Bis(tricyclic) Aromatic Enes That Exhibit Efficient Fluorescence in the Solid State
Abstract
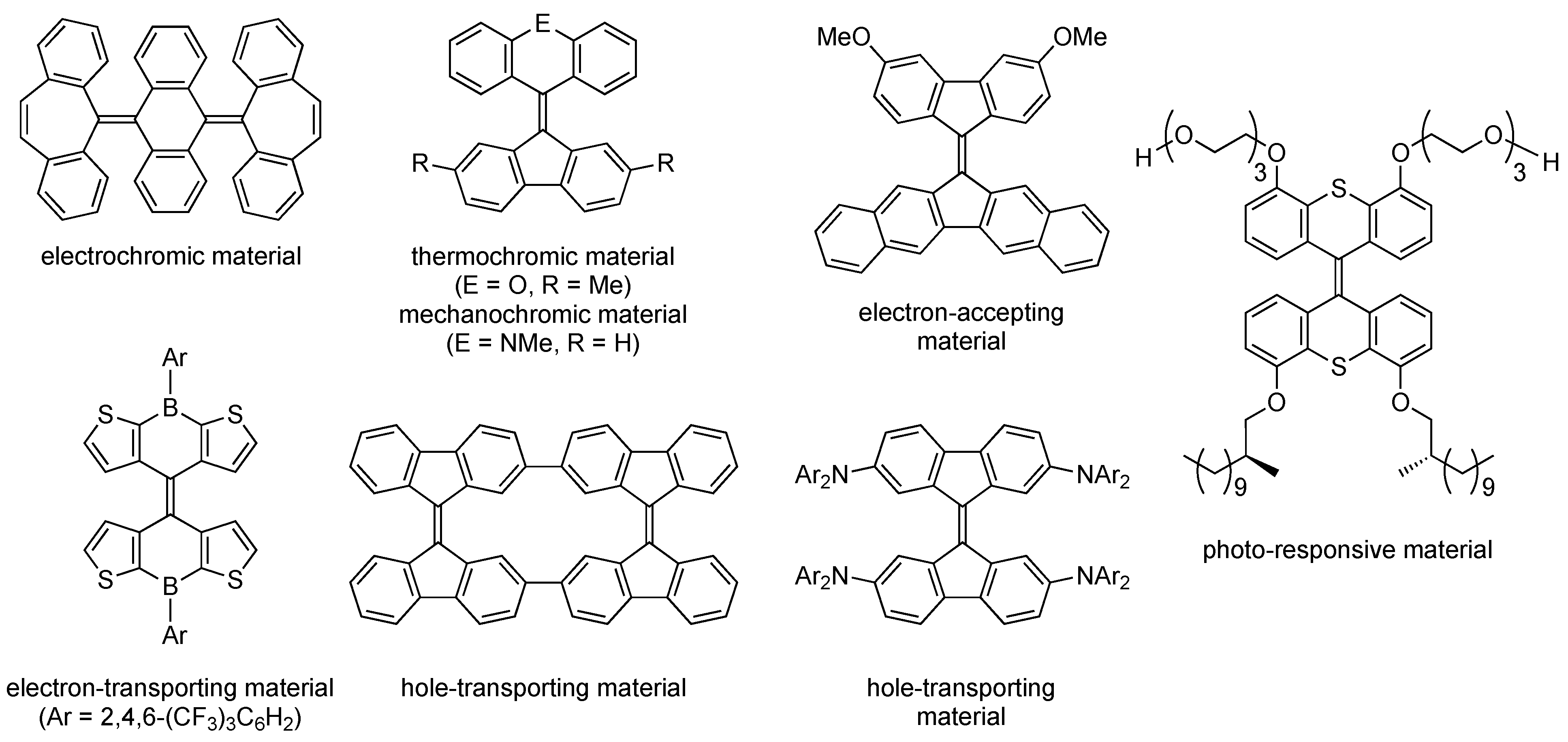
1. Introduction
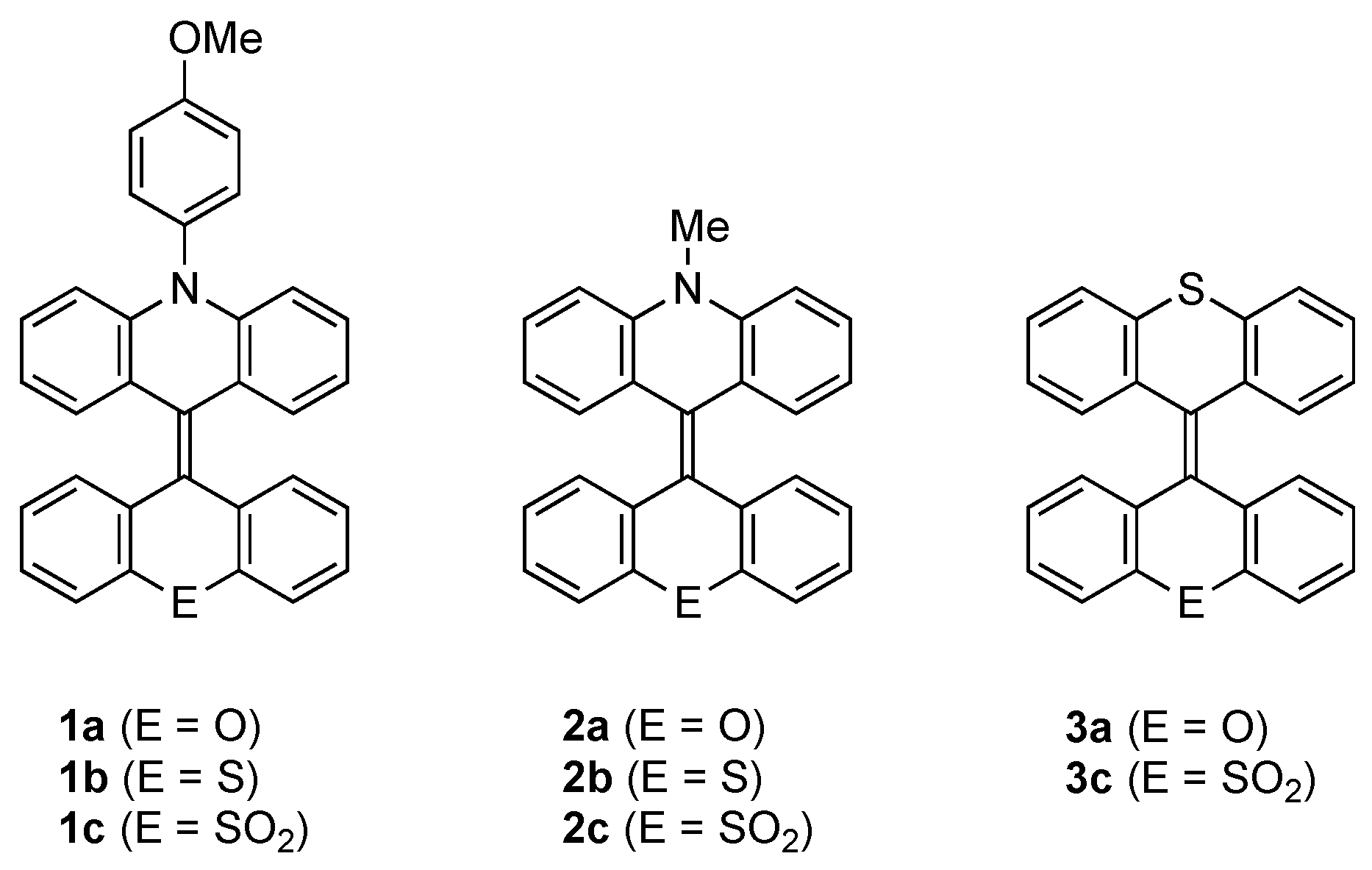
2. Results and Discussion
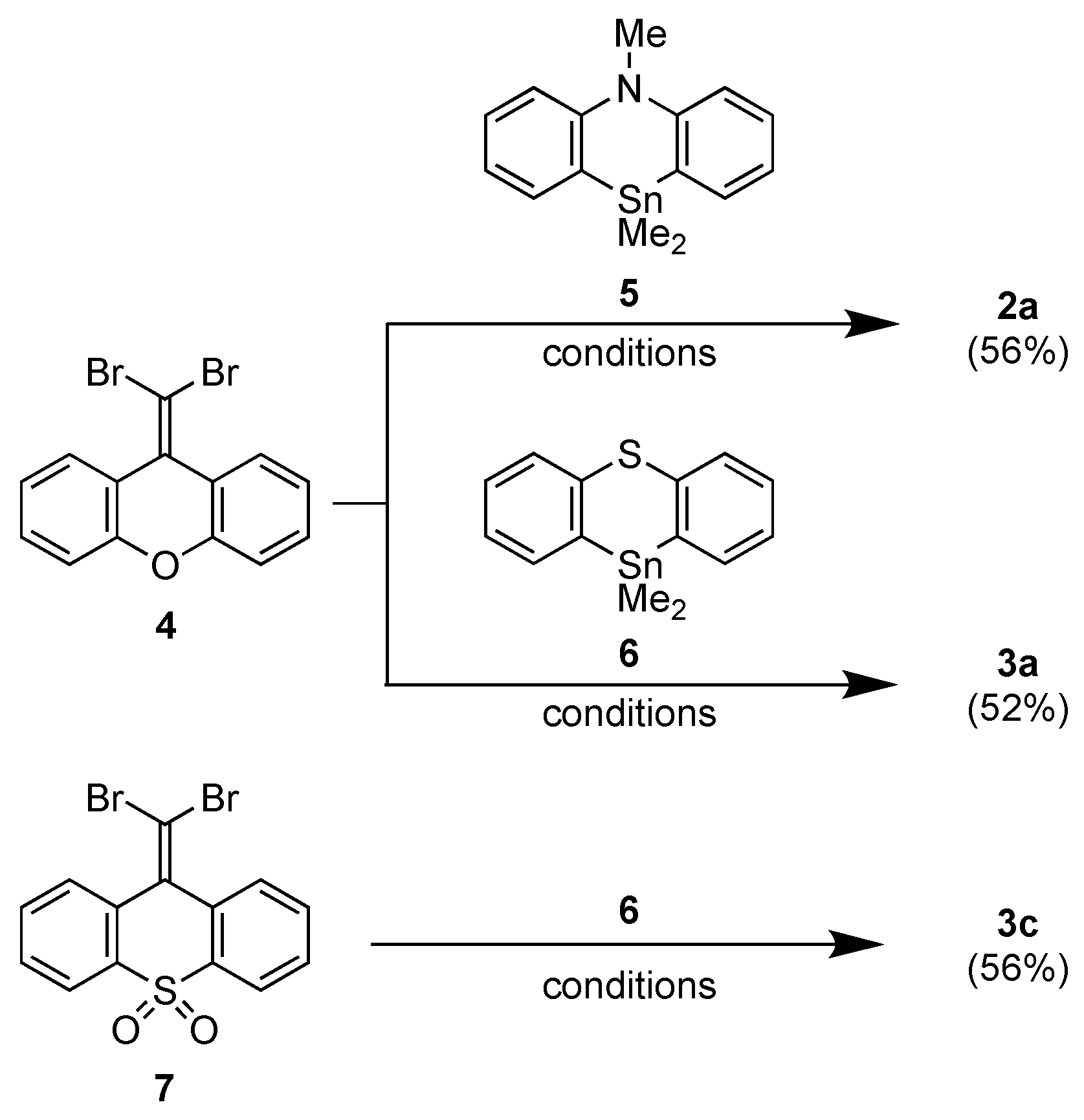
2.1. Synthesis
2.2. Single-Crystal Structures
2.3. Absorption Properties in Toluene
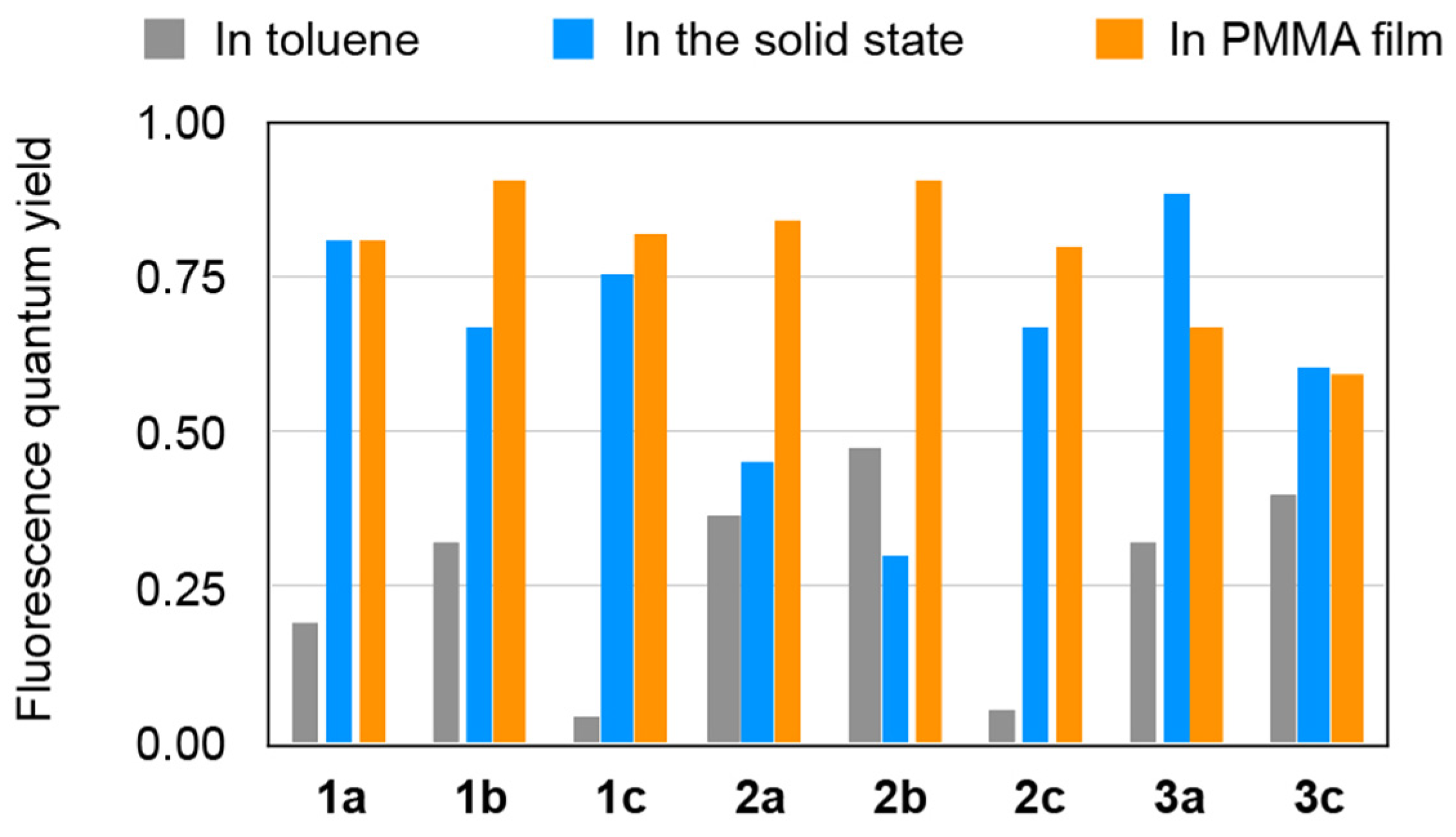
2.4. Fluorescence Properties in Toluene
2.5. Fluorescence Properties in the Solid State
2.6. Fluorescence Properties in PMMA Film
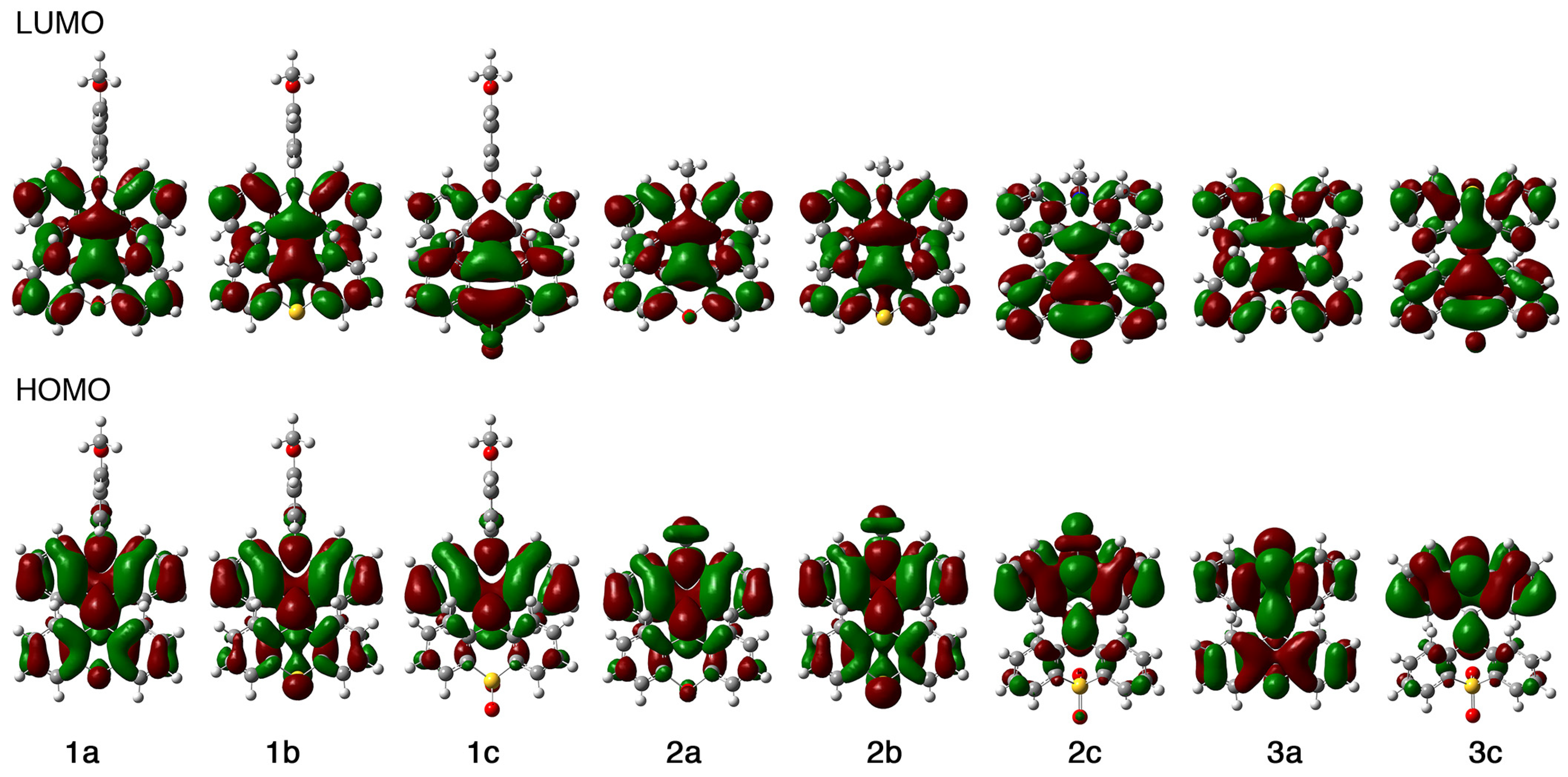
2.7. Theoretical Calculations
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Synthesis of 2a
3.2.2. Synthesis of 3a
3.2.3. Synthesis of 3c
3.3. X-Ray Crystallography of Single Crystals
3.4. Preparation of Doped PMMA Films
3.5. Measurement of Absorption and Photoluminescence Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browne, W.R.; Pollard, M.M.; de Lange, B.; Meetsma, A.; Feringa, B.L. Reversible Three-State Switching of Luminescence: A New Twist to Electro- and Photochromic Behavior. J. Am. Chem. Soc. 2006, 128, 12412–12413. [Google Scholar] [CrossRef] [PubMed]
- Wonink, M.B.S.; Corbet, B.P.; Kulago, A.A.; Boursalian, G.B.; de Bruin, B.; Otten, E.; Browne, W.R.; Feringa, B.L. Three-State Switching of an Anthracene Extended Bis-thiaxanthylidene with a Highly Stable Diradical State. J. Am. Chem. Soc. 2021, 143, 18020–18028. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Okada, H.; Nakagawa, T.; Komatsu, K.; Fujimoto, C.; Kagi, H.; Matsuo, Y. A fluorenylidene-acridane that becomes dark in color upon grinding–ground state mechanochromism by conformational change. Chem. Sci. 2018, 9, 475–482. [Google Scholar] [CrossRef]
- Matsuo, Y.; Wang, Y.; Ueno, H.; Nakagawa, T.; Okada, H. Mechanochromism, Twisted/Folded Structure Determination, and Derivatization of (N-Phenylfluorenylidene)acridane. Angew. Chem. Int. Ed. 2019, 58, 8762–8767. [Google Scholar] [CrossRef]
