Green Synthesis of 2-Mercapto 5,6-Dihydro-4H-1,3-Thiazines via Sequential C–S Couplings
Abstract
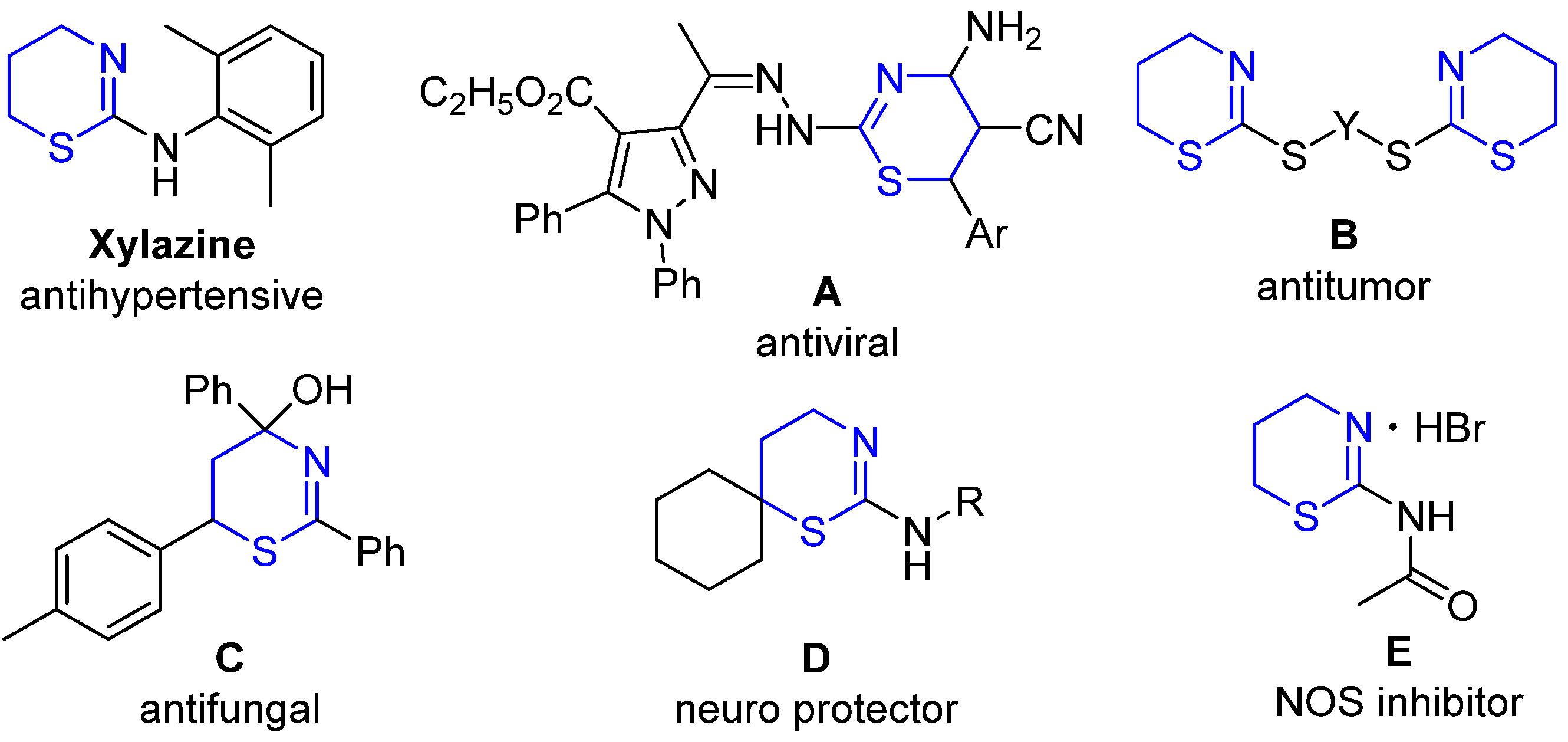
1. Introduction
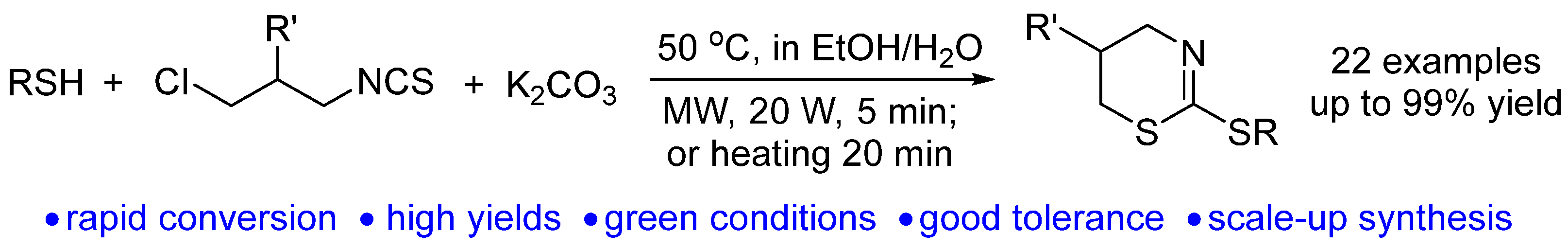
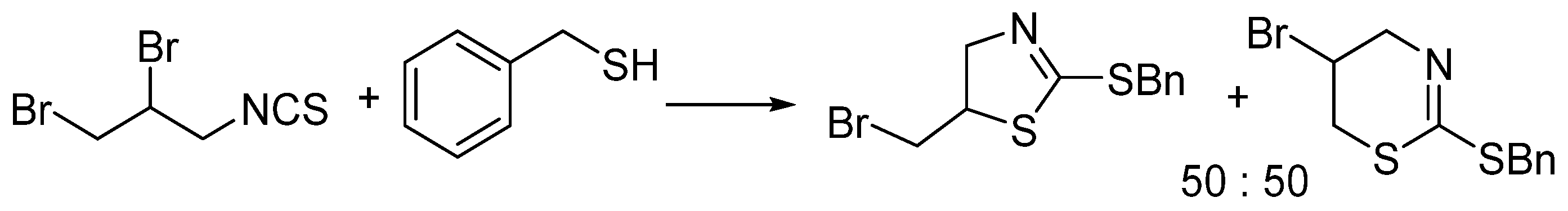
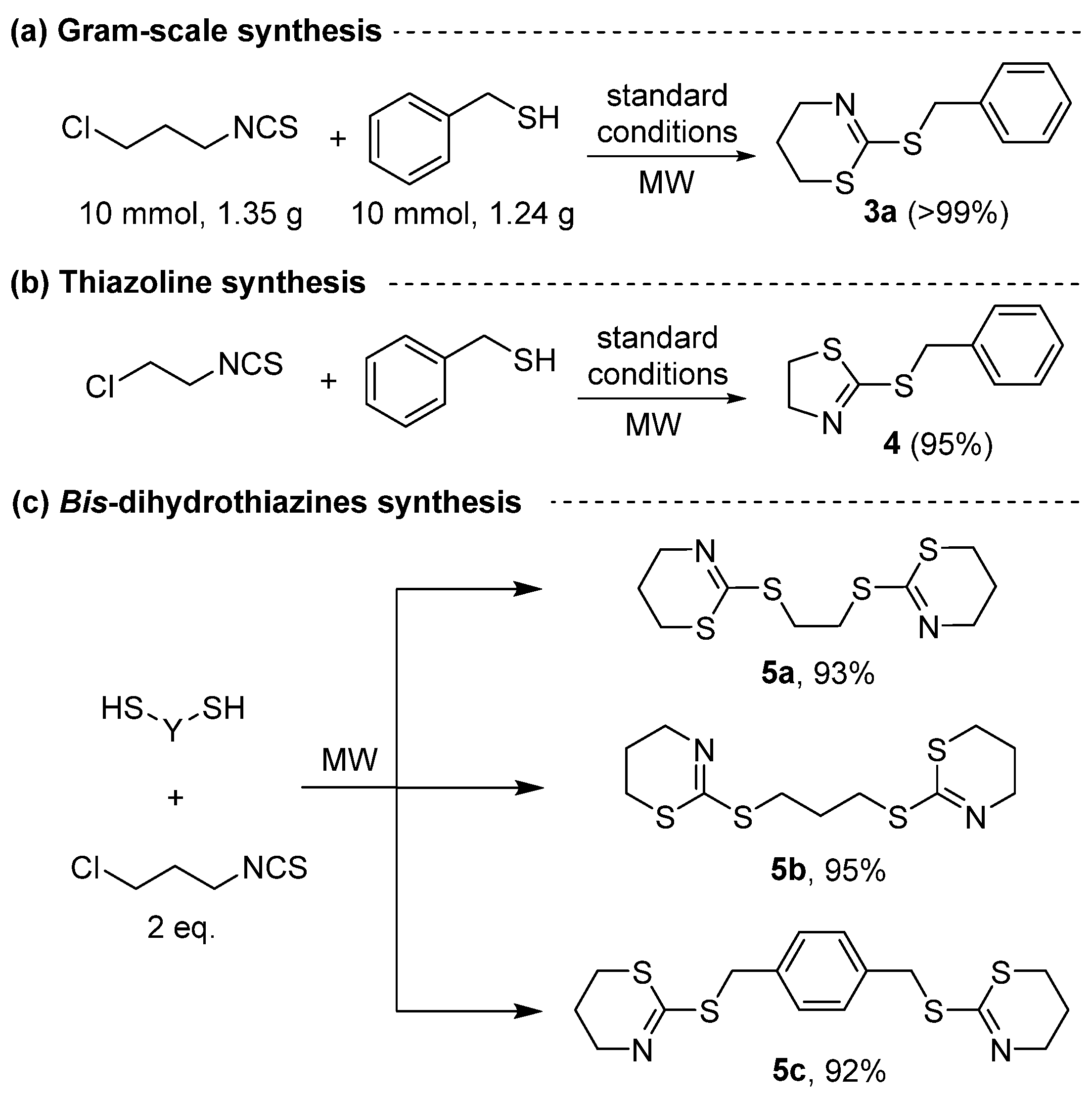
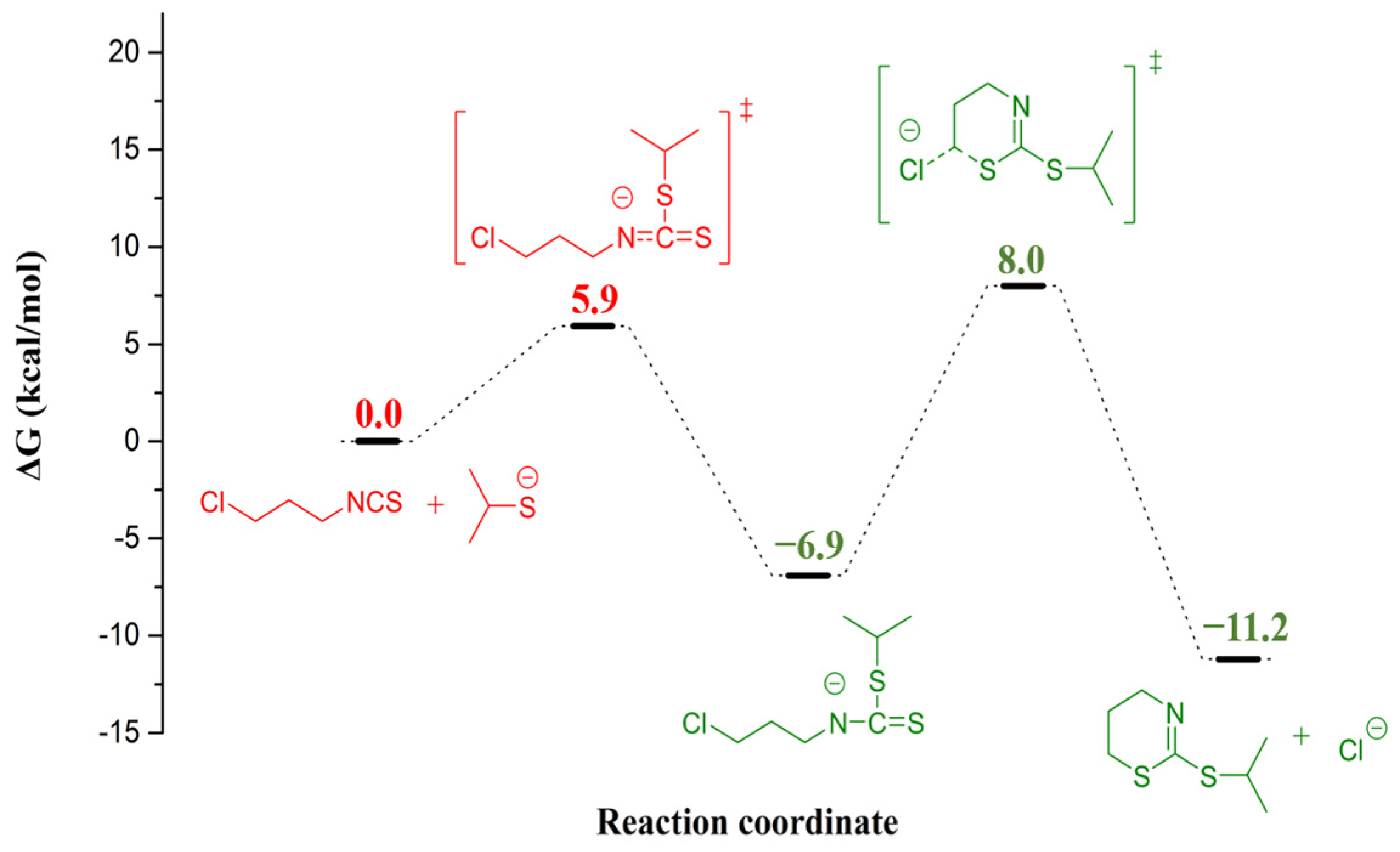
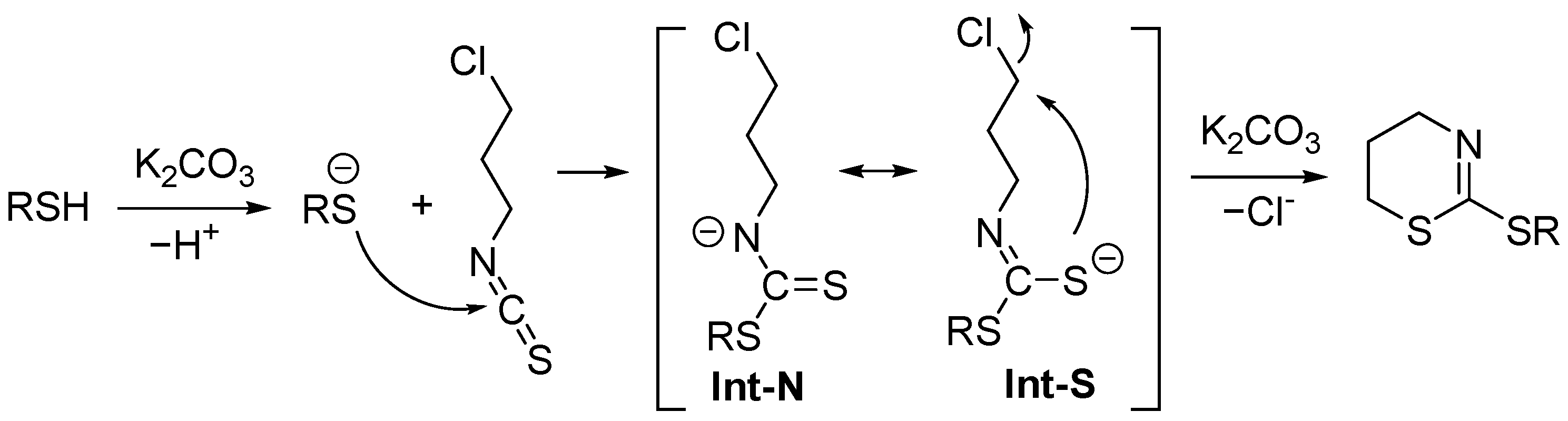
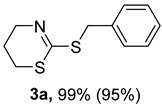
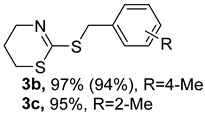
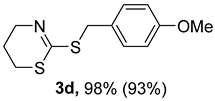
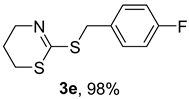
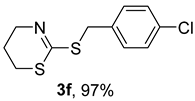
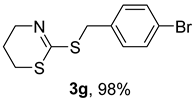
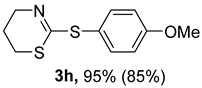
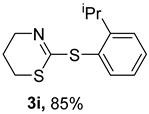
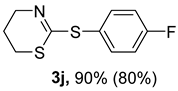
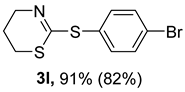
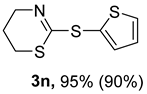
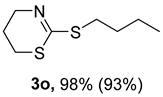
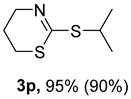
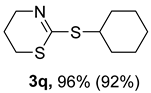
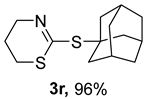
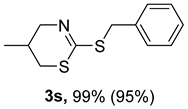
