Effects of Modified Oil Palm Kernel Expeller Fiber Enhanced via Enzymolysis Combined with Hydroxypropylation or Crosslinking on the Properties of Heat-Induced Egg White Protein Gel
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Different Modifications on Chemical Components of OPKEDF
2.2. Surface Area and Color Analysis of OPKEDF
2.3. Structural Analysis of OPKEDF
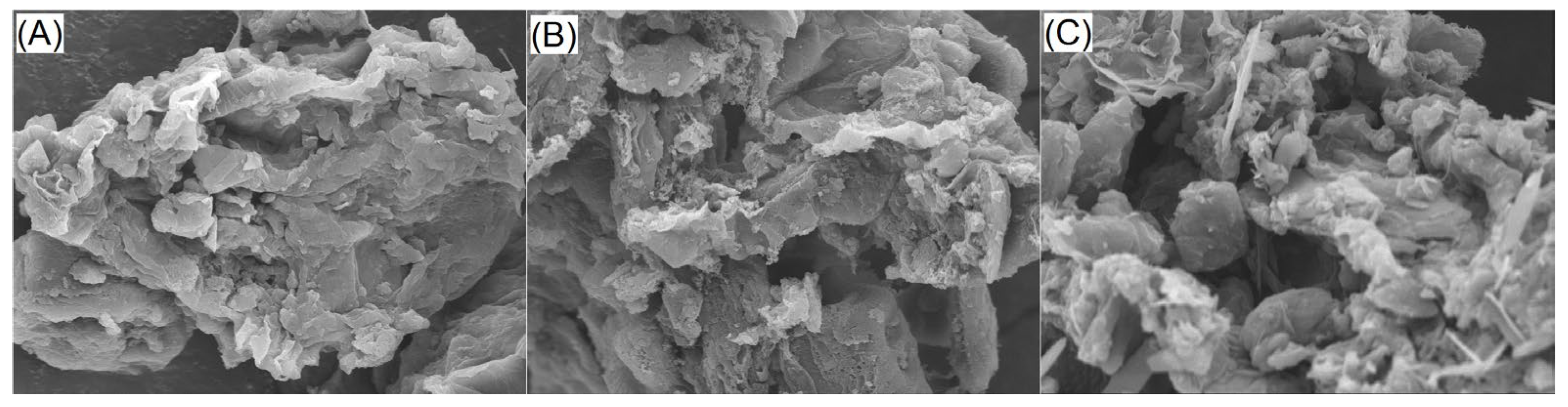
2.3.1. Surface Microstructure of OPKEDFs
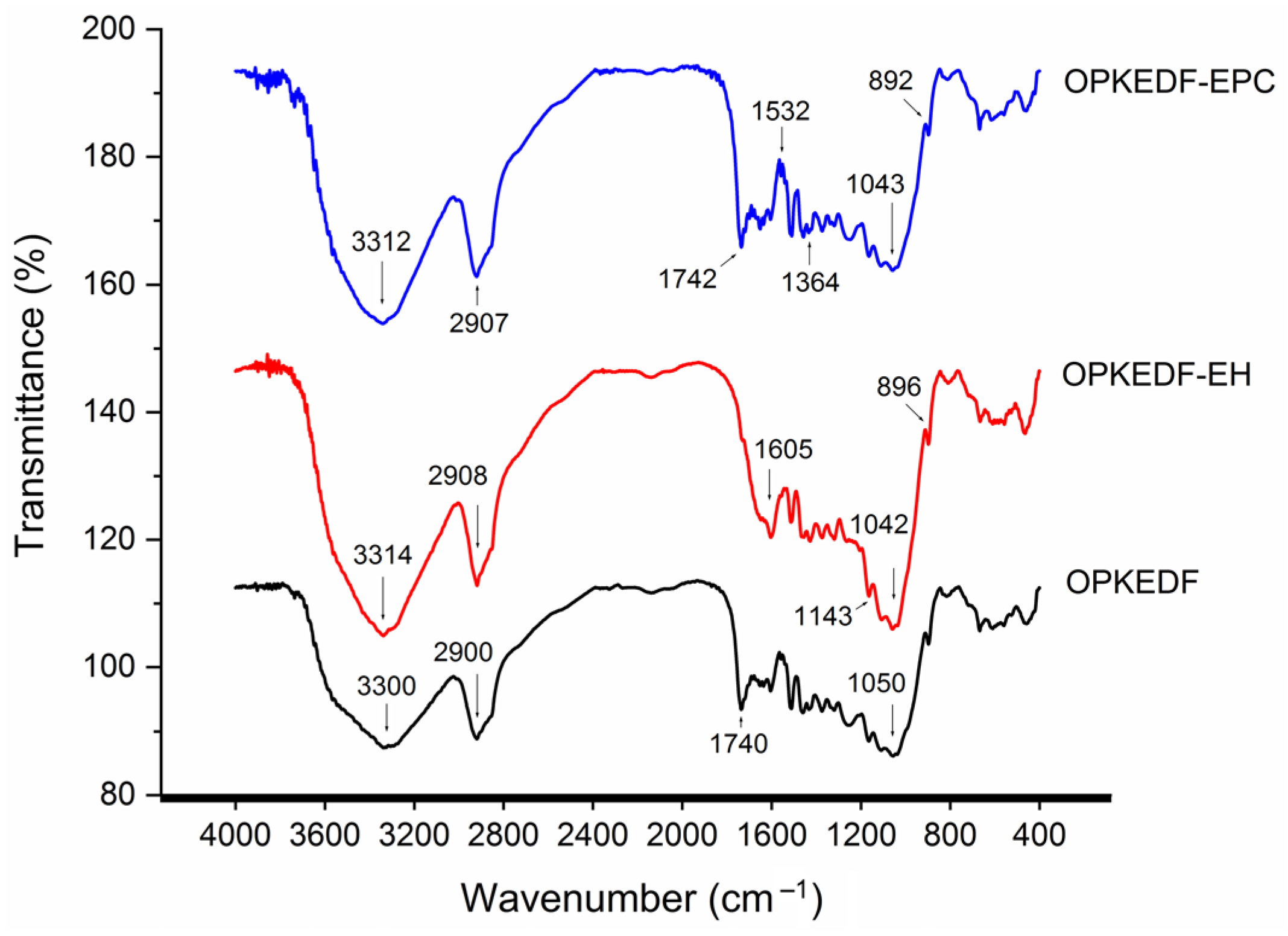
2.3.2. Fourier-Transform Infrared Spectra
2.4. Hydration Properties of OPKEDFs
2.5. Structural Characteristics of EWPGs
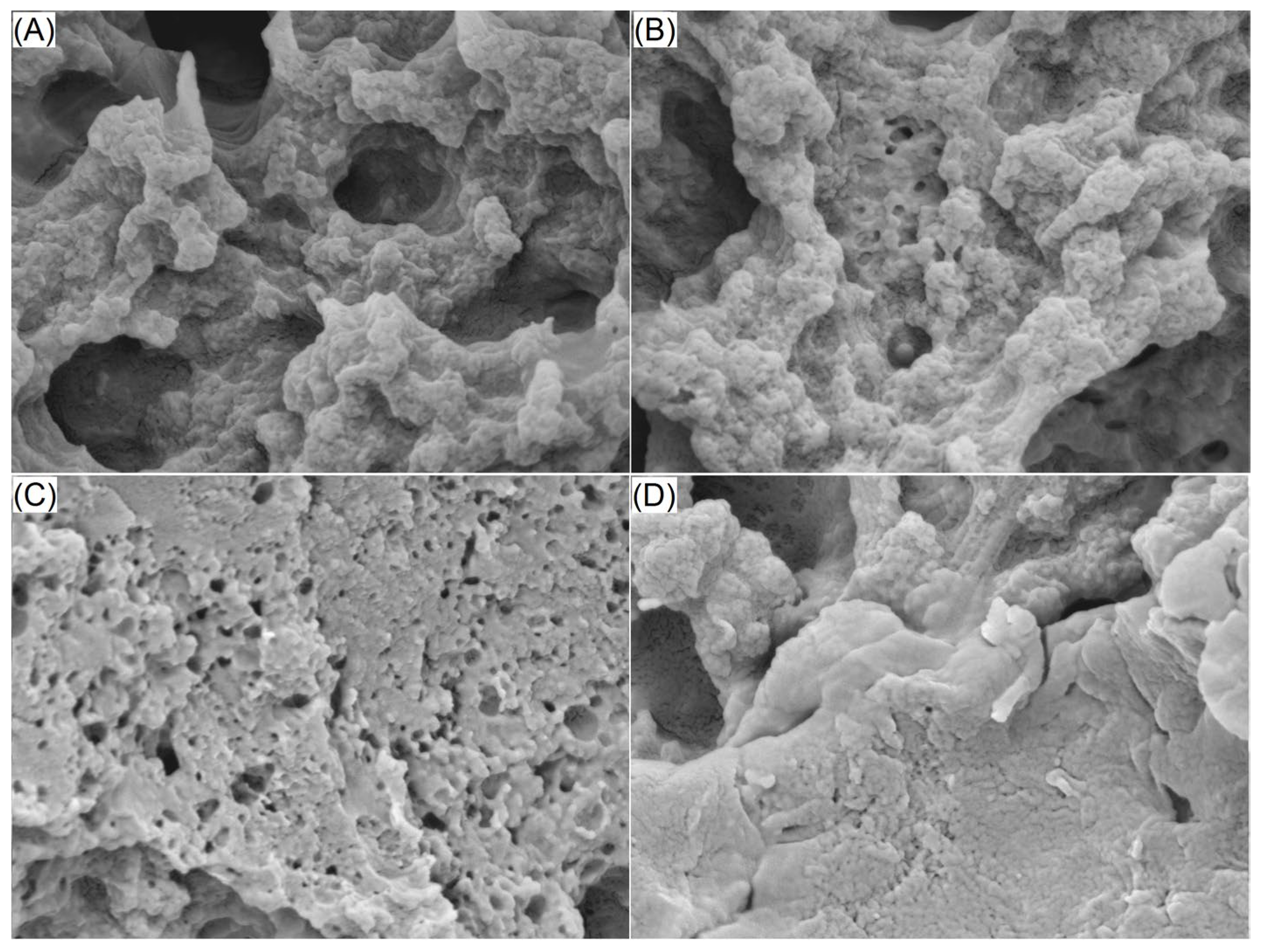
2.5.1. Surface Microstructure of EWPGs
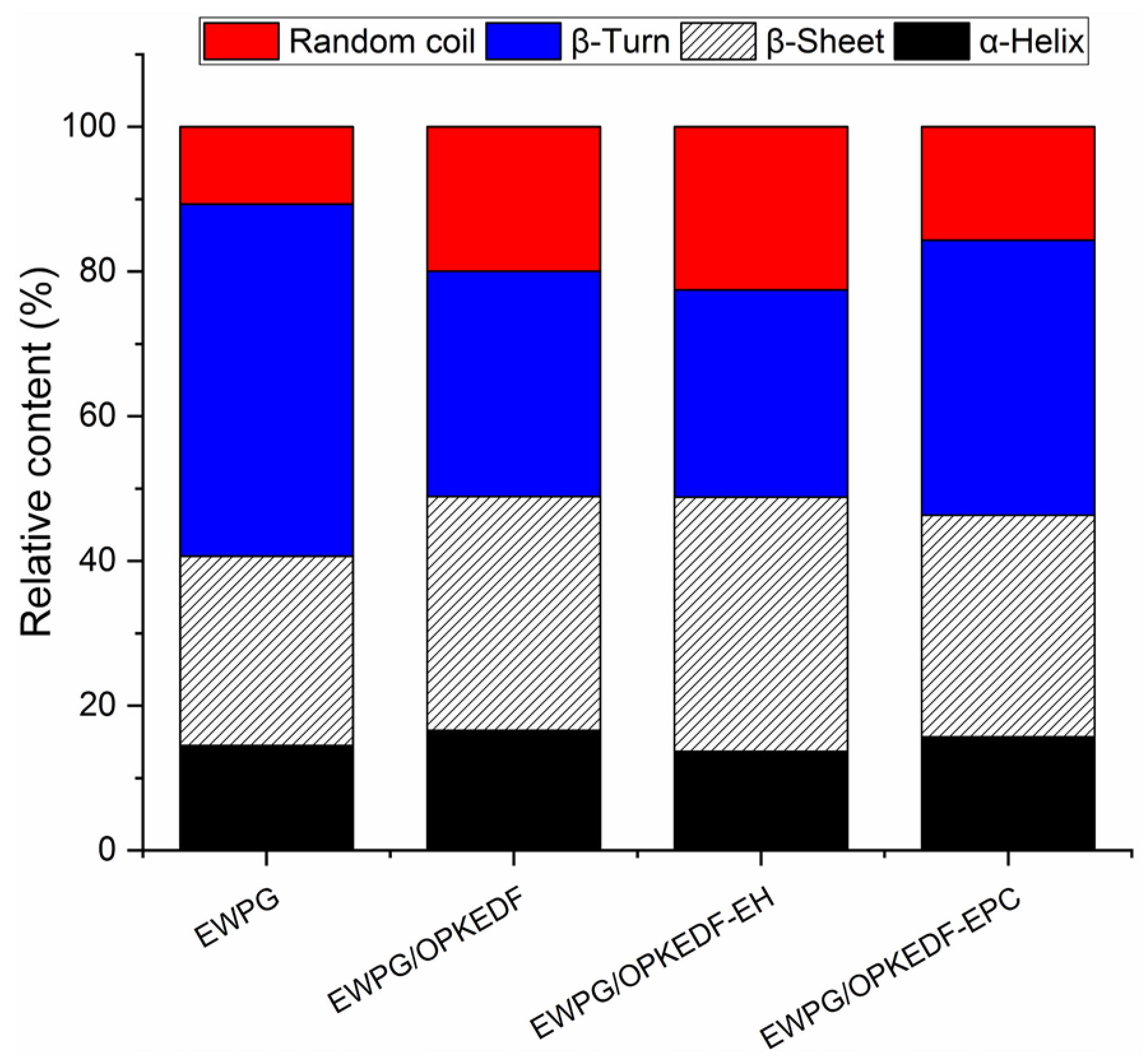
2.5.2. Secondary Structure
2.6. Gel Properties of EWPGs
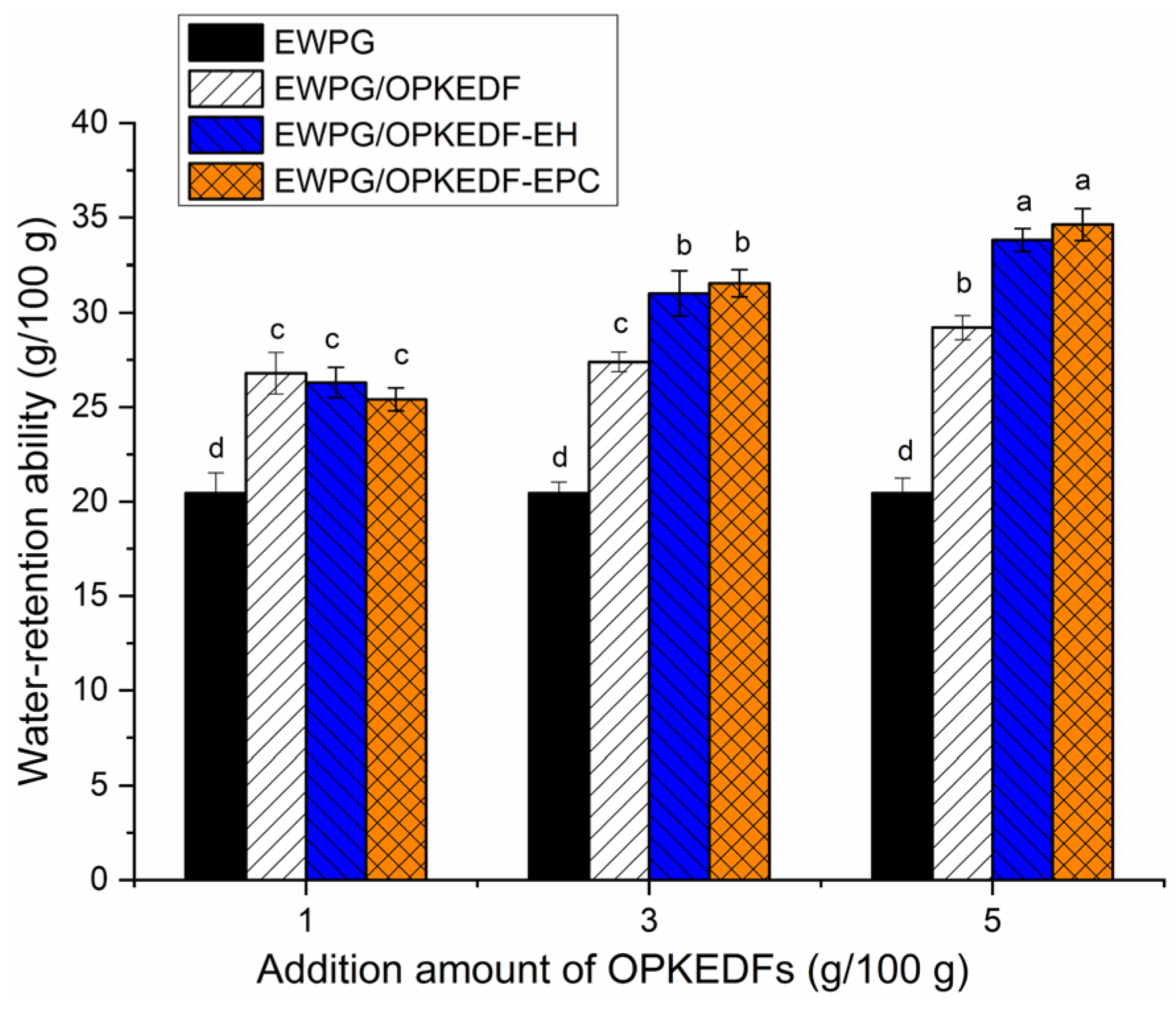
2.6.1. WRA of EWPGs
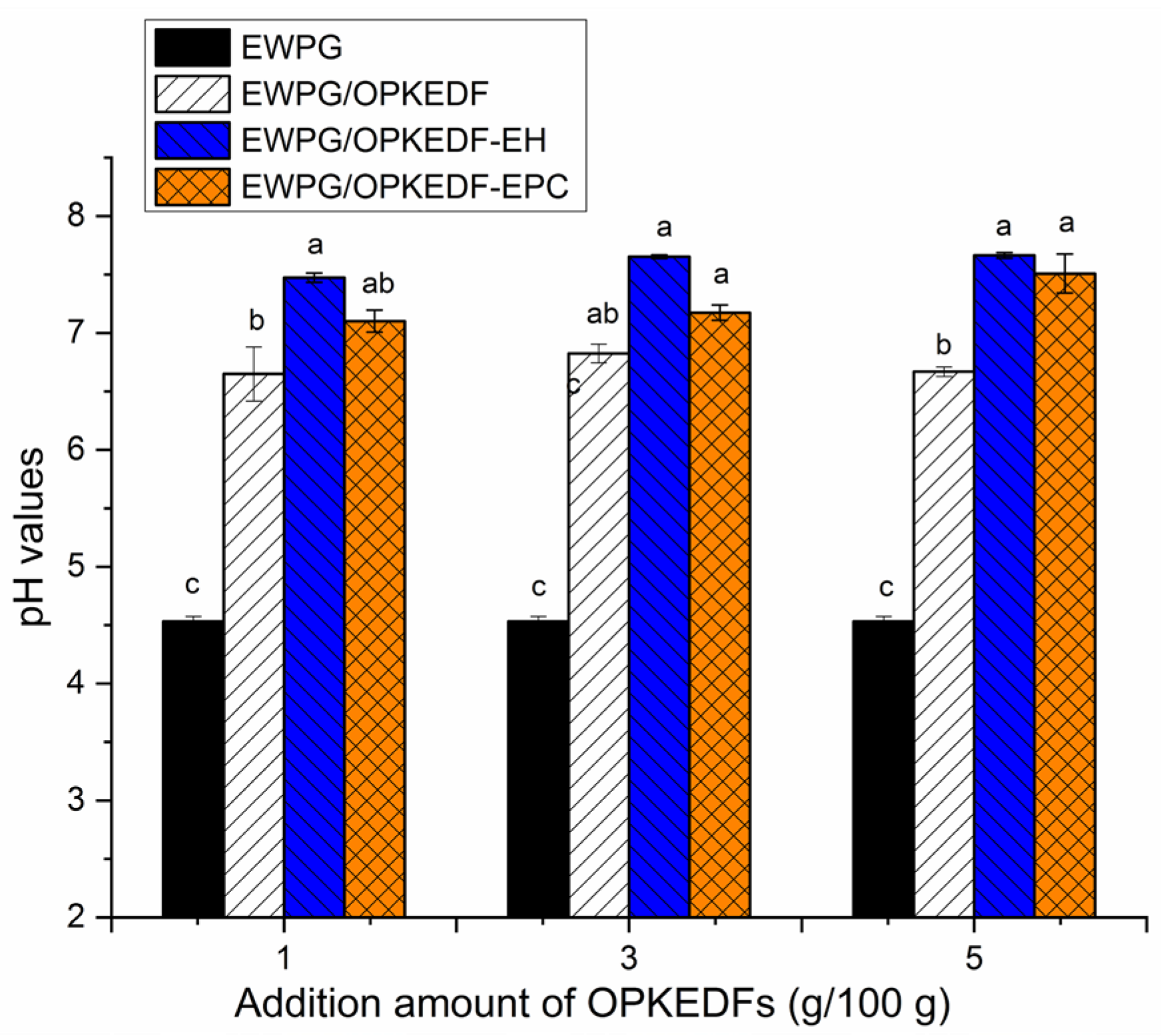
2.6.2. pH Value of EWPG
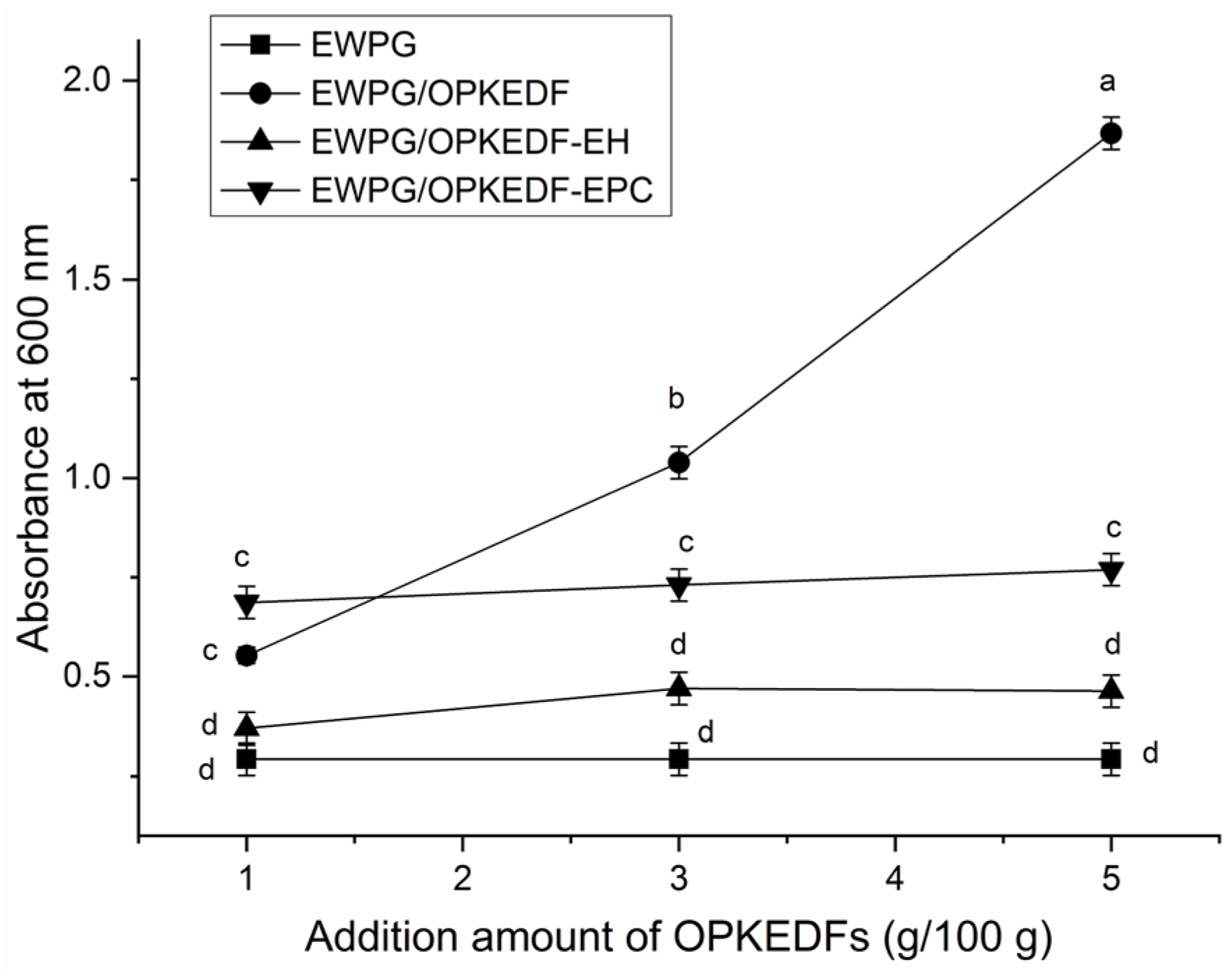
2.6.3. Optical Transparency of EWPG
2.7. Textural Properties of EWPG
3. Materials and Methods
3.1. Materials
3.2. Extraction and Cellulase Hydrolysis of OPKEDF
3.3. Hydroxypropylation of OPKEDF-E
3.4. Crosslinking of OPKEDF-E
3.5. Preparation of Egg White Protein OPKEDF Gel
3.6. Chemical Constituents, Surface Area, and Color Determinations
3.7. Structural Investigations
3.7.1. Microstructure Microscopy
3.7.2. Fourier-Transformed Infrared Spectroscopy
3.8. Hydration Properties
3.9. Characterization of Gels
3.9.1. Water-Retention Ability
3.9.2. Optical Transparency
3.10. Textural Properties of Gels
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Wang, X.; Chang, C.; Gu, L.; Su, Y.; Yang, Y.; Agyei, D.; Han, Q. Chicken Egg White Gels: Fabrication, Modification, and Applications in Foods and Oral Nutraceutical Delivery. Food 2024, 13, 1834. