Abstract
We report herein that bis(tricyclic) aromatic enes (BAEs) consisting of 6-6-6-membered frameworks such as acridine, xanthene, thioxanthene, and thioxanthene-S,S-dioxide act as a new class of organic luminophores that exhibit blue-to-green fluorescence in the solid state and in polymer film with good to excellent quantum yields. The BAEs were prepared by the palladium-catalyzed double cross-coupling reaction of phenazastannines or 10,10-dimethyl-10H-phenothiastannin with 9-(dibromomethylene)xanthene, 9-(dibromomethylene)thioxanthene, or 9-(dibromomethylene)-9H-thioxanthene-10,10-dioxide. Microcrystals or powder samples of the BAEs exhibited brilliant fluorescence with good to high quantum yields (Φ = 0.45–0.88). Furthermore, more efficient emission of blue-to-green light (Φ = 0.59–0.91) was observed for the BAEs dispersed in the poly(methyl methacrylate) (PMMA) films. Density functional theory (DFT) calculations suggest that the photo-absorption of the (thio)xanthene moiety-containing BAEs proceeds via π–π* transitions, whereas the optical excitation of 10,10-dioxido-9H-thioxanthene moiety-containing BAEs involves an intramolecular charge transfer from the acridine/thioxanthene part to the electron-accepting 10,10-dioxido-9H-thioxanthene moiety.
Keywords:
acridine; charge transfer; crystal; fluorescence; strained alkenes; thioxanthene; xanthene 1. Introduction
Bis(tricyclic) aromatic enes (BAEs) are attracting increasing interest as key frameworks for the development of functional organic materials. This is because BAEs exhibit unique electronic behavior and stimuli-responsiveness originating from their cross-conjugated electronic structures and the flexible conformations of the overcrowded alkene moieties. To date, several types of BAE-based functional materials have been developed that exhibit electrochromism [1,2], mechanochromism [3,4], thermochromism [5,6,7,8,9], charge transport [10,11,12,13,14,15], and photosensitivity [16,17,18,19,20,21,22] (Figure 1). However, there are few BAEs that exhibit efficient fluorescence, be that in a solution, in the solid state, or in a polymer film [23,24,25,26]. Organic luminophores that exhibit efficient solid-state emission are essential for the advancement of organic light-emitting diodes [27,28,29], organic light-emitting field-effect transistors [30,31,32,33], and organic solid-state lasers [34,35,36,37]. Furthermore, luminescent solar concentrators (LSCs) have received renewed attention in the field of renewable energy-generating devices [38,39,40,41,42,43,44]. Since LSCs consist of a transparent polymer waveguide, such as poly(methyl methacrylate) (PMMA), doped with a fluorophore, organic luminophores that exhibit efficient fluorophores in polymer film are very attractive as dopants for LSCs. This presents a significant opportunity to explore and develop highly fluorescent BAEs that efficiently fluoresce in the solid state and in a polymer film, expanding their potential as versatile multifunctional optoelectronic materials.
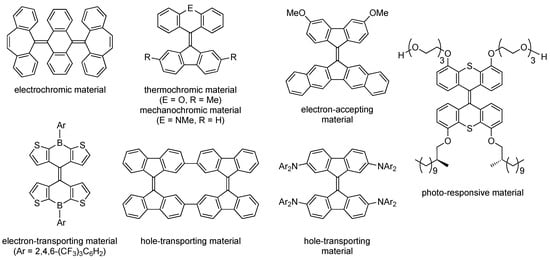
Figure 1.
Examples of functional bis(tricyclic) aromatic enes (BAEs).
We previously developed the palladium-catalyzed annulation of phenazastannines with 1,1-dibromoalkenes as a facile method for the synthesis of acridine moiety-containing heteromerous BAEs 1–3 (Figure 2) [45,46,47]. When we monitored the progress of the annulation reaction by thin-layer chromatography (TLC), we realized that the product spots on the TLC plates luminesced brilliantly on irradiation with a UV lamp (365 nm). This observation suggested that the synthesized BAEs 1–3 exhibit solid-state fluorescence and prompted us to investigate their luminescent properties. Herein, we report the single-crystal structures, absorption, and fluorescence properties and theoretical calculations of heteromerous BAEs 1–3 (Figure 2) and demonstrate that they are a promising new class of organic luminophores exhibiting efficient solid-state fluorescence in the blue-to-green region.
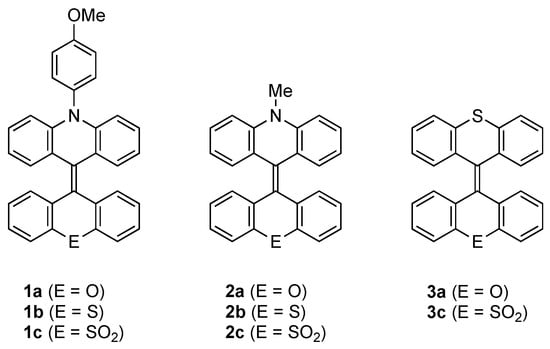
Figure 2.
Molecular structures of heteromerous BAEs 1–3.
2. Results and Discussion
2.1. Synthesis
The preparation of acridine moiety-containing BAEs 1a, 1b, 1c, 2b, and 2c has been reported previously and involves the Pd-catalyzed annulation of phenazastannines with the corresponding 1,1-dibromoalkenes [45]. Using the same protocol, 2a and 3a were synthesized in 56% and 52% yields from 9-(dibromomethylene)xanthene (4) [48] and 10,10-dimethyl-5-methylphenazastannine (5) [49] or 10,10-dimethyl-10H-phenothiastannin (6) [49], respectively (Scheme 1). The annulation of 9-(dibromomethylene)-9H-thioxanthene-10,10-dioxide (7) [45] with 6 afforded 3c in a 56% yield. The decomposition temperatures (Td) of 1–3, at which 5% of the mass was lost, were 291 (1a), 304 (1b), 322 (1c), 278 (2a), 282 (2b), 309 (2c), 256 (3a), and 303 °C (3c), indicating that the BAEs were thermally very stable.