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badshah, S.L.; Naeem, A. Bioactive Thiazine and Benzothiazine Derivatives: Green Synthesis Methods and Their Medicinal Importance. Molecules 2016, 21, 1054. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.A.; Constable, P.D.; Huhn, J.C.; Morin, D.E. Sedation with xylazine and lumbosacral epidural administration of lidocaine and xylazine for umbilical surgery in calves. J. Am. Vet. Med. Assoc. 1999, 214, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Gomha, S.; Elaziz, M.A.; Serag, N. Synthesis and evaluation of some novel thaizole and 1,3-thiazines as potent agents against the rabies virus. Turk. J. Chem. 2016, 40, 441–453. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, B.; Xu, C.; Wu, W.P. Synthesis and Antitumor Activity of the Thiazoline and Thiazine Multithioether. Int. J. Org. Chem. 2012, 2, 117–120. [Google Scholar] [CrossRef]
- Koketsu, M.; Tanaka, K.; Takenaka, Y.; Kwong, C.D.; Ishihara, H. Synthesis of 1,3-thiazine derivatives and their evaluation as potential antimycobacterial agents. Eur. J. Pharm. Sci. 2002, 15, 307–310. [Google Scholar] [CrossRef]
- Blokhina, S.V.; Volkova, T.V.; Ol’khovich, M.V.; Sharapova, A.V.; Proshin, A.N.; Bachurin, B.O.; Perlovich, G.L. Synthesis, biological activity, distribution and membrane permeability of novel spiro-thiazines as potent neuroprotectors. Eur. J. Med. Chem. 2014, 77, 8–17. [Google Scholar] [CrossRef]
- Trofimova, T.P.; Zefirova, O.N.; Mandrugin, A.A.; Fedoseev, V.M.; Peregud, D.I.; Onufriev, M.V.; Gulyaeva, N.V.; Proskuryakov, S.Y. Synthesis and Study of NOS-Inhibiting Activity of 2-N-Acylamino-5,6-dihydro-4H-1,3-thiazine. Mosc. Univ. Chem. Bull. 2008, 63, 274–277. [Google Scholar] [CrossRef]
- Liepa, A.J.; Saubern, S. 2-Substituted 5,6-Dihydro-1,3-thiazines from Nitriles and Thiocyanates: A Modification of Meyers’s Method. Aust. J. Chem. 1997, 50, 755–758. [Google Scholar] [CrossRef]
- Nishio, T.; Konno, Y.; Ori, M.; Sakamoto, M. Stereoselective Synthesis of 4H-5,6-Dihydro-1,3-thiazines by the Reaction of 3-N-Acylamino Alcohols with Lawesson’s Reagent. Eur. J. Org. Chem. 2001, 18, 3553–3557. [Google Scholar] [CrossRef]
