Preparation of Double-Networked Slow-Expanding Nanomicrospheres and Evaluation of Drive Modulation Performance
Abstract
1. Introduction
2. Results and Discussion
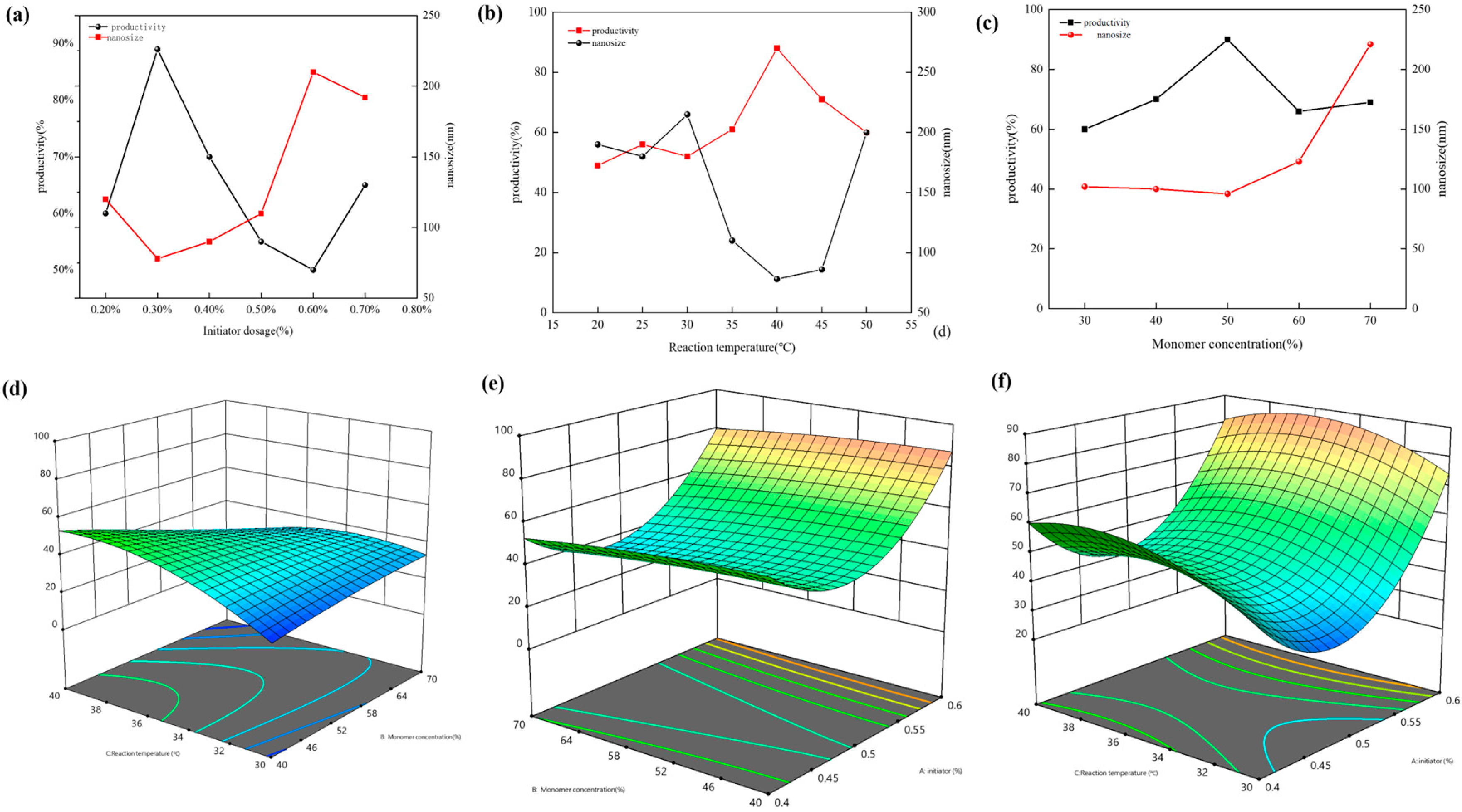
2.1. Effect of Synthesis Conditions on Gently Expanded Nanosized Double-Mesh Microspheres
2.2. Effect of Overall Crosslinking on the Structure of Two Nanospheres
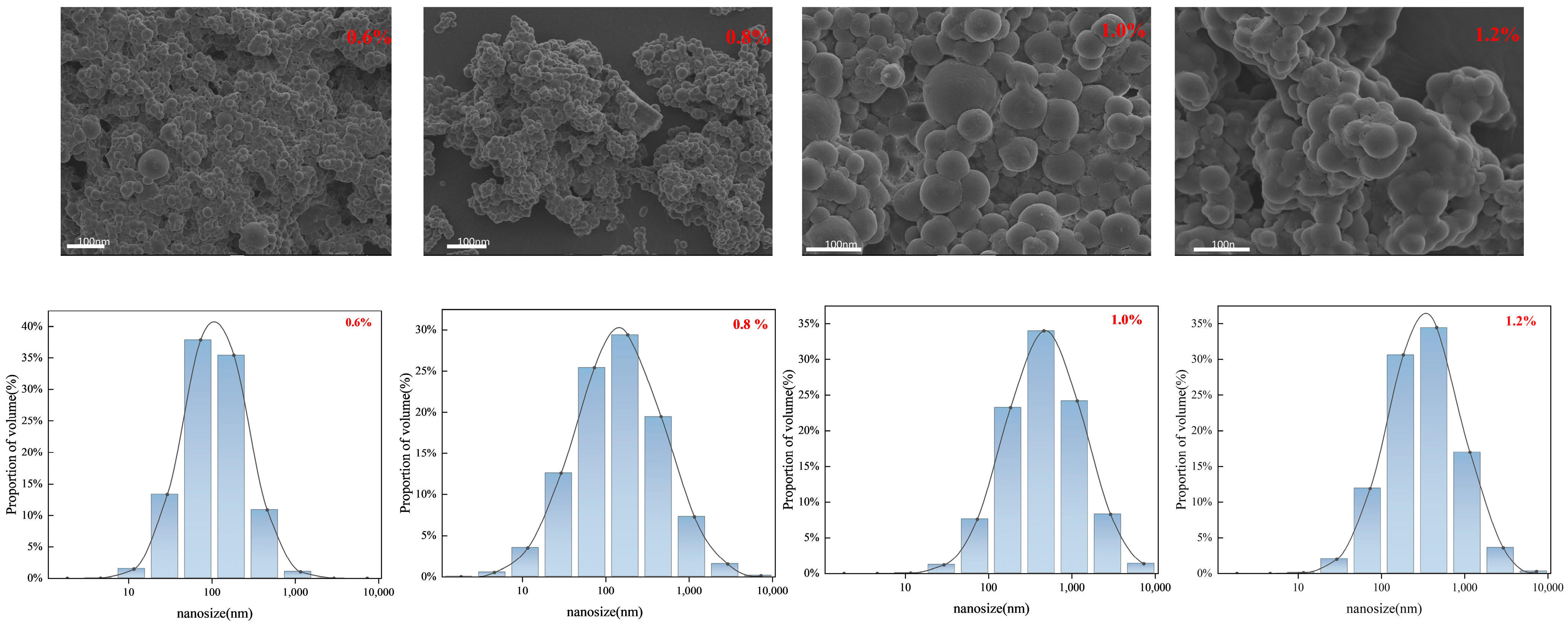
2.3. Characterization of Double-Mesh Nanospheres
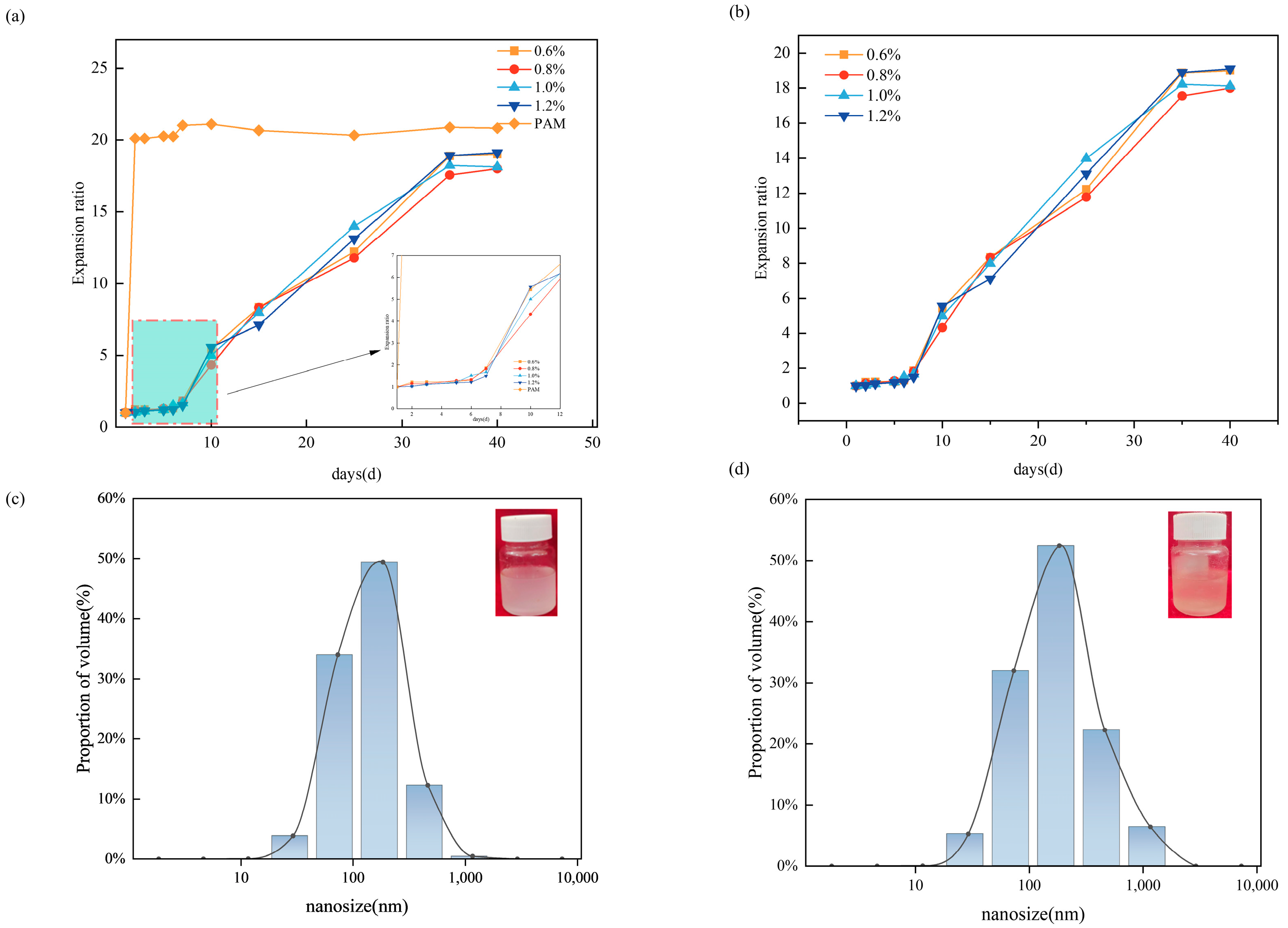
2.4. Slow-Swelling Properties of Double-Mesh Microspheres
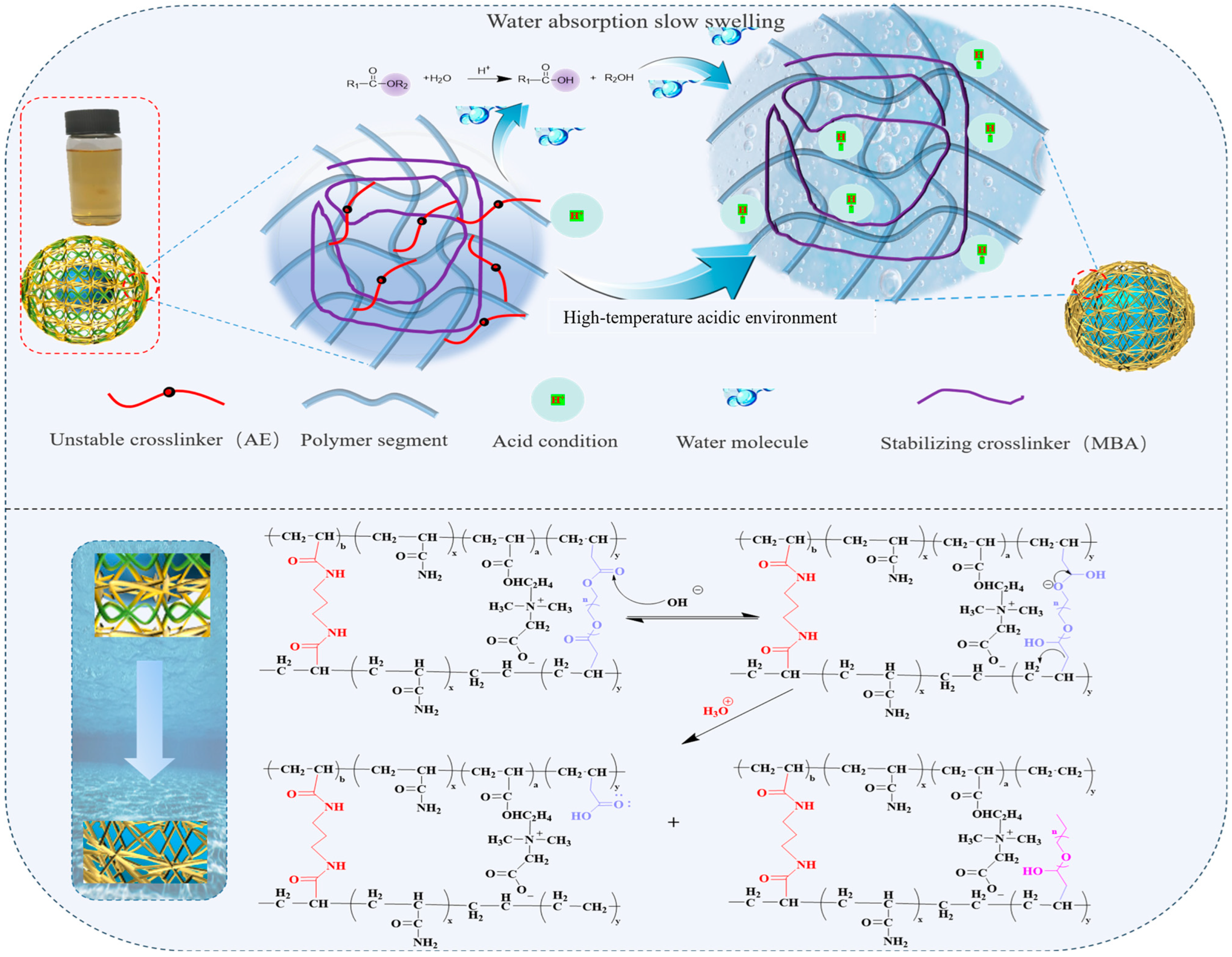
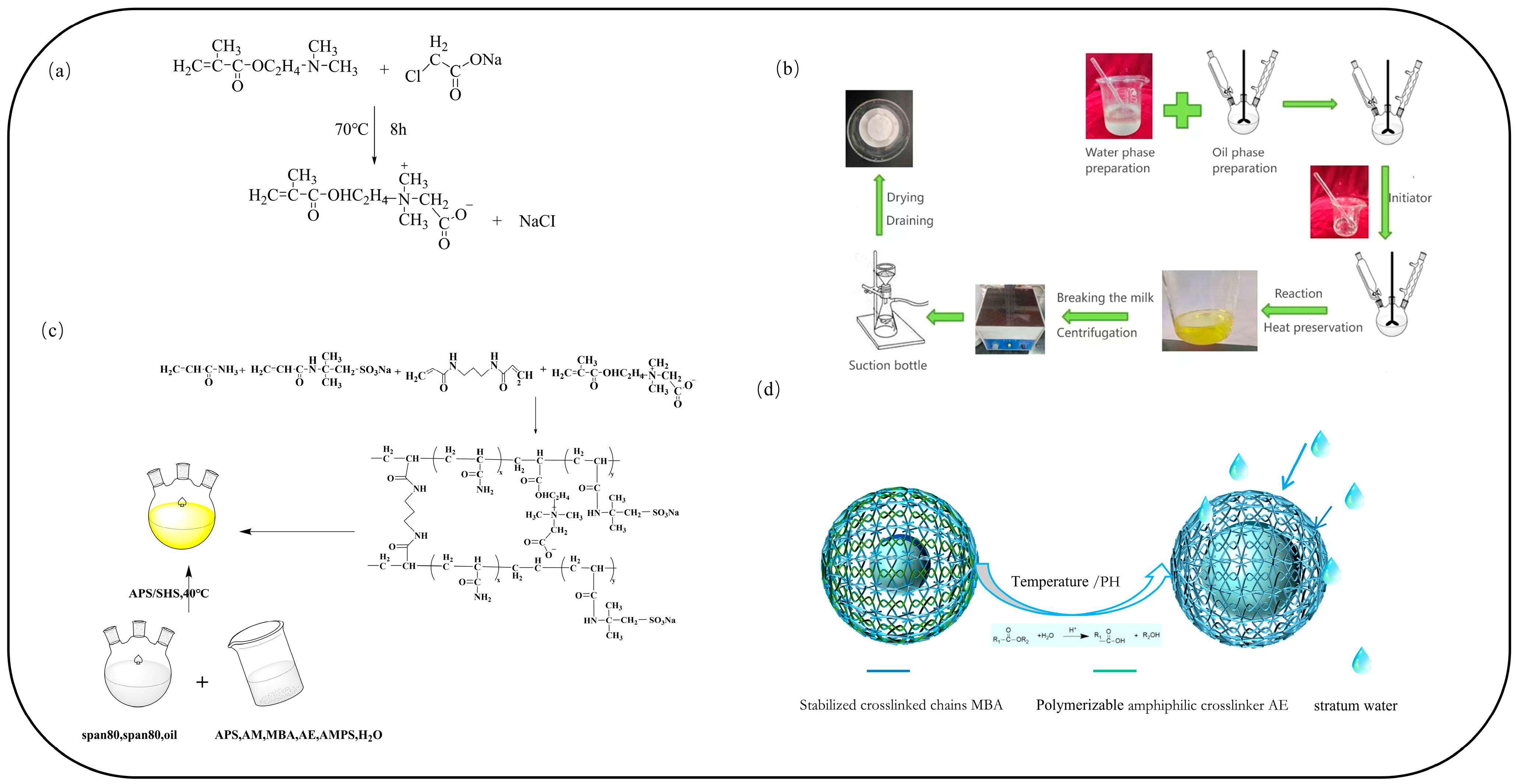
2.5. Analysis of Synthesis Mechanism and Slow-Swelling Mechanism of Microspheres
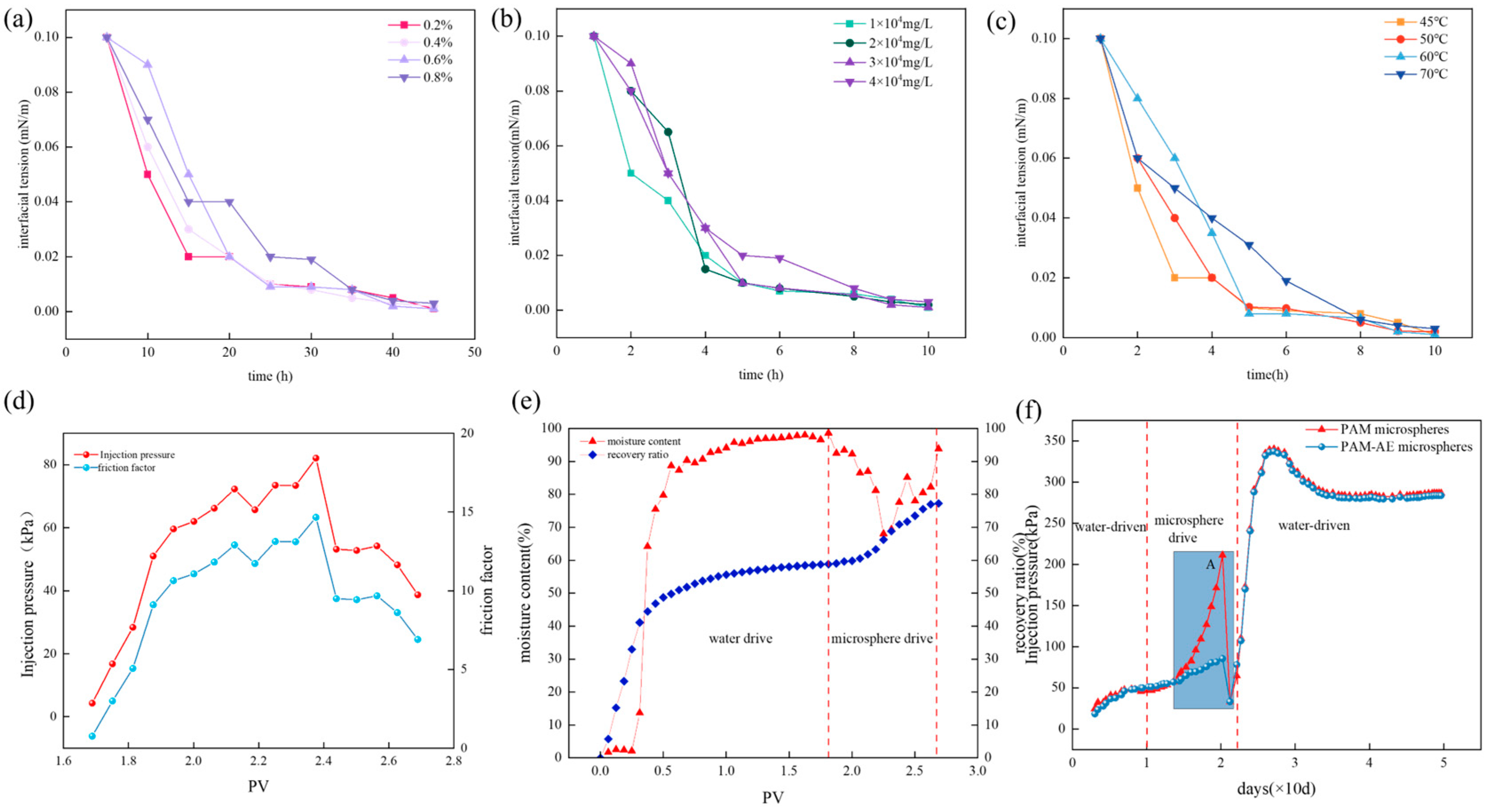
2.6. Analysis of Interfacial Tension and Drive Modulation Properties of Double-Mesh Microspheres
3. Experimental Section
3.1. Materials and Instruments
3.1.1. Materials
3.1.2. Experimental Equipment
3.2. Methods
3.2.1. Preparation of a Polymeric, Amphiphilic Crosslinker
3.2.2. Preparation of the Polymer Microsphere PAE
3.2.3. Preparation of the PAM for the Polymer Microspheres
3.3. Structural Characterization and Performance Tests
