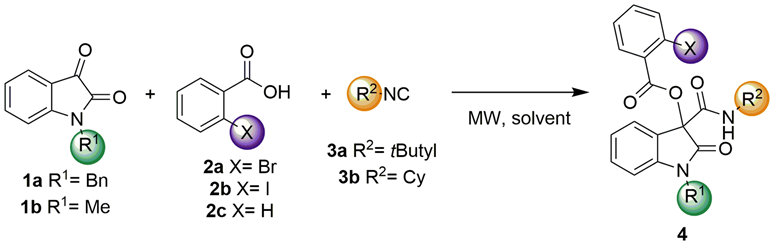
3.2. The General Procedure for the Synthesis of α-Acyloxyamide-Oxindole Hybrids 4 Using the 3CPR
Microwave Reactor: In a microwave vial with a magnetic stirrer, the corresponding isatin derivative 1a–l (0.4 mmol or 0.6 mmol), the carboxylic acid 2a–v, the isocyanide 3a–d, and the solvent (3 mL) were added. The vial was closed with a proper cap and the reaction mixture was left stirred at the indicated temperature for a certain time. The solvent was evaporated under reduced pressure, and the crude reaction mixture was purified in a short chromatographic glass column with SiO2 flash using hexane and AcOEt mixtures as the eluents.
Radley’s® 12-position Carousel: In a glass reactor tube with a magnetic stirrer, the corresponding isatin derivative 1a–l (1.0 mmol), the carboxylic acid 2a–v (2.0 mmol), the isocyanide 3a–d (2.0 mmol), powder 4Å MS (100 mg), and CH3CN (3 mL) were added. The vial was closed with a proper cap and the reaction mixture was left stirred at 100 °C for 24 h. The solvent was evaporated under reduced pressure, and the crude reaction mixture was purified in a short chromatographic glass column with SiO2 flash using hexane and AcOEt mixtures as the eluents.
3-(Cyclohexylcarbamoyl)-2-oxoindolin-3-yl benzoate (4ccb) [
20]:
1c (147.1 mg, 1.0 mmol),
2c (159 mg, 2.0 mmol, 2 equivalents),
3b (124 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4ccb as a white solid (69.4 mg, 16% yield), m.p. = 205.3–298.1 °C.
1H NMR (CDCl
3, 400 MHz) δ: 0.83–0.90 (m, 1H, CH
2), 1.28–1.42 (m, 4H, CH
2), 1.60–1.64 (m, 1H, CH
2), 1.71–1.76 (m, 2H, CH
2), 1.95–2.03 (m, 2H, CH
2), 3.81–3.89 (m, 1H, CH), 6.77 (d, 1H, NH), 6.87 (d,
J = 8 Hz, 1H, Ar), 7.01 (t,
J = 8 Hz, 1H, Ar), 7.24–7.32 (m, 2H, Ar), 7.47 (t,
J = 8 Hz, 2H, Ar), 7.61 (t,
J = 8 Hz, 1H, Ar), 7.99–8.02 (m, 2H, Ar), 8.48 (s br, 1H, NH).
13C APT NMR (CDCl
3, 100 MHz) δ: 24.75, 24.79, 25.55, 32.65, 32.88, 49.06, 81.27, 110.93, 123.15, 124.39, 125.47, 128.54, 128.80, 130.08, 130.83, 134.09, 142.39, 163.37, 163.90, 172.80. HRMS (ESI)
m/
z: calculated for C
22H
22O
4N
2Na [M]
+ 401.1472, found 401.1465.
3-(Cyclohexylcarbamoyl)-5-methyl-2-oxoindolin-3-yl benzoate (4dcb): 1d (100 mg, 0.62 mmol), 2c (152 mg, 2.0 mmol, 2 equivalents), 3b (149 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4dcb as a white solid (6 mg, <5% yield), m.p. = 139.5–142.1 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.24–1.36 (m, 5H, CH2), 1.60–1.65 (m, 1H, CH2), 1.69–1.78 (m, 2H, CH2), 1.96–2.05 (m, 2H, CH2), 2.26 (s, 3H, CH3), 3.82–3.87 (m, 1H, CH), 6.74–6.80 (m, 2H, NH + Ar), 7.08 (d, J = 8 Hz, 1H, Ar), 7.13 (s, 1H, Ar), 7.46–7.49 (m, 2H, Ar), 7.62 (t, J = 8 Hz, 1H, Ar), 8.01 (d, J = 8 Hz, 2H, Ar), 8.09–8.12 (s br, 1H, NH). 13C APT NMR (CDCl3, 100 MHz) δ: 21.22, 24.76, 24.81, 25.57, 32.68, 32.91, 49.05, 81.33, 110.51, 125.16, 125.41, 128.61, 128.81, 130.10, 131.26, 132.82, 134.08, 139.74, 163.42, 163.89, 172.75. HRMS (ESI) m/z: calculated for C23H24O4N2Na [M]+ 415.1628, found 415.1621.
5-Bromo-3-(cyclohexylcarbamoyl)-2-oxoindolin-3-yl benzoate (4hcb): 1h (100 mg, 0.44 mmol), 2c (108 mg, 2.0 mmol, 2 equivalents), 3b (110 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4hcb as a white solid (46 mg, 33% yield), m.p. = 147.3–149.1 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.32–1.46 (m, 5H, CH2), 1.62–1.65 (m, 1H, CH2), 1.72–1.78 (m, 2H, CH2), 1.95–2.00 (m, 2H, CH2), 3.80–3.87 (m, 1H, CH), 6.74 (d, J = 8 Hz, 1H, Ar), 6.80 (d, 1H, NH), 7.35–7.40 (m, 2H, Ar), 7.48 (t, J = 8 Hz, 2H, Ar), 7.63 (t, J = 8 Hz, 2H, Ar), 7.98–8.00 (m, 2H, Ar), 8.73 (s, 1H, NH). 13C APT NMR (CDCl3, 100 MHz) δ: 24.66, 24.71, 25.41, 32.53, 32.77, 49.18, 80.85, 112.42, 115.53, 127.22, 127.32, 128.81, 130.02, 133.56, 134.26, 141.47, 162.90, 163.75, 172.23. HRMS (ESI) m/z: calculated for C22H21O4N2BrNa [M]+ 479.0577, found 479.0568.
