New Asymmetric Gemini Triazole Surfactants with a Polar Triethylene Glycol Fragment: Synthesis and Physico-Chemical Properties
Abstract
1. Introduction
2. Results and Discussion
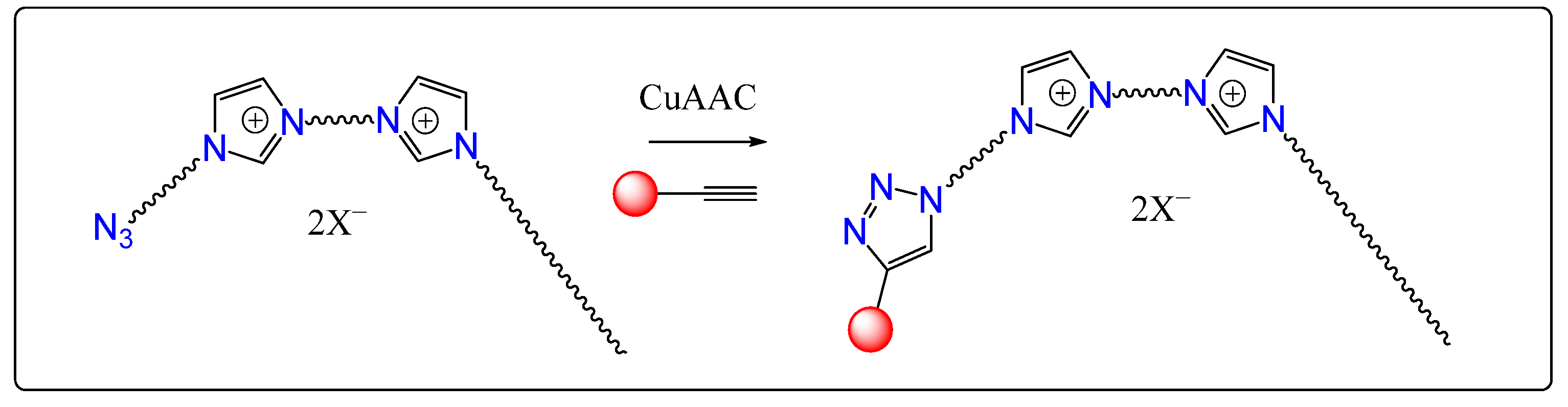
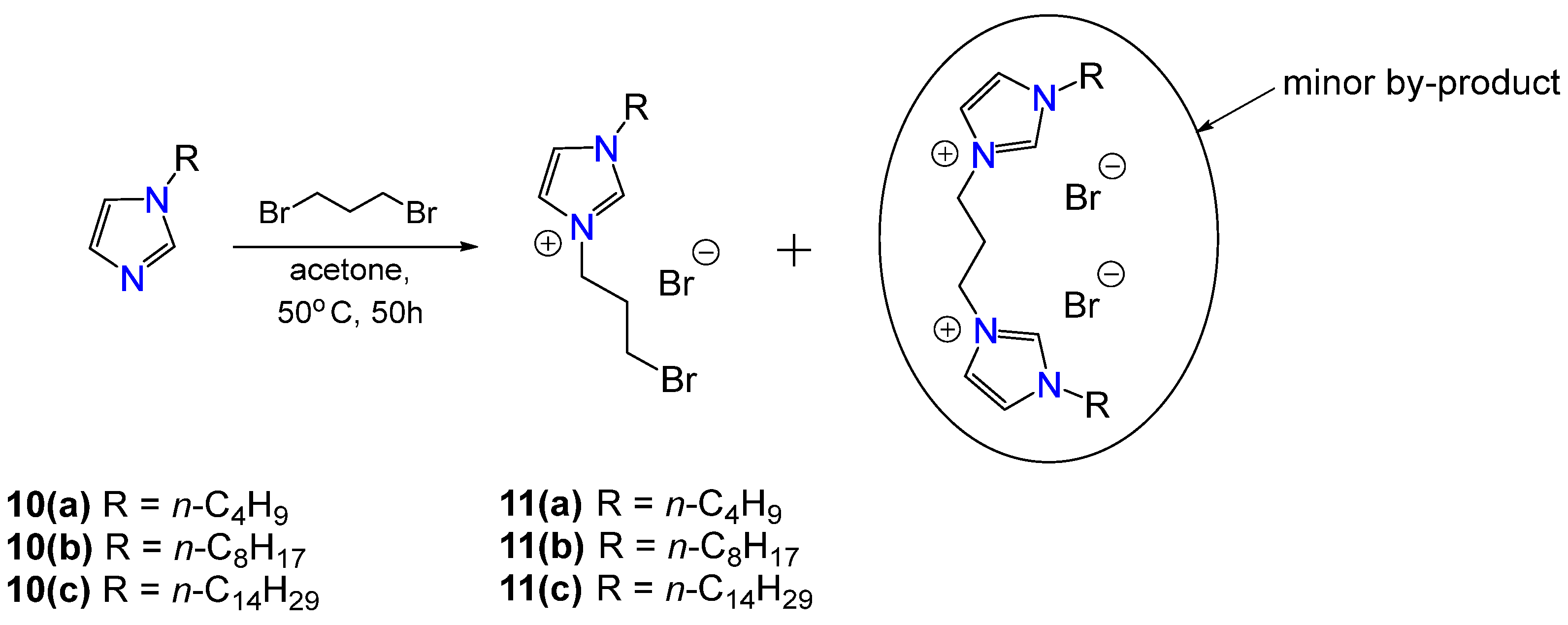
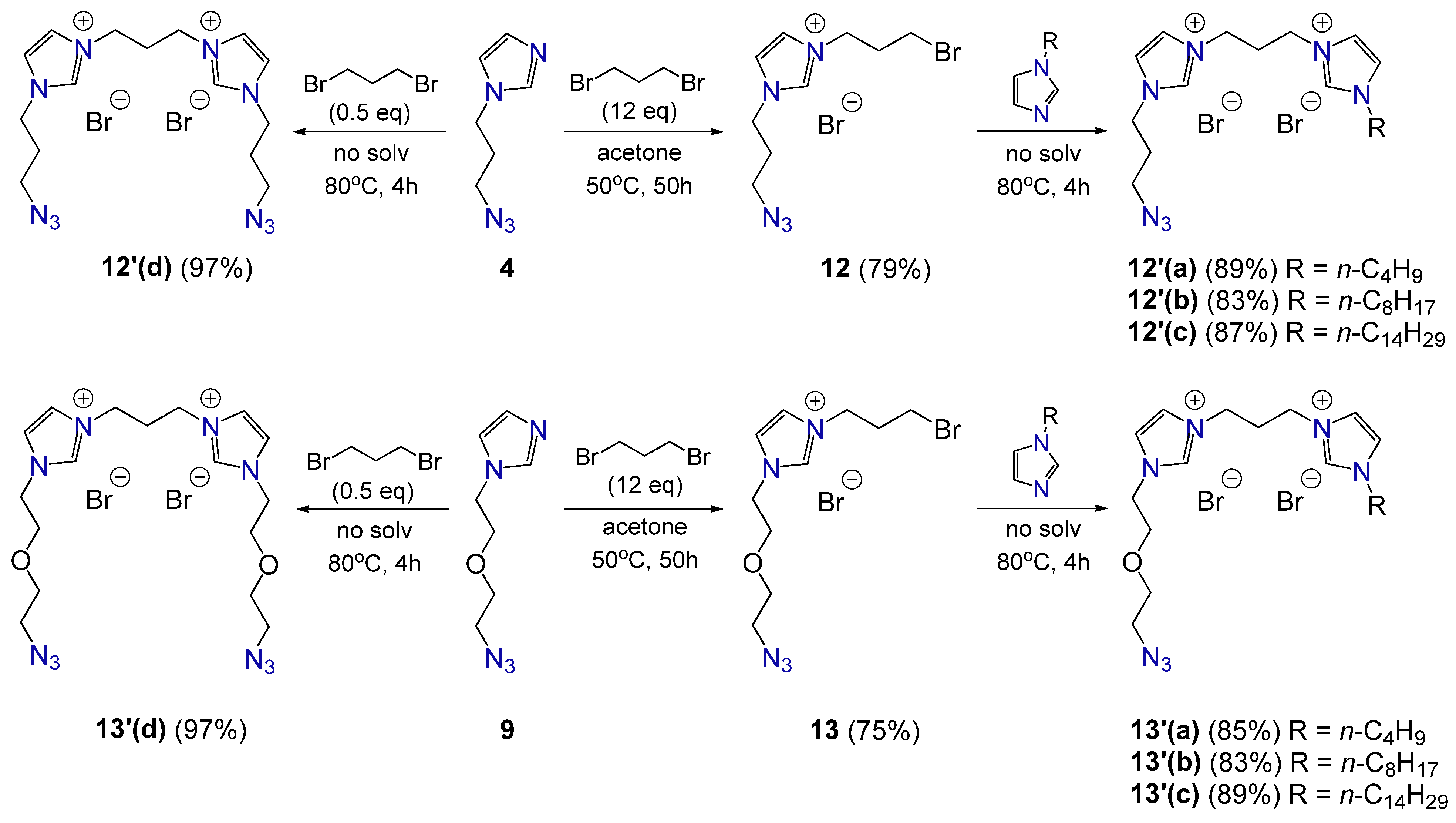
2.1. Synthesis of Gemini Surfactants
2.2. CAC Studies of Gemini Surfactant
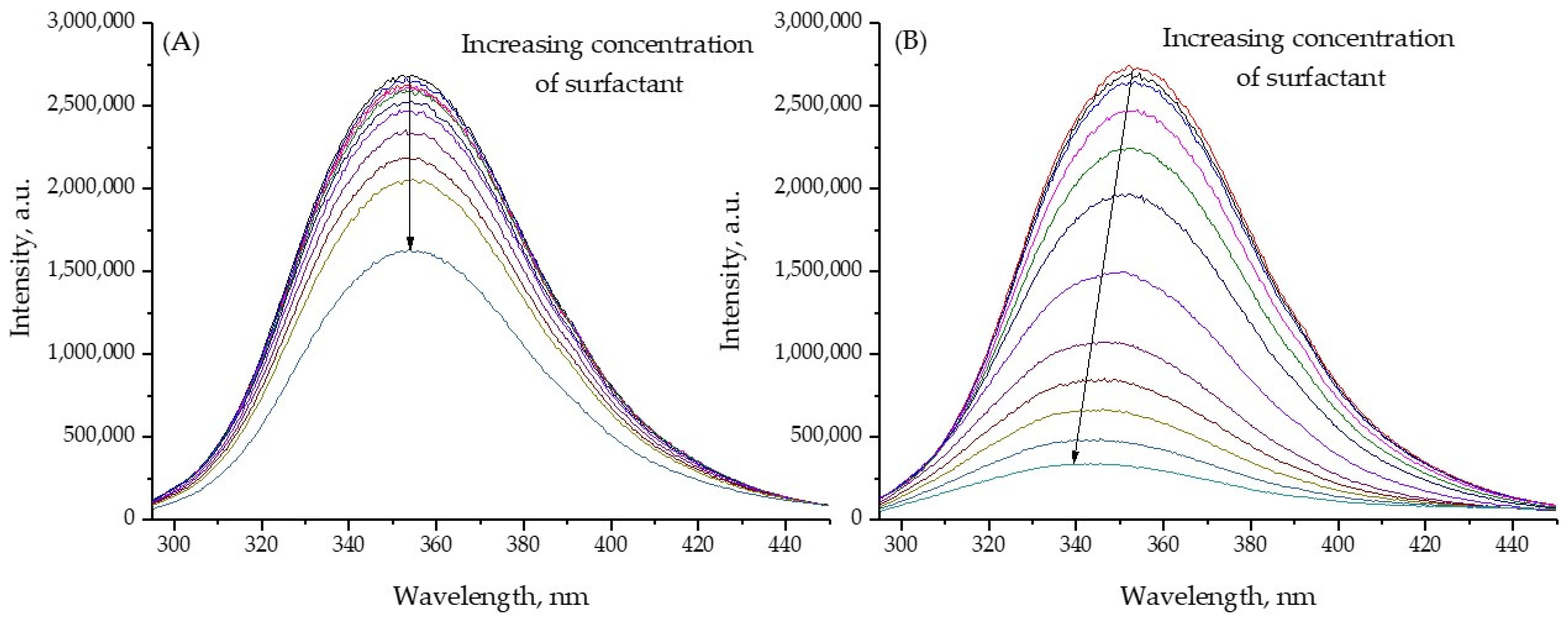
2.3. Fluorescence Spectra of BSA with Gemini Surfactant
2.4. Hydrodynamic Diameter and Zeta-Potential of BSA in Presence of Gemini Surfactant
3. Materials and Methods
3.1. Experimental Sections
3.2. Sample Preparation
3.3. General Procedure for Synthesis of Gemini Surfactants
3.4. Fluorescence Spectroscopy
3.5. Dynamic and Electrophoretic Light Scattering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, T.; Yuan, J.; Zhang, Z.; Xin, X.; Xu, G. The comparison of imidazolium Gemini surfactant [C14-4-C14im]Br2 and its corresponding monomer as corrosion inhibitors for A3 carbon steel in hydrochloric acid solutions: Experimental and quantum chemical studies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 57–65. [Google Scholar] [CrossRef]
- Gyani Devi, Y.; Koya Pulikkal, A.; Gurung, J. Research Progress on the Synthesis of Different Types of Gemini Surfactants with a Functionalized Hydrophobic Moiety and Spacer. ChemistrySelect 2022, 7, e202203485. [Google Scholar] [CrossRef]
- Sunitha, S.; Reddy, P.S.; Prasad, R.B.N.; Kanjilal, S. Synthesis and evaluation of new imidazolium-based aromatic ether functionalized cationic mono and gemini surfactants. Eur. J. Lipid Sci. Technol. 2011, 113, 756–762. [Google Scholar] [CrossRef]
- Chai, J.; Song, J.; Wang, D.; Chai, H.; Bai, T.; Liu, N. Comparison of the Composition and Structural Parameters of W/O Microemulsions Containing Gemini Imidazoliums with Those Containing Monomeric Analogues. J. Surfactants Deterg. 2015, 18, 287–295. [Google Scholar] [CrossRef]
