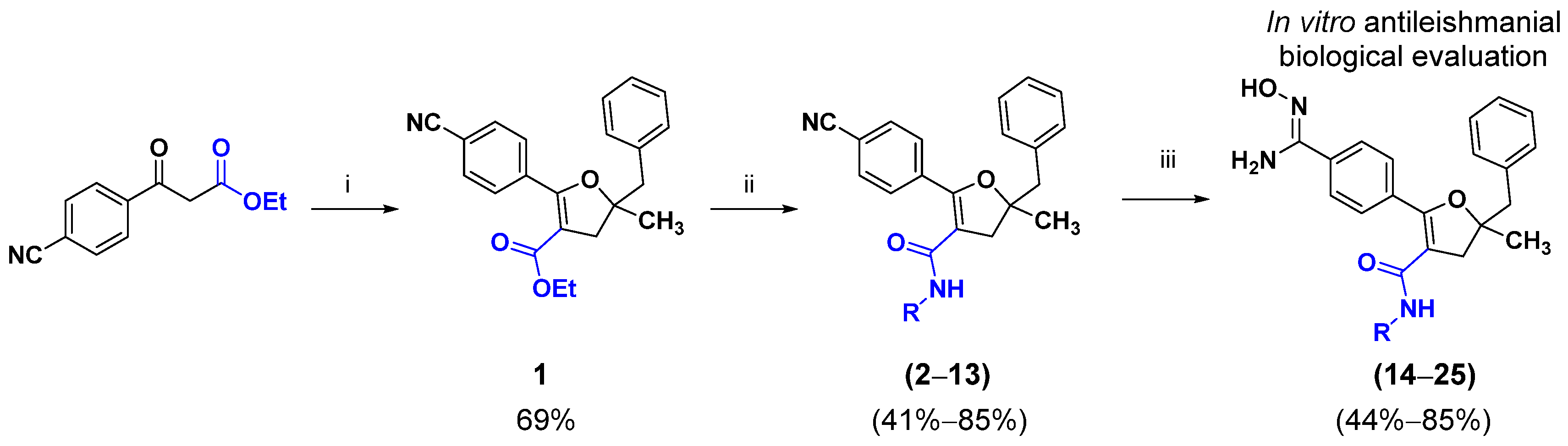
3.1. Chemistry
3.1.1. General
Reagents were purchased from Sigma-Aldrich (3050 Spruce Street St. Louis, MO, 63103, USA), Fluorochem (Unit 14 Graphite Way, Hadfield, Glossop SK13 1QH, UK), Fisher Scientific (168 3rd Ave, Waltham, MA 02451, USA), or TCI chemicals (9211 North Harborgate Street, Portland, OR 97203, USA) and used without further purification. Microwave reactions were performed using monomode reactors in a Biotage® Initiator Classic (Uppsala, Sweden) in sealed vials, with output powers ranged up to 400 W.
Reaction monitoring was conducted using thin-layer chromatography on aluminum plates (5 × 5 cm) precoated with silica gel 60 F254 nm (Merck-F254 or Alugram®: Macherey-Nagel) in an appropriate eluent. Visualization was performed using ultraviolet light under a UV lamp (VL-6.CL) at 254 nm (6 W) or 365 nm (6 W).
The LC-MS analysis was conducted on a Thermo Scientific Accela High-Speed LC System® (168 3rd Ave, Waltham, MA, USA) coupled with a single quadrupole mass spectrometer, the Thermo MSQ Plus® (168 3rd Ave, Waltham, MA, USA), with an HPLC column, the Thermo Hypersil Gold® (168 3rd Ave, Waltham, MA 02451, USA) 50 × 2.1 mm (C18-bonded), with particles of a diameter of 1.9 mm. The samples were injected into the column at a volume of 1 µL. A total of eight minutes was fixed for the chromatographic analysis, which was conducted using a gradient of the following solvents: methanol/water 50:50 at time zero; then, between zero and four minutes, a linear increase in the proportion of methanol to reach a methanol/water ratio of 95:5. Between four and six minutes, the methanol/water ratio was maintained at 95:5, and between six and seven minutes, a linear decrease in the proportion of methanol to return to a methanol/water ratio of 50:50. The water used was buffered with 5 mM ammonium acetate. The mobile phase had a flow rate of 0.3 mL/min. The LC-MS analysis was performed at the Faculty of Pharmacy in Marseille. The purity of the synthesized compounds was verified by the relative area in the LC chromatogram, with >90% for intermediates and >95% for tested compounds.
The melting points were determined using a Köfler melting point apparatus (Wagner & Munz GmbH, München, Germany) and were not corrected.
The following adsorbent was utilized for the column chromatography: silica gel 60 (Merck KGaA, Darmstadt, Germany, with a particle size of 0.063–0.200 mm; 70–230 mesh ASTM or Macherey-Nagel GmbH & Co. KG, Düren, Germany, with a particle size of 0.063–0.04 mm).
Flash chromatography was conducted on a PuriFlash® 5.020 apparatus (Interchim, Montluçon, France) using a solid deposit in dry-load PF-DEL-F004, which contained Celite® 545 CAS 68855-54-9 (Celite Corp., Lompoc, CA, USA). The maximum pressure was set at 15 bar and the flow rate was 15 mL/min. The ultraviolet–visible detector was configured at a wavelength of 254 nm.
The NMR spectra were recorded at the Faculty of Pharmacy in Marseille using a Bruker Avance NEO 400 MHz NanoBay spectrometer (Bruker, Billerica, MA, USA). Residual 1H and 13C signals in deuterated solvent (CDCl3) were used to calibrate the chemical shifts, obviating the need for an additional internal standard. The 1H NMR reference values were CDCl3 δ = 7.26 ppm and DMSO-d6 δ = 2.50 ppm. The 13C NMR reference values were CDCl3 δ = 77.16 ppm and DMSO-d6 δ = 39.52 ppm. The data for the 1H NMR are reported as follows: chemical shifts (δ) in parts per million (ppm), multiplicity (described as follows: s, singlet; bs, broad singlet; d, doublet; t, triplet; q, quadruplet; dd, doublet of doublet; ddd, doublet of doublet of doublet; m, multiplet), coupling constants (J) in Hertz (Hz), and integration. The data for the 13C NMR are reported as follows: chemical shifts (δ) in parts per million (ppm).