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Olenskyj, A.G.; Guerrero, M.G.; Graham, R.; Bornhorst, G.M. Interplay of egg white gel pH and intragastric pH: Impact on breakdown kinetics and mass transport processes. Food Res. Int. 2023, 173, 113290. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Zhang, Y.; Pan, X.; Peng, D.; Tu, Y.; Chen, J.; Zhang, Q.F.; Tang, D.B.; Yin, Z. Advances in the formation mechanism, influencing factors and applications of egg white gels: A review. Trends Food Sci. Tech. 2023, 138, 417–432. [Google Scholar] [CrossRef]
- Lv, Y.Q.; Wang, J.; Xu, L.L.; Tang, T.T.; Su, Y.J.; Gu, L.P.; Chang, C.H.; Zhang, M.; Yang, Y.J.; Li, J.H. Gel properties of okara dietary fiber-fortified soy protein isolate gel with/without NaCl. J. Sci. Food Agric. 2022, 103, 411–419. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.K.C.; Jeon, H.; Kim, Y.J.; Choi, Y.S.; Jung, S. Heat-induced gelation of egg white proteins depending on heating temperature: Insights into protein structure and digestive behaviors in the elderly in vitro digestion model. Int. J. Biol. Macromol. 2024, 262, 130053. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Liu, Y.; Peng, F.; Wang, X.; Wang, C.; Li, M.; Xv, D. Gel properties and formation mechanism of soy protein isolate gels improved by wheat bran cellulose. Food Chem. 2020, 324, 126876. [Google Scholar] [CrossRef]
- Liu, J.B.; Chai, J.J.; Yuan, Y.X.; Zhang, T.; Saini, R.K.; Yang, M.; Shang, X.M. Dextran sulfate facilitates egg white protein to form transparent hydrogel at neutral pH: Structural, functional, and degradation properties. Food Hydrocoll. 2022, 122, 107094. [Google Scholar] [CrossRef]
- Grootaert, C.; Monge-Morera, M.; Delcour, J.A.; Skirtach, A.G.; Rousseau, F.; Schymkowitz, J.; Dewettinck, K.; Van der Meeren, P. Impact of heat and enzymatic treatment on ovalbumin amyloid-like fibril formation and enzymeinduced gelation. Food Hydrocoll. 2022, 131, 107784. [Google Scholar]