3-(Cyclohexylcarbamoyl)-1-methyl-2-oxoindolin-3-yl benzoate (4bcb) [
19]:
1b (100 mg, 0.62 mmol),
2c (152 mg, 2.0 mmol, 2 equivalents),
3b (149 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4bcb as a yellow solid (96 mg, 40% yield), m.p. = 145.7–149.2 °C.
3-(Cyclohexylcarbamoyl)-1,5-dimethyl-2-oxoindolin-3-yl benzoate (4ecb): 1e (100 mg, 0.57 mmol), 2c (139 mg, 2.0 mmol, 2 equivalents), 3b (136 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4ecb as a white solid (12 mg, <10% yield), m.p. = 178.7–181.2 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.22–1.42 (m, 5H, CH2), 1.60–1.65 (m, 1H, CH2), 1.71–1.78 (m, 2H, CH2), 1.96–2.04 (m, 2H, CH2), 2.29 (s, 3H, CH3), 3.30 (s, 3H, CH3), 3.79–3.86 (m, 1H, CH), 6.78–6.82 (m, 2H, NH + Ar), 7.16–7.18 (m, 2H, Ar), 7.47 (t, J = 8 Hz, 2H, Ar), 7.61 (t, J = 8 Hz, 1H, Ar), 7.99 (d, J = 8 Hz, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 21.21, 24.75, 24.81, 25.59, 27.09, 32.68, 32.92, 49.03, 81.15, 108.66, 124.93, 125.12, 128.69, 128.78, 130.05, 131.18, 132.91, 134.01, 142.81, 163.45, 163.81, 171.48. HRMS (ESI) m/z: calculated for C24H26O4N2Na [M]+ 429.1785, found 429.1775.
5-Bromo-3-(cyclohexylcarbamoyl)-1-methyl-2-oxoindolin-3-yl benzoate (4fcb) [
18]:
1f (100 mg, 0.42 mmol),
2c (102 mg, 2.0 mmol, 2 equivalents),
3b (103 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding 4fcb as a pale yellow solid (52 mg, 22% yield), m.p. = 190.1–192.3 °C. HRMS (ESI)
m/
z: calculated for C
23H
23O
4N
2BrNa [M]
+ 493.07334, found 493.0725.
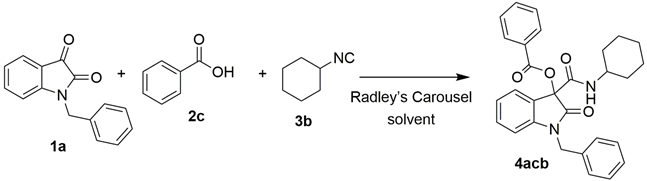
1-Benzyl-3-(cyclohexylcarbamoyl)-2-oxoindolin-3-yl benzoate (4acb): 1a (243 mg, 1.0 mmol), 2c (244 mg, 2.0 mmol, 2 equivalents), 3b (250 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4acb as a pale yellow solid (384 mg, 86% yield), m.p. = 140.0–142.1 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.28–1.40 (m, 5H, CH2), 1.62–1.66 (m, 1H, CH2), 1.74–1.79 (m, 2H, CH2), 2.02–2.04 (m, 2H, CH2), 3.84–3.93 (m, 1H, CH), 4.95 (d, J = 16 Hz, 1H, CH2), 5.14 (d, J = 16 Hz, 1H, CH2), 6.69 (d, J = 8 Hz, 1H, Ar), 6.79–6.81 (s br, 1H, NH), 7.01 (t, J = 8 Hz, 1H, Ar), 7.22 (t, J = 8 Hz, 1H, Ar), 7.26–7.38 (m, 4H, Ar), 7.44–7.51 (m, 4H, Ar), 7.63 (t, J = 8 Hz, 1H, Ar), 8.02 (d, J = 8 Hz, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 24.76, 24.78, 25.57, 32.76, 32.98, 44.47, 48.93, 81.25, 110.10, 123.24, 123.76, 127.15, 127.67, 128.81, 128.96, 130.06, 130.70, 134.07, 135.25, 144.26, 163.54, 163.64, 171.63. HRMS (ESI) m/z: calculated for C29H28O4N2Na [M]+ 491.1941, found 491.1931.
5-Bromo-3-(cyclohexylcarbamoyl)-1-(3-methoxybenzyl)-2-oxoindolin-3-yl benzoate (4gcb): 1g (100 mg, 0.28 mmol), 2c (70 mg, 2.0 mmol, 2 equivalents), 3b (72 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4gcb as a white solid (67 mg, 41% yield), m.p. = 154.6–157.3 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.24–1.45 (m, 5H, CH2), 1.62–1.66 (m, 1H, CH2), 1.75–1.78 (m, 2H, CH2), 2.00–2.05 (m, 2H, CH2), 3.74 (s, 3H, OCH3), 3.83–3.91 (m, 1H, CH), 5.09 (s, 2H, CH2), 6.68–6.73 (m, 2H, Ar), 6.81 (d, 1H, NH), 7.05 (t, J = 8 Hz, 1H, Ar), 7.14 (d, J = 4 Hz, 1H, Ar), 7.27 (t, J = 8 Hz, 1H, Ar), 7.32 (d, J = 8 Hz, 1H, Ar), 7.44–7.51 (m, 3H, Ar), 7.64 (t, J = 8 Hz, 1H, Ar), 7.98–8.01 (m, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 24.71, 24.75, 25.52, 32.70, 32.92, 44.59, 48.93, 55.76, 81.40, 109.93, 112.51, 112.58, 116.39, 123.43, 125.26, 128.43, 128.86, 129.91, 130.77, 133.29, 134.17, 135.03, 143.89, 159.72, 163.48, 163.58, 171.51. HRMS (ESI) m/z: calculated for C30H29O5N2BrNa [M]+ 599.1152, found 599.1143.