3.1.2. Procedure for Preparation of Ethyl 5-Benzyl-2-(4-cyanophenyl)-5-methyl-4,5-dihydrofuran-3-carboxylate (1)
In a 20 mL microwave vial equipped with a stirring bar, a solution of manganese (III) acetate dihydrate (2.1 equiv.) and copper(II) acetate (1 equiv.) in 10 mL of glacial acetic acid was heated at 80 °C under microwave irradiation at 100 W for 15 min. Subsequently, the reaction mixture was cooled, and a solution of ethyl 3-(4-cyanophenyl)-3-oxopropanoate (500 mg, 2.3 mmol, and 1 equiv.) and 2-methyl-3-phenyl-1-propene (608 mg, 4.6 mmol, and 2 equiv.) in 7 mL of acetic acid was added. The reaction mixture was monitored by thin-layer chromatography using cyclohexane/AcOEt (70/30), with an Rf value of 0.69. The reaction mixture was heated for 3 h under microwave irradiation using the same conditions. The resulting product was transferred to 50 mL of cold water and extracted with dichloromethane (3 × 40 mL). The organic extracts were subsequently collected and washed with saturated aqueous NaHCO
3 (3 × 40 mL) and then dried over Na
2SO
4. The filtrate solvent was evaporated under reduced pressure. The crude product was purified by flash chromatography using a PuriFlash
® IR-20SI-F0025 column and cyclohexane/AcOEt as eluent, employing a gradient from 100/0 to 80/20 over 20 column volumes. Furthermore, the UV–VIS detector was additionally configured at 307 nm, 235, nm, and 205 nm. The product was obtained in the form of an oily white solid. Yield: 69%. The analytical data are consistent with those in the previous literature [
23].
1H NMR (400 MHz, CDCl
3): δ (ppm) 7.85 (d,
3JH-H = 8.6 Hz, 2H, 2CH
Ar), 7.64 (d,
3JH-H = 8.6 Hz, 2H, 2CH
Ar), 7.30–7.19 (m, 5H, 5CH
Ar), 4.08 (q,
3JH-H = 7.1 Hz, 2H, CH
2), 3.10 (d,
2JH-H = 15.5 Hz, 1H, H-(CH
2)), 3.02 (d,
2JH-H = 15.3 Hz, 1H, H-(CH
2)), 2.98 (d,
2JH-H = 15.3 Hz, 1H, H-(CH
2)), 2.82 (d,
2JH-H = 15.5 Hz, 1H, H-(CH
2)), 1.45 (s, 3H, CH
3), and 1.17 (t,
3JH-H = 7.1 Hz, 3H, CH
3).
13C NMR (100 MHz, CDCl
3): δ (ppm) 164.6 (C), 161.0 (C), 136.1 (C), 134.5 (C), 131.1 (2CH
Ar), 130.2 (2CH
Ar), 129.8 (2CH
Ar), 128.0 (2CH
Ar), 126.6 (CH
Ar), 118.3 (C), 113.2 (C), 104.2 (C), 87.8 (C), 59.7 (CH
2), 46.5 (CH
2), 42.1 (CH
2), 26.4 (CH
3), and 14.0 (CH
3). HRMS (ESI +)
m/
z calcd for C
22H
21NO
3 [M + Na]
+ 370.1414; found 370.1413.
3.1.3. Procedure for Preparation of Compounds 2 to 13 by Amidation Reaction
A single-neck round-bottom flask of 5 mL equipped with a stirring bar was charged with the intermediate product, compound 1 (1.0 equiv.), the respective amine (1.5 equiv.), and then placed under nitrogen. The vial was subjected to three evacuation/backfilling cycles. Toluene (2 mL) and lithium bis(trimethylsilyl)amide (LiHMDS, 1.0 M in THF, and 2.5 equiv.) were added sequentially with vigorous stirring at room temperature. The reaction mixture was then stirred for 15 h at 70 °C. Subsequently, the reaction mixture was quenched with NH4Cl (1.0 M and 5 mL) and extracted with EtOAc (3 × 15 mL). The organic layers were combined, washed with water (1 × 20 mL) and brine (1 × 20 mL), dried over Na2SO4, and concentrated. Purification by flash chromatography using an appropriate solvent afforded the title product.
The compound 2 was synthesized following the previously described procedure from 5-bromopyridin-2-amine (112 mg, 0.65 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: AcOEt/cyclohexane 40/60; Rf of 0.33). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 80/20 over 25 column volumes. Yield: 45%.
The product was obtained as a light-brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.24 (d, 4JH-H = 2.4 Hz, 1H, CHAr), 8.12 (d, 3JH-H = 8.9 Hz, 1H, CHAr), 7.81 (d, 3JH-H = 8.7 Hz, 2H, 2CHAr), 7.75 (dd, 4JH-H = 2.4 Hz, 3JH-H = 8.9 Hz, 1H, CHAr), 7.68 (d, 3JH-H = 8.7 Hz, 2H, 2CHAr), 7.67 (br s, 1H, NH), 7.32–7.22 (m, 5H, 5CHAr), 3.23 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2), 3.08 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 3.04 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.92 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2), and 1.54 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 162.7 (C), 160.9 (C), 150.1 (C), 148.3 (CHAr), 141.1 (CHAr), 136.0 (C), 134.5 (C), 131.8 (2CHAr), 130.5 (2CHAr), 129.9 (2CHAr), 128.5 (2CHAr), 127.2 (CHAr), 118.5 (C), 115.5 (CHAr), 114.5 (C), 113.9 (C), 105.9 (C), 88.3 (C), 46.8 (CH2), 42.1 (CH2), and 27.0 (CH3). LC/MS ESI+ tr, 6.59 min, (m/z) calcd for C25H20BrN3O2 [M + H]+: found 474.96. HRMS (ESI +) m/z calcd for C25H20BrN3O2 [M + H]+ 476.0795; found 476.0798.