- Cao, H.; Hu, Y.; Xu, W.; Wang, Y.; Guo, X. Recent progress in the assembly behavior of imidazolium-based ionic liquid surfactants. J. Mol. Liq. 2020, 319, 114354. [Google Scholar] [CrossRef]
- Lu, G.; Mu, M.; Shu, Q.; Zhang, Y. Quaternary ammonium-based and imidazolium-based gemini surfactants: A comparison study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133023. [Google Scholar] [CrossRef]
- Zhou, T.; Ao, M.; Xu, G.; Liu, T.; Zhang, J. Interactions of bovine serum albumin with cationic imidazolium and quaternary ammonium gemini surfactants: Effects of surfactant architecture. J. Colloid. Interface Sci. 2013, 389, 175–181. [Google Scholar] [CrossRef]
- Hassanpour, M.; Torabi, S.M.; Afshar, D.; Kowsari, M.H.; Meratan, A.A.; Nikfarjam, N. Tracing the Antibacterial Performance of Bis-Imidazolium-based Ionic Liquid Derivatives. ACS Appl. Bio Mater. 2024, 7, 1558–1568. [Google Scholar] [CrossRef]
- Pinazo, A.; Pons, R.; Bustelo, M.; Manresa, M.Á.; Morán, C.; Raluy, M.; Pérez, L. Gemini histidine based surfactants: Characterization; surface properties and biological activity. J. Mol. Liq. 2019, 289, 111156. [Google Scholar] [CrossRef]
- Haque, R.A.; Nasri, S.F.; Iqbal, M.A.; Al-Rawi, S.S.; Jafari, S.F.; Ahamed, M.B.K.; Abdul Majid, A.M.S. Synthesis, Characterization, and Crystal Structures of Bis-Imidazolium Salts and Respective Dinuclear Ag(I) N-Heterocyclic Carbene Complexes: In Vitro Anticancer Studies against “Human Colon Cancer” and “Breast Cancer”. J. Chem. 2013, 2013, 804683. [Google Scholar] [CrossRef]