1-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)-3-(cyclohexylcarbamoyl)-2-oxoindolin-3-yl benzoate (4lcb): 1l (100 mg, 0.31 mmol), 2c (76 mg, 2.0 mmol, 2 equivalents), 3b (78 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4lcb as a pale yellow solid (90 mg, 52% yield), m.p. = 168.1–173.2 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.27–1.42 (m, 5H, CH2), 1.61–1.65 (m, 1H, CH2), 1.73–1.76 (m, 2H, CH2), 1.95–2.01 (m, 2H, CH2), 3.79–3.86 (m, 1H, CH), 5.17 (s br, 2H, CH2), 5.45–5.53 (m, 2H, CH2), 6.75 (d, 1H, NH), 6.92–6.93 (m, 1H, Ar), 7.02 (t, J = 8 Hz, 1H, Ar), 7.21–7.33 (m, 8H, CH + Ar), 7.48 (t, J = 8 Hz, 2H, Ar), 7.63 (t, J = 8 Hz, 1H, Ar), 7.91–7.94 (m, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 24.76, 24.78, 25.54, 32.69, 32.91, 49.00, 81.17, 110.13, 123.44, 123.58, 125.14, 128.16, 128.44, 128.71, 128.82, 129.11, 129.99, 130.85, 134.18, 134.58, 143.56, 163.42, 163.65, 171.11. HRMS (ESI) m/z: calculated for C32H31O4N5Na [M]+ 572.2268, found 572.2256.
1-Allyl-3-(cyclohexylcarbamoyl)-2-oxoindolin-3-yl benzoate (4icb): 1i (100 mg, 0.53 mmol), 2c (130 mg, 2.0 mmol, 2 equivalents), 3b (132 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4icb as a white solid (57 mg, 26% yield), m.p. = 136.5–138.9 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.21–1.43 (m, 5H, CH2), 1.60–1.64 (m, 1H, CH2), 1.72–1.77 (m, 2H, CH2), 1.96–2.04 (m, 2H, CH2), 3.80–3.89 (m, 1H, CH), 4.38–4.49 (m, 2H, CH2), 5.28 (d, J = 12 Hz, 1H, CH2), 5.46 (d, J = 20 Hz, 1H, CH2), 5.87–5.96 (m, 1H, CH), 6.77 (d, 1H, NH), 6.89 (d, J = 8 Hz, 1H, Ar), 7.04 (t, J = 8 Hz, 1H, Ar), 7.31–7.35 (m, 2H, Ar), 7.47 (t, J = 8 Hz, 2H, Ar), 7.61 (t, J = 8 Hz, 1H, Ar), 7.98–8.01 (m, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 24.72, 24.75, 25.53, 32.68, 32.91, 42.89, 48.92, 81.10, 109.84, 117.87, 123.14, 123.84, 125.22, 128.57, 128.76, 129.99, 130.66, 134.01, 144.34, 163.44, 163.60, 171.23. HRMS (ESI) m/z: calculated for C25H26O4N2Na [M]+ 441.1785, found 441.1776.
(E)-1-(But-2-en-1-yl)-3-(cyclohexylcarbamoyl)-2-oxoindolin-3-yl benzoate (4jcb): 1j (100 mg, 0.49 mmol), 2c (121 mg, 2.0 mmol, 2 equivalents), 3b (123 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4jcb as a white solid (105 mg, 50% yield), m.p. = 130.1–133.2 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.24–1.43 (m, 5H, CH2), 1.60–1.64 (m, 1H, CH2), 1.71–1.77 (m, 5H, CH3 + CH2), 1.95–2.02 (m, 2H, CH2), 3.80–3.87 (m, 1H, CH), 4.31–4.43 (m, 2H, CH2), 5.50–5.57 (m, 1H, CH), 5.82–5.88 (m, 1H, CH), 6.76 (d, 1H, NH), 6.91 (d, J = 8 Hz, 1H, Ar), 7.04 (t, J = 8 Hz, 1H, Ar), 7.31–7.35 (m, 2H, Ar), 7.45–7.48 (m, 2H, Ar), 7.61 (t, J = 8 Hz, 1H, Ar), 7.99 (d, J = 8 Hz, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 17.91, 24.73, 24.76, 25.57, 32.69, 32.92, 42.46, 48.92, 81.07, 109.93, 123.06, 123.72, 124.03, 125.20, 128.66, 128.76, 129.34, 130.03, 130.66, 133.98, 144.52, 163.42, 163.67, 171.17. HRMS (ESI) m/z: calculated for C26H28O4N2Na [M]+ 455.1941, found 455.1934.
3-(Cyclohexylcarbamoyl)-2-oxo-1-(prop-2-yn-1-yl)indolin-3-yl benzoate (4kcb): 1k (100 mg, 0.54 mmol), 2c (132 mg, 2.0 mmol, 2 equivalents), 3b (134 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4kcb as a white solid (81 mg, 35% yield), m.p. = 145.7–147.2 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.23–1.40 (m, 5H, CH2), 1.59–1.63 (m, 1H, CH2), 1.70–1.74 (m, 2H, CH2), 1.94–2.01 (m, 2H, CH2), 2.33–2.34 (m, 1H, CH), 3.80–3.87 (m, 1H, CH), 4.53–4.62 (m, 2H, CH2), 6.75 (d, 1H, NH), 7.08 (t, J = 8 Hz, 1H, Ar), 7.13 (d, J = 8 Hz, 1H, Ar), 7.35–7.47 (m, 4H, Ar), 7.57–7.61 (m, 1H, Ar), 7.99 (d, J = 8 Hz, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 24.59, 24.62, 25.39, 29.92, 32.49, 32.71, 48.87, 72.93, 76.11, 80.77, 109.94, 123.47, 124.03, 124.91, 128.26, 128.65, 129.91, 130.70, 133.97, 143.23, 162.95, 163.62, 170.54. HRMS (ESI) m/z: calculated for C25H24O4N2Na [M]+ 439.1628, found 439.1622.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl benzoate (4bca) [
19]:
1b (100 mg, 0.62 mmol),
2c (152 mg, 1.2 mmol, 2 equivalents),
3a (140 μL, 1.2 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4bca as a pale yellow solid (142 mg, 63% yield), m.p. = 144.3–146.7 °C.
3-(Benzylcarbamoyl)-1-methyl-2-oxoindolin-3-yl benzoate (4bcc): 1b (108.8 mg, 0.67 mmol), 2c (152 mg, 1.2 mmol, 2 equivalents), 3c (151 μL, 1.2 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bcc as a white solid (177 mg, 66% yield), m.p. = 180.3–182.9 °C. 1H NMR (CDCl3, 400 MHz) δ: 3.33 (s, 3H, CH3), 4.52 (dd, J = 6 Hz, 2H, Ar), 4.64 (dd, J = 6 Hz, 2H, Ar), 6.94 (d, J = 8 Hz, 1H, Ar), 7.06–7.10 (m, 1H, Ar), 7.31–7.45 (m, 10H, Ar + NH), 7.57–7.61 (m, 1H, Ar), 7.95–7.98 (m, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.06, 43.93, 81.12, 109.03, 123.36, 124.32, 124.88, 127.66, 127.79, 128.39, 128.74, 128.93, 130.03, 131.00, 134.06, 137.57, 145.24, 163.79, 164.46, 171.36. HRMS (ESI) m/z: calculated for C24H20O4N2Na [M]+ 423.1315, found 423.1306.
