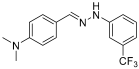
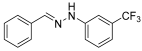
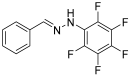
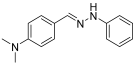
Solvent- and Catalyst-Free Environmentally Benign High Hydrostatic Pressure-Assisted Synthesis of Bioactive Hydrazones and the Evaluation of Their Stability Under Various Storage Conditions
Abstract
1. Introduction
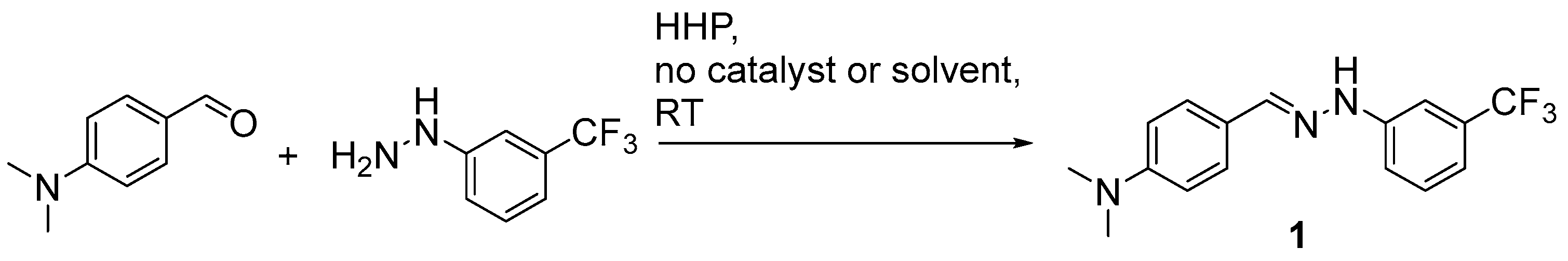
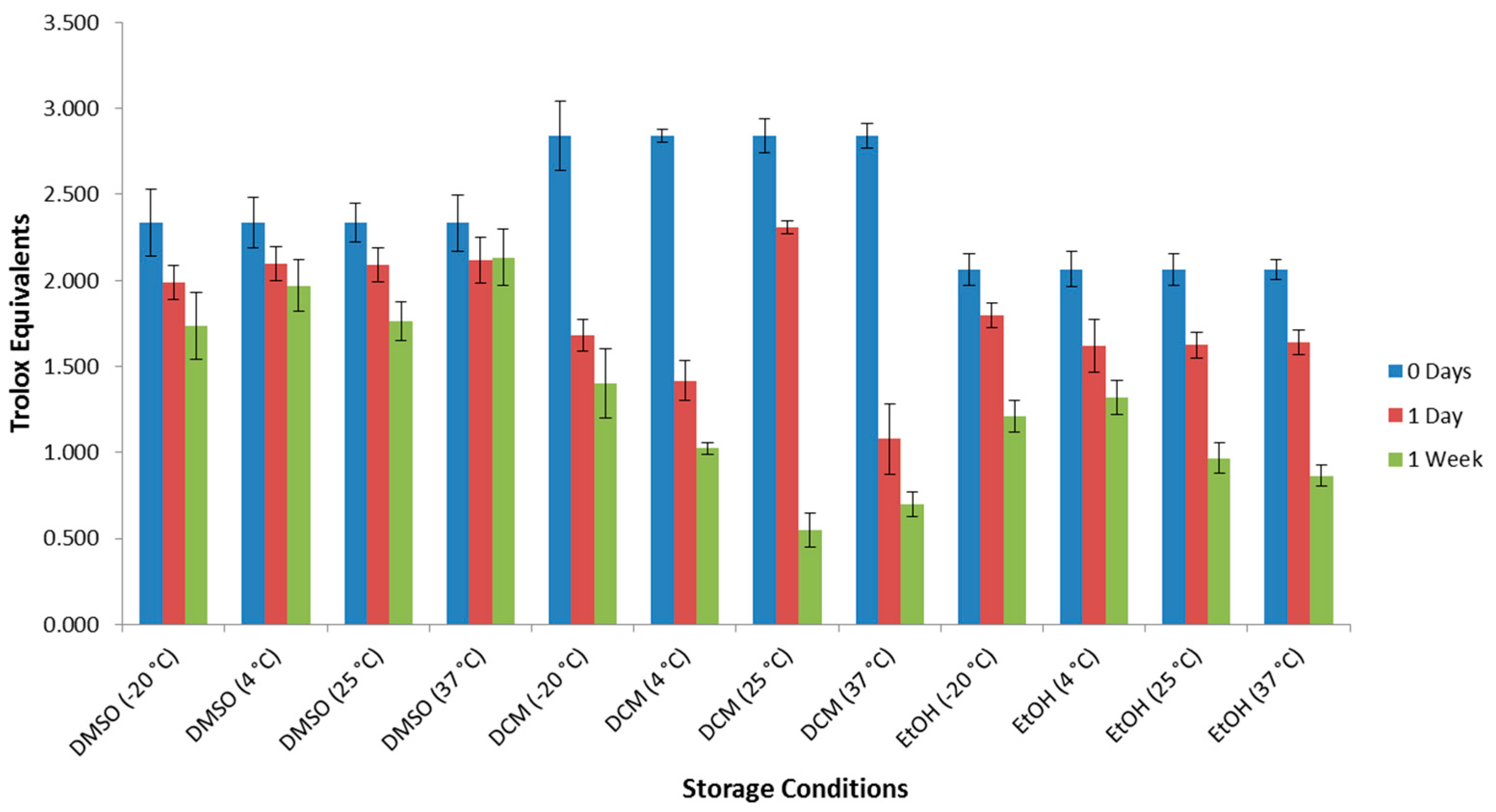
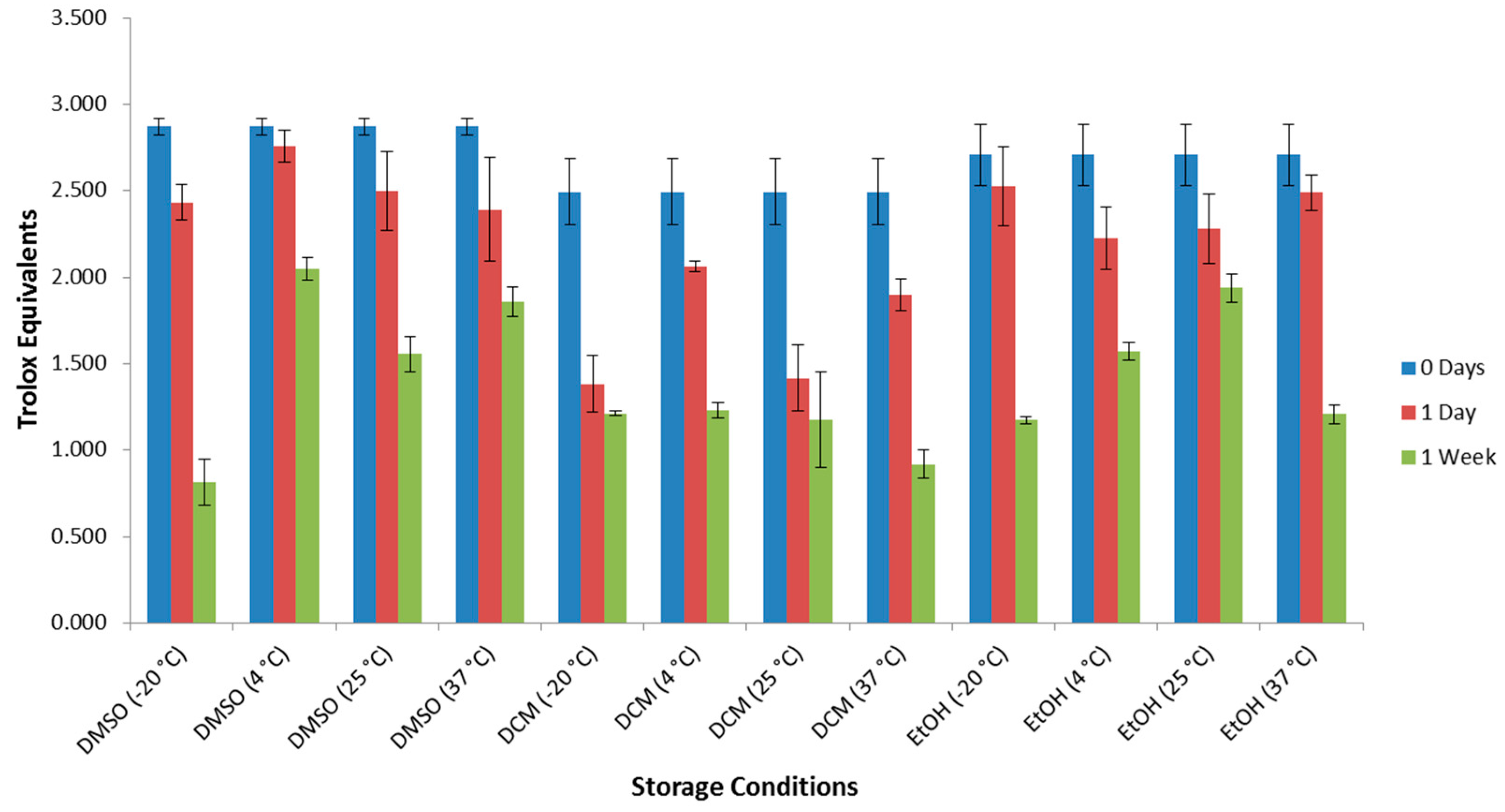
2. Results and Discussion
3. Materials and Methods
3.1. General Information
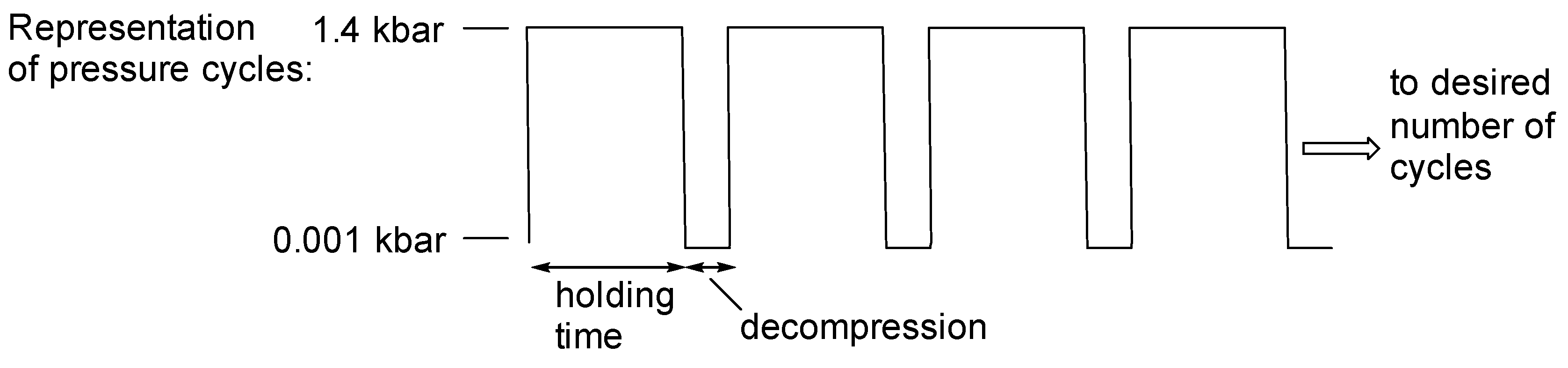
3.2. General Synthesis of the Hydrazones Under High Hydrostatic Pressure
3.3. Preparation of Samples for Decomposition Tests by Antioxidant Assays
3.4. Preparation of Samples for Decomposition Tests by GC-QTOFMS Analysis
3.5. Separation of Sample from DMSO