- Ullah, I.; Hu, Y.; You, J.; Yin, T.; Xiong, S.; Din, Z.U.; Huang, Q.L.; Liu, R. Influence of okara dietary fiber with varying particle sizes on gelling properties, water state and microstructure of tofu gel. Food Hydrocoll. 2019, 89, 512–522. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, B.; Liu, T.; Cai, Y.; Huang, L.; Zhao, M.; Zhao, Q. The formation, structural and rheological properties of emulsion gels stabilized by egg white protein-insoluble soybean fiber complex. Food Hydrocoll. 2023, 134, 108035. [Google Scholar] [CrossRef]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Huyst, A.M.R.; Deleu, L.J.; Luyckx, T.; Van der Meeren, L.; Housmans, J.A.J.; Iqbal, S.; Tirpanalan-Staben, Ö.; Franke, K. Modification of Dietary Fibers to Valorize the By-Products of Cereal, Fruit and Vegetable Industry—A Review on Treatment Methods. Plants 2022, 11, 3466. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Kang, J.; Wang, L.; Dong, L.; Yin, M.; Wei, S.; Luo, P. Agrocybe cylindracea Dietary Fiber Modification: Sodium Hydroxide Treatment Outperforms High-Temperature, Cellulase, and Lactobacillus Fermentation. Molecules 2024, 29, 3519. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, P.; Yadav, R.B.; Yadav, B.S. Influence of chemical modification approaches on physicochemical and structural properties of dietary fiber from oat. J. Cereal Sci. 2023, 111, 103688. [Google Scholar] [CrossRef]
- Tian, Y.; Sheng, Y.; Wu, T.; Quan, Z.; Wang, C. Effects of cavitation jet combined with ultrasound, alkaline hydrogen peroxide and Bacillus subtilis treatment on the properties of dietary fiber. Food Biosci. 2024, 59, 103895. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Li, Y.; Tian, H.L. Effects of carboxymethylation, acidic treatment, hydroxypropylation and heating combined with enzymatic hydrolysis on structural and physicochemical properties of palm kernel expeller dietary fiber. LWT-Food Sci. Technol. 2020, 133, 109909. [Google Scholar] [CrossRef]