3-((2-Ethoxy-2-oxoethyl)carbamoyl)-1-methyl-2-oxoindolin-3-yl benzoate (4bcd) [
19]:
1b (100 mg, 0.62 mmol),
2c (152 mg, 1.2 mmol, 2 equivalents),
3d (135 μL, 1.2 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4bcd as a white solid (57 mg, 23% yield), m.p. = 132.5–135.9 °C.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-bromobenzoate (4baa) [
19]:
1b (172 mg, 1.1 mmol),
2a (402 mg, 2.0 mmol, 2 equivalents),
3a (200 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4baa as a white solid (163 mg, 35% yield), m.p. = 158.9–160.1 °C.
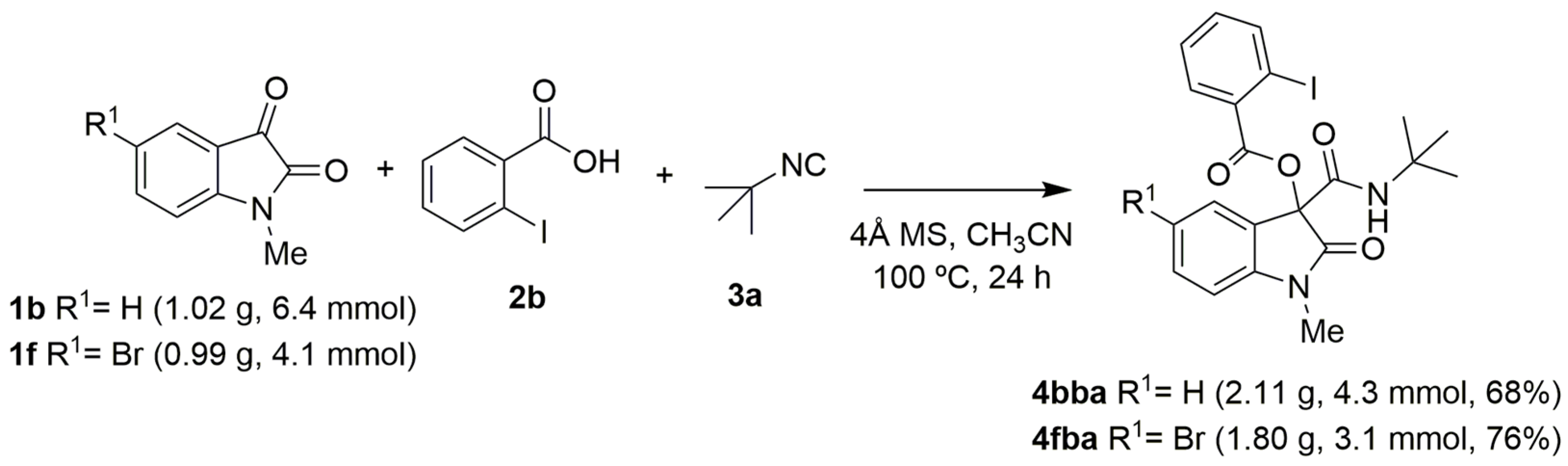
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-iodobenzoate (4bba): 1b (170 mg, 1.1 mmol), 2b (496 mg, 2.0 mmol, 2 equivalents), 3a (200 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bba as a white solid (209 mg, 56% yield), m.p. = 192.4–196.3 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.41 (s, 9H, 3H), 3.31 (s, 3H, CH3), 6.91 (d, J = 8 Hz, 1H, Ar), 7.01 (s br, 1H, NH), 7.09 (t, J = 8 Hz, 1H, Ar), 7.20 (t, J = 8 Hz, 1H, Ar), 7.35–7.44 (m, 3H, Ar), 7.74 (d, J = 8 Hz, 1H, Ar), 7.98 (d, J = 8 Hz, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.02, 28.76, 52.43, 82.12, 93.24, 109.01, 123.19, 124.01, 125.18, 128.38, 130.84, 132.39, 132.57, 133.44, 141.35, 145.55, 163.26, 163.73, 171.12. HRMS (ESI) m/z: calculated for C21H21O4N2INa [M]+ 515.0438, found 515.0433.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-chlorobenzoate (4bda) [
19]:
1b (100 mg, 0.62 mmol),
2d (307 mg, 1.2 mmol, 2 equivalents),
3a (140 μL, 1.2 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4bda as a white solid (80 mg, 32% yield), m.p. = 149.1–152.4 °C.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-fluorobenzoate (4bea) [
19]:
1b (100 mg, 0.62 mmol),
2e (174 mg, 1.2 mmol, 2 equivalents),
3a (140 μL, 1.2 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding 4bea as a white solid (92 mg, 39% yield), m.p. = 161.5–163.4 °C.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 3-hydroxybenzoate (4bpa): 1b (100 mg, 0.62 mmol), 2p (171 mg, 1.2 mmol, 2 equivalents), 3a (140 μL, 1.2 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bpa as a white solid (196 mg, 83% yield), m.p. = 245.7–247.2 °C. 1H NMR (DMSO-d6, 400 MHz) δ: 1.34 (s, 9H, CH3), 3.18 (s, 3H, CH3), 7.02–7.12 (m, 3H, Ar), 7.36–7.40 (m, 3H, Ar), 7.46–7.52 (m, 2H, Ar), 7.62 (s br, 1H, NH), 10.03 (s br, 1H, OH). 13C APT NMR (DMSO-d6, 100 MHz) δ: 26.53, 28.28, 51.55, 81.25, 109.06, 116.25, 120.66, 121.39, 122.62, 122.92, 125.89, 129.18, 130.12, 130.44, 145.21, 157.61, 163.26, 164.08, 170.98. HRMS (ESI) m/z: calculated for C21H22O5N2Na [M]+ 405.1421, found 405.1413.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 3-bromobenzoate (4boa): 1b (100 mg, 0.62 mmol), 2o (250 mg, 1.24 mmol, 2 equivalents), 3a 140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4boa as a white solid (234 mg, 88% yield), m.p. = 201.7–204.7 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.42 (s, 9H, CH3), 3.31 (s, 3H, CH3), 6.75 (s br, 1H, NH), 6.91 (d, J = 8 Hz, 1H, Ar), 7.08 (t, J = 8 Hz, 1H, Ar), 7.36–7.40 (m, 2H, Ar), 7.59–7.61 (m, 2H, Ar), 7.82–7.84 (m, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.05, 28.66, 52.28, 81.25, 108.95, 123.39, 124.49, 124.77, 127.49, 129.32, 130.93, 131.40, 132.17, 145.14, 162.87, 163.18, 171.58. HRMS (ESI) m/z: calculated for C21H21O4N2BrNa [M]+ 467.0577, found 467.0571.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-aminobenzoate (4bta): 1b (100 mg, 0.62 mmol), 2t (170 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bta as a pale yellow solid (78 mg, 33% yield), m.p. = 264.8–267.1 °C. 1H NMR (DMSO-d6, 400 MHz) δ: 1.32 (s, 9H, CH3), 3.18 (s, 3H, CH3), 6.53 (s br, 2H, NH2), 6.62 (t, J = 8 Hz, 1H, Ar), 6.76 (d, J = 8 Hz, 1H, Ar), 7.02–7.08 (m, 2H, Ar), 7.28–7.33 (m, 1H, Ar), 7.37 (t, J = 8 Hz, 1H, Ar), 7.49 (d, J = 8 Hz, 1H, Ar), 7.53 (s br, 1H, NH). 13C APT NMR (DMSO-d6, 100 MHz) δ: 26.52, 28.26, 51.49, 80.79, 106.76, 108.99, 114.94, 116.69, 122.52, 122.78, 126.27, 130.23, 131.37, 135.08, 145.10, 152.07, 164.30, 164.56, 171.29. HRMS (ESI) m/z: calculated for C21H23O4N3Na [M]+ 404.1581, found 404.1573.