3.6. Gas Chromatography Mass Spectroscopy of the Samples
3.7. DPPH Radical Scavenging Assay of the Samples
3.8. ABTS Radical Scavenging Assay of the Samples
- 1H NMR (400 MHz, DMSO-d6) δ (ppm): 10.32 (s, 1H, NH), 7.85 (s, 1H, CH=), 7.51 (d, 2H, J = 8.0 Hz), 7.39 (t, 1H, J = 7.8 Hz), 7.34 (s, 1H), 7.27 (d, 1H, J = 7.9 Hz), 6.99 (d, 1H, J = 7.8 Hz), 6.71 (d, 2H, J = 4.1 Hz), 2.91 (s, 6H).
- 13C NMR (100.58 MHz, DMSO-d6) δ (ppm): 151.2, 147.1, 140.2, 130.7 (q, C-CF3, J = 31 Hz), 130.6, 127.8, 125.2 (q, CF3, J = 273 Hz), 123.7, 115.9, 114.4, 112.6, 108.1 (q, C-C-CF3, J = 4 Hz), 41.3
- 19F NMR (376 MHz, DMSO-d6) δ (ppm): −61.55
- HRMS C16H16F3N3: Calc.: 307.12963 Found: 307.12853
- (E)-1-benzylidene-2-phenylhydrazine (2) (ref. [8])
- 1H NMR (400 MHz, DMSO-d6), δ (ppm): 10.33 (s, 1H, NH), 7.86 (s, 1H, CH=), 7.63 (d, 2H, J = 4.1 Hz), 7.36 (t, 2H, J = 7.2 Hz), 7.27–7.19 (m, 3H), 7.08 (d, 2H, J = 4.0 Hz) 6.73 (t, 1H, J = 7.8 Hz)