- Biedermann, P.U.; Stezowski, J.J.; Agranat, I. Thermochromism of overcrowded bistricyclic aromatic enes (BAEs). A theoretical study. Chem. Commun. 2001, 37, 954–955. [Google Scholar] [CrossRef]
- Biedermann, P.U.; Stezowski, J.J.; Agranat, I. Polymorphism Versus Thermochromism: Interrelation of Color and Conformation in Overcrowded Bistricyclic Aromatic Enes. Chem. Eur. J. 2006, 12, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Pogodin, S.; Cohen, S.; Agranat, I. Thermochromism at Room Temperature in Overcrowded Bistricyclic Aromatic Enes: Closely Populated Twisted and Folded Conformations. Eur. J. Org. Chem. 2007, 2007, 5198–5211. [Google Scholar] [CrossRef]
- Hirao, Y.; Hamamoto, Y.; Kubo, T. Tunable Solid-State Thermochromism: Alkyl Chain Length-Dependent Conformational Isomerization of Bianthrones. Chem. Asian J. 2022, 17, e202200121. [Google Scholar] [CrossRef]
- Yamada, K.; Adachi, Y.; Ohshita, J. Synthesis and Properties of Boron-Containing Heteromerous Bistricyclic Aromatic Enes: Structural Effects on Thermodynamic Stability and Photoreactivity. Chem. Eur. J. 2023, 29, e202302370. [Google Scholar] [CrossRef]
- Brunetti, F.G.; Gong, X.; Tong, M.; Heeger, A.J.; Wudl, F. Strain and Hückel Aromaticity: Driving Forces for a Promising New Generation of Electron Acceptors in Organic Electronics. Angew. Chem. Int. Ed. 2010, 49, 532–536. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Wang, H.; Brunetti, F.G.; Wudl, F.; Hawker, C.J. Twisted but Conjugated: Building Blocks for Low Bandgap Polymers. Angew. Chem. Int. Ed. 2014, 53, 3996–4000. [Google Scholar] [CrossRef]
- Rakstys, K.; Saliba, M.; Gao, P.; Gratia, P.; Kamarauskas, E.; Paek, S.; Jankauskas, V.; Nazeeruddin, M.K. Highly Efficient Perovskite Solar Cells Employing an Easily Attainable Bifluorenylidene-Based Hole-Transporting Material. Angew. Chem. Int. Ed. 2016, 55, 7464–7468. [Google Scholar] [CrossRef]