- Gan, S.; Chen, R.S.; Padzil, F.N.M.; Moosavi, S.; Tarawneh, M.A.; Loh, S.K.; Idris, Z. Potential valorization of oil palm fiber in versatile applications towards sustainability: A review. Ind. Crops Prod. 2023, 199, 116763. [Google Scholar] [CrossRef]
- Zhang, Z.; Ruan, Q.; Sun, X.; Yuan, J. Optimization of Enzymolysis Modification Conditions of Dietary Fiber from Bayberry Pomace and Its Structural Characteristics and Physicochemical and Functional Properties. Molecules 2024, 29, 3415. [Google Scholar] [CrossRef]
- Abdul Manap, N.R.; Shamsudin, R.; Maghpor, M.N.; Abdul Hamid, M.A.; Jalar, A. Adsorption isotherm and kinetic study of gas-solid system of formaldehyde on oil palm mesocarp bio-char: Pyrolysis effect. J Environ. Chem. Eng. 2018, 6, 970–983. [Google Scholar] [CrossRef]
- Iberahim, N.; Sethupathi, S.; Bashir, M.J.K.; Kanthasamy, R.; Ahmad, T. Evaluation of oil palm fiber biochar and activated biochar for sulphur dioxide adsorption. Sci. Total Environ. 2022, 805, 150421. [Google Scholar] [CrossRef] [PubMed]
- Harahap, M.; Perangin-Angin, Y.A.; Purwandari, V.; Goei, R.; Tok, A.L.Y.; Gea, S. Acetylated lignin from oil palm empty fruit bunches and its electrospun nanofibres with PVA: Potential carbon fibre precursor. Heliyon 2023, 9, 14556. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.; Kato, C.G.; Corrêa, R.C.G.; Moreira, R.F.P.M.; Peralta, R.A.; Barros, L.; Ferreira, I.G.F.R.; Zanin, G.M.; Bracht, A.; Peralta, R.M. Laccases in food processing: Current status, bottlenecks and perspectives. Trends Food Sci. Technol. 2021, 115, 445–460. [Google Scholar] [CrossRef]
- Torres-Pérez, R.; Martínez-García, E.; Siguero-Tudela, M.M.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Enhancing Gluten-Free Bread Production: Impact of Hydroxypropyl Methylcellulose, Psyllium Husk Fiber, and Xanthan Gum on Dough Characteristics and Bread Quality. Foods 2024, 13, 1691. [Google Scholar] [CrossRef]