The compound 3 was synthesized following the previously described procedure from 5-chloropyridin-2-amine (112 mg, 0.86 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 30/40/30; Rf of 0.51). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. Yield: 48%.
The product was obtained as a light-yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.18 (d, 3JH-H = 8.9 Hz, 1H, CHAr), 8.14 (d, 4JH-H = 2.4 Hz, 1H, CHAr), 7.81 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.73 (br s, 1H, NH), 7.68 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.62 (dd, 4JH-H = 2.4 Hz, 3JH-H = 8.9 Hz, 1H, CHAr), 7.33–7.23 (m, 5H, 5CHAr), 3.23 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2), 3.08 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 3.04 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.93 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2), and 1.54 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 162.7 (C), 160.9 (C), 149.7 (C), 146.0 (CHAr), 138.4 (CHAr), 136.0 (C), 134.5 (C), 131.8 (2CHAr), 130.5 (2CHAr), 129.9 (2CHAr), 128.5 (2CHAr), 127.2 (CHAr), 126.7 (C), 118.5 (C), 115.0 (CHAr), 113.9 (C), 105.9 (C), 88.3 (C), 46.8 (CH2), 42.1 (CH2), and 27.0 (CH3). LC/MS ESI+ tr, 6.54 min, (m/z) calcd for C25H20ClN3O2 [M + H]+: found 430.06. HRMS (ESI+) m/z calcd for C25H20ClN3O2 [M + H]+ 430.1317; found 413.1314.
The compound 4 was synthesized following the previously described procedure from 5-methylpyridin-2-amine (74 mg, 0.68 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 40/30/30; Rf of 0.55). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. Yield: 66%. The product was obtained as a light-yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.13 (d, 3JH-H = 8.6 Hz, 1H, CHAr), 8.01 (m, 2H, NH, CHAr), 7.82 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.67 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.53 (dd, 4JH-H = 2.0 Hz, 3JH-H = 8.6 Hz, 1H, CHAr), 7.34–7.20 (m, 5H, 5CHAr), 3.27 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2), 3.08 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 3.04 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2), 2.96 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 2.28 (s, 3H, CH3), and 1.53 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 162.8 (C), 160.9 (C), 149.0 (C), 145.5 (CHAr), 140.5 (CHAr), 136.1 (C), 134.7 (C), 131.8 (2CHAr), 130.5 (2CHAr), 130.0 (2CHAr), 129.3 (C), 128.5 (2CHAr), 127.2 (CHAr), 118.6 (C), 114.4 (CHAr), 113.8 (C), 106.1 (C), 88.3 (C), 46.8 (CH2), 42.3 (CH2), 27.0 (CH3), and 17.9 (CH3). LC/MS ESI+ tr, 6.10 min, (m/z) calcd for C26H23N3O2 [M + H]+: found 410.11. HRMS (ESI+) m/z calcd for C26H23N3O2 [M + H]+ 410.1863; found 410.1870.
The compound 5 was synthesized following the previously described procedure from pyridin-2-amine (41 mg, 0.43 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 30/40/30; Rf of 0.80). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configurated additionally at 222 nm and 230 nm. Yield: 85%. The product was obtained as a light-brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.24–8.17 (m, 2H, 2CHAr), 7.89 (br s, 1H, NH), 7.83 (d, 3JH-H = 8.6 Hz, 2H, 2CHAr), 7.70–7.67 (m, 3H, 3CHAr), 7.33–7.23 (m, 5H, 5CHAr), 7.05–7.01 (m, 1H, CHAr), 3.26 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 3.09 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 3.05 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.96 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), and 1.54 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 162.9 (C), 160.7 (C), 151.3 (C), 146.9 (CHAr), 139.1 (CHAr), 136.1 (C), 134.7 (C), 131.8 (2CHAr), 130.5 (2CHAr), 130.0 (2CHAr), 128.5 (2CHAr), 127.2 (CHAr), 119.7 (CHAr), 118.6 (C), 114.5 (CHAr), 113.8 (C), 106.2 (C), 88.2 (C), 46.8 (CH2), 42.3 (CH2), and 27.0 (CH3). Analysis calculated for C25H21N3O2: HRMS: m/z [M + H]+ calculated 396.1707; found 396.1703.
The compound 6 was synthesized following the previously described procedure from and pyridin-4-amine (40 mg, 0.43 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: dichloromethane/methanol 96/4; Rf of 0.38). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with dichloromethane/methanol in a gradient from 100/0 to 95/5 over 25 column volumes. A UV–VIS detector was configurated additionally at 222 nm and 230 nm. Yield: 50%. The product was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.35 (d, 3JH-H = 6.5 Hz, 2H, 2CHAr), 7.78 (d, 3JH-H = 8.6 Hz, 2H, 2CHAr), 7.68 (d, 3JH-H = 8.6 Hz, 2H, 2CHAr), 7.44 (d, 3JH-H = 6.5 Hz, 2H, 2CHAr), 7.39 (br s, 1H, NH), 7.32–7.23 (m, 5H, 5CHAr), 3.24 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2), 3.07 (d, 2JH-H = 13.9 Hz, 1H, H-(CH2), 3.04 (d, 2JH-H = 13.9 Hz, 1H, H-(CH2), 2.98 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2)), and 1.55 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 163.2 (C), 159.9 (C), 150.5 (2CHAr), 145.1 (C), 136.1 (C), 134.4 (C), 132.0 (2CHAr), 130.5 (2CHAr), 129.8 (2CHAr), 128.5 (2CHAr), 127.2 (CHAr), 118.4 (C), 114.1 (C), 113.5 (2CHAr), 106.5 (C), 88.3 (C), 46.9 (CH2), 42.3 (CH2), and 27.1 (CH3). Analysis calculated for C25H21N3O2: HRMS: m/z [M + H]+ calculated 396.1707; found 396.1702.