- Borisov, A.V.; Osmanov, V.K.; Sokolov, I.G.; Borisova, G.N.; Matsulevich, Z.V. Unusual Transformation in the Cycloaddition of Aroyliminochloromethanesulfenyl Chlorides to 3,3-Dimethyl-2-butene. Chem. Heterocycl. Compd. 2002, 38, 1150–1151. [Google Scholar] [CrossRef]
- Murai, T.; Niwa, H.; Kimura, T.; Shibahara, F. Iodo-Cyclization of N-Homoallyl Thioamides Leading to 2,4-Diaryl-5,6-dihydro-4H-1,3-thiazines. Chem. Lett. 2004, 33, 508–509. [Google Scholar] [CrossRef]
- Marchand, A.H.; Collet, S.; Guingant, A.; Pradère, J.P.; Toupet, L. Highly diastereoselective cycloaddition reactions of variously substituted 1-thia- and 1-thia-3-aza-buta-1,3-dienes. Synthesis of enantiomerically pure 5,6-dihydro-4H-[1,3]thiazines and 3,4-dihydro-2H-thiopyrans. Tetrahedron 2004, 60, 1827–1839. [Google Scholar] [CrossRef]
- Leflemme, N.; Dallemangne, P.; Rault, S. A convenient synthesis of dihydro- and tetrahydro-1,3-thiazine derivatives from β-aryl-β-amino acids. Tetrahedron Lett. 2004, 45, 1503–1505. [Google Scholar] [CrossRef]
- Kodama, Y.; Ori, M.; Nishio, T. Thionation of N-(ω-Halogenoalkyl)-Substituted Amides with Lawesson’s Reagent: Facile Synthesis of 4,5-Dihydro-1,3-thiazoles and 5,6-Dihydro-4H-1,3-thiazines. Helv. Chim. Acta 2005, 88, 187–193. [Google Scholar] [CrossRef]
- Li, Y.Y.; Kong, S.F.; Yang, F.J.; Xu, W.Q. Protective Effects of 2-Amino-5,6-dihydro-4H-1,3-thiazine and Its Derivative against Radiation-Induced Hematopoietic and Intestinal Injury in Mice. Int. J. Mol. Sci. 2018, 19, 1530. [Google Scholar] [CrossRef]
- Wan, J.P.; Pan, Y.H.; Mao, H.; Chen, Y.H.; Pan, Y.J. Microwave assisted three-component reaction for rapid synthesis of some 5, 6-dihydro-4H-1,3-thiazine derivatives under solvent-free conditions. Synth. Commun. 2010, 40, 709–716. [Google Scholar] [CrossRef]