- 13C NMR (100.58 MHz, DMSO-d6) δ (ppm): 145.7, 136.8, 136.3, 129.6, 129.1, 128.3, 126.1, 119. 2, 112.4.
- HRMS C13H12N2: Calc.: 196.10005, Found: 196.09898
- (E)-1-benzylidene-2-(3-(trifluoromethyl)-phenylhydrazine (3) (ref. [12])
- 1H NMR (400 MHz, DMSO-d6) δ 10.68 (s, 1H, NH), 7.93 (s, 1H, CH=), 7.66 (d, 2H, J = 4.1 Hz), 7.41–7.25 (m, 6H), 7.02 (d, 1H, J = 4.5 Hz).
- 13C NMR (100.58 MHz, DMSO-d6) δ 146.4, 138.7, 135.8, 130.5 (q, C-CF3, J = 30 Hz), 130.4, 129.0, 128.8, 126.3, 124.9 (q, CF3, J = 272 Hz), 116.0, 115.1, 108.3.
- 19F NMR (376 MHz, DMSO-d6) δ (ppm): −61.6
- HRMS C14H11F3N2: Calc.: 264.08743, Found: 264.09200
- (E)-1-benzylidene-2-(1,2,3,4,5-pentafluoro)-phenylhydrazine (4)
- 1H NMR (400 MHz, DMSO-d6) δ 10.28 (s, 1H, NH), 8.08 (s, 1H, CH=), 7.56 (d, 2H, J = 4.1 Hz), 7.36 (t, 2H, J = 8 Hz), 7.30 (t, 1H, J = 7.9 Hz).