- Xu, J.; Takai, A.; Bannaron, A.; Nakagawa, T.; Matsuo, Y.; Sugimoto, M.; Matsushita, Y.; Takeuchi, M. A helically-twisted ladder based on 9,9′-bifluorenylidene: Synthesis, characterization, and carrier-transport properties. Mater. Chem. Front. 2018, 2, 780–784. [Google Scholar] [CrossRef]
- Adachi, Y.; Nomura, T.; Tazuhara, S.; Naito, H.; Ohshita, J. Thiophene-based twisted bistricyclic aromatic ene with tricoordinate boron: A new n-type semiconductor. Chem. Commun. 2021, 57, 1316–1319. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, Y.; Chen, S.; Zou, Y.; Chen, X.; Liu, K.; Wang, N.; Qiao, Y.; Yin, X. Fluorenylidene-Cyclopentadithiophene Based Asymmetric Bistricyclic Aromatic Ene Compounds: Synthesis and Substituents Effects. Chem. Eur. J. 2023, 29, e202301055. [Google Scholar] [CrossRef] [PubMed]
- Huck, N.P.M.; Jager, W.F.; de Lange, B.; Feringa, B.L. Dynamic Control and Amplification of Molecular Chirality by Circular Polarized Light. Science 1996, 273, 1686–1688. [Google Scholar] [CrossRef]
- Ivashenko, O.; Logtenberg, H.; Areephong, J.; Coleman, A.C.; Wesenhagen, P.V.; Geertsema, E.M.; Heureux, N.; Feringa, B.L.; Rudolf, P.; Browne, W.R. Remarkable Stability of High Energy Conformers in Self-Assembled Monolayers of a Bistable Electro- and Photoswitchable Overcrowded Alkene. J. Phys. Chem. C 2011, 115, 22965–22975. [Google Scholar] [CrossRef]
- Luo, J.; Song, K.; Gu, F.L.; Miao, Q. Switching of non-helical overcrowded tetrabenzoheptafulvalene derivatives. Chem. Sci. 2011, 2, 2029–2034. [Google Scholar] [CrossRef]
- Filatov, M. Theoretical Study of the Photochemistry of a Reversible Three-State Bis-Thiaxanthylidene Molecular Switch. ChemPhysChem 2011, 12, 3348–3353. [Google Scholar] [CrossRef]
- Tran, M.N.; Chenoweth, D.M. Photoelectrocyclization as an Activation Mechanism for Organelle-Specific Live-Cell Imaging Probes. Angew. Chem. Int. Ed. 2015, 54, 6442–6446. [Google Scholar] [CrossRef]