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 3-aminobenzoate (4bsa): 1b (100 mg, 0.62 mmol), 2s (170 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bsa as a pale yellow solid (105 mg, 44% yield), m.p. = 174.7–176.9 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.42 (s, 9H, CH3), 3.30 (s, 3H, CH3), 6.78 (s br, 1H, NH), 6.87–6.91 (m, 2H, Ar), 7.05 (t, J = 8 Hz, 1H, Ar), 7.20–7.24 (m, 2H, Ar), 7.32–7.38 (m, 3H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.03, 28.71, 52.22, 81.17, 108.88, 116.04, 119.79, 120.38, 123.27, 124.13, 125.22, 129.52, 129.65, 130.78, 145.28, 146.84, 163.39, 163.93, 171.73. HRMS (ESI) m/z: calculated for C21H23O4N3Na [M]+ 404.1581, found 404.1575.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2,4-dibromobenzoate (4bna): 1b (100 mg, 0.62 mmol), 2n (347 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bna as a white solid (290 mg, 89% yield), m.p. = 193.2–195.7 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.43 (s, 9H, CH3), 3.31 (s, 3H, CH3), 6.76 (s br, 1H, NH), 6.92 (d, J = 8 Hz, 1H, Ar), 7.10 (t, J = 8 Hz, 1H, Ar), 7.38–7.41 (m, 1H, Ar), 7.87–7.88 (m, 1H, Ar), 8–02 (s, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.11, 28.65, 29.83, 52.44, 81.53, 109.06, 123.38, 123.60, 124.35, 124.88, 131.14, 131.72, 131.74, 131.86, 138.85, 139.25, 145.12, 161.56, 162.49, 171.36. HRMS (ESI) m/z: calculated for C21H20O4N2Br2Na [M]+ 544.9682, found 544.9672.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 4-aminobenzoate (4bra): 1b (100 mg, 0.62 mmol), 2r (170 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bra as a white solid (48 mg, 20% yield), m.p. = 169.3–170.1 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.42 (s, 9H, CH3), 3.29 (s, 3H, CH3), 4.27 (s br, 2H, NH2), 6.58 (d, J = 8 Hz, 2H, Ar), 6.78 (s br, 1H, NH), 6.88 (d, J = 8 Hz, 1H, Ar), 7.04 (t, J = 8 Hz, 1H, Ar), 7.29 (d, J = 8 Hz, 1H, Ar), 7.35 (t, J = 8 Hz, 1H, Ar), 7.72 (d, J = 8 Hz, 2H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 26.97, 28.69, 52.11, 80.90, 108.83, 113.84, 117.16, 123.12, 123.73, 125.77, 130.58, 132.12, 145.24, 152.11, 163.62, 163.84, 172.11. HRMS (ESI) m/z: calculated for C21H23O4N3Na [M]+ 404.1581, found 404.1574.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 4-fluoro-2-iodobenzoate (4bfa): 1b (100 mg, 0.62 mmol), 2f (330 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bfa as a pale yellow solid (278 mg, 88% yield), m.p. = 166.1–169.5 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.41 (s, 9H, CH3), 3.32 (s, 3H, CH3), 6.91 (d, J = 8 Hz, 1H, Ar), 6.97 (s br, 1H, NH), 7.07–7.14 (m, 2H, Ar), 7.36–7.43 (m, 2H, Ar), 7.71 (dd, J = 8 Hz, 1H, Ar), 7.78–7.82 (m, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.08, 28.78, 52.51, 82.15, 93.85, 93.94, 109.09, 115.69, 115.90, 123.33, 124.24, 125.01, 128.65, 128.89, 130.94, 134.19, 134.28, 145.48, 162.66, 162.86, 163.06, 165.24, 171.22. HRMS (ESI) m/z: calculated for C21H20O4N2FINa [M]+ 533.0344, found 533.0334.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-bromo-4-(trifluoromethyl)benzoate (4bga): 1b (100 mg, 0.62 mmol), 2g (334 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bga as a pale yellow solid (197 mg, 61% yield), m.p. = 149.8–152.1 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.40 (s, 9H, CH3), 3.32 (s, 3H, CH3), 6.93 (d, J = 8 Hz, 1H, Ar), 6.97 (s br, 1H, NH), 7.10 (t, J = 8 Hz, 1H, Ar), 7.35–7.40 (m, 2H, Ar), 7.65 (d, J = 8 Hz, 1H, Ar), 7.89 (d, J = 8 Hz, 1H, Ar), 7.94 (s, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 26.99, 28.60, 29.72, 52.40, 82.28, 109.08, 121.46, 123.33, 123.76, 124.56, 124.69, 131.04, 131.38, 132.17, 133.07, 145.46, 162.26, 162.89, 170.78. HRMS (ESI) m/z: calculated for C22H20O4N2BrF3Na [M]+ 535.0451, found 535.0443.