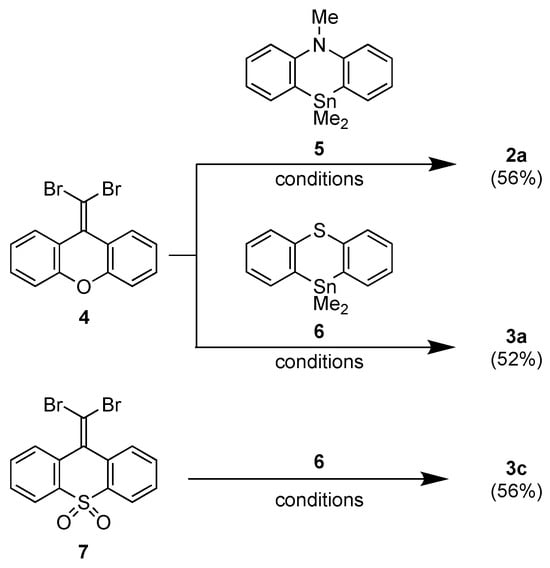
Scheme 1.
Synthesis of 2a, 3a, and 3c (conditions: Pd2(dba)3 (5 mol%), PCy3 (20 mol%), CsF (10 eq), and 1,4-dioxane, 130 °C).
2.2. Single-Crystal Structures
Single crystals of 1a and 3a suitable for X-ray diffraction analysis were obtained by recrystallization from a solution of ethyl acetate and dichloromethane, respectively. CCDC numbers of 1a and 3a are CCDC 2180600 and 2180603. Compound 1a crystallized in the triclinic space group P-1. As shown in Figure 3a,b, the tetraarylated ethene moiety (C2(C4)C1=C6(C7)C9) was flat, having dihedral angles of 0.10° (∠C2–C1–C6–C7) and 1.16° (∠C4–C1–C6–C9). The length of the C1=C6 bond is 1.358 Å, slightly longer than a standard value of the C=C bond (1.34 Å) [50], and the ∠C2–C1–C6, ∠C4–C1–C6, ∠C2–C1–C4, ∠C1–C6–C7, ∠C1–C6–C9, and ∠C7–C6–C9 bond angles are 124.92°, 125.72°, 109.36°, 124.11°, 125.12°, and 110.75°, respectively, which are significantly deviated from the ideal sp2 carbon angle of 120°. The unusual bond angles probably reduced the steric crowding around the tetraarylated ethene moiety. The central six-membered rings of the acridine and xanthene moieties adopted a boat-like conformation. The methoxyphenyl group was almost perpendicular to the acridine moiety, having a dihedral angle of 79.06° (∠C11–N1–C13–C14), suggesting that the N-aryl group did not participate in the conjugated system of the acridine moiety. Probably due to the bent molecular framework, there is no close packing between the BAE cores (Figure 3c), which is an attractive characteristic for the realization of efficient solid-state emissions. Compound 3a crystallized in the orthorhombic space group Pca21. As observed for 1a, the tetraarylated ethene moiety (C2(C4)C1=C6(C7)C9) of 3a was also flat, having dihedral angles of 3.16° (∠C2–C1–C6–C7) and 0.05° (∠C4–C1–C6–C9), and the bond angles at the sp2 carbons differed significantly from 120° (125.37° for ∠C2–C1–C6, 124.72° for ∠C4–C1–C6, 109.88° for ∠C2–C1–C4, 124.05° for ∠C1–C6–C7, 123.58° for ∠C1–C6–C9, and 112.36° for ∠C7–C6–C9) (Figure 3d). The conformations of the pyran and thiopyran rings were characterized as boat conformations (Figure 3e). Each molecule in the crystal forms a loose packing structure, which is advantageous for avoiding concentration quenching in the solid state (Figure 3f).
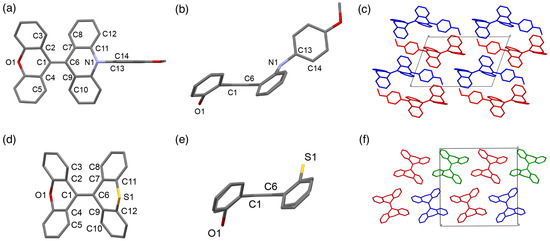
Figure 3.
(a) Top view of the molecular structure of 1a. (b) Side view of the molecular structure of 1a. Hydrogen atoms are omitted for clarity. Selected bond lengths [Å], angles [°], and dihedral angles [°]: C1–C6 1.358 Å, ∠C2–C1–C6 124.92°, ∠C4–C1–C6 125.72°, ∠C2–C1–C4 109.36°, ∠C1–C6–C7 124.11°, ∠C1–C6–C9 125.12°, ∠C7–C6–C9 110.75°, ∠C2–C1–C6–C7 0.10°, ∠C4–C1–C6–C9 1.16°, and ∠C11–N1–C13–C14 79.06°. (c) Crystal structure of 1a [space group: P-1; unit cell dimensions: a = 8.3291(9), b = 10.3629(12), c = 15.2598(17), α = 107.212(2), β = 94.588(2), and γ = 108.454(2)]. Each molecule in packing diagram is color-coded by symmetry equivalence. (d) Top view of the molecular structure of 3a. (e) Side view of the molecular structure of 3a. Hydrogen atoms are omitted for clarity. Selected bond lengths [Å] angles [°], and dihedral angles [°]: C1–C6 1.346 Å, ∠C2–C1–C6 125.37°, ∠C4–C1–C6 124.72°, ∠C2–C1–C4 109.88°, ∠C1–C6–C7 124.05°, ∠C1–C6–C9 123.58°, ∠C7–C6–C9 112.36°, ∠C2–C1–C6–C7 3.16°, and ∠C4–C1–C6–C9 0.05°. (f) Crystal structure of 3a [space group: Pca21; unit cell dimensions: a = 18.282(2), b = 5.4902(7), and c = 18.355(2)]. Each molecule in the packing diagram is color-coded by symmetry equivalence.