- Agustin, M.; Morais de Carvalho, D.; Lahtinen, M.; Hilden, K.; Lundell, T.; Mikkonen, K.S. Laccase as a tool in building advanced lignin-based materials. ChemSusChem 2021, 14, 4615–4635. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohyd. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, B.; Shi, P.; Tian, H.; Li, Y.; Wang, X.; Wu, S.; Liang, P. The influences of acetylation, hydroxypropylation, enzymatic hydrolysis and crosslinking on improved adsorption capacities and in vitro hypoglycemic properties of millet bran dietary fibre. Food Chem. 2022, 368, 130883. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, J.; Yu, M.; Zhang, R.; Lin, H.; Yan, H.; Li, C.; Jia, J.M.; Hu, Y. Insight into the mechanism of water-insoluble dietary fiber from star anise (Illicium verum Hook. f.) on water-holding capacity of myofibrillar protein gels. Food Chem. 2023, 423, 136348. [Google Scholar] [CrossRef]
- Ma, Z.; Yao, J.; Wang, Y.; Jia, J.; Liu, F.; Liu, X. Polysaccharide-based delivery system for curcumin: Fabrication and characterization of carboxymethylated corn fiber gum/chitosan biopolymer particles. Food Hydrocoll. 2022, 125, 107367. [Google Scholar] [CrossRef]
- Khemakhem, M.; Attia, H.; Ayadi, M.A. The effect of pH, sucrose, salt and hydrocolloid gums on the gelling properties and water holding ability of egg white gel. Food Hydrocoll. 2019, 87, 11–19. [Google Scholar] [CrossRef]
- Saini, P.; Sinha, A.S.K.; Prasad, K. Wet refining: A novel approach for modification of wheat bran fiber. Innov. Food Sci. Emerg. 2023, 90, 103508. [Google Scholar] [CrossRef]
- Han, T.F.; Xue, H.; Hu, X.; Li, R.L.; Liu, H.L.; Tu, Y.G.; Zhao, Y. Combined effects of NaOH, NaCl, and heat on the gel characteristics of duck egg white. LWT-Food Sci. Technol. 2022, 159, 113178. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Shan, A.S.; Wang, R.H.; Zhao, Y.; Chi, Y.J. Characterization of egg white powder gel structure and its relationship with gel properties influenced by pretreatment with dry heat. Food Hydrocolloid. 2021, 110, 106149. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Ma, S.; Yang, M.; Zhang, H.; Zhang, T.; Yu, Y.; Du, Z. Co-assembly of egg white-derived peptides and protein-polysaccharide complexes for curcumin encapsulation: The enhancement of stability, redispersibility, and bioactivity. Food Chem. 2022, 394, 133496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiong, Z.; Walayat, N.; Lorenzo, J.M.; Liu, J.; Nawaz, A.; Xiong, H. Influence of the Mixture of Carrageenan Oligosaccharides and Egg White Protein on the Gelation Properties of Culter alburnus Myofibrillar Protein under Repeated Freezing–Thawing Cycles. Antioxidants 2022, 11, 32. [Google Scholar] [CrossRef]
- Ning, X.; Wang, L.; Jin, S.; Fu, X.; Sun, X.; Cao, J. A Strategy for Sample Preparation: Using Egg White Gel to Promote the Determination of Aflatoxin M1 Content in Milk Samples. Molecules 2022, 27, 5039. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, Z.; Wu, R.; Guo, D.; Xu, W.; Wang, H.; Yi, Y. Fabrication and Characterization of the Egg-White Protein Chitosan Double-Layer Emulsion. Molecules 2022, 27, 6036. [Google Scholar] [CrossRef]
- Kleemann, C.; Zink, J.; Selmer, I.; Smirnova, I.; Kulozik, U. Effect of Ethanol on the Textural Properties of Whey Protein and Egg White Protein Hydrogels during Water-Ethanol Solvent Exchange. Molecules 2020, 25, 4417. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Bashash, M.; Varidi, M.; Varshosaz, J. Ultrasound-triggered transglutaminase-catalyzed egg white-bovine gelatin composite hydrogel: Physicochemical and rheological studies. Innov. Food Sci. Emerg. 2022, 76, 102936. [Google Scholar] [CrossRef]
| Constituent | OPKEDF | OPKEDF-EH | OPKEDF-EPC |
|---|---|---|---|
| Moisture (g∙100 g−1) | 6.75 ± 0.67 c | 7.49 ± 0.27 c | 8.35 ± 0.36 c |