The compound 7 was synthesized following the previously described procedure from aniline (40 mg, 0.43 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 3/3/4; Rf of 0.92). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured at 242 nm, 290 nm and 327 nm. Yield: 41%. The product was obtained as an oily yellow solid. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.81 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.67 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.52–7.43 (m, 1H, CHAr), 7.34–7.27 (m, 8H, 8CHAr), 7.08 (t, 3JH-H = 7.2 Hz, 1H, CHAr), 6.70 (br s, 1H, NH), 3.19 (d, 2JH-H = 14.7 Hz, 1H, H-(CH2), 3.08 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2), 3.03 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2), 2.95 (d, 2JH-H = 14.7 Hz, 1H, H-(CH2), and 1.55 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 169.2 (C), 157.9 (C), 137.8 (C), 136.4 (C), 134.6 (C), 132.0 (2CHAr), 130.5 (2CHAr), 129.7 (2CHAr), 129.2 (2CHAr), 128.5 (2CHAr), 127.1 (CHAr), 124.5 (CHAr), 120.0 (2CHAr), 118.5 (C), 113.7 (C), 107.3 (C), 87.6 (C), 47.0 (CH2), 42.8 (CH2), and 27.1 (CH3). Analysis calculated for C26H22N2O2: HRMS: m/z [M + H]+ calculated 395.1754; found 395.1753.
The compound 8 was synthesized following the previously described procedure from 4-fluoroaniline (45 mg, 0.40 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt 50/00; Rf of 0.29). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/ethyl acetate in a gradient from 100/0 to 60/40 over 25 column volumes. A UV–VIS detector configured additionally at 225 nm and 230 nm. Yield: 68%. The product was obtained as a light-brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.81 (d, 3JH-H = 8.6 Hz, 2H, 2CHAr), 7.67 (d, 3JH-H = 8.6 Hz, 2H, 2CHAr), 7.34–7.25 (m, 7H, 7CHAr), 7.02–6.95 (m, 2H, 2CHAr), 6.66 (br s, 1H, NH), 3.18 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2), 3.08 (d, 2JH-H = 13.7 Hz, 1H, H-(CH2), 3.03 (d, 2JH-H = 13.7 Hz, 1H, H-(CH2), 2.93 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2), and 1.55 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 162.9 (C), 159.5 (d, JC-F = 246.3 Hz, C), 158.3 (C), 136.8 (C), 136.3 (C), 133.7 (d, JC-F = 3.1 Hz, C), 130.5 (2CHAr), 129.8 (2CHAr), 128.5 (2CHAr), 127.3 (2CHAr), 127.2 (CHAr), 121.1 (d, JC-F = 8.6 Hz, 2CHAr), 118.5 (C), 115.9 (d, JC-F = 21.6 Hz, 2CHAr), 113.7 (C), 106.9 (C), 87.6 (C), 47.0 (CH2), 42.8 (CH2), and 27.1 (CH3). 19F NMR (376.5 MHz, CDCl3): δ (ppm) −103.3. LC/MS ESI+ tr, 5.10 min, (m/z) calcd for C26H21FN2O2 [M + H]+: found 413.06. HRMS (ESI +) m/z calcd for C26H21FN2O2 [M + H]+ 413.1660; found 413.1662.
The compound 9 was synthesized following the previously described procedure from (4-(trifluoromethyl)phenyl)methanamine (76 mg, 0.4 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt 70/30; Rf of 0.19). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/ethyl acetate in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured additionally in 225 nm and 230 nm. Yield: 47%. The product was obtained as a light-brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.81 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.62 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.58 (d, 3JH-H = 8.1 Hz, 2H, 2CHAr), 7.32 (d, 3JH-H = 8.1 Hz, 2H, 2CHAr), 7.27–7.22 (m, 5H, 5CHAr), 5.44 (br s, 1H, NH), 4.50–4.42 (m, 2H, CH2), 3.07 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2)), 3.04 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.99 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.82 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2)), and 1.51 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 164.7 (C), 158.0 (C), 142.4 (C), 136.3 (C), 134.7 (C), 131.7 (2CHAr), 130.5 (2CHAr), 130.2 (q, 2JC-F = 34.6 Hz, C), 129.8 (2CHAr), 128.4 (2CHAr), 128.1 (2CHAr), 127.1 (CHAr), 125.8 (q, 3JC-F = 3.7 Hz, 2CHAr), 124.2 (q, 1JC-F = 271.8 Hz, CF3), 118.6 (C), 113.5 (C), 106.1 (C), 87.4 (C), 47.0 (CH2), 43.0 (CH2), 42.7 (CH2), and 27.1 (CH3). 19F NMR (376.5 MHz, CDCl3): δ (ppm) −62.5. Analysis calculated for C28H23F3N2O2: HRMS: m/z [M + H]+ calculated 477.1784; found 477.1786.
The compound 10 was synthesized following the previously described procedure from pyridazin-3-amine (41 mg, 0.43 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 2/6/2; Rf of 0.35). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/ethyl acetate in a gradient from 100/0 to 60/40 over 25 column volumes. A UV–VIS detector was configured to 241 nm, 212 nm, and 315 nm. Yield: 43%. The product was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.84 (d, 3JH-H = 12.1 Hz, 1H, CHAr), 8.68 (br s, 1H, NH), 8.50 (d, 3JH-H = 8.7 Hz, 1H, CHAr), 7.81 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.68 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.61 (dd, 3JH-H = 12.1 Hz, 3JH-H = 8.7 Hz, 1H, CHAr), 7.35–7.20 (m, 5H, 5CHAr), 3.32 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2)), 3.10 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 3.06 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 3.02 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2)), and 1.56 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 163.6 (C), 162.1 (C), 155.3 (C), 152.0 (C), 148.2 (CHAr), 135.9 (C), 134.5 (C), 131.8 (2CHAr), 130.5 (2CHAr), 130.0 (2CHAr), 128.5 (2CHAr), 128.1 (CHAr), 127.2 (CHAr), 119.7 (CHAr), 118.4 (C), 105.7 (C), 88.7 (C), 46.8 (CH2), 41.9 (CH2), and 27.0 (CH3). Analysis calculated for C24H20N4O2: HRMS: m/z [M + H]+ calculated 397.1659; found 397.1657.