2.3. Absorption Properties in Toluene
The absorption data and spectra of 1–3 in toluene are shown in Table 1 and Figure 4, respectively. The absorption maxima of 1a, 1b, and 1c are almost the same as those of 2a, 2b, and 2c, respectively, indicating that the nitrogen substituent does not influence the energy gaps between the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs). The maxima of 1a–1c and 2a–2c were red-shifted in the order 1b, 1a, 1c, 2b, 2a, and 2c. The maxima of 3a and 3c were significantly blue-shifted compared to those of 1 and 2, which is reasonable considering that the electron-donating nature of a sulfur atom is less than that of a nitrogen atom.

Table 1.
Absorption data of 1–3 in toluene.
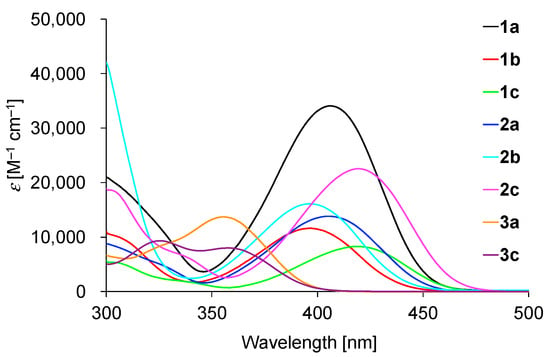
Figure 4.
Absorption spectra of 1–3 in toluene.
2.4. Fluorescence Properties in Toluene
Toluene solutions of 1–3 were faintly or moderately fluorescent (Table 2). The spectra are shown in Figure 5. The emission maxima of 1a–1c and 2a–2c appeared at 475–494 nm, whereas 3a and 3c fluoresced at 435 and 441 nm. The shorter wavelengths of the emission maxima of 3a and 3c compared to those of 1a–1c and 2a–2c are consistent with the trend in the absorption maxima. In contrast, the fluorescence maxima of 1a and 2a were almost the same as those of 1b and 2b, respectively, which is inconsistent with the absorption data. These results suggest that the electronic structures of the lowest singlet excited states (S1 states) of isolated 1a and 1b as well as 2a and 2b are quite similar (see Theoretical Calculations). Except for those of 1c and 2c (0.04 and 0.06, respectively), the quantum yields of 1–3 in toluene were moderate. Since the radiative decay constants (kr) of 1c and 2c were comparable to those of the others, the primary cause of the very low quantum yields of an SO2 moiety-containing 1c and 2c in toluene is the large nonradiative decay constants (knr) of 32.0 and 23.5, respectively, which are an order of magnitude larger than those of the others (see Table S1 in Supplementary Material).

Table 2.
Fluorescence data of 1–3 in toluene, in the solid state, and in PMMA film.
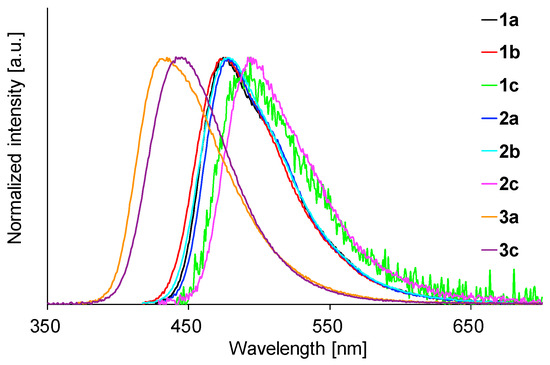
Figure 5.
Fluorescence spectra of 1–3 in toluene.
2.5. Fluorescence Properties in the Solid State
In sharp contrast to the weak fluorescence in toluene, brilliant green fluorescence (λem = 494–568 nm) was observed for the solid samples of 1 and 2. In contrast, 3a and 3c efficiently emitted blue fluorescence at 443 and 448 nm, respectively, in microcrystalline solid form (Figure 6a). The fluorescence data and spectra are summarized in Table 2 and shown in Figure 7. The fluorescence quantum yields were good to excellent (Φ = 0.45–0.88) and were higher than those in toluene, except for that of 2b (Φ = 0.30). Until now, only 0.308 and 0.347 have been reported as solid-state quantum yields of BAEs for 9-(9H-xanthen-9-ylidene)-9H-xanthene [23] and tetrabenzo[5.7]fulvalene [24]. Thus, these results demonstrate that the developed BAEs are a novel class of organic fluorophores that exhibit efficient fluorescence in the solid state [51,52,53,54,55,56,57]. The fluorescence maximum and quantum yield of 2b were 568 nm and 0.30, respectively. Compared to other BAEs, the maximum was significantly red-shifted, and the yield was much lower, suggesting that 2b was densely aggregated in the solid state, and thus, suffered from severe concentration quenching.
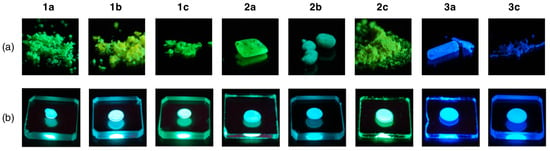
Figure 6.
Fluorescence images of 1–3 in the solid state (a) and in PMMA films (b) upon irradiation with a UV lamp (365 nm).
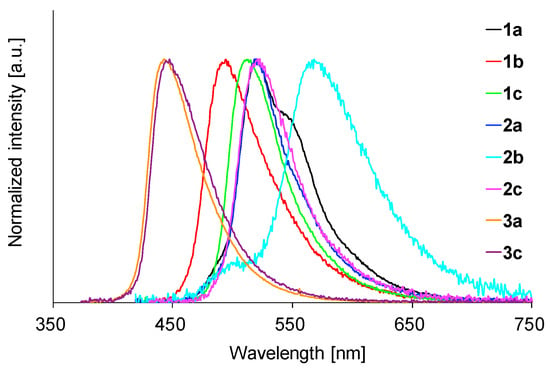
Figure 7.
Fluorescence spectra of 1–3 in the solid state.