- Adak, S.; Datta, S.; Bhattacharya, S.; Banerjee, R. Role of spacer length in interaction between novel gemini imidazolium surfactants and Rhizopus oryzae lipase. Int. J. Biol. Macromol. 2015, 81, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Kang, Q.; Wang, T.; Xiao, J.; Yu, L. Alignment of nematic liquid crystals decorated with gemini surfactants and interaction of proteins with gemini surfactants at fluid interfaces. J. Colloid. Interface Sci. 2018, 518, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Gospodarczyk, W.; Szutkowski, K.; Kozak, M. Interaction of Bovine Serum Albumin (BSA) with Novel Gemini Surfactants Studied by Synchrotron Radiation Scattering (SR-SAXS), Circular Dichroism (CD), and Nuclear Magnetic Resonance (NMR). J. Phys. Chem. B 2014, 118, 8652–8661. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, X. Asymmetric Gemini surfactants as corrosion inhibitors for carbon steel in acidic medium: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130850. [Google Scholar] [CrossRef]
- Cao, G.; Gao, M.; Shen, T.; Zhao, B.; Zeng, H. Comparison between Asymmetric and Symmetric Gemini Surfactant-Modified Novel Organo-vermiculites for Removal of Phenols. Ind. Eng. Chem. Res. 2019, 58, 12927–12938. [Google Scholar] [CrossRef]
- Muslim, A.A.; Ayyash, D.; Gujral, S.S.; Mekhail, G.M.; Rao, P.P.N.; Wettig, S.D. Synthesis and characterization of asymmetrical gemini surfactants. Phys. Chem. Chem. Phys. 2017, 19, 1953–1962. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, X.; Jia, L.; Zhao, Z.; Yang, R.; Zhang, Y.; Sun, H. Novel asymmetrical bis-surfactants with naphthalene and two amide groups: Synthesis, foamability and foam stability. J. Mol. Liq. 2021, 329, 115534. [Google Scholar] [CrossRef]
- Kumar, V.; Lal, K.; Naveen; Tittal, R.K. The fate of heterogeneous catalysis & click chemistry for 1,2,3-triazoles: Nobel prize in chemistry 2022. Catal. Commun. 2023, 176, 106629. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Tiwari, V.K. Growing Impact of Intramolecular Click Chemistry in Organic Synthesis. Chem. Rec. 2023, 23, e202300167. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Rivera-Monroy, Z.J.; Maldonado, M. Copper(I)-Catalyzed Alkyne–Azide Cycloaddition (CuAAC) “Click” Reaction: A Powerful Tool for Functionalizing Polyhydroxylated Platforms. ACS Omega 2023, 8, 3650–3666. [Google Scholar] [CrossRef]