| Fat (g∙100 g−1) | 3.28 ± 0.21 c | 2.54 ± 0.63 c | 2.08 ± 0.27 c |
| Ash (g∙100 g−1) | 3.19 ± 0.31 c | 3.86 ± 0.16 c | 4.05 ± 0.11 c |
| Protein (g∙100 g−1) | 3.38 ± 0.28 c | 3.39 ± 0.28 c | 3.89 ± 0.39 c |
| Total dietary fiber (g∙100 g−1) | 74.58 ± 5.56 c | 72.37 ± 3.73 c | 72.26 ± 3.57 c |
| Insoluble dietary fiber (g∙100 g−1) | 70.61 ± 4.52 c | 66.15 ± 3.33 d | 64.51 ± 2.73 d |
| Soluble dietary fiber (g∙100 g−1) | 2.97 ± 0.11 e | 5.25 ± 1.09 d | 7.79 ± 0.15 c |
| Cellulose (g∙100 g−1) | 43.15 ± 2.98 a | 27.9 ± 0.92 c | 27.01 ± 3.04 c |
| Lignin (g∙100 g−1) | 34.31 ± 1.99 a | 22.85 ± 1.94 b | 20.73 ± 2.23 b |
| Hemicellulose (g∙100 g−1) | 21.07 ± 2.43 a | 14.95 ± 0.54 b | 13.47 ± 1.13 b |
| D3,2 (μm) | 115.87 ± 2.58 c | 79.68 ± 0.67 e | 90.36 ± 2.47 d |
| Specific surface area (cm2/cm3) | 1704.57± 7.85 e | 2034.44 ± 9.87 c | 1874.47 ± 12.53 d |
| L | 42.7 ± 2.14 c | 34.83 ± 2.02 d | 30.25 ± 0.67 d |
| a | 5.73 ± 0.23 d | 8.79 ± 0.27 c | 9.06 ± 0.44 c |
| b | 8.62 ± 0.26 d | 13.48 ± 0.36 c | 14.91 ± 1.02 c |
| ΔE | Control | 9.37 | 30.90 |
| Water-retention ability (g/g) | 2.34 ± 0.09 d | 5.22 ± 0.18 c | 4.91 ± 0.46 c |
| Water-expansion ability (mL/g) | 0.92 ± 0.23 e | 2.15 ± 0.17 d | 3.43 ± 0.20 c |
| Viscosity (cP) | 4.78 ± 0.27 e | 7.71 ± 0.35 d | 9.78 ± 1.65 c |
| Gels | Amount (g/100 g) | Hardness (g) | Adhesiveness | Springiness | Gumminess | Chewiness (g) | Resilience |
|---|---|---|---|---|---|---|---|
| H-EWG | 0 | 97.96 ± 7.50 e | −30.63 ± 2.17 b | 0.77 ± 0.03 a | 84.63 ± 3.65 e | 78.65 ± 6.63 e | 0.297 ± 0.012 d |
| H-EWG/OPKEDF | 1 | 93.07 ± 5.08 e | −35.43 ± 1.57 c | 0.82 ± 0.00 a | 76.74 ±4.71 f | 74.19 ± 5.69 ef | 0.343 ± 0.007 b |
| 3 | 113.04 ± 6.19d e | −30.37 ± 1.44 b | 0.83 ± 0.01 a | 83.82 ± 1.49 e | 84.36 ± 4.45 ef | 0.347 ± 0.007 b | |
| 5 | 124.49± 7.18 c | −26.22 ± 0.64 a | 0.82 ± 0.00 a | 104.00 ± 3.27 d | 92.71 ± 3.36 c | 0.318 ± 0.003 c | |