2.6. Fluorescence Properties in PMMA Film
We next investigated the fluorescence properties of the BAEs in polymer film to explore their potential as LSC-applicable emitters. First, four polymer films were prepared from PMMA, polystyrene, poly(bisphenol A carbonate), and poly(vinyl acetate) using 1b and 2c as the dopants. The fluorescence spectra were almost the same regardless of the host polymer (Figure S7 for 1b and Figure S8 for 2c). The highest fluorescence quantum yields (0.91 for 1b and 0.80 for 2c) were observed with the PMMA films (the data are shown in Table S2). Therefore, PMMA was chosen as the host polymer and the fluorescence properties of the other BAEs in PMMA were investigated. The fluorescence data and spectra are shown in Table 2 and Figure 8. The films efficiently emitted fluorescence at 429–506 nm, which were like those in the solution case, indicating that each molecule dispersed in the PMMA film was spatially and electronically isolated, as in the solution case. The fluorescence quantum yields in the PMMA film were high to excellent (Φ = 0.59–0.91), probably because of the isolated and rigid environment of each luminophore. Consequently, 1–3 are considered very attractive fluorophores for LSC applications.
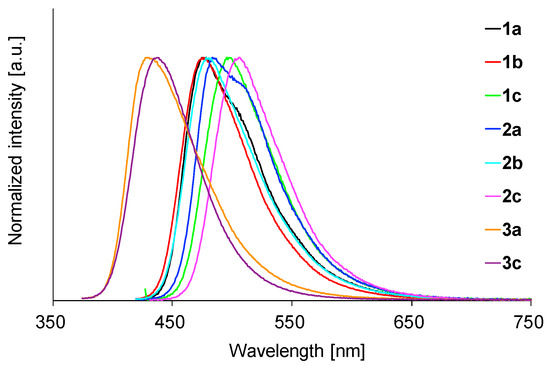
Figure 8.
Fluorescence spectra of 1–3 in PMMA film.
2.7. Theoretical Calculations
To gain insight into the electronic structures and photoluminescence mechanisms of 1–3, density functional theory (DFT) calculations of the BAEs were carried out using the Gaussian 09 package [58]. Initially, DFT calculations were performed at the B3LYP/6-31+G(d) level of theory. However, the calculated HOMO–LUMO energy gaps did not correlate well with the experimentally estimated gaps from absorption spectra, suggesting that this functional and basis set combination was inadequate. In contrast, calculations at the B3LYP/cc-pVDZ level of theory yielded results that were consistent with the experimental data, with the exception of 2a. Therefore, we used the B3LYP/cc-pVDZ levels of theory for theoretical calculation study. Initial structures for structural optimization were generated from the single-crystal structures of 1a and 3a. The calculated HOMO and LUMO energies (EHOMO and ELUMO) and their energy gaps (ΔELUMO–HOMO) are summarized in Table 3 along with the experimentally determined HOMO–LUMO gaps (ΔEexp), which were estimated from the absorption maxima in toluene. The magnitude of the relationship between the calculated HOMO–LUMO energy gaps is consistent with that of the experimentally estimated energy gaps except for that of 2a. The discrepancy between the calculated and experimental HOMO-LUMO gaps for compound 2a remains unclear. One possible explanation is the difference in phases: The calculated value is for the gas phase, while the experimental value is for the solution phase. The HOMOs of 1a, 1b, 2a, 2b, and 3a were distributed over the entire BAE framework with a large contribution from the nitrogen and sulfur atoms despite their bent structures (Figure 9). The absence of the HOMOs over the methoxyphenyl group of 1a and 1b is probably due to the perpendicular orientation of the methoxyphenyl group to the BAE plane. The HOMOs of 1c, 2c, and 3c containing an SO2 moiety are localized over the acridine skeleton and the central C=C bond, but not over the SO2 group nor over the two benzene rings bridged by the SO2 group. Meanwhile, the LUMOs of 1a, 1b, 2a, and 2b were delocalized over the BAE skeleton with little or no contribution from the sulfur or nitrogen atom. In the cases of 1c, 2c, and 3c, which contain an SO2 moiety, the LUMOs were mostly accumulated over the 10,10-dioxido-9H-thioxanthene moiety. In short, these orbital distributions suggest that 1a, 1b, 2a, 2b, and 3a are optically excited via the π–π* transitions of the BAE skeletons, while the photoexcitation of 1c, 2c, and 3c occurs through a weak intramolecular charge transfer (ICT) from the acridine/thioxanthene moiety to the electron-accepting 10,10-dioxido-9H-thioxanthene moiety. The Lippert–Mataga plots of 1c and 2c were prepared with seven solvents (CHCl3, Et2O, EtOAc, THF, CH2Cl2, PhCN, and DMF) [59,60,61,62,63]. The plots fit the calibration lines defined by a linear approximation with moderate correlation coefficients of 0.701 and 0.731 (Figures S10 and S12), which experimentally supports ICT as the mechanism for the excitation process of the SO2-containing BAEs.

Table 3.
HOMO and LUMO energies of 1–3 a.
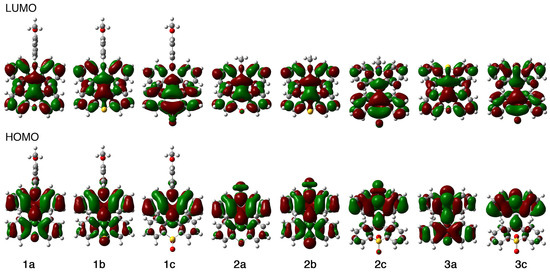
Figure 9.
HOMO and LUMO drawings of 1–3.