- Meng, G.; Guo, T.; Ma, T.; Zhang, J.; Shen, Y.; Sharpless, K.B.; Dong, J. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 2019, 574, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.W.; Heidelberg, T.; Duali Hussen, R.S.; Salman, A.A. Imidazolium-Linked Azido-Functionalized Guerbet Glycosides: Multifunctional Surfactants for Biofunctionalization of Vesicles. ACS Omega 2019, 4, 17039–17047. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Haque, R.A.; Iqbal, M.A.; Ahmad, A.; Hassan, L.E.A.; Taleb-Agha, M.; Ahamed, M.B.K.; Majid, A.M.S.A.; Razali, M.R. Tetra N-heterocyclic carbene dinuclear silver(I) complexes as potential anticancer agents: Synthesis and in vitro anticancer studies. J. Organomet. Chem. 2017, 853, 122–135. [Google Scholar] [CrossRef]
- Zhao, X.; An, D.; Ye, Z. Adsorption and thermodynamic properties of dissymmetric gemini imidazolium surfactants with different spacer length. J. Dis. Sci. Technol. 2017, 38, 296–302. [Google Scholar] [CrossRef]
- Gogolieva, G.; Durand, J.; Dechy-Cabaret, O.; Gras, E. Uncommon approach toward NHC–triazole complexes of palladium: Synthesis and applications. J. Organomet. Chem. 2014, 769, 7–10. [Google Scholar] [CrossRef]
- Messali, M. An efficient and green sonochemical synthesis of some new eco-friendly functionalized ionic liquids. Arab. J. Chem. 2014, 7, 63–70. [Google Scholar] [CrossRef]
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of ionic liquids towards protein stability: A comprehensive overview on the current understanding and their implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef]