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 3-hydroxy-4-methoxybenzoate (4bqa): 1b (100 mg, 0.62 mmol), 2q (208 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bqa as a pale yellow solid (196 mg, 83% yield), m.p. = 148.8–151.1 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.44 (s, 9H, CH3), 3.30 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 5.99 (s br, 1H, OH), 6.82–6.84 (m, 2H, NH + Ar), 6.89 (d, J = 8 Hz, 1H, Ar), 7.05 (t, J = 8 Hz, 1H, Ar), 7.31 (d, J = 8 Hz, 1H, Ar), 7.35 (t, J = 8 Hz, 1H, Ar), 7.46 (d, J = 4 Hz, 1H, Ar), 7.49–7.52 (m, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.03, 28.70, 52.22, 56.19, 81.21, 108.87, 110.16, 115.78, 121.45, 123.25, 123.51, 123.98, 125.42, 130.70, 145.25, 145.56, 151.36, 163.35, 163.49, 171.90. HRMS (ESI) m/z: calculated for C22H24O6N2Na [M]+ 435.1527, found 435.1519.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-phenylacetate (4bha): 1b (100 mg, 0.62 mmol), 2h (168 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bha as a white solid (36 mg, <5% yield), m.p. = 210.8–203.6 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.27 (s, 9H, CH3), 3.23 (s, 3H, CH3), 3.73 (d, J = 4 Hz, 2H, CH2), 6.29 (s br, 1H, NH), 6.84 (d, J = 8 Hz, 1H, Ar), 7.02 (d, J = 8 Hz, 1H, Ar), 7.07–7.09 (m, 1H, Ar), 7.25–7.27 (m, 2H, Ar), 7.28–7.39 (m, 4H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 26.91, 28.55, 41.10, 51.98, 81.10, 108.92, 123.06, 123.11, 125.28, 127.62, 128.99, 129.35, 130.76, 133.13, 145.39, 163.34, 168.01, 171.25. HRMS (ESI) m/z: calculated for C22H24O4N2Na [M]+ 403.1628, found 403.1619.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl formate (4bia): 1b (100 mg, 0.62 mmol), 2i (46.8 µL, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bia as a white solid (53 mg, 29% yield), m.p. = 163.4–165.7 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.38 (s, 9H, CH3), 3.25 (s, 3H, CH3), 6.67 (s br, 1H, NH), 6.87 (d, J = 8 Hz, 1H, Ar), 7.07 (t, J = 8 Hz, 1H, Ar), 7.25–7.27 (m, 1H, Ar), 7.34–7.39 (m, 1H, Ar), 7.97 (s, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 26.97, 28.60, 52.30, 80.77, 109.05, 123.34, 123.61, 124.78, 131.01, 145.22, 157.20, 162.92, 170.79. HRMS (ESI) m/z: calculated for C15H18O4N2Na [M]+ 313.1159, found 313.1153.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl tetradecanoate (4bja): 1b (100 mg, 0.62 mmol), 2j (283 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bja as a white solid (233 mg, 82% yield), m.p. = 136.3–138.8 °C. 1H NMR (CDCl3, 400 MHz) δ: 0.86–0.89 (m, 3H, CH3), 1.24–1.25 (m, 20H, CH2), 1.38 (s, 9H, CH3), 2.34–2.48 (m, 2H, CH2), 3.25 (s, 3H, CH3), 6.64 (s br, 1H, NH), 6.85 (d, J = 8 Hz, 1H, Ar), 7–05 (t, J = 8 Hz, 1H, Ar), 7.23 (d, J = 8 Hz, 1H, Ar), 7.34 (t, J = 8 Hz, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 14.25, 22.80, 24.72, 26.93, 28.63, 28.95, 29.32, 29.46, 29.50, 29.68, 29.75, 29.77, 32.03, 33.93, 52.10, 80.88, 108.89, 123.14, 123.38, 125.54, 130.65, 145.30, 163.42, 170.69, 171.71. HRMS (ESI) m/z: calculated for C28H44O4N2Na [M]+ 495.3193, found 495.3186.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl picolinate (4bka) [
19]:
1b (100 mg, 0.62 mmol),
2k (153 mg, 1.24 mmol, 2 equivalents),
3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4bka as a white solid (83 mg, 32% yield), m.p. = 161.5–163.0 °C.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl furan-2-carboxylate (4bla) [
19]:
1b (100 mg, 0.62 mmol),
2l (139 mg, 1.24 mmol, 2 equivalents),
3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH
3CN (3 mL) were used to obtain the corresponding
4bla as a pale yellow solid (154 mg, 70% yield), m.p. = 193.0–195.6 °C.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 5-nitrofuran-2-carboxylate (4bma): 1b (100 mg, 0.62 mmol), 2m (190 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bma as a pale yellow solid (133 mg, 58% yield), m.p. = 210.1–212.8 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.44 (s, 9H, CH3), 3.29 (s, 3H, CH3), 6.86 (s br, 1H, NH), 6.92 (d, J = 8 Hz, 1H, Ar), 7.08 (t, J = 8 Hz, 1H, Ar), 7.29–7.34 (m, 3H, Ar), 7.40 (t, J = 8 Hz, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.05, 28.59, 52.42, 81.66, 109.18, 111.66, 120.65, 123.50, 123.83, 124.27, 131.39, 143.01, 145.50, 153.56, 162.58, 170.32. HRMS (ESI) m/z: calculated for C19H19O7N3Na [M]+ 424.1115, found 424.1104.