| H-EWG/OPKEDF-EC | 1 | 126.34 ± 11.38 c | −35.93 ± 3.27c | 0.82 ± 0.02 a | 106.51 ± 6.32 d | 73.54 ± 3.98 e | 0.335 ± 0.010 b |
| 3 | 119.88 ± 9.65 d | −47.74 ± 4.78 e | 0.79 ± 0.03 a | 113.22 ± 7.33 c | 89.44± 1.05 cd | 0.337 ± 0.007 b | |
| 5 | 167.92 ± 3.95 b | −49.57 ± 4.39 e | 0.83 ± 0.01 a | 152.27 ± 7.69 a | 102.23± 4.54 b | 0.359 ± 0.003 a | |
| H-EWG/OPKEDF-EPC | 1 | 84.22 ± 7.27 f | −45.48 ±2.82 de | 0.79 ± 0.01 a | 88.56 ± 3.39 e | 63.45 ± 2.44 f | 0.232 ± 0.003 e |
| 3 | 174.91 ± 5.07 b | −43.36 ± 4.15 d | 0.75 ± 0.03 a | 119.16 ± 8.12 c | 91.04 ± 4.06 c | 0.195 ± 0.020 f | |
| 5 | 195.00 ± 7.65 a | −51.36 ± 3.82 e | 0.75 ± 0.00 a | 140.72 ± 9.18 b | 147.39 ± 2.63 a | 0.152 ± 0.010 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Gu, Y.; Zhang, W. Effects of Modified Oil Palm Kernel Expeller Fiber Enhanced via Enzymolysis Combined with Hydroxypropylation or Crosslinking on the Properties of Heat-Induced Egg White Protein Gel. Molecules 2024, 29, 5224. https://doi.org/10.3390/molecules29225224
Jin Z, Gu Y, Zhang W. Effects of Modified Oil Palm Kernel Expeller Fiber Enhanced via Enzymolysis Combined with Hydroxypropylation or Crosslinking on the Properties of Heat-Induced Egg White Protein Gel. Molecules. 2024; 29(22):5224. https://doi.org/10.3390/molecules29225224
Chicago/Turabian StyleJin, Zhiqiang, Yaoguang Gu, and Wen Zhang. 2024. "Effects of Modified Oil Palm Kernel Expeller Fiber Enhanced via Enzymolysis Combined with Hydroxypropylation or Crosslinking on the Properties of Heat-Induced Egg White Protein Gel" Molecules 29, no. 22: 5224. https://doi.org/10.3390/molecules29225224
APA StyleJin, Z., Gu, Y., & Zhang, W. (2024). Effects of Modified Oil Palm Kernel Expeller Fiber Enhanced via Enzymolysis Combined with Hydroxypropylation or Crosslinking on the Properties of Heat-Induced Egg White Protein Gel. Molecules, 29(22), 5224. https://doi.org/10.3390/molecules29225224