- Kumar, P.K.; Jha, I.; Venkatesu, P.; Bahadur, I.; Ebenso, E.E. A comparative study of the stability of stem bromelain based on the variation of anions of imidazolium-based ionic liquids. J. Mol. Liq. 2017, 246, 178–186. [Google Scholar] [CrossRef]
- Kumar, A.; Venkatesu, P. Overview of the stability of α-chymotrypsin in different solvent media. Chem. Rev. 2012, 112, 4283–4307. [Google Scholar] [CrossRef]
- Alves, M.M.S.; Araújo, J.M.M.; Martins, I.C.; Pereiro, A.B.; Archer, M. Insights into the interaction of Bovine Serum Albumin with Surface-Active Ionic Liquids in aqueous solution. J. Mol. Liq. 2021, 322, 114537. [Google Scholar] [CrossRef]
- Geng, F.; Zheng, L.; Liu, J.; Yu, L.; Tung, C. Interactions between a surface active imidazolium ionic liquid and BSA. Colloid Polym. Sci. 2009, 287, 1253–1259. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Sun, L.; Yu, L.; Jiao, J.; Wang, R. Interaction of Bovine Serum Albumin with Ester-Functionalized Anionic Surface-Active Ionic Liquids in Aqueous Solution: A Detailed Physicochemical and Conformational Study. J. Phys. Chem. B 2012, 116, 12479–12488. [Google Scholar] [CrossRef] [PubMed]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Clujul Med. 2014, 87, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Das, N.K.; Pawar, L.; Kumar, N.; Mukherjee, S. Quenching interaction of BSA with DTAB is dynamic in nature: A spectroscopic insight. Chem. Phys. Lett. 2015, 635, 50–55. [Google Scholar] [CrossRef]
- Ghosh, S.; Dey, J. Binding of Fatty Acid Amide Amphiphiles to Bovine Serum Albumin: Role of Amide Hydrogen Bonding. J. Phys. Chem. B 2015, 119, 7804–7815. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Rostami, E. Spectroscopic Investigation of the Interaction of BSA with Cationic Surfactants. Chem. Eng. Technol. 2008, 31, 1265–1271. [Google Scholar] [CrossRef]