- 13C NMR (100.58 MHz, DMSO-d6), δ 142.4, 139.3 (m), 136.7 (m), 135.2, 132.9 (m), 129.3, 129.1, 126.4, 121.7.
- 19F NMR (376 MHz, DMSO-d6) δ (ppm): −156.0, −164.5, −170.3
- HRMS C13H7F5N2: Calc.: 286.05294; Found: 286.04846
- (E)-N,N-dimethyl-4-((2-phenyl)hydrazynylidene)methyl)aniline (5)
- 1H NMR (400 MHz, DMSO-d6) δ 9.04 (s, 1H, NH), 6.87 (s, 1H, CH=), 6.55 (d, 2H, J = 3.9 Hz), 6.27(t, 2H, J = 7.5 Hz), 6.13 (d, 2H, J = 4.0 Hz), 5.78 (m, 3H), 1.98 (s, 6H, CH3).
- 13C NMR (100.58 MHz, DMSO-d6), δ 149.8, 145.4, 137.3, 126.7, 126.4, 123.2 117.4, 111.6, 111.2, 39.4.
- HRMS C15H17N3: Calc.: 239.14225 Found: 239.14263
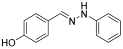
- (E)-4-((2-phenylhydrazono)methyl)phenol (6) (ref. [8])
- 1H NMR (400 MHz, DMSO-d6), δ (ppm) 9.98 (s, 1H, NH), 9.61 (s, broad, 1H, OH), 7.76 (s, 1H, CH=), 7.45 (d, 1H, J = 4.0 Hz), 7.16 (t, 3H, J = 4.1 Hz), 7.00 (d, 3H, J = 3.9 Hz), 6.78 (d, 1H, J = 4.2 Hz), 6.66 (t, 1H, J = 4.1 Hz).
- 13C NMR (100.58 MHz, DMSO-d6), δ (ppm) 158.1, 146.1, 137.5, 129.4, 127.6, 127.4, 118.5, 115.9, 112.1.
- HRMS C13H12N2O: Calc.: 212.09469; Found: 212.10139
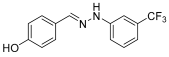
- (E)-1-(4-hydroxybenzylidene)-2-(3-(trifluoromethyl)-phenylhydrazine (7)
- 1H NMR (400 MHz, DMSO-d6) δ 10.39 (s, 1H, NH), 9.69 (s, 1H, OH), 7.85 (s, 1H, CH=), 7.51 (d, 2H, J = 4 Hz), 7.36 (t, 1H, J = 8.0 Hz), 7.31 (s, 1H), 7.24 (d, 1H, J = 3.9 Hz), 6.97 (d, 1H, J = 4.1 Hz), 6.82 (d, 2H, J = 8.3 Hz).
- 13C NMR (100.58 MHz, DMSO-d6), δ 158.6, 146.7, 139.4, 130.4 (q, C-CF3, J = 30 Hz), 130.4, 128.0, 124.9 (q, CF3, J = 271 Hz), 126.8, 116.1, 115.8, 114.4, 107.9.
- 19F NMR (376 MHz, DMSO-d6) δ (ppm): –61.6.
- HRMS C14H11F3N2O: Calc.: 280.08235; Found: 280.08752
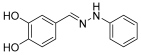
- (E)-1-(3,4-dihydroxybenzylidene)-2-phenylhydrazine (8)
- 1H NMR (400 MHz, DMSO-d6) δ 9.07 (s, 1H, NH), 8.93 (s, 2H, OH), 7.73 (s, 1H, CH=), 7.21–7.19 (s, 1H), 7.14 (m, 4H), 7.12 (d, 1H, J = 8.2 Hz), 6.97 (t, 1H, J = 8.0 Hz), 6.76 (t, 1H, J = 7.9 Hz)
- 13C NMR (100.58 MHz, DMSO-d6) δ 146.6, 146.2, 146.0, 137.9, 129.5, 127.8, 118.9, 118.5, 116.0, 112.3, 112.1.
- HRMS C13H10N2O2: Calc.: 226.07423; Found: 226.07351
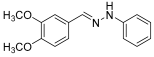
- (E)-1-(3,4-dimethoxybenzylidene)-2-phenylhydrazine (9) (ref. [8])
- 1H NMR (400 MHz, DMSO-d6), δ (ppm) 10.15 (s, 1H, NH), 7.80 (s, 1H, CH=), 7.30 (s, 1H), 7.19 (t, 1H, J = 4.1 Hz), 7.06 (d, 3H, J = 4 Hz), 6.92 (d, 2H, J = 4 Hz), 6.70 (t, 1H, J = 8.2 Hz), 3.79 (s, 3H), 3.74 (s, 3H).
- 13C NMR (100.58 MHz, DMSO-d6), δ (ppm) 149.6, 149.5, 146.0, 137.2, 129.5, 129.2, 119.9, 118.8, 112.3, 112.0, 108.1, 56.3, 55.9.