- van Dijken, D.J.; Štacko, P.; Stuart, M.C.A.; Browne, W.R.; Feringa, B.L. Chirality controlled responsive self-assembled nanotubes in water. Chem. Sci. 2017, 8, 1783–1789. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Hayashi, Y.; Suzuki, T. Photo- and Thermal Interconversion of Multiconfigurational Strained Hydrocarbons Exhibiting Completely Switchable Oxidation to Stable Dicationic Dyes. J. Am. Chem. Soc. 2019, 141, 18293–18300. [Google Scholar] [CrossRef]
- Shi, J.; Chang, N.; Li, C.; Mei, J.; Deng, C.; Luo, X.; Liu, Z.; Bo, Z.; Dong, Y.Q.; Tang, B.Z. Locking the phenyl rings of tetraphenylethene step by step: Understanding the mechanism of aggregation-induced emission. Chem. Commun. 2012, 48, 10675–10677. [Google Scholar] [CrossRef] [PubMed]
- Crocker, R.D.; Zhang, B.; Pace, D.P.; Wong, W.W.H.; Nguyen, T.V. Tetrabenzo [5.7]fulvalene: A forgotten aggregation induced-emission luminogen. Chem. Commun. 2019, 55, 11591–11594. [Google Scholar] [CrossRef]
- Yin, X.; Low, J.Z.; Fallon, K.J.; Paley, D.W.; Campos, L.M. The butterfly effect in bisfluorenylidene-based dihydroacenes: Aggregation induced emission and spin switching. Chem. Sci. 2019, 10, 10733–10739. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Ogumi, K.; Wang, B.; Nakagawa, T.; Fu, Y.; Matsuo, Y. Equilibrium and thermodynamic studies of chromic overcrowded fluorenylidene-acridanes with modified fluorene moieties. Commun. Chem. 2020, 3, 93. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Herron, N. Organic Small Molecule Materials for Organic Light-Emitting Diodes. In Organic Light-Emitting Materials and Devices; Li, Z., Meng, H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 295–412. [Google Scholar]
- Mitschke, U.; Bäuerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Chen, D.; Li, W.; Gan, L.; Wang, Z.; Li, M.; Su, S.-J. Non-noble-metal-based organic emitters for OLED applications. Mater. Sci. Eng. R Rep. 2020, 142, 100581. [Google Scholar] [CrossRef]