- Mandeville, J.-S.; Froehlich, E.; Tajmir-Riahi, H.A. Study of curcumin and genistein interactions with human serum albumin. J. Pharm. Biomed. Anal. 2009, 49, 468–474. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into Protein-Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, M.; Zhao, Y.; Wu, Y.; Liu, J.; Cai, C.; Shi, Y.; Han, J. Spectroscopic and cytotoxicity studies on the combined interaction of (−)-epigallocatechin-3-gallate and anthracycline drugs with human serum albumin. Spectrochim. Acta Part A 2019, 222, 117213. [Google Scholar] [CrossRef]
- Cheng, L.-Y.; Yang, C.-Z.; Li, H.-Z.; Li, M.; Bai, A.-M.; Ouyang, Y.; Hu, Y.-J. Probing the interaction of cephalosporin with bovine serum albumin: A structural and comparative perspective. Luminescence 2018, 33, 209–218. [Google Scholar] [CrossRef]
- Peters, T., Jr. Serum albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [CrossRef] [PubMed]
- Khalili, L.; Dehghan, G.; Akbar Moosavi-Movahedi, A.; Yoon, Y.; Khataee, A. In vitro and in silico insights into the molecular interaction mechanism of acetylshikonin with bovine serum albumin. J. Mol. Liq. 2022, 365, 120191. [Google Scholar] [CrossRef]
- Khachatrian, A.A.; Mukhametzyanov, T.A.; Salikhov, R.Z.; Safin, M.V.; Yakhvarov, D.G.; Gafurov, Z.N.; Garifullin, B.F.; Rakipov, I.T.; Mironova, D.A.; Solomonov, B.N. A good and bad aggregation: Effect of imidazolium- and cholinium-based ionic liquids on the thermal stability of bovine serum albumin. J. Mol. Liq. 2023, 381, 121787. [Google Scholar] [CrossRef]
- Wolf, N.; Kersting, L.; Herok, C.; Mihm, C.; Seibel, J. High-Yielding Water-Soluble Asymmetric Cyanine Dyes for Labeling Applications. J. Org. Chem. 2020, 85, 9751–9760. [Google Scholar] [CrossRef]