- HRMS C15H16N2O2: Calc.: 256.12118 Found: 256.12112
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacKay, A.P.; Berg, C.J.; Atrash, H.K. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet. Gynecol. 2001, 97, 533–538. [Google Scholar] [PubMed]
- American Coll. Gynecologists, Task Force on Hypertension in PE. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Zsengellér, Z.K.; Rajakumar, A.; Hunter, J.T.; Salahuddin, S.; Rana, S.; Stillman, I.E.; Karumanchi, A.S. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.E.; Lecarpentier, E.; Lo, A.; Salahuddin, S.; Gray, K.J.; Karumanchi, S.A.; Zsengellér, Z.K. AP39, a Modulator of Mitochondrial Bioenergetics, Reduces Antiangiogenic Response and Oxidative Stress in Hypoxia-Exposed Trophoblasts: Relevance for Preeclampsia Pathogenesis. Am. J. Pathol. 2019, 189, 104–114. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Horváth, B.; Zsengellėr, Z.; Bátkai, S.; Cao, Z.; Kechrid, M.; Holovac, E.; Erdėlyi, K.; Tanchian, G.; Liaudet, L.; et al. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: Therapeutic potential of mitochondrially targeted antioxidants. Free Radic. Biol. Med. 2012, 53, 1123–1138. [Google Scholar] [CrossRef]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The Antioxidant Therapy: New Insights in the Treatment of Hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.W.; Cindrova-Davies, T.; Spiroski, A.M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef]
- Török, B.; Sood, A.; Bag, S.; Tulsan, T.; Ghosh, S.; Borkin, D.; Kennedy, A.R.; Melanson, M.; Madden, R.; Zhou, W.; et al. Diaryl Hydrazones as Multifunctional Inhibitors of Amyloid Self-Assembly. Biochemistry 2013, 52, 1137–1148. [Google Scholar] [CrossRef]
- Peerannawar, S.; Horton, W.; Kokel, A.; Török, F.; Török, B.; Török, M. Theoretical and Experimental Analysis of the Antioxidant Features of Diarylhydrazones. Struct. Chem. 2017, 28, 391–402. [Google Scholar] [CrossRef]
- Kareem, H.S.; Ariffin, A.; Nordin, N.; Heidelberg, T.; Abdul-Aziz, A.; Kong, K.W.; Yehye, W.A. Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3, 4, 5-trimethoxy benzyl moiety. Eur. J. Med. Chem. 2015, 103, 497–505. [Google Scholar] [CrossRef]
- Carradori, F.; Ortuso, A.; Petzer, D.; Bagetta, C.; De Monte, D.; Secci, D.; De Vita, P.; Guglielmi, G.; Zengin, A.; Aktumsek, S.; et al. Design, synthesis and biochemical evaluation of novel multi-target inhibitors as potential anti-Parkinson agents. Eur. J. Med. Chem. 2018, 143, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Baier, A.; Kokel, A.; Horton, W.; Gizinska, E.; Pandey, G.; Szyszka, R.; Török, B.; Török, M. Organofluorine Hydrazone Derivatives as Multifunctional Anti-Alzheimer’s Agents with CK2 Inhibitory and Antioxidant Features. ChemMedChem 2021, 16, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Küçükgüzel, G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Domalaon, R.; Idowu, T.; Zhanel, G.G.; Schweizer, F. Antibiotic Hybrids: The Next Generation of Agents and Adjuvants against Gram-Negative Pathogens? Clin. Microbiol. Rev. 2018, 31, e00077-17. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Moi, D.; Balboni, G.; Vertuani, S.; Manfredini, S.; Onnis, V. Benzofuran hydrazones as potential scaffold in the development of multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity. Eur. J. Med. Chem. 2018, 156, 118–125. [Google Scholar] [CrossRef]