- Liu, C.-F.; Liu, X.; Lai, W.-Y.; Huang, W. Organic Light-Emitting Field-Effect Transistors: Device Geometries and Fabrication Techniques. Adv. Mater. 2018, 30, 1802466. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Hu, W. Organic Light-Emitting Transistors: Materials, Device Configurations, and Operations. Small 2016, 12, 1252–1294. [Google Scholar] [CrossRef]
- Hotta, S.; Yamao, T.; Bisri, S.Z.; Takenobu, T.; Iwasa, Y. Organic single-crystal light-emitting field-effect transistors. J. Mater. Chem. C 2014, 2, 965–980. [Google Scholar] [CrossRef]
- Cicoira, F.; Santato, C. Organic light emitting field effect transistors: Advances and perspectives. Adv. Funct. Mater. 2007, 17, 3421–3434. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.-Y.; Liu, X.; Lin, H.; Gao, K.; Lai, W.-Y.; Huang, W. Organic solid-state lasers: A materials view and future development. Chem. Soc. Rev. 2020, 49, 5885–5944. [Google Scholar] [CrossRef]
- Wei, G.-Q.; Wang, X.-D.; Liao, L.-S. Recent Advances in 1D Organic Solid-State Lasers. Adv. Funct. Mater. 2019, 29, 1902981. [Google Scholar] [CrossRef]
- Gierschner, J.; Varghese, S.; Park, S.Y. Organic Single Crystal Lasers: A Materials View. Adv. Opt. Mater. 2016, 4, 348–364. [Google Scholar] [CrossRef]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments on Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef]
- Zhang, B.; Lyu, G.; Kelly, E.A.; Evans, R.C. Förster Resonance Energy Transfer in Luminescent Solar Concentrators. Adv. Sci. 2022, 9, 2201160. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, I.; Portnoi, M.; Debije, M.G. The Hidden Potential of Luminescent Solar Concentrators. Adv. Energy Mater. 2021, 11, 2002883. [Google Scholar] [CrossRef]
- Roncali, J. Luminescent Solar Collectors: Quo Vadis? Adv. Energy Mater. 2020, 10, 2001907. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, Y.; Dong, R.; Luscombe, C.K. Review on the Role of Polymers in Luminescent Solar Concentrators. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 201–215. [Google Scholar] [CrossRef]
- Pucci, A. Luminescent Solar Concentrators Based on Aggregation Induced Emission. Isr. J. Chem. 2018, 58, 837–844. [Google Scholar] [CrossRef]