3. Materials and Methods
3.1. General
Melting and decomposition points were determined with a TG/DTA6200 Seiko Instrument (Seiko Instruments Inc., Chiba, Japan). 1H NMR spectra were recorded with a Varian Mercury 400 (400 MHz) spectrometer (Las Vegas, NV, USA). The chemical shifts of 1H NMR signals are expressed in parts per million, relative to the internal tetramethylsilane standard (δ = 0 ppm) or chloroform (δ = 7.26 ppm). Splitting patterns are indicated as follows: s, singlet; d, doublet; t, triplet; and m, multiplet. 13C NMR spectra were recorded with a Varian Mercury 400 (100 MHz) spectrometer with tetramethylsilane (δ = 0 ppm) or chloroform-d (δ = 77.0 ppm) as internal standard. Chemical shifts are given in parts per million, relative to the internal standards. IR attenuated total reflectance (IR-ATR) spectra were recorded by using a Shimadzu FTIR-8400 spectrometer (Kyoto, Japan) equipped with a Specac® Quest ATR diamond accessory (Pleasantville, NY, USA). Fast atom bombardment high-resolution mass spectrometry (FAB–HRMS) analyses were performed with a JEOL JMS-700 spectrometer (Tokyo, Japan). TLC analyses were performed by using Merck Kieselgel 60 F254 silica plates (Rahway, NJ, USA). Silica gel column chromatography was carried out by using Merck Kieselgel 60 (230–400 mesh). 1,4-Dioxane was distilled from Na before use. All reactions were carried out under argon atmosphere.
3.2. Synthesis
3.2.1. Synthesis of 2a
In a glovebox, 5 (0.25 g, 0.75 mmol), 4 (0.18 g, 0.50 mmol), Pd2(dba)3 (23 mg, 0.025 mmol), PCy3 (28 mg, 0.10 mmol), CsF (0.76 g, 5.0 mmol), and 1,4-dioxane (25 mL) were added to a 50 mL vial. The vial was sealed, removed from the glovebox, and heated at 130 °C for 15 h. The reaction mixture was then filtered through a pad of Celite, and the filtrate was concentrated in vacuo. The crude product was purified using silica gel column chromatography (eluent: hexane/EtOAc 10:1) to give 2a (0.10 g, 0.28 mmol, 56%) as a yellow solid. Tm (dec.) = 328 °C; TLC: Rf 0.40 (hexane/EtOAc 10:1); 1H NMR (CDCl3, 400 MHz): δ = 3.56 (s, 3H), 6.76–6.84 (m, 4H), 7.00 (d, J = 7.6 Hz, 2H), 7.09 (d, J = 7.6 Hz, 2H), 7.14–7.23 (m, 8H); 13C NMR (CDCl3, 100 MHz): δ = 33.4, 112.6, 116.9, 119.7, 120.2, 122.2, 123.8, 125.6 (2C), 127.6, 127.7, 128.0, 128.3, 144.8, 155.6; IR (neat): ν = 2958, 1591, 1550, 1494, 1452, 1433, 1386, 1338, 1288, 1265, 1161, 1134, 1120, 1058, 1028, 879, 844, 754, 742, 700 cm−1; HRMS (FAB) m/z [M+] calcd for C27H19NO: 373.1467, found: 373.1459.
3.2.2. Synthesis of 3a
The same procedure for the synthesis of 2a was applied to 4 and 6, affording 3a in 52% yield as a colorless solid. Tm (dec.) = 295 °C; TLC: Rf 0.25 (hexane); 1H NMR (CDCl3, 400 MHz): δ = 6.77–6.78 (m, 4H), 7.01 (t, J = 7.6 Hz, 2H), 7.11 (d, J = 7.6 Hz, 2H), 7.15–7.23 (m, 6H), 7.58 (d, J = 7.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz): δ = 116.7, 122.3, 123.9, 124.0, 126.0, 126.7, 127.8, 128.4, 128.8, 129.3, 130.3, 136.5, 136.7, 154.8; IR (neat): ν = 3913, 3709, 3616, 3564, 3047, 2349, 1579, 1556, 1467, 1435, 1394, 1373, 1238, 1228, 1195, 1095, 1062, 1026, 945, 883, 839, 790, 742, 727, 698 cm−1; HRMS (FAB) m/z [M+] calcd for C26H16OS: 376.0922, found: 376.0918.
3.2.3. Synthesis of 3c
The same procedure for the synthesis of 2a was applied to 6 and 7, affording 3c in 56% yield as a colorless solid. Tm (dec.) = 366 °C; TLC: Rf 0.25 (hexane/EtOAc 10:1); 1H NMR (CDCl3, 400 MHz): δ = 6.89 (d, J = 7.6 Hz, 2H), 6.98 (t, J = 7.6 Hz, 2H), 7.07 (d, J = 7.6 Hz, 2H), 7.14–7.22 (m, 4H), 7.37 (t, J = 7.6 Hz, 2H), 7.59 (d, J = 7.6 Hz, 2H), 8.08 (d, J = 7.6 Hz, 2H); 13C NMR (CDCl3, 100 MHz): δ = 124.0, 126.1, 127.2, 127.5, 127.6 (2C), 130.2, 130.2, 130.8, 134.6, 135.2, 137.1, 138.7, 139.1; IR (neat): ν = 1454, 1437, 1311, 1294, 1267, 1165, 1141, 1116, 1066, 1049, 742, 723, 688 cm−1; HRMS (FAB) m/z [M+] calcd for C26H16O2S2: 424.0592, found: 424.0602.
3.3. X-Ray Crystallography of Single Crystals
Data were measured on a Bruker (Bremen, Germany) SMART APEX diffractometer (MoKa radiation, l = 0.71073 Å) at 300 K. The structures were solved by direct methods using SHELXTL programs (ver 6.1) and refined with full-matrix least-squares on F2. CCDC numbers of 1a and 3a are CCDC 2180600 and 2180603.
3.4. Preparation of Doped PMMA Films
The sample (1 mg) was dissolved in a CH2Cl2 (1 mL) solution of PMMA (99 mg). The resulting solution was dropped onto a quartz plate (10 mm × 10 mm) and spin-coated at 300 rpm for 40 s and then at 1000 rpm for 60 s. The deposited film was dried at room temperature in air for 12 h and under reduced pressure for 6 h.
3.5. Measurement of Absorption and Photoluminescence Spectra
The spectroscopic-grade toluene for photoluminescence measurements was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan) and degassed with argon before use. The absorption spectra were obtained using a Shimadzu UV-2550 spectrometer. The fluorescence spectra and absolute quantum yields were recorded using a calibrated integrating sphere with a Hamamatsu Photonics C9920-02 Absolute PL Quantum Yield Measurement System (Hamamatsu, Japan), and the fluorescence lifetimes were measured with a Hamamatsu Photonics Quantaurus-Tau.