- Lari, J.; Moradgholi, F.; Vahedi, H.; Massoudi, A. Synthesis of Novel di Benzo Spiro Bis-Crown-Ether. Lett. Org. Chem. 2015, 12, 668–673. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Bai, S.; Wang, Y.-Y.; Han, Y.-F. Process-tracing study on the post-assembly modification of poly-NHC-based metallosupramolecular cylinders with tunable aggregation-induced emission. ChemComm 2019, 55, 13689–13692. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, T.; Xiao, J.; Wang, T.; Yu, L. Aggregation behavior of pyrrolidinium-based surface active ionic liquids in H2O-EAN binary solvents. J. Mol. Liq. 2017, 225, 50–55. [Google Scholar] [CrossRef]
- Tosoni, M.; Laschat, S.; Baro, A. Synthesis of Novel Chiral Ionic Liquids and Their Phase Behavior in Mixtures with Smectic and Nematic Liquid Crystals. Helv. Chim. Acta 2004, 87, 2742–2749. [Google Scholar] [CrossRef]
- Chandra, P.; Jonas, A.M.; Fernandes, A.E. Sequence and Surface Confinement Direct Cooperativity in Catalytic Precision Oligomers. J. Am. Chem. Soc. 2018, 140, 5179–5184. [Google Scholar] [CrossRef]



| Surfactant | CAC 1, mM | Concentration, mM | d, nm | PDI | ζ, mV |
|---|---|---|---|---|---|
| 15b | 3.2 | 3.5 | 495 ± 25 | 0.47 | +29 ± 4 |
| 15c | 1.1 | 1.2 | 445 ± 10 | 0.34 | +35 ± 1 |
| 14b | 2.7 | 3.0 | 410 ± 32 | 0.53 | +16 ± 2 |
| 14c | 0.9 | 1.1 | 210 ± 20 | 0.45 | +36 ± 3 |
| BSA * | Temperature, K | ΔH, kJ mol−1 | ΔS, J mol−1 K−1 | n | ||
|---|---|---|---|---|---|---|
| 14a | 298.15 | 0.37 | 0.73 | 24 | 136 | 1.1 |
| 303.15 | 0.42 | 0.90 | ||||
| 310.15 | 0.48 | 1.07 | ||||
| 14b | 298.15 | 1.70 | 2.0 | 27 | 174 | 1.1 |
| 303.15 | 1.62 | 2.55 | ||||
| 310.15 | 1.59 | 3.18 | ||||
| 14c | 298.15 | 4.88 | 4.49 | 39 | 219 | 1.3 |
| 1.36 | 1.48 b | 0.9 | ||||
| 4.54 | 6.39 a | 1.2 | ||||
| 303.15 | 4.68 | 6.05 | ||||
| 310.15 | 4.45 | 8.25 | ||||
| 14d | 298.15 | 1.13 | 3.48 | 19 | 152 | 1.2 |
| 303.15 | 1.15 | 4.24 | ||||
| 310.15 | 1.13 | 4.74 | ||||
| 15a | 298.15 | 0.29 | 0.79 | 8 | 82 | 1.1 |
| 303.15 | 0.32 | 0.84 | ||||
| 310.15 | 0.35 | 0.89 | ||||
| 15b | 298.15 | 1.34 | 1.0 | 58 | 270 | 1.4 |