3.4. Water Absorption and Expansion Performance
3.5. Physical Transfer Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Song, H.; Zhou, F. Geometric characteristics of diverting fractures for multi-stage dynamic temporary plugging and diverting fracturing in fractured reservoir. Phys. Fluids 2024, 36, 037107. [Google Scholar] [CrossRef]
- Cao, W.; Xie, K.; Lu, X.; Liu, Y.; Zhang, Y. Effect of profile-control oil-displacement agent on increasing oil recovery and its mechanism. Fuel 2019, 237, 1151–1160. [Google Scholar] [CrossRef]
- Xu, L.T. Chemical water shutoff profile research status and development trends. IOP Conf. Ser. Earth Environ. Sci. 2017, 82, 012061. [Google Scholar] [CrossRef]
- Wu, G.; Wang, L.; Zhao, C.; Zhang, Z.; Yin, J.; Anwaier, M.; Ren, H.; Yang, D.; Yin, S.; Cai, Z.; et al. Experimental study of reservoir damage of water-based fracturing fluids prepared by different polymers. Pet. Sci. 2024, 21, 3298–3306. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Y.; Li, Y.; Liu, C.; Mou, S. Study on oil displacement efficiency of offshore sandstone reservoir with big bottom water. J. Pet. Explor. Prod. Technol. 2021, 11, 3289–3299. [Google Scholar] [CrossRef]