4. Conclusions
We demonstrated that heteromerous BAEs consisting of two different tricyclic moieties (in this study, acridine, xanthene, thioxanthene, or thioxanthene-S,S-dioxide) are thermally very stable, adopt bent conformations, and efficiently fluoresce in the solid state in the blue-to-green region. As summarized in Figure 10, the fluorescence quantum yields in the solid state, especially in PMMA film form, were good to excellent and significantly higher than those in the toluene solution. Given that to date there have been no reports of BAEs exhibiting solid-state emissions with high efficiency (Φ > 0.40), this study represents the first demonstration of BAEs that are highly luminescent in the solid state and add solid-state luminescent properties to the material functions exhibited by BAEs. We hope that the findings of this study will encourage materials chemists and scientists to use BAEs as solid-state fluorescent materials.
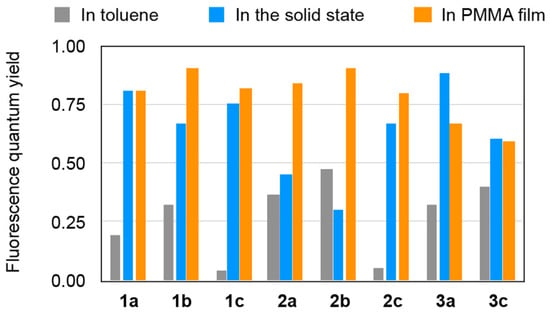
Figure 10.
Graphical summary of the fluorescence quantum yields of 1–3.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29225361/s1, Figures S1–S6: 1H and 13C NMR spectra of 2a, 3a, and 3c; Figure S7: Fluorescence spectra of 1b in polymer film; Figure S8: Fluorescence spectra of 2c in polymer film; Figure S9: Absorption and fluorescence spectra of 1c in various solvents; Figure S10: Lippert–Mataga plots of 1c; Figure S11: Absorption and fluorescence spectra of 2c in various solvents; Figure S12: Lippert–Mataga plots of 2c; Table S1: Fluorescence data of 1, 2, and 3; Table S2: Fluorescence data of 1b and 2c in polymer films; Table S3: Orientational polarizability (Δf), wavelength of absorption maximum (λabs), wavelength of fluorescence maximum (λF), wave number of absorption maximum (νa), wave number of fluorescence maximum (νF), and Stokes shift (νa–νF) of 1c; Table S4: Orientational polarizability (Δf), wavelength of absorption maximum (λabs), wavelength of fluorescence maximum (λF), wave number of absorption maximum (νa), wave number of fluorescence maximum (νF), and Stokes shift (νa–νF) of 2c.
Author Contributions
Conceptualization, M.S.; methodology, M.S.; validation, M.S., K.N., M.M., R.T., T.S. and H.S.; formal analysis, M.S. and T.S.; investigation, M.S., K.N., M.M., R.T. and T.S.; resources, H.S.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, M.S., T.S. and H.S.; visualization, M.S. and T.S.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ZE Research Program, IAE (Kyoto University), grant numbers ZE2021A-29 and ZE2022A-27.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
The NMR and MS measurements were performed with research equipment shared via the MEXT Project for promoting the public utilization of advanced research infrastructure (Program for supporting the introduction of the new sharing system; Grant Number JPMXS0421800221).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Browne, W.R.; Pollard, M.M.; de Lange, B.; Meetsma, A.; Feringa, B.L. Reversible Three-State Switching of Luminescence: A New Twist to Electro- and Photochromic Behavior. J. Am. Chem. Soc. 2006, 128, 12412–12413. [Google Scholar] [CrossRef] [PubMed]
- Wonink, M.B.S.; Corbet, B.P.; Kulago, A.A.; Boursalian, G.B.; de Bruin, B.; Otten, E.; Browne, W.R.; Feringa, B.L. Three-State Switching of an Anthracene Extended Bis-thiaxanthylidene with a Highly Stable Diradical State. J. Am. Chem. Soc. 2021, 143, 18020–18028. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Okada, H.; Nakagawa, T.; Komatsu, K.; Fujimoto, C.; Kagi, H.; Matsuo, Y. A fluorenylidene-acridane that becomes dark in color upon grinding–ground state mechanochromism by conformational change. Chem. Sci. 2018, 9, 475–482. [Google Scholar] [CrossRef]
- Matsuo, Y.; Wang, Y.; Ueno, H.; Nakagawa, T.; Okada, H. Mechanochromism, Twisted/Folded Structure Determination, and Derivatization of (N-Phenylfluorenylidene)acridane. Angew. Chem. Int. Ed. 2019, 58, 8762–8767. [Google Scholar] [CrossRef]
- Biedermann, P.U.; Stezowski, J.J.; Agranat, I. Thermochromism of overcrowded bistricyclic aromatic enes (BAEs). A theoretical study. Chem. Commun. 2001, 37, 954–955. [Google Scholar] [CrossRef]
- Biedermann, P.U.; Stezowski, J.J.; Agranat, I. Polymorphism Versus Thermochromism: Interrelation of Color and Conformation in Overcrowded Bistricyclic Aromatic Enes. Chem. Eur. J. 2006, 12, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Pogodin, S.; Cohen, S.; Agranat, I. Thermochromism at Room Temperature in Overcrowded Bistricyclic Aromatic Enes: Closely Populated Twisted and Folded Conformations. Eur. J. Org. Chem. 2007, 2007, 5198–5211. [Google Scholar] [CrossRef]
- Hirao, Y.; Hamamoto, Y.; Kubo, T. Tunable Solid-State Thermochromism: Alkyl Chain Length-Dependent Conformational Isomerization of Bianthrones. Chem. Asian J. 2022, 17, e202200121. [Google Scholar] [CrossRef]
- Yamada, K.; Adachi, Y.; Ohshita, J. Synthesis and Properties of Boron-Containing Heteromerous Bistricyclic Aromatic Enes: Structural Effects on Thermodynamic Stability and Photoreactivity. Chem. Eur. J. 2023, 29, e202302370. [Google Scholar] [CrossRef]