- Bhowmik, S.; Mishra, A.; Batra, S. A novel stereoselective one-pot synthesis of 2-susbstituted amino-5,6-dihydro-4H-1,3-thiazines via primary allylamines afforded from Morita–Baylis–Hillman acetates. RSC Adv. 2011, 1, 1237–1244. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ohnaka, K.; Endo, T. Reprocessable Aliphatic Polydithiourethanes Based on the Reversible Addition Reaction of Diisothiocyanates and Dithiols. Macromolecules 2019, 52, 6080–6087. [Google Scholar] [CrossRef]
- Hanek, K.; Duch, A.; Frąckowiak, D.; Żak, P. Mechanochemical Synthesis of β-Sulfenylated Ketones and Esters. ChemSusChem 2024, 2024, e202401591. [Google Scholar] [CrossRef]
- Bołt, M.; Delaude, L.; Żak, P. Rhodium catalysts with superbulky NHC ligands for the selective α-hydrothiolation of alkynes. Dalton Trans. 2022, 51, 4429–4434. [Google Scholar] [CrossRef]
- Canudo, G.; Herrera, R.P.; Gimeno, C.M. New Twists in the Chemistry of Thioureas: 1,3-Thiazolidines as a Vector of Sustainability. Chem. Eur. J. 2024, 30, e202402812. [Google Scholar] [CrossRef] [PubMed]
- HG/T2354-2010; Chromatographic Silica Gel. Qingdao Haiyang Chemical Co., Ltd.: Qingdao, China, 2024.