| 303.15 | 1.29 | 1.43 | ||||
| 310.15 | 1.22 | 2.54 | ||||
| 15c | 298.15 | 4.82 | 1.0 | −5 | 61 | 1.1 |
| 303.15 | 4.12 | 1. | ||||
| 310.15 | 3.54 | 1. | ||||
| 15d | 298.15 | 1.4 | 1.0 | 25 | 162 | 1.3 |
| 303.15 | 1.27 | 1.3 | ||||
| 310.15 | 1.03 | 1.5 |
| BSA * | d, nm | PDI | Zeta-Potential, mV |
|---|---|---|---|
| - | 10 ± 1 | 0.25 | −6.7 ± 0.4 |
| 14a | 9 ± 1 | 0.24 | −10 ± 0.8 |
| 14b | 11 ± 1 | 0.36 | −7.7 ± 0.2 |
| 14c | 16 ± 1 | 0.36 | +20.3 ± 0.9 |
| 14d | 9 ± 1 | 0.29 | −9.3 ± 0.2 |
| 15a | 8 ± 1 | 0.21 | −14.2 ± 0.7 |
| 15b | 11 ± 1 | 0.36 | −11.6 ± 0.3 |
| 15c | 151 ± 2 | 0.34 | +8.3 ± 1.3 |
| 15d | 10 ± 1 | 0.34 | −9.7 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, I.; Mironova, D.; Sultanova, E.; Burilov, V.; Solovieva, S.; Antipin, I. New Asymmetric Gemini Triazole Surfactants with a Polar Triethylene Glycol Fragment: Synthesis and Physico-Chemical Properties. Molecules 2024, 29, 5420. https://doi.org/10.3390/molecules29225420
Bogdanov I, Mironova D, Sultanova E, Burilov V, Solovieva S, Antipin I. New Asymmetric Gemini Triazole Surfactants with a Polar Triethylene Glycol Fragment: Synthesis and Physico-Chemical Properties. Molecules. 2024; 29(22):5420. https://doi.org/10.3390/molecules29225420
Chicago/Turabian StyleBogdanov, Ilshat, Diana Mironova, Elza Sultanova, Vladimir Burilov, Svetlana Solovieva, and Igor Antipin. 2024. "New Asymmetric Gemini Triazole Surfactants with a Polar Triethylene Glycol Fragment: Synthesis and Physico-Chemical Properties" Molecules 29, no. 22: 5420. https://doi.org/10.3390/molecules29225420
APA StyleBogdanov, I., Mironova, D., Sultanova, E., Burilov, V., Solovieva, S., & Antipin, I. (2024). New Asymmetric Gemini Triazole Surfactants with a Polar Triethylene Glycol Fragment: Synthesis and Physico-Chemical Properties. Molecules, 29(22), 5420. https://doi.org/10.3390/molecules29225420