- Zsengeller, Z.K.; Mastyugin, M.; Lo, A.; Salahuddin, S.; Karumanchi, A.; Török, M.; Török, B. Organofluorine Hydrazones Preventing Oxidant Stress in an in vitro Model of Preeclampsia. Hypertension 2021, 78, AP236. [Google Scholar] [CrossRef]
- Török, B.; Schäfer, C. (Eds.) Non-Traditional Activation Methods in Green and Sustainable Applications: Microwaves, Ultrasounds, Photo, Electro and Mechanochemistry and High Hydrostatic Pressure; Elsevier: Cambridge, MA, USA; Oxford, UK, 2021. [Google Scholar]
- Holzapfel, W.; Isaacs, N. High Pressure Techniques in Chemistry and Physics: A Practical Approach; Oxford University Press: Oxford, UK, 1997; p. 400. [Google Scholar]
- Chataigner, I.; Maddaluno, J. High-Pressure Synthesis: An Eco-friendly Chemistry. In Activation Methods: Sonochemistry and High Pressure, 1st ed.; Goddard, J.-P., Malacria, M., Ollivier, C., Eds.; ISTE Ltd.: London UK; John Wiley & Sons, Inc.: New York, NY, USA, 2019; pp. 95–149. [Google Scholar]
- Yamamoto, K. High hydrostatic pressure in food industry applications. In Non-Traditional Activation Methods in Green and Sustainable Applications: Microwaves, Ultrasounds, Photo, Electro and Mechanochemistry and High Hydrostatic Pressure; Török, B., Schäfer, C., Eds.; Elsevier: Cambridge, MA, USA; Oxford, UK, 2021; pp. 559–574. [Google Scholar]
- Dauben, W.G.; Krabbenhoft, H.O. Organic Reactions at High Pressure. Cycloadditions with Furans. J. Am. Chem. Soc. 1976, 98, 1992–1993. [Google Scholar] [CrossRef]
- Tomin, A.; Lazarev, A.; Bere, M.P.; Redjeb, H.; Török, B. Selective Reduction of Ketones using Water as a Hydrogen Source under High Hydrostatic Pressure. Org. Biomol. Chem. 2012, 10, 7321–7326. [Google Scholar] [CrossRef]
- Horinouchi, R.; Kamei, K.; Watanabe, R.; Hieda, N.; Tatsumi, N.; Nakano, K.; Ichikawa, Y.; Kotsuki, H. Enantioselective Synthesis of Quaternary Carbon Stereogenic Centers through the Primary Amine-Catalyzed Michael Addition Reaction of α-Substituted Cyclic Ketones at High Pressure. Eur. J. Org. Chem. 2015, 20, 4457–4463. [Google Scholar] [CrossRef]
- Hayashi, Y.; Tsuboi, W.; Shoji, M.; Suzuki, N. Application of high pressure induced by water-freezing to the direct catalytic asymmetric three-component List− Barbas− Mannich reaction. J. Am. Chem. Soc. 2003, 125, 11208–11209. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y. The Asymmetric Catalytic Mannich Reaction Catalyzed by Organocatalyst. A Personal Account. J. Synt. Org. Chem. 2014, 72, 1228–1238. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. Enhanced synthesis of isoamyl acetate using an ionic liquid–alcohol biphasic system at high hydrostatic pressure. J. Mol. Catal. B Enzym. 2010, 67, 36–40. [Google Scholar] [CrossRef]
- Misumi, Y.; Matsumoto, K. Diastereoselective Asymmetric Nitro-Aldol Reaction of α-Amino Aldehydes under High Pressure without Catalyst. Angew. Chem. Int. Ed. 2002, 41, 1031–1033. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Dudzinski, K.; Łyżwa, D. Effect of high pressure on the organocatalytic asymmetric Michael reaction: Highly enantioselective synthesis of γ-nitroketones with quaternary stereogenic centers. Org. Lett. 2011, 13, 3624–3627. [Google Scholar] [CrossRef]