- Renny, A.; Yang, C.; Anthony, R.; Lunt, R.R. Luminescent Solar Concentrator Paintings: Connecting Art and Energy. J. Chem. Educ. 2018, 95, 1161–1166. [Google Scholar] [CrossRef]
- Meinardi, F.; Bruni, F.; Brovelli, S. Luminescent solar concentrators for building-integrated photovoltaics. Nat. Rev. Mater. 2017, 2, 17072. [Google Scholar] [CrossRef]
- Shimizu, M.; Nishimura, K.; Mineyama, M.; Fuji, H. Palladium-Catalyzed Annulation of Phenazastannines with 9-(Dibromomethylene)fluorene and -(thio)xanthenes: Facile Synthesis of Acridine Moiety-Containing Bis(tricyclic) Aromatic Enes. Org. Process Res. Dev. 2019, 23, 1740–1745. [Google Scholar] [CrossRef]
- Biedermann, P.U.; Agranat, I. Stereochemistry of Bistricyclic Aromatic Enes and Related Polycyclic Systems. In Polyarenes II; Siegel, J.S., Wu, Y.-T., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 177–277. [Google Scholar]
- Agranat, I. Comment on “Asymmetric Bistricyclic Aromatic Enes”. Chem. Eur. J. 2024, 30, e202302864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.; Xin, B.; Wang, Y.-L.; Li, C.; Chen, Z.-Q.; Yu, Q.; Huang, Z.-L.; Zhu, M.-Q. Geminal cross-coupling synthesis, ion-induced emission and lysosome imaging of cationic tetraarylethene oligoelectrolytes. Chem. Commun. 2018, 54, 3617–3620. [Google Scholar] [CrossRef]
- Stanoppi, M.; Lorbach, A. Boron-based donor-spiro-acceptor compounds exhibiting thermally activated delayed fluorescence (TADF). Dalton Trans. 2018, 47, 10394–10398. [Google Scholar] [CrossRef]
- Emsley, J. The Elements, 3rd ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Shimizu, M.; Hiyama, T. Organic Fluorophores Exhibiting Highly Efficient Photoluminescence in the Solid State. Chem. Asian J. 2010, 5, 1516–1531. [Google Scholar] [CrossRef]