The compound 11 was synthesized following the previously described procedure from 6-chloropyridazin-3-amine (86 mg, 0.67 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 6/2/2; Rf of 0.41). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured additionally to 210 nm and 230 nm. Yield: 59%. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.53 (d, 3JH-H = 9.5 Hz, 1H, CHAr), 7.80 (d, 3JH-H = 8.6 Hz, 2H, 2CHAr), 7.69 (d, 3JH-H = 8.6 Hz, 2H, 2CHAr), 7.48 (d, 3JH-H = 9.5 Hz, 1H, CHAr), 7.35–7.20 (m, 5H, 5CHAr), 3.31 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2), 3.11 (d, 2JH-H = 13.9 Hz, 1H, H-(CH2), 3.05 (d, 2JH-H = 13.9 Hz, 1H, H-(CH2), 3.01 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2), and 1.58 (s, 3H, CH3). NH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.3 (C), 154.4 (C), 152.0 (C), 135.9 (2C), 134.4 (C), 131.9 (2CHAr), 130.6 (CHAr), 130.5 (2CHAr), 130.0 (2CHAr), 128.5 (2CHAr), 127.3 (CHAr), 122.0 (CHAr), 118.4 (C), 114.2 (C), 105.3 (C), 89.0 (C), 46.8 (CH2), 41.7 (CH2), and 27.1 (CH3). Analysis calculated for C24H19ClN4O2: HRMS: m/z [M + Na]+ calculated 431.1183; found 431.1184.
The compound 12 was synthesized following the previously described procedure from pyrazin-2-amine (41 mg, 0.43 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 3/4/3; Rf of 0.57). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured additionally to 210 nm and 230 nm. Yield: 59%. The product was obtained as light-yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 9.48 (s, 1H, NH), 8.28 (d, 3JH-H = 2.4 Hz, 1H, CHAr), 8.17 (d, 3JH-H = 2.4 Hz, 1H, CHAr), 7.82 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.70 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.49 (br s, 1H, CHAr), 7.34–7.20 (m, 5H, 5CHAr), 3.24 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2)), 3.10 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 3.04 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.95 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2)), and 1.56 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 162.5 (C), 161.2 (C), 148.2 (C), 141.9 (CHAr), 140.0 (CHAr), 137.2 (CHAr), 135.9 (C), 134.4 (C), 132.0 (2CHAr), 130.5 (2CHAr), 130.0 (2CHAr), 128.5 (2CHAr), 127.3 (CHAr), 118.4 (C), 114.2 (C), 105.6 (C), 88.4 (C), 46.8 (CH2), 42.1 (CH2), and 27.1 (CH3). Analysis calculated for C24H20N4O2: HRMS: m/z [M + Na]+ calculated 419.1478; found 419.1480.
The compound 13 was synthesized following the previously described procedure from 1,5-dimethyl-1H-pyrazol-3-amine (48 mg, 0.43 mmol, and 1.5 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 3/4/3; Rf of 0.29). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/ethyl acetate in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured to 238 nm and 320 nm. Yield: 66%. The product was obtained as a light-brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.84 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.64 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.60 (br s, 1H, NH), 7.30–7.18 (m, 5H, 5CHAr), 6.43 (s, 1H, CHAr), 3.62 (s, 3H, CH3), 3.15 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2), 3.04 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2), 3.00 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2), 2.85 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2), 2.21 (s, 3H, CH3), and 1.49 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ (ppm) 161.7 (C), 159.2 (C), 145.3 (C), 139.9 (C), 136.1 (C), 134.7 (C), 131.7 (2CHAr), 130.4 (2CHAr), 129.8 (2CHAr), 128.4 (2CHAr), 127.0 (CHAr), 118.6 (C), 113.4 (C), 106.0 (C), 97.4 (CHAr), 87.6 (C), 46.7 (CH2), 42.4 (CH2), 35.6 (CH3), 26.9 (CH3), and 11.4 (CH3). Analysis calculated for C25H24N4O2: HRMS: m/z [M + H]+ calculated 413.1972; found 413.1972.
3.1.4. General Procedure for Amidoxime Synthesis
Hydroxylamine hydrochloride (10 equiv.) in a solvent mixture of dimethyl sulfoxide was stirred under an inert atmosphere of nitrogen while being cooled to 0 °C. Gradual addition of potassium tert-butoxide (10 equiv.) followed, and was subsequently stirred for 30 min. Then, one equivalent of the required nitrile was added, and the reaction mixture was stirred to reach room temperature for 18 h. Upon completion of the reaction, the mixture was poured into cold water and extracted with EtOAc (3 × 15 mL). The combined organic phases were washed sequentially with water (1 × 20 mL) and brine (2 × 20 mL), then dried over Na2SO4 and concentrated. The final product was obtained through purification via flash or column chromatography, employing an appropriate solvent.
The compound 14 was synthesized following the previously described procedure from 2 (82 mg, 0.17 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 4/3/3; Rf of 0.26). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured additionally to 222 nm and 230 nm. Yield: 68%. The product was obtained as a light brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.21 (d, 4JH-H = 2.5 Hz, 1H, CHAr), 8.12 (d, 3JH-H = 8.9 Hz, 1H, CHAr), 7.80 (br s, 1H, NH), 7.72 (dd, 4JH-H = 2.5 Hz, 3JH-H = 8.9 Hz, 1H, CHAr), 7.69 (d, 3JH-H = 8.7 Hz, 2H, 2CHAr), 7.66 (d, 3JH-H = 8.7 Hz, 2H, 2CHAr), 7.33–7.19 (m, 5H, 5CHAr), 5.10 (br s, 2H, NH2), 3.17 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2), 2.99 (s, 2H, CH2), 2.87 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2), and 1.46 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.4 (C), 161.5 (C), 152.6 (C), 150.4 (C), 148.6 (CHAr), 140.8 (CHAr), 136.3 (C), 133.9 (C), 131.9 (C), 130.5 (2CHAr), 129.6 (2CHAr), 128.4 (2CHAr),127.1 (CHAr), 126.0 (2CHAr), 115.5 (CHAr), 114.2 (C), 105.2 (C), 87.8 (C), 46.8 (CH2), 42.3 (CH2), and 26.8 (CH3). Analysis calculated for C25H23BrN4O3: HRMS: m/z [M + H]+ calculated 509.1010; found 509.1010.