1-Benzyl-3-(benzylcarbamoyl)-5-bromo-2-oxoindolin-3-yl 2-bromobenzoate (4mac): 1m (171 mg, 0.5 mmol), 2a (201 mg, 1.0 mmol, 2 equivalents), 3c (123 μL, 1.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4mac as a pale yellow solid (229 mg, 67% yield), m.p. = 133.8–135.0 °C. 1H NMR (CDCl3, 400 MHz) δ: 4.52–4.64 (m, 2H, CH2), 4.96–5.12 (m, 2H, CH2), 6.57 (d, J = 8 Hz, 1H, Ar), 7.29–7.40 (m, 10H, Ar), 7.44–7.45 (m, 3H, Ar), 7.51–7.53 (m, 1H, Ar), 7.64–7.66 (m, 1H, Ar), 7.83–7.85 (m, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 44.18, 44.66, 81.82, 111.77, 115.89, 121.58, 126.77, 127.06, 127.20, 127.81, 127.88, 127.97, 128.03, 129.05, 130.11, 133.23, 133.68, 134.04, 134.69, 134.72, 137.13, 143.70, 163.05, 163.99, 170.44. HRMS (ESI) m/z: calculated for C30H22O4N2Br2Na [M]+ 654.9839, found 654.9830.
3-(tert-Butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 1H-pyrrole-2-carboxylate (4bua): 1b (100 mg, 0.62 mmol), 2u (138 mg, 1.24 mmol, 2 equivalents), 3a (140 μL, 1.24 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4bua as a white solid (196 mg, 83% yield), m.p. = 196.1–199.2 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.41 (s, 9H, CH3), 3.25 (s, 3H, CH3), 6.24–6.26 (m, 1H, Ar), 6.77 (s br, 1H, NH), 6.85–6.87 (m, 2H, Ar), 6.95–6.96 (m, 1H, Ar), 7.02–7.06 (m, 1H, Ar), 7.28–7.37 (m, 2H, Ar), 9.49 (s br, 1H, NH). 13C APT NMR (CDCl3, 100 MHz) δ: 26.94, 28.64, 52,15, 80.88, 108.81, 110.81, 114.89, 116.71, 120.67, 123.18, 123.59, 123.79, 125.23, 125.57, 130.70, 145.74, 158.24, 163.47, 171.74. HRMS (ESI) m/z: calculated for C19H21O4N3Na [M]+ 378.1424, found 378.1417.
1-Benzyl-3-(tert-butylcarbamoyl)-2-oxoindolin-3-yl 2-iodobenzoate (4aba): 1a (102 mg, 0.4 mmol), 2b (209 mg, 0.8 mmol, 2 equivalents), 3a (95 μL, 0.8 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4aba as a pale yellow solid (74 mg, 30% yield), m.p. = 144.0–145.8 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.43 (s, 9H, CH3), 5.05 (q, J = 16 Hz, 2H, CH2), 6.68 (d, J = 8 Hz, 1H, Ar), 7.02–7.06 (m, 2H, NH + Ar), 7.19–7.24 (m, 2H, Ar), 7.28 (d, J= 8 Hz, 1H, Ar), 7.33–7.37 (m, 2H, Ar), 7.42–7.47 (m, 4H, Ar), 7.80 (dd, J = 8 Hz, 1H, Ar), 8.00 (dd, J = 8 Hz, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 28.85, 44.51, 52.49, 82.35, 93.37, 110.21, 123.22, 123.89, 125.31, 127.27, 127.64, 128.43, 128.92, 130.69, 132.49, 133.62, 134.45, 135.27, 141.43, 144.61, 163.33, 163.73, 171.28. HRMS (ESI) m/z: calculated for C27H25O4N2INa [M]+ 591.0751, found 591.0743.
1-Benzyl-3-(cyclohexylcarbamoyl)-2-oxoindolin-3-yl 2-iodobenzoate (4abb): 1a (113 mg, 0.47 mmol), 2b (209 mg, 0.8 mmol, 2 equivalents), 3b (104 μL, 0.8 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4abb as a pale yellow solid (91 mg, 31% yield), m.p. = 155.2–157.4 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.22–1.39 (m, 5H, CH2), 1.61–1.64 (m, 1H, CH2), 1.73–1.76 (m, 2H, CH2), 1.98–2.00 (m, 2H, CH2), 3.82–3.89 (m, 1H, CH), 4.96 (d, J = 16 Hz, 1H, CH2), 5.14 (d, J = 16 Hz, 1H, CH2), 6.67 (d, J = 8 Hz, 1H, Ar), 7.01–7.08 (m, 2H, NH + Ar), 7.20–7.28 (m, 3H, Ar), 7.33–7.37 (m, 2H, Ar), 7.40–7.48 (m, 4H, Ar), 7.80 (dd, J = 8 Hz, 1H, Ar), 8.01 (dd, J = 8 Hz, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 24.89, 24.91, 25.56, 32.94, 33.20, 44.50, 49.14, 82.25, 93.34, 110.22, 123.18, 123.76, 125.30, 127.20, 127.64, 128.45, 128.94, 130.75, 132.61, 133.66, 134.47, 135.25, 141.43, 144.61, 163.50, 163.72, 171.10. HRMS (ESI) m/z: calculated for C29H27O4N2INa [M]+ 617.0908, found 617.0895.
1-Benzyl-3-(tert-butylcarbamoyl)-2-oxoindolin-3-yl 2-bromobenzoate (4aaa): 1a (101 mg, 0.4 mmol), 2a (169 mg, 0.8 mmol, 2 equivalents), 3a (95 μL, 0.8 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4aaa as a pale yellow solid (115 mg, 52% yield), m.p. = 175.8–176.0 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.43 (s, 9H, CH3), 5.05 (s, 2H, CH2), 6.67 (d, J = 8 Hz, 1H, Ar), 7.03 (t, J = 8 Hz, 1H, Ar), 7.13 (s br, 1H, NH), 7.21 (dt, J = 8 Hz, 1H, Ar), 7.28 (d, J = 8 Hz, 1H, Ar), 7.33–7.37 (m, 3H, Ar), 7.40–7.42 (m, 2H, Ar), 7.45–7.47 (m, 2H, Ar), 7.69–7.71 (m, 1H, Ar), 7.83–7.86 (m, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 28.82, 44.52, 52.40, 82.41, 110.23, 121.30, 123.23, 123.39, 125.45, 127.24, 127.63, 127.83, 128.93, 130.71, 130.78, 133.27, 133.83, 134.60, 135.29, 144.70, 163.16, 163.53, 171.21. HRMS (ESI) m/z: calculated for C27H25O4N2BrNa [M]+ 543.0889, found 543.0883.