- Brunetti, F.G.; Gong, X.; Tong, M.; Heeger, A.J.; Wudl, F. Strain and Hückel Aromaticity: Driving Forces for a Promising New Generation of Electron Acceptors in Organic Electronics. Angew. Chem. Int. Ed. 2010, 49, 532–536. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Wang, H.; Brunetti, F.G.; Wudl, F.; Hawker, C.J. Twisted but Conjugated: Building Blocks for Low Bandgap Polymers. Angew. Chem. Int. Ed. 2014, 53, 3996–4000. [Google Scholar] [CrossRef]
- Rakstys, K.; Saliba, M.; Gao, P.; Gratia, P.; Kamarauskas, E.; Paek, S.; Jankauskas, V.; Nazeeruddin, M.K. Highly Efficient Perovskite Solar Cells Employing an Easily Attainable Bifluorenylidene-Based Hole-Transporting Material. Angew. Chem. Int. Ed. 2016, 55, 7464–7468. [Google Scholar] [CrossRef]
- Xu, J.; Takai, A.; Bannaron, A.; Nakagawa, T.; Matsuo, Y.; Sugimoto, M.; Matsushita, Y.; Takeuchi, M. A helically-twisted ladder based on 9,9′-bifluorenylidene: Synthesis, characterization, and carrier-transport properties. Mater. Chem. Front. 2018, 2, 780–784. [Google Scholar] [CrossRef]
- Adachi, Y.; Nomura, T.; Tazuhara, S.; Naito, H.; Ohshita, J. Thiophene-based twisted bistricyclic aromatic ene with tricoordinate boron: A new n-type semiconductor. Chem. Commun. 2021, 57, 1316–1319. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, Y.; Chen, S.; Zou, Y.; Chen, X.; Liu, K.; Wang, N.; Qiao, Y.; Yin, X. Fluorenylidene-Cyclopentadithiophene Based Asymmetric Bistricyclic Aromatic Ene Compounds: Synthesis and Substituents Effects. Chem. Eur. J. 2023, 29, e202301055. [Google Scholar] [CrossRef] [PubMed]
- Huck, N.P.M.; Jager, W.F.; de Lange, B.; Feringa, B.L. Dynamic Control and Amplification of Molecular Chirality by Circular Polarized Light. Science 1996, 273, 1686–1688. [Google Scholar] [CrossRef]
- Ivashenko, O.; Logtenberg, H.; Areephong, J.; Coleman, A.C.; Wesenhagen, P.V.; Geertsema, E.M.; Heureux, N.; Feringa, B.L.; Rudolf, P.; Browne, W.R. Remarkable Stability of High Energy Conformers in Self-Assembled Monolayers of a Bistable Electro- and Photoswitchable Overcrowded Alkene. J. Phys. Chem. C 2011, 115, 22965–22975. [Google Scholar] [CrossRef]
- Luo, J.; Song, K.; Gu, F.L.; Miao, Q. Switching of non-helical overcrowded tetrabenzoheptafulvalene derivatives. Chem. Sci. 2011, 2, 2029–2034. [Google Scholar] [CrossRef]
- Filatov, M. Theoretical Study of the Photochemistry of a Reversible Three-State Bis-Thiaxanthylidene Molecular Switch. ChemPhysChem 2011, 12, 3348–3353. [Google Scholar] [CrossRef]
- Tran, M.N.; Chenoweth, D.M. Photoelectrocyclization as an Activation Mechanism for Organelle-Specific Live-Cell Imaging Probes. Angew. Chem. Int. Ed. 2015, 54, 6442–6446. [Google Scholar] [CrossRef]
- van Dijken, D.J.; Štacko, P.; Stuart, M.C.A.; Browne, W.R.; Feringa, B.L. Chirality controlled responsive self-assembled nanotubes in water. Chem. Sci. 2017, 8, 1783–1789. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Hayashi, Y.; Suzuki, T. Photo- and Thermal Interconversion of Multiconfigurational Strained Hydrocarbons Exhibiting Completely Switchable Oxidation to Stable Dicationic Dyes. J. Am. Chem. Soc. 2019, 141, 18293–18300. [Google Scholar] [CrossRef]
- Shi, J.; Chang, N.; Li, C.; Mei, J.; Deng, C.; Luo, X.; Liu, Z.; Bo, Z.; Dong, Y.Q.; Tang, B.Z. Locking the phenyl rings of tetraphenylethene step by step: Understanding the mechanism of aggregation-induced emission. Chem. Commun. 2012, 48, 10675–10677. [Google Scholar] [CrossRef] [PubMed]
- Crocker, R.D.; Zhang, B.; Pace, D.P.; Wong, W.W.H.; Nguyen, T.V. Tetrabenzo [5.7]fulvalene: A forgotten aggregation induced-emission luminogen. Chem. Commun. 2019, 55, 11591–11594. [Google Scholar] [CrossRef]
- Yin, X.; Low, J.Z.; Fallon, K.J.; Paley, D.W.; Campos, L.M. The butterfly effect in bisfluorenylidene-based dihydroacenes: Aggregation induced emission and spin switching. Chem. Sci. 2019, 10, 10733–10739. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Ogumi, K.; Wang, B.; Nakagawa, T.; Fu, Y.; Matsuo, Y. Equilibrium and thermodynamic studies of chromic overcrowded fluorenylidene-acridanes with modified fluorene moieties. Commun. Chem. 2020, 3, 93. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Herron, N. Organic Small Molecule Materials for Organic Light-Emitting Diodes. In Organic Light-Emitting Materials and Devices; Li, Z., Meng, H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 295–412. [Google Scholar]
- Mitschke, U.; Bäuerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Chen, D.; Li, W.; Gan, L.; Wang, Z.; Li, M.; Su, S.-J. Non-noble-metal-based organic emitters for OLED applications. Mater. Sci. Eng. R Rep. 2020, 142, 100581. [Google Scholar] [CrossRef]
- Liu, C.-F.; Liu, X.; Lai, W.-Y.; Huang, W. Organic Light-Emitting Field-Effect Transistors: Device Geometries and Fabrication Techniques. Adv. Mater. 2018, 30, 1802466. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Hu, W. Organic Light-Emitting Transistors: Materials, Device Configurations, and Operations. Small 2016, 12, 1252–1294. [Google Scholar] [CrossRef]