The compound 15 was synthesized following the previously described procedure from 3 (90 mg, 0.21 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 4/3/3; Rf of 0.59). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured additionally to 222 nm and 230 nm. Yield: 55%. The product was obtained as a light-yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.17 (d, 3JH-H = 8.9 Hz, 1H, CHAr), 8.12 (d, 4JH-H = 2.6 Hz, 1H, CHAr), 7.88 (br s, 1H, NH), 7.69 (d, 3JH-H = 8.9 Hz, 2H, 2CHAr), 7.66 (d, 3JH-H = 8.9 Hz, 2H, 2CHAr), 7.58 (dd, 4JH-H = 2.6 Hz, 3JH-H = 8.9 Hz, 1H, CHAr), 7.31–7.18 (m, 5H, 5CHAr), 5.06 (br s, 2H, NH2), 3.15 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2), 3.00 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2), 2.95 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2), 2.86 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2), and 1.43 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.4 (C), 161.4 (C), 152.5 (C), 150.3 (C), 146.3 (CHAr), 138.1 (CHAr), 136.3 (C), 134.0 (C), 131.8 (C), 130.5 (2CHAr), 129.6 (2CHAr), 128.4 (2CHAr),127.0 (CHAr), 126.4 (C), 125.9 (2CHAr), 115.5 (CHAr), 105.1 (C), 87.8 (C), 46.8 (CH2), 42.3 (CH2), and 26.8 (CH3). Analysis calculated for C25H23ClN4O3: HRMS: m/z [M + H]+ calculated 463.1531; found 463.1531.
The compound 16 was synthesized following the previously described procedure from 5 (85 mg, 0.21 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 4/3/3; Rf of 0.23). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector was configured additionally to 222 nm and 230 nm. Yield: 85%. The product was obtained as a light-yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.22–8.10 (m, 2H, 2CHAr), 8.04 (br s, 1H, NH), 7.74 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.68 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.49 (d, 3JH-H = 8.8 Hz, 1H, CHAr), 7.32–7.18 (m, 5H, 5CHAr), 4.98 (br s, 2H, NH2), 3.15 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 2.96 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.88 (d, 2JH-H = 13.4 Hz, 1H, H-(CH2)), 2.86 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 2.28 (s, 3H, CH3), and 1.36 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.6 (C), 160.9 (C), 152.3 (C), 149.5 (C), 147.1 (CHAr), 139.3 (CHAr), 136.4 (C), 134.2 (C), 131.9 (C), 130.5 (2CHAr), 129.5 (2CHAr), 128.8 (C), 128.3 (2CHAr), 126.9 (CHAr), 125.8 (2CHAr), 114.2 (CHAr), 105.3 (C), 87.4 (C), 46.7 (CH2), 42.6 (CH2), 26.5 (CH3), and 17.9 (CH3). Analysis calculated for C26H26N4O3: HRMS: m/z [M + H]+ calculated 443.2078; found 443.2076.
The compound 17 was synthesized following the previously described procedure from 5 (74 mg, 0.19 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: dichloromethane/MeOH 97/3; Rf of 0.23). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with dichloromethane/MeOH in a gradient from 100/0 to 98/2 over 25 column volumes. A UV–VIS detector was configured additionally to 222 nm and 230 nm. Yield: 55%. The product was obtained as a light-gray oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.46 (br s, 1H, NH), 7.23 (d, 3JH-H = 8.6 Hz, 1H, CHAr), 8.17–8.14 (m, 1H, CHAr), 7.75–7.62 (m, 5H, 5CHAr), 7.31–7.19 (m, 5H, 5CHAr), 7.04–6.97 (m, 1H, CHAr), 5.65 (br s, 2H, NH2), 3.24 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 3.01 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2)), 2.98 (d, 2JH-H = 14.2 Hz, 1H, H-(CH2)), 2.96 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), and 1.45 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.8 (C), 161.9 (C), 157.3 (C), 151.2 (C), 141.7 (C), 139.3 (CHAr), 136.3 (C), 132.3 (C), 130.6 (2CHAr), 129.7 (2CHAr), 128.4 (2CHAr), 127.0 (CHAr), 126.8 (CHAr), 126.3 (2CHAr), 119.5 (CHAr), 115.1 (CHAr), 105.2 (C), 88.1 (C), 46.8 (CH2), 42.3 (CH2), and 26.8 (CH3). Analysis calculated for C25H24N4O3: HRMS: m/z [M + H]+ calculated 429.1921; found 429.1925.
The compound 18 was synthesized following the previously described procedure from 6 (67 mg, 0.17 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: dichloromethane/methanol 95/5; Rf of 0.19). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with dichloromethane/MeOH in a gradient 100/0 to 96/4 over 25 column volumes. A UV–VIS detector was configured to 254 nm, 375 nm, and 327 nm. Yield: 70%. The product was obtained as a white oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.34 (d, 3JH-H = 6.4 Hz, 2H, 2CHAr), 7.72 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.59 (d, 3JH-H = 8.5 Hz, 2H, 2CHAr), 7.34–7.22 (m, 5H, 5CHAr), 7.17 (d, 3JH-H = 6.4 Hz, 2H, 2CHAr), 7.08 (br s, 1H, NH), 4.95 (br s, 2H, NH2), 3.19 (d, 2JH-H = 14.8 Hz, 1H, H-(CH2), 3.06 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2), 3.01 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2), 2.93 (d, 2JH-H = 14.8 Hz, 1H, H-(CH2)), and 1.52 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.8 (C), 160.0 (C), 151.7 (C), 150.3 (2CHAr), 145.3 (C), 136.4 (C), 135.1 (C), 131.3 (C), 130.6 (2CHAr), 129.3 (2CHAr), 128.4 (2CHAr), 127.0 (CHAr), 126.2 (2CHAr), 113.3 (2CHAr), 106.5 (C), 88.1 (C), 46.9 (CH2), 42.3 (CH2), and 27.0 (CH3). Analysis calculated for C25H24N4O3: HRMS: m/z [M + H]+ calculated 429.1921; found 419.1920.