- Yusupova, L.F.; Khalikova, K.M.; Khusnutdinova, R.R. Technological feature of water shutoff operations. IOP Conf. Ser. Earth Environ. Sci. 2021, 868, 012086. [Google Scholar] [CrossRef]
- Clarke, A.; Howe, A.M.; Mitchell, J.; Staniland, J.; Hawkes, L.A. How viscoelastic-polymer flooding enhances displacement efficiency. SPE J. 2016, 21, 675–687. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Zhou, Y. Flow behavior and displacement mechanisms of nanoparticle stabilized foam flooding for enhanced heavy oil recovery. Energies 2017, 10, 560. [Google Scholar] [CrossRef]
- Lai, N.; Tian, Y.; Wang, J.; Xu, H.; Tang, L. Establishment of temperature-strength response prediction model of supramolecular gel temporary plugging agent by response surface method analysis. J. Appl. Polym. Sci. 2023, 141, e55004. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Y.; Yi, P.; Zhou, S.; Wang, Y.; Yang, D. Design, preparation and properties of new polyacrylamide based composite nano-microspheres with like “ball in ball” structure. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 130037. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Lin, M.Q.; Li, H.K.; Dong, Z.X.; Zhang, J.; Yang, Z.H. Plugging property and displacement characters of a novel high-temperature resistant polymer nanoparticle. Pet. Sci. 2021, 19, 387–396. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, Y.; Liu, T.; Mao, L.; Kang, R.; Wei, Q.; Zhou, Y. Synthesis of small-crystal y zeolites via regulating na2o/al2o3 and superior catalytic performance of corresponding niws-supported catalysts for hydrocracking of naphthalene. New J. Chem. 2023, 47, 20157–20170. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Negash, B.M.; Rahman, M.M.; Haroun, M.; Al-Shami, T.M. Perspective review of polymers as additives in water-based fracturing fluids. ACS Omega 2022, 7, 7431–7443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, H.; Pan, R.; Ren, Z.; Kang, W.; Zhang, J.; Pan, S.; Sarsenbekuly, B. Performance and enhanced oil recovery efficiency of an acid-resistant polymer microspheres of anti-co2 channeling in low-permeability reservoirs. Pet. Sci. 2024, 21, 2420–2432. [Google Scholar] [CrossRef]
- Liu, P.; Niu, L.; Tao, X.; Li, X.; Zhang, Z. Facile preparation of superhydrophobic quartz sands with micro-nano-molecule hierarchical structure for controlling the permeability of oil and water phase. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 1–9. [Google Scholar] [CrossRef]