- Gierschner, J.; Park, S.Y. Luminescent distyrylbenzenes: Tailoring molecular structure and crystalline morphology. J. Mater. Chem. C 2013, 1, 5818–5832. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Design Aspects of Luminescent Organic Crystals. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2014, 84, 131–149. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.K.; Pal, P.; Malik, S. Solid-state emissive organic chromophores: Design, strategy and building blocks. J. Mater. Chem. C 2020, 8, 788–802. [Google Scholar] [CrossRef]
- Gierschner, J.; Shi, J.; Milián-Medina, B.; Roca-Sanjuán, D.; Varghese, S.; Park, S. Luminescence in Crystalline Organic Materials: From Molecules to Molecular Solids. Adv. Opt. Mater. 2021, 9, 2002251. [Google Scholar] [CrossRef]
- Shimizu, M. The Journey to Precious-Metal-Free Organic Phosphors from Single-Benzene-Cored Fluorophores. Chem. Rec. 2021, 21, 1489–1505. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Valeur, B. Molecular Fluorescence–Principles and Applications; Wiley–VCH: Weinheim, Germany, 2002; pp. 208–213. [Google Scholar]
- Lippert, E. Dipolmoment und Elektronenstruktur von angeregten Molekülen. Naturforsch 1955, 10, 541–545. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. The Solvent Effect on Fluorescence Spectrum, Change of Solute-Solvent Interaction during the Lifetime of Excited Solute Molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Lippert, E. Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Z. Elektrochem. Ber. Bunsenges. Phys. Chem. 1957, 61, 962–975. [Google Scholar] [CrossRef]

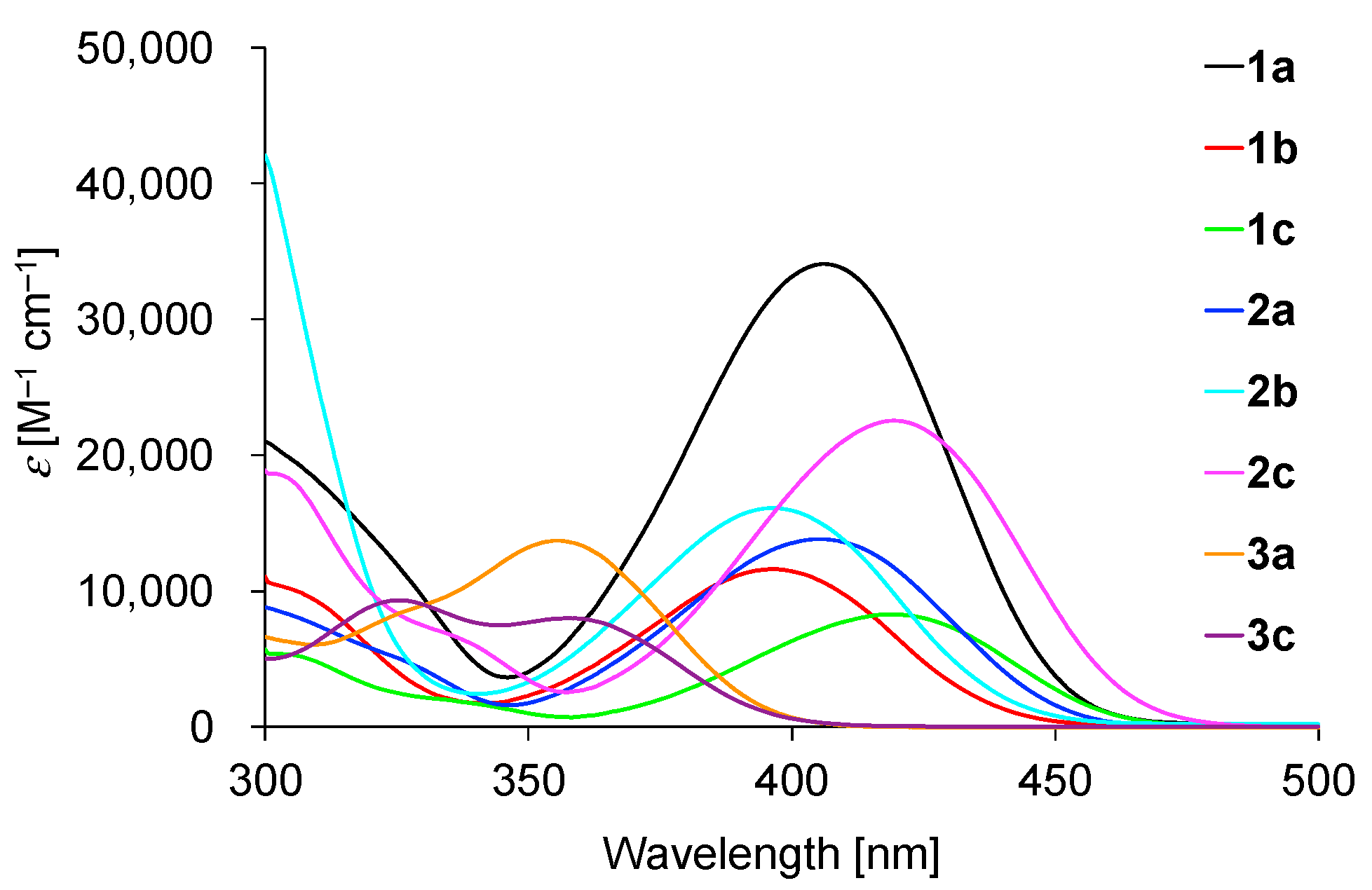
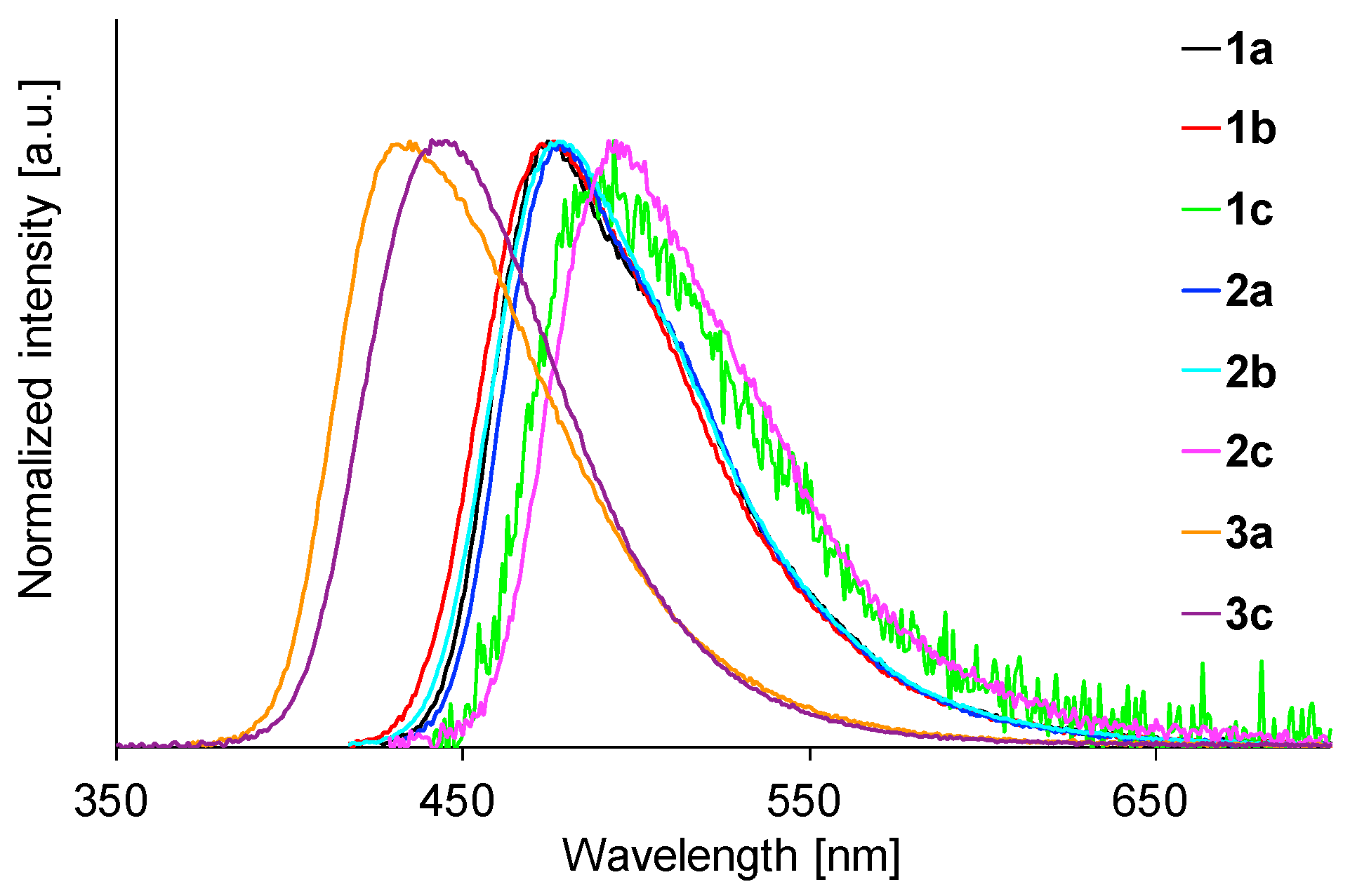
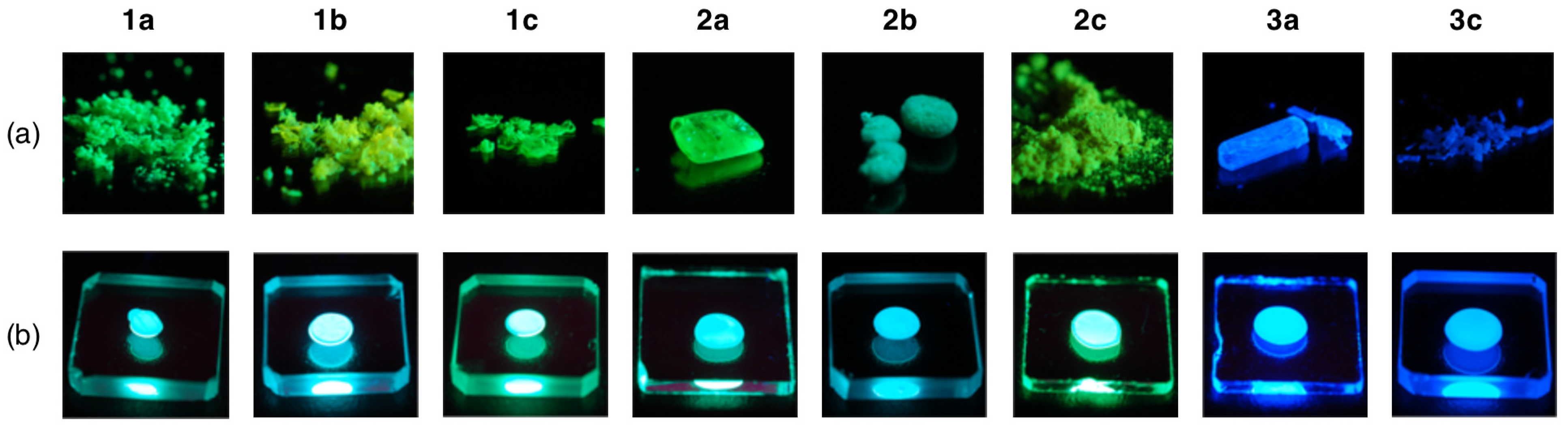
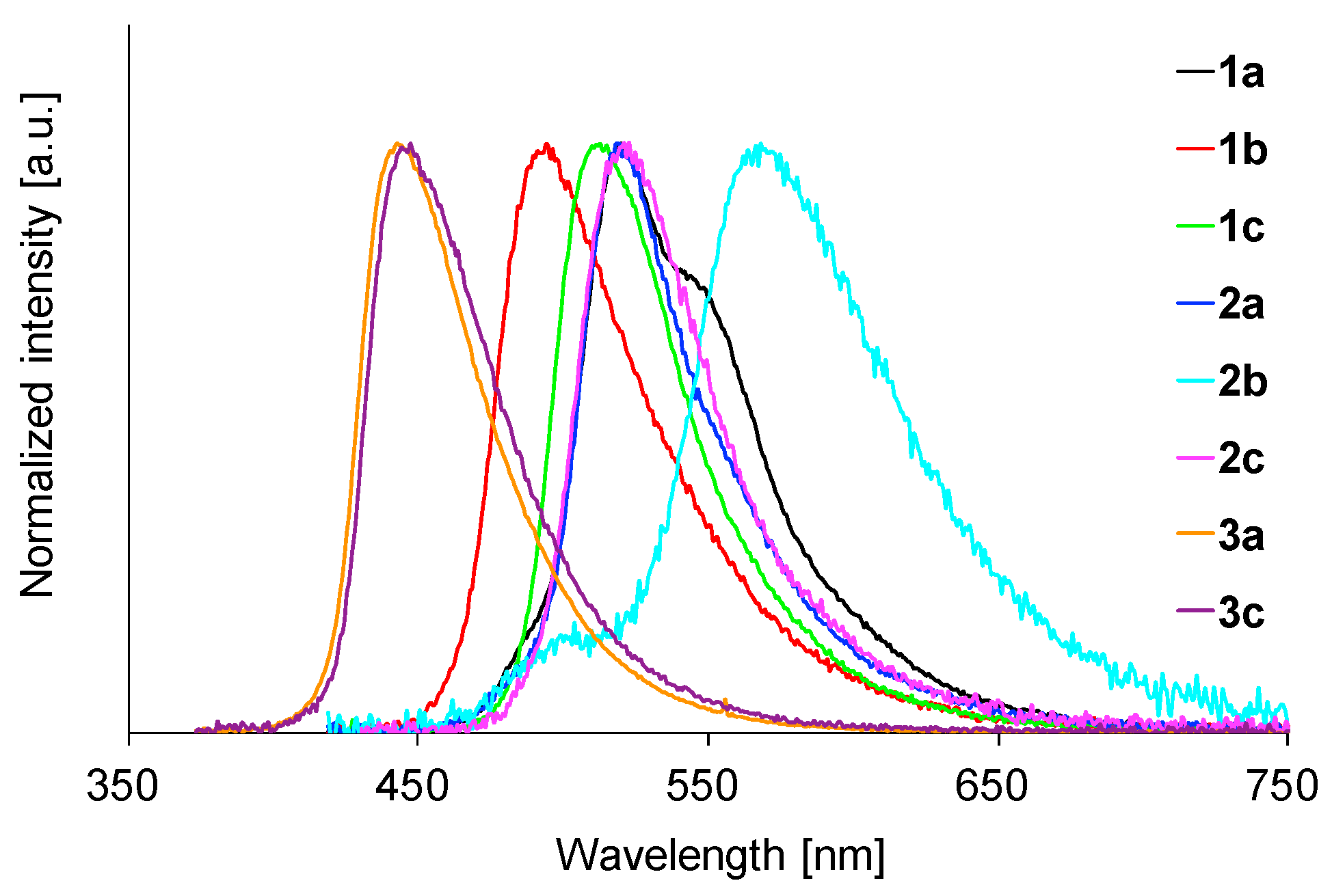
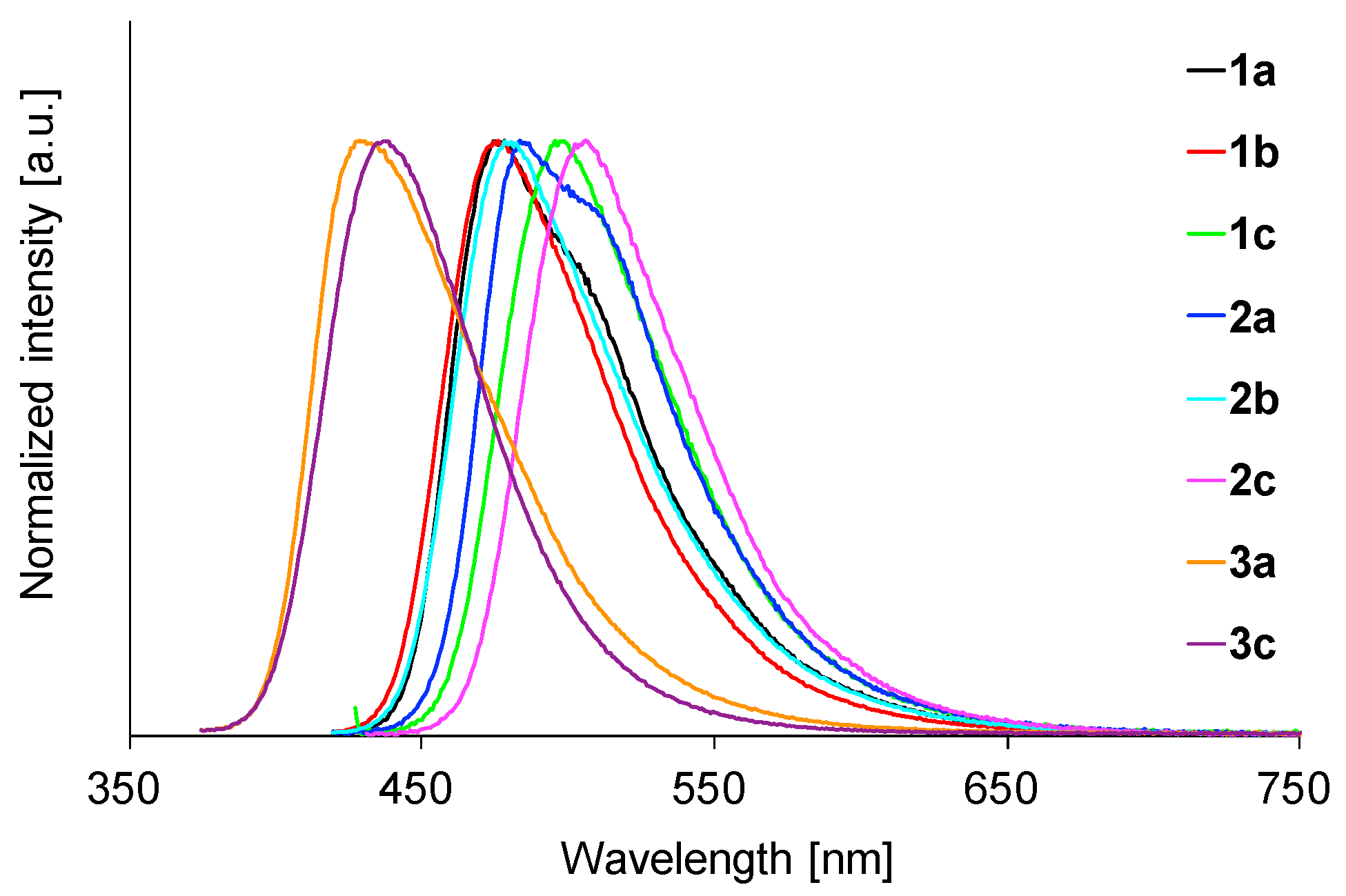
| Comp | λmax (nm) a | ε (M−1 cm−1) b |
|---|---|---|
| 1a | 406 | 34,100 |
| 1b | 396 | 11,600 |
| 1c | 419 | 8300 |
| 2a | 405 | 13,800 |
| 2b | 396 | 16,100 |
| 2c | 419 | 22,500 |
| 3a | 356 | 13,700 |
| 3c | 359 | 8000 |
| Comp | In Toluene a | In the Solid State b | In PMMA c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| λem d | Φ e | τ f | λem d | Φ e | τ f | λem d | Φ e | τ f | |