The compound 19 was synthesized following the previously described procedure from 7 (60 mg, 0.15 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: dichloromethane/MeOH 96/4; Rf of 0.47). The crude product was purified by column chromatography (eluent: dichloromethane/MeOH 98/2). Yield: 44%. The product was obtained as an oily yellow solid. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.69 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.67 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.32–7.22 (m, 9H, 9CHAr), 7.04 (t, 3JH-H = 6.9 Hz, 1H, CHAr), 6.83 (br s, 1H, NH), 4.96 (s, 2H, NH2), 3.19 (d, 2JH-H = 14.8 Hz, 1H, H-(CH2), 3.06 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2), 3.03 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2), 2.95 (d, 2JH-H = 14.8 Hz, 1H, H-(CH2), and 1.53 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.5 (C), 158.3 (C), 152.1 (C), 138.0 (C), 136.6 (C), 134.1 (C), 131.8 (C), 130.6 (2CHAr), 129.4 (2CHAr), 129.1 (2CHAr), 128.3 (2CHAr), 126.9 (CHAr), 126.0 (2CHAr), 124.0 (CHAr), 119.6 (2CHAr), 106.7 (C), 87.4 (C), 47.0 (CH2), 42.7 (CH2), and 27.0 (CH3). Analysis calculated for C26H25N3O3: HRMS: m/z [M + H]+ calculated 428.1969; found 428.1967.
The compound 20 was synthesized following the previously described procedure from 8 (70 mg, 0.17 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: dichloromethane/MeOH 96/4; Rf of 0.47). The crude product was purified by column chromatography (eluent: dichloromethane/MeOH 98/2). Yield: 49%. The product was obtained as an oily brown solid. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.70–7.59 (m, 4H, 4CHAr), 7.29–7.17 (m, 7H, 7CHAr), 6.91 (t, 3JH-H = 8.6 Hz, 2H, 2CHAr), 6.73 (br s, 1H, NH), 4.92 (br s, 2H, NH2), 3.15 (d, 2JH-H = 14.8 Hz, 1H, H-(CH2), 3.04 (d, 2JH-H = 13.9 Hz, 1H, H-(CH2), 2.99 (d, 2JH-H = 13.9 Hz, 1H, H-(CH2), 2.91 (d, 2JH-H = 14.8 Hz, 1H, H-(CH2), and 1.50 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.4 (C), 159.3 (d, JC-F = 243.0 Hz, C), 158.6 (C), 158.1 (C), 136.6 (C), 134.2 (C), 134.0 (d, JC-F = 2.8 Hz, C), 131.8 (C), 130.6 (2CHAr), 129.4 (2CHAr), 128.4 (2CHAr), 127.0 (CHAr), 126.0 (2CHAr), 121.4 (d, JC-F = 7.7 Hz, 2CHAr), 115.7 (d, JC-F = 22.5 Hz, 2CHAr), 106.4 (C), 87.5 (C), 47.0 (CH2), 42.7 (CH2), and 27.0 (CH3). 19F NMR (376.5 MHz, CDCl3): δ (ppm) −104.5. Analysis calculated for C26H24FN3O3: HRMS: m/z [M + H]+ calculated 446.1874; found 446.1874.
The compound 21 was synthesized following the previously described procedure from 9 (70 mg, 0.15 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt 70/30; Rf of 0.21). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with cyclohexane/AcOEt in a gradient from 100/0 to 70/30 over 25 column volumes. A UV–VIS detector configured additionally to 225 nm and 230 nm. Yield: 57%. The product was obtained as a brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.65–7.52 (m, 6H, 6CHAr), 7.33–7.16 (m, 7H, 7CHAr), 5.42 (t, 3JH-H = 5.8 Hz, 1H, NH), 4.87 (br s, 2H, NH2), 4.46–4.35 (m, 2H, CH2), 3.09 (d, 2JH-H = 14.7 Hz, 1H, H-(CH2)), 3.03 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.99 (d, 2JH-H = 13.8 Hz, 1H, H-(CH2)), 2.85 (d, 2JH-H = 14.7 Hz, 1H, H-(CH2)), and 1.49 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 165.4 (C), 158.5 (C), 152.0 (C), 142.6 (C), 136.6 (C), 134.1 (C), 131.9 (C), 130.5 (2CHAr), 129.3 (2CHAr), 129.7 (q, 2JC-F = 32.5 Hz, C), 128.3 (2CHAr), 128.1 (2CHAr), 126.9 (CHAr), 125.7 (2CHAr), 125.6 (q, 3JC-F = 3.7 Hz, 2CHAr), 124.5 (q, 1JC-F = 271.5 Hz, CF3), 105.4 (C), 82.7 (C), 46.9 (CH2), 42.9 (CH2), 42.7 (CH2), and 26.9 (CH3). 19F NMR (376.5 MHz, CDCl3): δ (ppm) -63.1. Analysis calculated for C28H26F3N3O3: HRMS: m/z [M + H]+ calculated 510.1999; found 510.1997.
