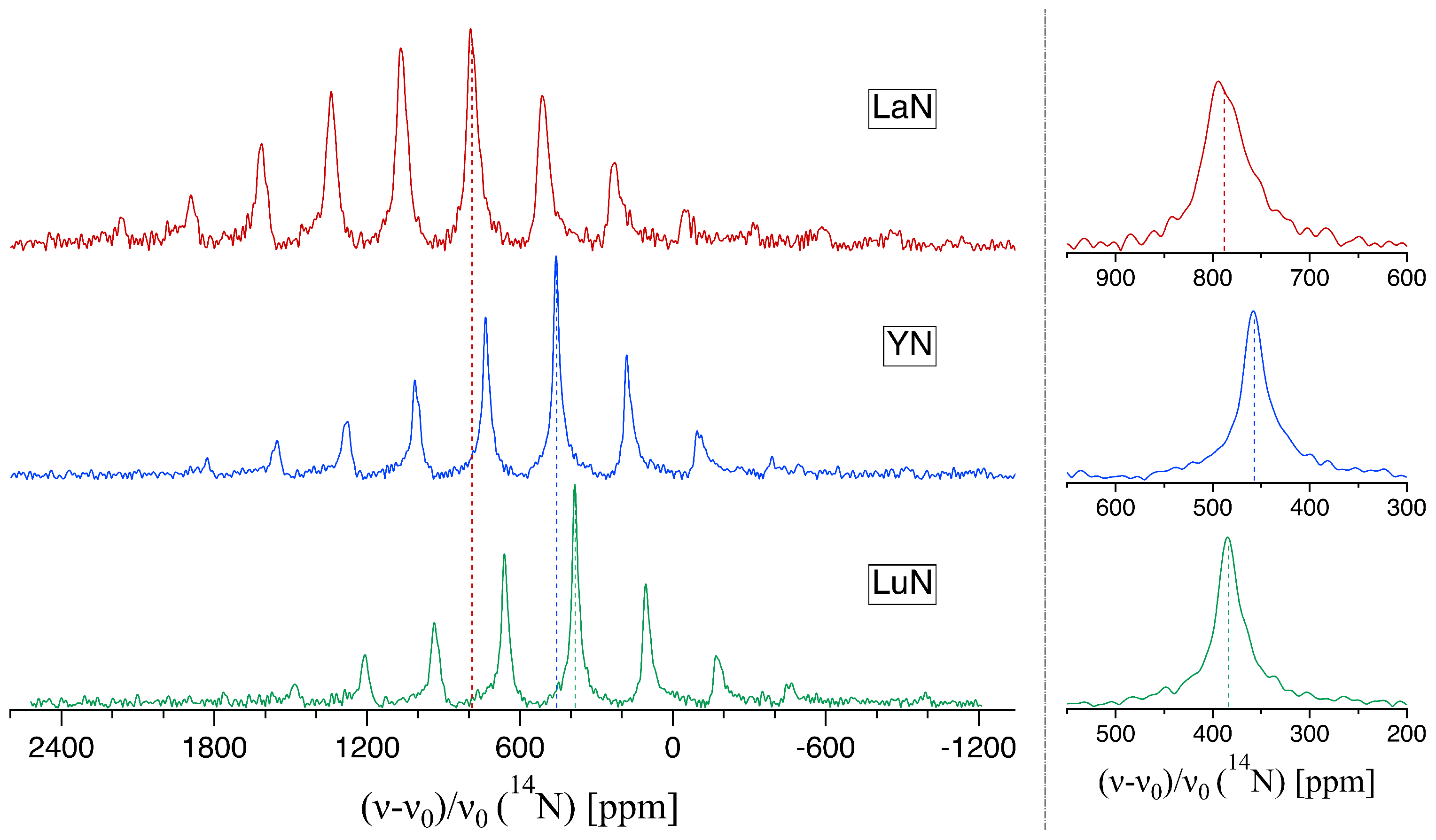
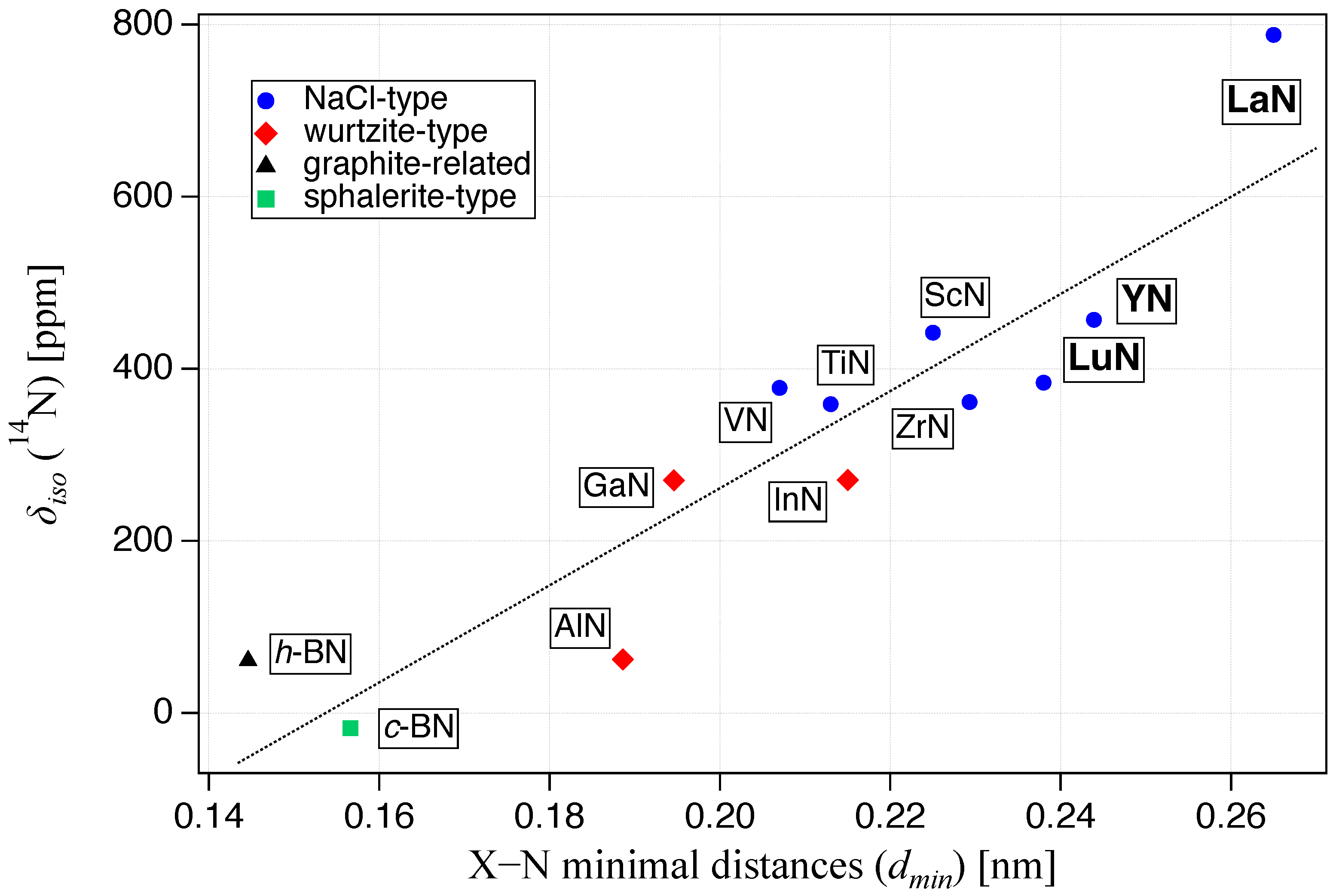


| Compound | Structure Type | Crystallogr. Data Ref. | /ppm | NMR Ref. |
|---|---|---|---|---|
| c-BN | sphalerite | [47] | [24] | |
| h-BN | graphite-related | [48] | [24] | |
| AlN * | wurtzite | [49] | [27] | |
| GaN * | wurtzite | [50] | [26] | |
| InN * | wurtzite | [51] | [26] | |
| TiN | rock-salt | [52] | 359 | [25] |
| ZrN * | rock-salt | [53] | [26] | |
| VN | rock-salt | [54] | 378 | [28] |
| LuN | rock-salt | [55] | 384 | this work |
| ScN | rock-salt | [56] | 442 | [28] |
| YN | rock-salt | [57] | 457 | this work |
| LaN | rock-salt | [58] | 788 | this work |
| 89YN | 139LaN | Y14N | La14N | Lu14N | |
|---|---|---|---|---|---|
| d1/s | 240 | 1 | 180 | 15 | 240 |
| ns | 512 | 1000 | 960 | 6256 | 320 |
| lb/Hz | 50 | 500 | 0 | 0 | 0 |
| fwhm/kHz | 2.2 | 15.5 | 1.2 | 2.0 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinadler, J.; Krach, G.; Schnick, W.; Bräuniger, T. Investigation of the Binary Nitrides YN, LaN and LuN by Solid-State NMR Spectroscopy. Molecules 2024, 29, 5572. https://doi.org/10.3390/molecules29235572
Steinadler J, Krach G, Schnick W, Bräuniger T. Investigation of the Binary Nitrides YN, LaN and LuN by Solid-State NMR Spectroscopy. Molecules. 2024; 29(23):5572. https://doi.org/10.3390/molecules29235572
Chicago/Turabian StyleSteinadler, Jennifer, Georg Krach, Wolfgang Schnick, and Thomas Bräuniger. 2024. "Investigation of the Binary Nitrides YN, LaN and LuN by Solid-State NMR Spectroscopy" Molecules 29, no. 23: 5572. https://doi.org/10.3390/molecules29235572
APA StyleSteinadler, J., Krach, G., Schnick, W., & Bräuniger, T. (2024). Investigation of the Binary Nitrides YN, LaN and LuN by Solid-State NMR Spectroscopy. Molecules, 29(23), 5572. https://doi.org/10.3390/molecules29235572