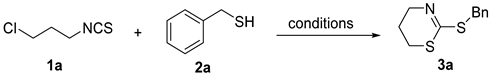 | |||||
|---|---|---|---|---|---|
| Entry | Base (eq.) | Solvent | Temp. (°C) | Time (min) | 3a (%) b |
| 1 | K2CO3 (0.6) | neat | 25 | 60 | 30 |
| 2 | K2CO3 (0.6) | H2O | 25 | 60 | 49 |
| 3 | K2CO3 (0.6) | EtOH | 25 | 60 | 31 |
| 4 | K2CO3 (0.6) | EtOH/H2O | 25 | 60 | 78 |
| 5 | K2CO3 (0.6) | EtOH/H2O | 50 | 60 | 96 |
| 6 | K2CO3 (0.6) | EtOH/H2O | 50 | 20 | 95 |
| 7 | NaOH (1.2) | EtOH/H2O | 50 | 20 | 94 |
| 8 | Na2CO3 (0.6) | EtOH/H2O | 50 | 20 | 94 |
| 9 | NaHCO3 (1.2) | EtOH/H2O | 50 | 20 | 92 |
| 10 | Et3N (1.2) | EtOH/H2O | 50 | 20 | 94 |
| 11 | DBU (1.2) | EtOH/H2O | 50 | 20 | 94 |
| 12 | K2CO3 (0.6) | Acetone | 50 | 20 | 91 |
| 13 | K2CO3 (0.5) | EtOH/H2O | 50 | 20 | 92 |
| 14 | K2CO3 (0.75) | EtOH/H2O | 50 | 20 | 95 |
| 15 c | K2CO3 (0.6) | EtOH/H2O | 50 | 5 | 99 |
| 16 c | K2CO3 (0.6) | EtOH/H2O | 80 | 5 | 95 |
| 17 d | K2CO3 (0.6) | EtOH/H2O | 50 | 5 | 98 |
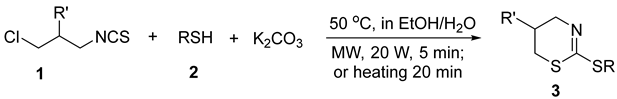 | ||
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, S.; Pan, L.; Bi, X.; Shi, E. Green Synthesis of 2-Mercapto 5,6-Dihydro-4H-1,3-Thiazines via Sequential C–S Couplings. Molecules 2024, 29, 5255. https://doi.org/10.3390/molecules29225255
Liu W, Wang S, Pan L, Bi X, Shi E. Green Synthesis of 2-Mercapto 5,6-Dihydro-4H-1,3-Thiazines via Sequential C–S Couplings. Molecules. 2024; 29(22):5255. https://doi.org/10.3390/molecules29225255
Chicago/Turabian StyleLiu, Wenjie, Shuo Wang, Li Pan, Xiaojing Bi, and Enxue Shi. 2024. "Green Synthesis of 2-Mercapto 5,6-Dihydro-4H-1,3-Thiazines via Sequential C–S Couplings" Molecules 29, no. 22: 5255. https://doi.org/10.3390/molecules29225255
APA StyleLiu, W., Wang, S., Pan, L., Bi, X., & Shi, E. (2024). Green Synthesis of 2-Mercapto 5,6-Dihydro-4H-1,3-Thiazines via Sequential C–S Couplings. Molecules, 29(22), 5255. https://doi.org/10.3390/molecules29225255