1-Benzyl-3-(benzylcarbamoyl)-2-oxoindolin-3-yl 2-iodobenzoate (4abc): 1a (420 mg, 1.8 mmol), 2b (892 mg, 3.6 mmol, 2 equivalents), 3c (438 μL, 3.6 mmol, 2 equivalents), powder 4Å MS (200 mg), and CH3CN (6 mL) were used to obtain the corresponding 4abc as a white solid (152 mg, 14% yield), m.p. = 142.8–145.3 °C. 1H NMR (CDCl3, 400 MHz) δ: 4.49–4.54 (m, 1H, CH2), 4.62–4.67 (m, 1H, CH2), 5.06 (q, J = 16 Hz, 2H, CH2), 6.71 (d, J = 8 Hz, 1H, Ar), 7.05 (t, J = 8 Hz, 1H, Ar), 7.18 (t, J = 8 Hz, 1H, Ar), 7.24–7.43 (m, 11H, Ar), 7.48 (d, J = 8 Hz, 2H, Ar), 7.77–7.80 (m, 1H, Ar), 7.95 (d, J = 8 Hz, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 44.06, 44.57, 93.46, 110.30, 123.29, 123.96, 125.03, 127.25, 127.69, 127.92, 128.06, 128.36, 128.96, 130.90, 132.47, 133.63, 134.18, 135.17, 137.37, 141.44, 144.61, 163.64, 164.45, 170.97. HRMS (ESI) m/z: calculated for C30H23O4N2INa [M]+ 625.0595, found 625.0584.
1-Benzyl-3-(benzylcarbamoyl)-2-oxoindolin-3-yl 2-bromoacetate (4avc): 1a (565 mg, 2.4 mmol), 2v (667 mg, 4.8 mmol, 2 equivalents), 3c (585 μL, 4.8 mmol, 2 equivalents), powder 4Å MS (200 mg), and CH3CN (6 mL) were used to obtain the corresponding 4avc as a white solid (167 mg, 14% yield), m.p. = 196.7–197.2 °C. 1H NMR (CDCl3, 400 MHz) δ: 3.89 (q, J = 16 Hz, 2H, CH2), 4.47–4.62 (m, 2H, CH2), 5.00 (q, J = 16 Hz, 2H, CH2), 6.68 (d, J = 8 Hz, 1H, Ar), 7.05 (t, J = 8 Hz, 1H, Ar), 7.16 (s br, 1H, NH), 7.22–7.41 (m, 12H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 24.84, 43.94, 44.55, 81.98, 110.38, 123.49, 123.53, 124.25, 127.16, 127.78, 127.93, 128.97, 129.00, 131.20, 134.93, 137.33, 144.48, 163.85, 164.08, 170.60. HRMS (ESI) m/z: calculated for C25H21O4N2BrNa [M]+ 515.0577, found 515.0571.
3-(Benzylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-bromobenzoate (4bac): 1b (300 mg, 2.0 mmol), 2a (804 mg, 4.0 mmol, 2 equivalents), 3c (487 μL, 4.0 mmol, 2 equivalents), powder 4Å MS (200 mg), and CH3CN (6 mL) were used to obtain the corresponding 4bac as a pale yellow solid (314 mg, 33% yield), m.p. = 142.8–145.3 °C. 1H NMR (CDCl3, 400 MHz) δ: 3.33 (s, 3H, CH3), 4.46–4.51 (m, 1H, CH2), 4.57–4.63 (m, 1H, CH2), 6.93 (d, J = 8 Hz, 1H, Ar), 7.09 (t, J = 8 Hz, 1H, Ar), 7.30–7.42 (m, 9H, Ar), 7.49 (s br, 1H, NH), 7.61–7.63 (m, 1H, Ar), 7.77–7.79 (m, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.09, 44.02, 82.12, 109.14, 121.42, 123.30, 123.79, 124.97, 127.70, 127.86, 127.92, 128.93, 130.47, 131.07, 132.94, 133.77, 134.57, 137.34, 145.61, 163.09, 164.44, 170.80. HRMS (ESI) m/z: calculated for C24H19O4N2BrNa [M]+ 501.0420, found 501.0410.
5-Bromo-3-(tert-butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-bromobenzoate (4faa): 1f (259 mg, 1.1 mmol), 2a (402 mg, 2.0 mmol, 2 equivalents), 3a (200 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4faa as a white solid (328 mg, 58% yield), m.p. = 195.9–198.1 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.41 (s, 9H, CH3), 3.29 (s, 3H, CH3), 6.79 (d, J = 8 Hz, 1H, Ar), 7.09 (s br, 1H, NH), 7.39–7.43 (m, 3H, Ar), 7.50 (dd, J = 8 Hz, 1H, Ar), 7.69–7.71 (m, 1H, Ar), 7.79–7.82 (m, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.14, 28.72, 52.55, 81.62, 110.46, 115.74, 121.44, 126.69, 127.22, 127.86, 130.22, 133.23, 133.65, 134.04, 134.72, 144.72, 162.83, 163.11, 170.62. HRMS (ESI) m/z: calculated for C21H20O4N2Br2Na [M]+ 544.9682, found 544.9675.
5-Bromo-3-(tert-butylcarbamoyl)-1-methyl-2-oxoindolin-3-yl 2-iodobenzoate (4fba): 1f (268 mg, 1.1 mmol), 2b (496 mg, 2.0 mmol, 2 equivalents), 3a (200 μL, 2.0 mmol, 2 equivalents), powder 4Å MS (100 mg), and CH3CN (3 mL) were used to obtain the corresponding 4fba as a white solid (311 mg, 48% yield), m.p. = 196.7–202.4 °C. 1H NMR (CDCl3, 400 MHz) δ: 1.41 (s, 9H, CH3), 3.29 (s, 3H, CH3), 6.79 (d, J = 8 Hz, 1H, Ar), 6.99 (s br, 1H, NH), 7.21 (dt, J = 8 Hz, 1H, Ar), 7.43 (t, J = 8 Hz, 1H, Ar), 7.48–7.52 (m, 2H, Ar), 7.74 (dd, J = 8 Hz, 1H, Ar), 7.98–8.01 (m, 1H, Ar). 13C APT NMR (CDCl3, 100 MHz) δ: 27.14, 28.75, 52.61, 81.54, 93.41, 110.45, 115.72, 127.06, 127.18, 128.44, 132.44, 133.60, 133.77, 133.99, 141.48, 144.63, 162.62, 163.69, 170.67. HRMS (ESI) m/z: calculated for C21H20O4N2BrINa [M]+ 592.9543, found 592.9536.