- Fedotova, A.; Crousse, B.; Chataigner, I.; Maddaluno, J.; Rulev, A.Y.; Legros, J. Benefits of a dual chemical and physical activation: Direct aza-Michael addition of anilines promoted by solvent effect under high pressure. J. Org. Chem. 2015, 80, 10375–10379. [Google Scholar] [CrossRef]
- Rulev, A.Y.; Kotsuki, H.; Maddaluno, J. High pressure promoted aza-Michael addition of primary and secondary amines to α-substituted acrylates. Green Chem. 2012, 14, 503–508. [Google Scholar] [CrossRef]
- Kumamoto, K.; Fukada, I.; Kotsuki, H. Diels-Alder reaction of thiophene: Dramatic effects of high-pressure/solvent-free conditions. Angew. Chem. Int. Ed. 2004, 43, 2015–2017. [Google Scholar] [CrossRef]
- Loco, D.; Spezia, R.; Cartier, F.; Chataigner, I.; Piquemal, J.-P. Solvation effects drive the selectivity in Diels–Alder reaction under hyperbaric conditions. Chem. Commun. 2020, 56, 6632–6635. [Google Scholar] [CrossRef]
- Łyżwa, D.; Dudzinski, K.; Kwiatkowski, P. High-Pressure Accelerated Asymmetric Organocatalytic Friedel–Crafts Alkylation of Indoles with Enones: Application to Quaternary Stereogenic Centers Construction. Org. Lett. 2012, 14, 1540–1543. [Google Scholar] [CrossRef]
- Xie, G.; Lazarev, A.; Török, B. High Pressure Initiated Solvent and Catalyst-free Instant Paal-Knorr Reactions. Green Chem. 2023, 25, 1582–1587. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Baron, M.; Chamayou, A.; André-Barrès, C.; Guidetti, B.; Baltas, M. Solvent-free mechanochemical route for green synthesis of pharmaceutically attractive phenol-hydrazones. RSC Adv. 2014, 4, 56736–56742. [Google Scholar] [CrossRef]
- Al-Ajely, H.M. Green synthesis of new hippuric hydrazones. Asian J. Green Chem. 2020, 4, 142–148. [Google Scholar]
- Sayed, A.R.; Gomha, S.M.; Abd El-lateef, H.M.; Abolibda, T.Z. L-proline catalyzed green synthesis and anticancer evaluation of novel bioactive benzil bis-hydrazones under grinding technique. Green Chem. Lett. Rev. 2021, 14, 180–189. [Google Scholar] [CrossRef]
- Ameen, S.T.S.; Vilvanathan, A.; Khader, S.Z.A.; Mahalingam, G. Microwave-assisted Green Synthesis of β-Diketone Hydrazone Derivatives and Evaluation of their Antioxidant and Antibacterial Activities. Curr. Microw. Chem. 2020, 7, 222–229. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, H.M.; Matar, I.K.; El-Sayed, A.A. Green synthesis, molecular docking and anticancer activity of novel 1,4-dihydropyridine-3,5-Dicarbohydrazones under grind-stone chemistry. Green Chem. Lett. Rev. 2020, 13, 6–17. [Google Scholar] [CrossRef]
- Bamoniri, A.; Mirjalili, B.B.F.; Moshtael-Arani, N. Nano BF3·SiO2: A green heterogeneous solid acid for synthesis of formazan dyes under solvent-free condition. J. Mol. Catal. A Chem. 2014, 393, 272–278. [Google Scholar] [CrossRef]
- Brahmachari, G.; Karmakar, I. sp2-C–H Acetoxylation of Diversely Substituted (E)-1-(Arylmethylene)-2-phenylhydrazines Using PhI(OAc)2 as Acetoxy Source at Ambient Conditions. Eur. J. Org. Chem. 2019, 2019, 5925–5933. [Google Scholar] [CrossRef]
- Datta, S.; Sood, A.; Török, M. Steps Toward Green Peptide Synthesis. Curr. Org. Synth. 2011, 8, 262–280. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| Entry | P (kbar) | Holding Time (min) | Decompression Time (s) | Number of Cycles | Yield b (%) |
| 1 | 1.4 | 0.5 | 5 | 10 | 79 |
| 2 | 1.4 | 0.5 | 5 | 5 | 81 |
| 3 | 1.4 | 0.5 | 5 | 2 | 83 |
| 4 | 1.4 | 0.5 | 5 | 1 | 79 |