| 1a | 475 | 0.20 | 1.3 | 520 | 0.81 | 5.92 | 478 | 0.81 | 5.49 |
| 1b | 476 | 0.32 | 1.9 | 494 | 0.67 | 4.42 | 476 | 0.91 | 5.56 |
| 1c | 493 | 0.04 | 0.3 | 513 | 0.76 | 4.95 | 496 | 0.82 | 4.87 |
| 2a | 478 | 0.37 | 2.4 | 519 | 0.45 | 6.22 | 484 | 0.84 | 6.52 |
| 2b | 478 | 0.48 | 3.2 | 568 | 0.30 | 6.49 | 478 | 0.91 | 5.98 |
| 2c | 494 | 0.06 | 0.4 | 521 | 0.67 | 5.34 | 506 | 0.80 | 5.95 |
| 3a | 435 | 0.32 | 1.8 | 443 | 0.88 | 4.66 | 429 | 0.67 | 4.02 |
| 3c | 441 | 0.40 | 2.2 | 448 | 0.60 | 3.54 | 437 | 0.59 | 4.13 |
| Comp | EHOMO [eV] | ELUMO [eV] | ΔELUMO−HOMO [eV] | ΔEexp [eV] b |
|---|---|---|---|---|
| 1a | −4.96 | −1.45 | 3.51 | 3.05 |
| 1b | −5.05 | −1.40 | 3.65 | 3.13 |
| 1c | −5.45 | −2.05 | 3.40 | 2.96 |
| 2a | −5.21 | −1.47 | 3.74 | 3.06 |
| 2b | −5.10 | −1.42 | 3.68 | 3.13 |
| 2c | −5.58 | −2.15 | 3.43 | 2.96 |
| 3a | −5.54 | −1.67 | 3.87 | 3.48 |
| 3c | −5.94 | −2.07 | 3.87 | 3.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, M.; Nishimura, K.; Mineyama, M.; Terao, R.; Sakurai, T.; Sakaguchi, H. Bis(tricyclic) Aromatic Enes That Exhibit Efficient Fluorescence in the Solid State. Molecules 2024, 29, 5361. https://doi.org/10.3390/molecules29225361
Shimizu M, Nishimura K, Mineyama M, Terao R, Sakurai T, Sakaguchi H. Bis(tricyclic) Aromatic Enes That Exhibit Efficient Fluorescence in the Solid State. Molecules. 2024; 29(22):5361. https://doi.org/10.3390/molecules29225361
Chicago/Turabian StyleShimizu, Masaki, Kenta Nishimura, Mizuki Mineyama, Rin Terao, Tsuneaki Sakurai, and Hiroshi Sakaguchi. 2024. "Bis(tricyclic) Aromatic Enes That Exhibit Efficient Fluorescence in the Solid State" Molecules 29, no. 22: 5361. https://doi.org/10.3390/molecules29225361
APA StyleShimizu, M., Nishimura, K., Mineyama, M., Terao, R., Sakurai, T., & Sakaguchi, H. (2024). Bis(tricyclic) Aromatic Enes That Exhibit Efficient Fluorescence in the Solid State. Molecules, 29(22), 5361. https://doi.org/10.3390/molecules29225361