The compound 22 was synthesized following the previously described procedure from 10 (62 mg, 0.16 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: dichloromethane/MeOH 95/5; Rf of 0.32). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with dichloromethane/MeOH in a gradient from 100/0 to 98/2 over 25 column volumes. A UV–VIS detector was configured to 241 nm, 212 nm, and 315 nm. Yield: 60%. The product was obtained as a light-yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.80 (d, 3JH-H = 5.2 Hz, 1H, CHAr), 8.53 (br s, 1H, NH), 8.40 (d, 3JH-H = 9.6 Hz, 1H, CHAr), 7.67 (d, 3JH-H = 8.8 Hz, 2H, 2CHAr), 7.67 (d, 3JH-H = 8.8 Hz, 2H, 2CHAr), 7.38 (dd, 3JH-H = 9.6 Hz, 3JH-H = 5.2 Hz, 1H, CHAr), 7.28–7.19 (m, 5H, 5CHAr), 5.17 (br s, 2H, NH2), 3.19 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 3.00 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2)), 2.96 (d, 2JH-H = 14.3 Hz, 1H, H-(CH2)), 2.90 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), and 1.43 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 164.0 (C), 162.2 (C), 155.5 (C), 152.4 (C), 148.1 (CHAr), 136.2 (C), 134.5 (C), 131.5 (C), 130.5 (2CHAr), 129.4 (2CHAr), 128.4 (3CHAr), 127.0 (CHAr), 126.1 (2CHAr), 119.2 (CHAr), 104.9 (C), 88.0 (C), 46.7 (CH2), 42.1 (CH2), and 27.6 (CH3). Analysis calculated for C24H23N5O3: HRMS: m/z [M + H]+ calculated 430.1874; found 430.1875.
The compound 23 was synthesized following the previously described procedure from 11 (55 mg, 0.33 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: AcOEt/cyclohexane/dichloromethane 4/3/3; Rf of 0.19). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with AcOEt/cyclohexane in a gradient from 10/90 to 60/40 over 25 column volumes. A UV–VIS detector was configured to 241 nm, 212 nm, and 315 nm. Yield: 83%. The product was obtained as a light-brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 8.65 (s, 1H, NH), 8.47 (d, 3JH-H = 9.2 Hz, 1H, CHAr), 7.66 (s, 4H, 4CHAr), 7.42 (d, 3JH-H = 9.2 Hz, 1H, CHAr), 7.30–7.20 (m, 5H, 5CHAr), 5.08 (br s, 2H, NH2), 3.19 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 3.00 (s, 2H, CH2), 2.90 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), and 1.46 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.8 (C), 162.6 (C), 154.6 (C), 151.9 (C), 148.2 (C), 136.2 (C), 134.0 (C), 132.0 (C), 130.7 (2CHAr), 130.0 (CHAr), 129.6 (2CHAr), 128.5 (2CHAr), 127.2 (2CHAr), 126.4 (CHAr), 121.5 (CHAr), 105.0 (C), 88.4 (C), 46.8 (CH2), 42.0 (CH2), and 26.9 (CH3). Analysis calculated for C24H22ClN5O3: HRMS: m/z [M + H]+ calculated 464.1484; found 464.1484.
The compound 24 was synthesized following the previously described procedure from 12 (77 mg, 0.19 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: cyclohexane/AcOEt/dichloromethane 30/40/30; Rf of 0.15). The crude product was purified by column chromatography (eluent: cyclohexane/ethyl acetate/dichloromethane 30/40/30). Yield: 59%. The product was obtained as an oily yellow solid. 1H NMR (400 MHz, CDCl3): δ (ppm) 9.50 (s, 1H, NH), 8.27 (d, 3JH-H = 2.7 Hz, 1H, CHAr), 8.14 (d, 3JH-H = 2.7 Hz, 1H, CHAr), 7.78 (s, 1H, CHAr), 7.70 (s, 4H, 4CHAr), 7.31–7.24 (m, 5H, 5CHAr), 5.14 (br s, 2H, NH2), 3.20 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), 3.02 (s, 2H, CH2), 2.91 (d, 2JH-H = 14.4 Hz, 1H, H-(CH2)), and 1.48 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 163.2 (C), 161.9 (C), 152.4 (C), 148.4 (C), 141.9 (CHAr), 139.7 (CHAr), 137.3 (CHAr), 136.2 (C), 134.2 (C), 131.7 (C), 130.5 (2CHAr), 129.6 (2CHAr), 128.4 (2CHAr), 127.1 (CHAr), 126.1 (2CHAr), 104.9 (C), 88.0 (C), 46.8 (CH2), 42.3 (CH2), and 26.8 (CH3). Analysis calculated for C24H23N5O3: HRMS: m/z [M + H]+ calculated 430.1874; found 430.1871.
The compound 25 was synthesized following the previously described procedure from 13 (88 mg, 0.21 mmol, and 1 equiv.). The reaction progress was monitored by TLC (eluent: dichloromethane/MeOH 30/40/30; Rf of 0.31). The crude product was purified by flash chromatography using a PuriFlash® IR-50SI-F0025 column and eluted with dichloromethane/MeOH in a gradient from 100/0 to 90/10 over 25 column volumes. A UV–VIS detector was configured at 238 nm and 320 nm. Yield: 60%. The product was obtained as a light-brown oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.90 (s, 1H, NH), 7.71 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.64 (d, 3JH-H = 8.4 Hz, 2H, 2CHAr), 7.30–7.18 (m, 5H, 5CHAr), 6.47 (s, 1H, CHAr), 5.00 (br s, 2H, NH2), 3.63 (s, 3H, CH3), 3.06 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2)), 2.93 (d, 2JH-H = 13.7 Hz, 1H, H-(CH2)), 2.86 (d, 2JH-H = 13.7 Hz, 1H, H-(CH2)), 2.78 (d, 2JH-H = 14.5 Hz, 1H, H-(CH2)), 2.21 (s, 3H, CH3), and 1.33 (s, 3H, CH3). OH not observed. 13C NMR (100 MHz, CDCl3): δ (ppm) 162.5 (C), 159.7 (C), 152.2 (C), 145.8 (C), 139.5 (C), 136.5 (C), 134.2 (C), 131.7 (C), 130.5 (2CHAr), 129.3 (2CHAr), 128.2 (2CHAr), 126.8 (CHAr), 125.7 (2CHAr), 105.0 (C), 97.5 (CHAr), 87.0 (C), 46.6 (CH2), 42.7 (CH2), 35.5 (CH3), 26.5 (CH3), and 11.4 (CH3). Analysis calculated for C25H27N5O3: HRMS: m/z [M + H]+ calculated 446.2187; found 446.2187.