| 5 | 1.4 | 1 | 5 | 10 | 96 |
| 6 | 1.4 | 1 | 5 | 5 | 86 |
| 7 | 1.4 | 1 | 5 | 2 | 79 |
| 8 | 1.4 | 1 | 5 | 1 | 75 |
| 9 | 1.4 | 2 | 5 | 10 | 88 |
| 10 | 1.4 | 2 | 5 | 5 | 93 |
| 11 | 1.4 | 2 | 5 | 2 | 87 |
| 12 | 1.4 | 2 | 5 | 1 | 80 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | Product | Time (min) | HHP Yield b (%) | Control Yield c (%) |
| 1 | 4-N(CH3)2 | 3-CF3 | 1 | 10 | 96 | 75 |
| 2 d | H | H | 2 | 60 | 96 | 86 |
| 3 | H | 3-CF3 | 3 | 10 | 98 | 98 |
| 4 | H | 2,3,4,5,6-F | 4 | 10 | 96 | 77 |
| 5 | 4-N(CH3)2 | H | 5 | 10 | 77 | 70 |
| 6 | 4-OH | H | 6 | 10 | 75 | 64 |
| 7 | 4-OH | 3-CF3 | 7 | 10 | 77 | 37 |
| 8 d | 3,4-(OH)2 | H | 8 | 60 | 54 | 24 |
| 9 d | 3,4-(OCH3)2 | H | 9 | 60 | 76 | 70 |
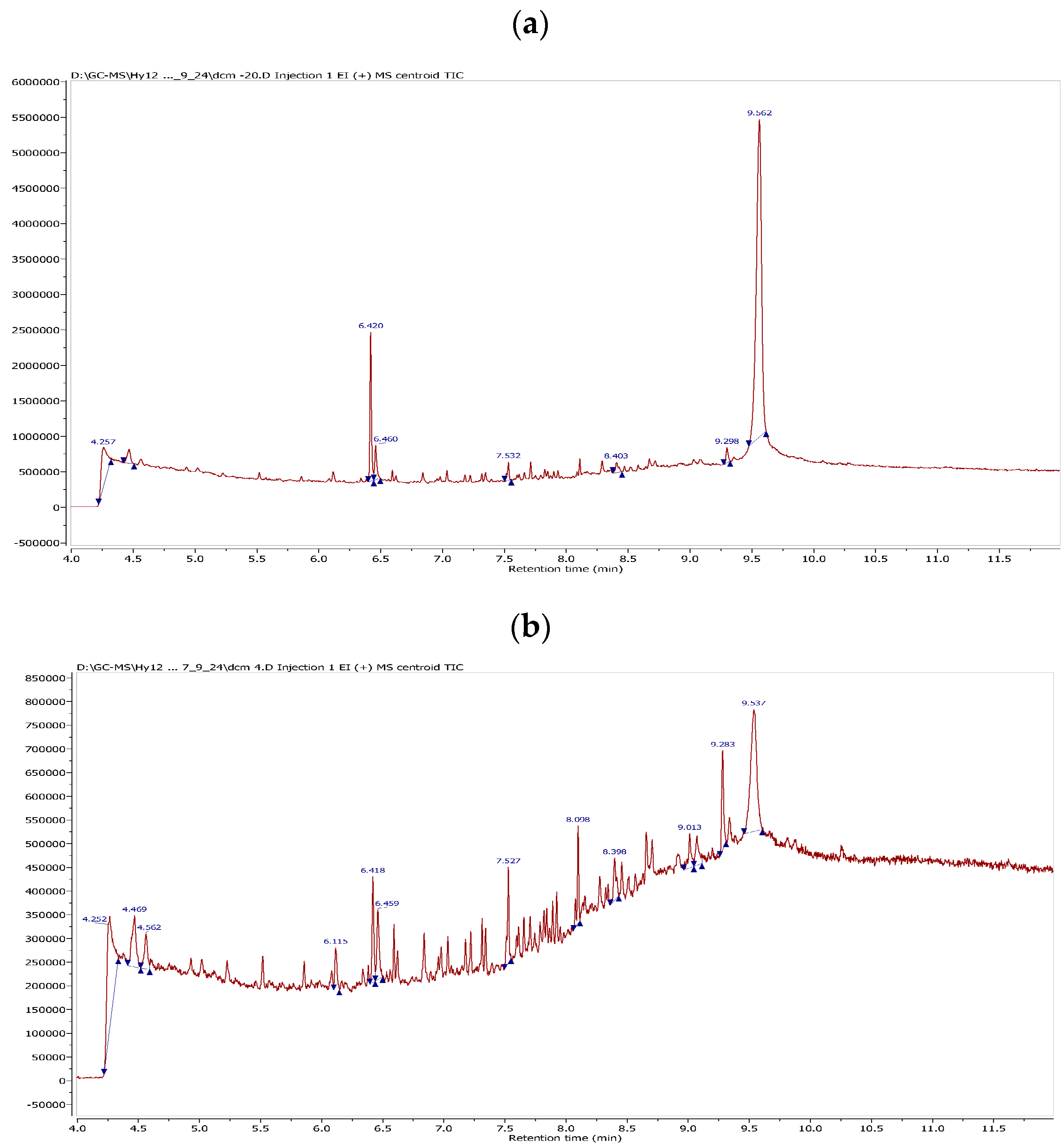
| Entry | Storage Conditions b | Remaining Antioxidant Activity by DPPH Assay (%) c | Remaining Antioxidant Activity by ABTS Assay (%) d | 1 Concentration by GC-QTOF MS Analysis (%) e | Notes |
|---|---|---|---|---|---|
| 1 | DMSO/−20 °C | 74 | 28 | 18 | multiple products |
| 2 | DMSO/4 °C | 84 | 71 | 8 | multiple products |
| 3 | DMSO/25 °C | 76 | 54 | 8 | multiple products |
| 4 | DMSO/37 °C | 92 | 65 | 13 | multiple products |
| 5 | DCM/−20 °C | 49 | 49 | 72 | hydrolysis products |
| 6 | DCM/4 °C | 36 | 49 | 25 | hydrolysis and many related products |
| 7 | DCM/25 °C | 19 | 47 | 64 | mainly hydrolysis products |
| 8 | DCM/37 °C | 25 | 37 | 47 | hydrolysis products dominate, with many small peaks |
| 9 | EtOH/−20 °C | 59 | 43 | 12 | multiple products |
| 10 | EtOH/4 °C | 64 | 58 | 7 | hydrolysis products dominate, with many small peaks |
| 11 | EtOH/25 °C | 47 | 72 | 7 | hydrolysis products dominate, with many small peaks |
| 12 | EtOH/37 °C | 42 | 45 | 15 | multiple products |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.; Adhamidhi, F.; Mastyugin, M.; Fusco, A.R.; Lazarev, A.; Zsengeller, Z.K.; Török, M.; Török, B. Solvent- and Catalyst-Free Environmentally Benign High Hydrostatic Pressure-Assisted Synthesis of Bioactive Hydrazones and the Evaluation of Their Stability Under Various Storage Conditions. Molecules 2024, 29, 5287. https://doi.org/10.3390/molecules29225287
Costa M, Adhamidhi F, Mastyugin M, Fusco AR, Lazarev A, Zsengeller ZK, Török M, Török B. Solvent- and Catalyst-Free Environmentally Benign High Hydrostatic Pressure-Assisted Synthesis of Bioactive Hydrazones and the Evaluation of Their Stability Under Various Storage Conditions. Molecules. 2024; 29(22):5287. https://doi.org/10.3390/molecules29225287
Chicago/Turabian StyleCosta, Maximilian, Frances Adhamidhi, Maxim Mastyugin, Adrianna R. Fusco, Alexander Lazarev, Zsuzsanna K. Zsengeller, Marianna Török, and Béla Török. 2024. "Solvent- and Catalyst-Free Environmentally Benign High Hydrostatic Pressure-Assisted Synthesis of Bioactive Hydrazones and the Evaluation of Their Stability Under Various Storage Conditions" Molecules 29, no. 22: 5287. https://doi.org/10.3390/molecules29225287
APA StyleCosta, M., Adhamidhi, F., Mastyugin, M., Fusco, A. R., Lazarev, A., Zsengeller, Z. K., Török, M., & Török, B. (2024). Solvent- and Catalyst-Free Environmentally Benign High Hydrostatic Pressure-Assisted Synthesis of Bioactive Hydrazones and the Evaluation of Their Stability Under Various Storage Conditions. Molecules, 29(22), 5287. https://doi.org/10.3390/molecules29225287