- Davarpanah, A. Parametric study of polymer-nanoparticles-assisted injectivity performance for axisymmetric two-phase flow in eor processes. Nanomaterials 2020, 10, 1818. [Google Scholar] [CrossRef]
- Yang, L.; Ou, Z.; Jiang, G. Research progress of elastomer materials and application of elastomers in drilling fluid. Polymers 2023, 15, 918. [Google Scholar] [CrossRef]
- Jin, F.; Yang, L.; Li, X.; Song, S.; Du, D. Migration and plugging characteristics of polymer microsphere and eor potential in produced-water reinjection of offshore heavy oil reservoirs. Chem. Eng. Res. Des. 2021, 172, 291–301. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Pu, H.; Ding, Y.; Du, J.; Wang, Y.; Shan, Y. Effect of the sio2 nanoparticle size on application of enhanced oil recovery in medium-permeability formations. Energy Fuels 2023, 37, 5143–5153. [Google Scholar] [CrossRef]
- Wei, J.; Wang, X.; Li, Q.; Yang, J. Rivet-like iron oxide nanoparticles and their catalytic effect on extra heavy oil upgrading. Fuel 2021, 293, 120458. [Google Scholar] [CrossRef]
- Hanbin, L.; Hao, B.; Baoqiang, L.; Chengzheng, L.; Jin, L.; Guopeng, H.; Erdong, Y.; Fujian, Z. Parameter optimization and field test of nano variable-viscosity slick water fracturing in changqing shale reservoir. IOP Conf. Ser. Earth Environ. Sci. 2022, 984, 012004. [Google Scholar] [CrossRef]
- Jian, Z.; Dan, L.; Liqi, W.; Engao, T.; Xianjie, L.; Wensen, Z. Application of horizontal well discontinuous chemical flooding technology in bohai oilfield. Front. Energy Res. 2023, 10, 1036835. [Google Scholar] [CrossRef]
- Ye, Y.; Song, H.; Zhu, J.; Zheng, W.; Zhou, F.; Zhou, G.; Zhang, Q. Mechanism study and performance evaluation of nano-materials used to improve wellbore stability. Sustainability 2023, 15, 5530. [Google Scholar] [CrossRef]
- Song, Z.; Hu, J.; Liu, P.; Sun, Y. Synthesis and performance evaluation of alginate-coated temperature-sensitive polymer gel microspheres. Gels 2023, 9, 480. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, Q.; Li, M.; Li, A.; Zhan, D.; Li, Y.; Mo, Z.; Sun, S.; Zhang, Y. Green synthesis of natural nanocomposite with synergistically tunable sorption/desorption for solar-driven all-weather moisture harvesting. Nano Energy 2024, 124, 109471. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, Z.; Li, H.; Zhao, Y.; Pan, Y.; Liu, Y.; Yuan, W.; Hou, J. Evaluation of profile control and oil displacement effect of starch gel and nano-mos2 combination system in high-temperature heterogeneous reservoir. Gels 2024, 10, 127. [Google Scholar] [CrossRef]
- Shagymgereyeva, S.; Sarsenbekuly, B.; Kang, W.; Yang, H.; Turtabayev, S. Advances of polymer microspheres and its applications for enhanced oil recovery. Colloids Surf. B Biointerfaces 2024, 233, 113622. [Google Scholar] [CrossRef]
- Diwu, P.; Jiang, B.; Hou, J.; You, Z.; Wang, J.; Sun, L.; Ju, Y.; Zhang, Y.; Liu, T. Experimental study on expansion characteristics of core-shell and polymeric microspheres. J. Nanotechnol. 2018, 2018, 7602982. [Google Scholar] [CrossRef]
- Cheng, T.; Hou, J.; Yang, Y.; You, Z.; Liu, Y.; Zhao, F.; Li, J. Study on the plugging performance of bilayer-coating microspheres for in-depth conformance control: Experimental study and mathematical modeling. Ind. Eng. Chem. Res. 2019, 58, 6796–6810. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, T.; Liu, H.; You, Z.; Hou, J. Oil displacement performance using bilayer-coating microspheres. Ind. Eng. Chem. Res. 2021, 60, 2300–2313. [Google Scholar] [CrossRef]