- Hotta, S.; Yamao, T.; Bisri, S.Z.; Takenobu, T.; Iwasa, Y. Organic single-crystal light-emitting field-effect transistors. J. Mater. Chem. C 2014, 2, 965–980. [Google Scholar] [CrossRef]
- Cicoira, F.; Santato, C. Organic light emitting field effect transistors: Advances and perspectives. Adv. Funct. Mater. 2007, 17, 3421–3434. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.-Y.; Liu, X.; Lin, H.; Gao, K.; Lai, W.-Y.; Huang, W. Organic solid-state lasers: A materials view and future development. Chem. Soc. Rev. 2020, 49, 5885–5944. [Google Scholar] [CrossRef]
- Wei, G.-Q.; Wang, X.-D.; Liao, L.-S. Recent Advances in 1D Organic Solid-State Lasers. Adv. Funct. Mater. 2019, 29, 1902981. [Google Scholar] [CrossRef]
- Gierschner, J.; Varghese, S.; Park, S.Y. Organic Single Crystal Lasers: A Materials View. Adv. Opt. Mater. 2016, 4, 348–364. [Google Scholar] [CrossRef]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments on Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef]
- Zhang, B.; Lyu, G.; Kelly, E.A.; Evans, R.C. Förster Resonance Energy Transfer in Luminescent Solar Concentrators. Adv. Sci. 2022, 9, 2201160. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, I.; Portnoi, M.; Debije, M.G. The Hidden Potential of Luminescent Solar Concentrators. Adv. Energy Mater. 2021, 11, 2002883. [Google Scholar] [CrossRef]
- Roncali, J. Luminescent Solar Collectors: Quo Vadis? Adv. Energy Mater. 2020, 10, 2001907. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, Y.; Dong, R.; Luscombe, C.K. Review on the Role of Polymers in Luminescent Solar Concentrators. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 201–215. [Google Scholar] [CrossRef]
- Pucci, A. Luminescent Solar Concentrators Based on Aggregation Induced Emission. Isr. J. Chem. 2018, 58, 837–844. [Google Scholar] [CrossRef]
- Renny, A.; Yang, C.; Anthony, R.; Lunt, R.R. Luminescent Solar Concentrator Paintings: Connecting Art and Energy. J. Chem. Educ. 2018, 95, 1161–1166. [Google Scholar] [CrossRef]
- Meinardi, F.; Bruni, F.; Brovelli, S. Luminescent solar concentrators for building-integrated photovoltaics. Nat. Rev. Mater. 2017, 2, 17072. [Google Scholar] [CrossRef]
- Shimizu, M.; Nishimura, K.; Mineyama, M.; Fuji, H. Palladium-Catalyzed Annulation of Phenazastannines with 9-(Dibromomethylene)fluorene and -(thio)xanthenes: Facile Synthesis of Acridine Moiety-Containing Bis(tricyclic) Aromatic Enes. Org. Process Res. Dev. 2019, 23, 1740–1745. [Google Scholar] [CrossRef]
- Biedermann, P.U.; Agranat, I. Stereochemistry of Bistricyclic Aromatic Enes and Related Polycyclic Systems. In Polyarenes II; Siegel, J.S., Wu, Y.-T., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 177–277. [Google Scholar]
- Agranat, I. Comment on “Asymmetric Bistricyclic Aromatic Enes”. Chem. Eur. J. 2024, 30, e202302864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.; Xin, B.; Wang, Y.-L.; Li, C.; Chen, Z.-Q.; Yu, Q.; Huang, Z.-L.; Zhu, M.-Q. Geminal cross-coupling synthesis, ion-induced emission and lysosome imaging of cationic tetraarylethene oligoelectrolytes. Chem. Commun. 2018, 54, 3617–3620. [Google Scholar] [CrossRef]
- Stanoppi, M.; Lorbach, A. Boron-based donor-spiro-acceptor compounds exhibiting thermally activated delayed fluorescence (TADF). Dalton Trans. 2018, 47, 10394–10398. [Google Scholar] [CrossRef]
- Emsley, J. The Elements, 3rd ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Shimizu, M.; Hiyama, T. Organic Fluorophores Exhibiting Highly Efficient Photoluminescence in the Solid State. Chem. Asian J. 2010, 5, 1516–1531. [Google Scholar] [CrossRef]
- Gierschner, J.; Park, S.Y. Luminescent distyrylbenzenes: Tailoring molecular structure and crystalline morphology. J. Mater. Chem. C 2013, 1, 5818–5832. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Design Aspects of Luminescent Organic Crystals. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2014, 84, 131–149. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.K.; Pal, P.; Malik, S. Solid-state emissive organic chromophores: Design, strategy and building blocks. J. Mater. Chem. C 2020, 8, 788–802. [Google Scholar] [CrossRef]
- Gierschner, J.; Shi, J.; Milián-Medina, B.; Roca-Sanjuán, D.; Varghese, S.; Park, S. Luminescence in Crystalline Organic Materials: From Molecules to Molecular Solids. Adv. Opt. Mater. 2021, 9, 2002251. [Google Scholar] [CrossRef]
- Shimizu, M. The Journey to Precious-Metal-Free Organic Phosphors from Single-Benzene-Cored Fluorophores. Chem. Rec. 2021, 21, 1489–1505. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Valeur, B. Molecular Fluorescence–Principles and Applications; Wiley–VCH: Weinheim, Germany, 2002; pp. 208–213. [Google Scholar]
- Lippert, E. Dipolmoment und Elektronenstruktur von angeregten Molekülen. Naturforsch 1955, 10, 541–545. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. The Solvent Effect on Fluorescence Spectrum, Change of Solute-Solvent Interaction during the Lifetime of Excited Solute Molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Lippert, E. Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Z. Elektrochem. Ber. Bunsenges. Phys. Chem. 1957, 61, 962–975. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).