| Ion Type(mg/L) | Na+ | Ca2+ | CI− | Total |
|---|---|---|---|---|
| Concentration | 18.48 × 103 | 1.08 × 103 | 30.45 × 103 | 5 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Q.; Fan, Z.; Liu, Q.; Fu, Y.; Cui, L.; Yang, J. Preparation of Double-Networked Slow-Expanding Nanomicrospheres and Evaluation of Drive Modulation Performance. Molecules 2024, 29, 5378. https://doi.org/10.3390/molecules29225378
Zuo Q, Fan Z, Liu Q, Fu Y, Cui L, Yang J. Preparation of Double-Networked Slow-Expanding Nanomicrospheres and Evaluation of Drive Modulation Performance. Molecules. 2024; 29(22):5378. https://doi.org/10.3390/molecules29225378
Chicago/Turabian StyleZuo, Qiaolin, Zhenzhong Fan, Qingwang Liu, Yuanfeng Fu, Luoqi Cui, and Junfeng Yang. 2024. "Preparation of Double-Networked Slow-Expanding Nanomicrospheres and Evaluation of Drive Modulation Performance" Molecules 29, no. 22: 5378. https://doi.org/10.3390/molecules29225378
APA StyleZuo, Q., Fan, Z., Liu, Q., Fu, Y., Cui, L., & Yang, J. (2024). Preparation of Double-Networked Slow-Expanding Nanomicrospheres and Evaluation of Drive Modulation Performance. Molecules, 29(22), 5378. https://doi.org/10.3390/molecules29225378





