Design and Optimization of MoS2@rGO@NiFeS Nanocomposites for Hybrid Supercapattery Performance and Sensitive Electrochemical Detection
Abstract
1. Introduction
2. Experimental Section
2.1. Material
2.2. Process of Cleaning Ni Foam (NF)
2.3. Synthesis Process
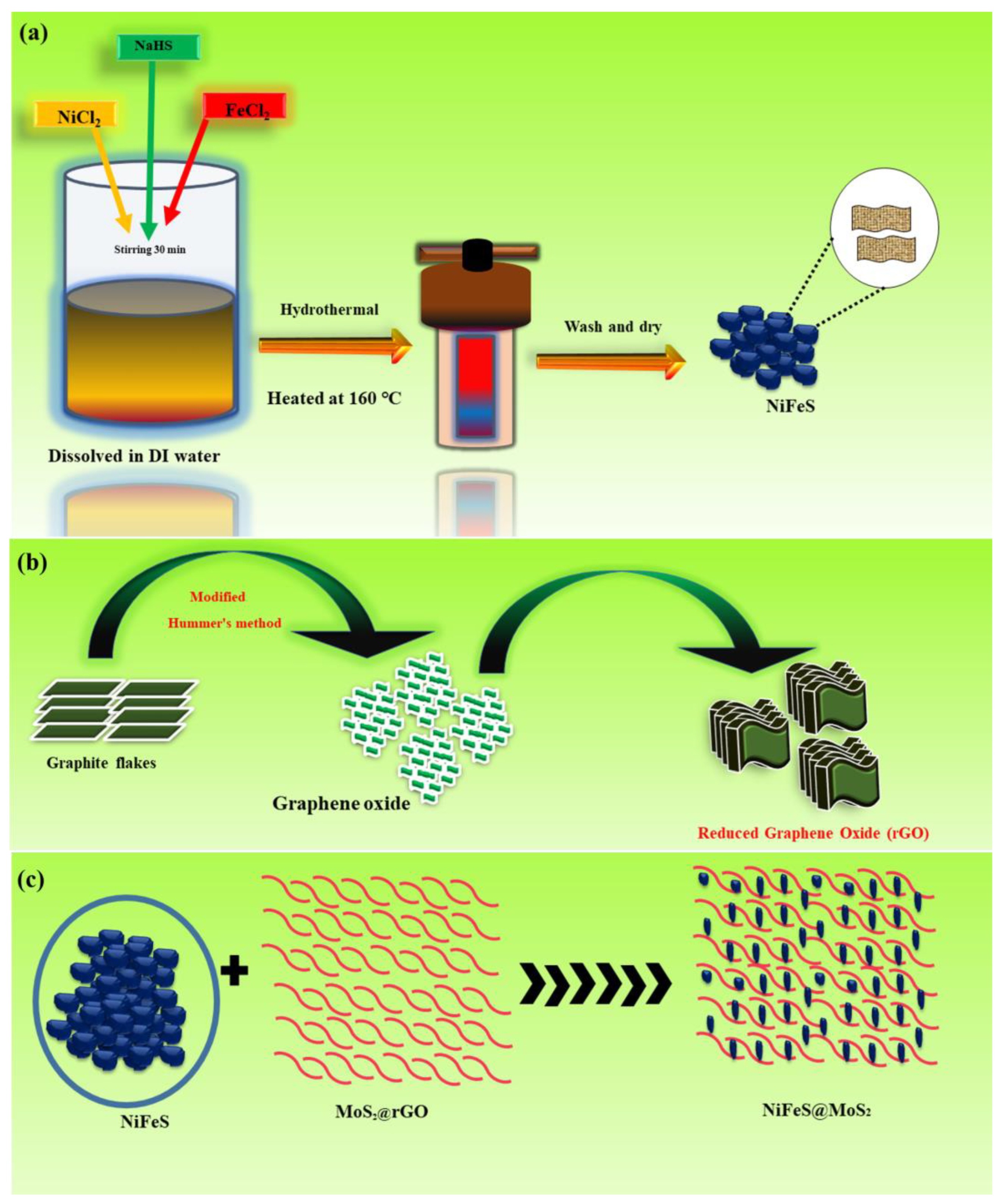
2.3.1. Preparation of Nickel–Iron Sulfide
2.3.2. Synthesis of rGO
2.3.3. Preparation of Nickel–Iron Sulfide@MoS2@rGO Composite
2.3.4. Synthesis of Electrode Materials and Fabrication of Electrodes
2.4. Electrochemical Measurements
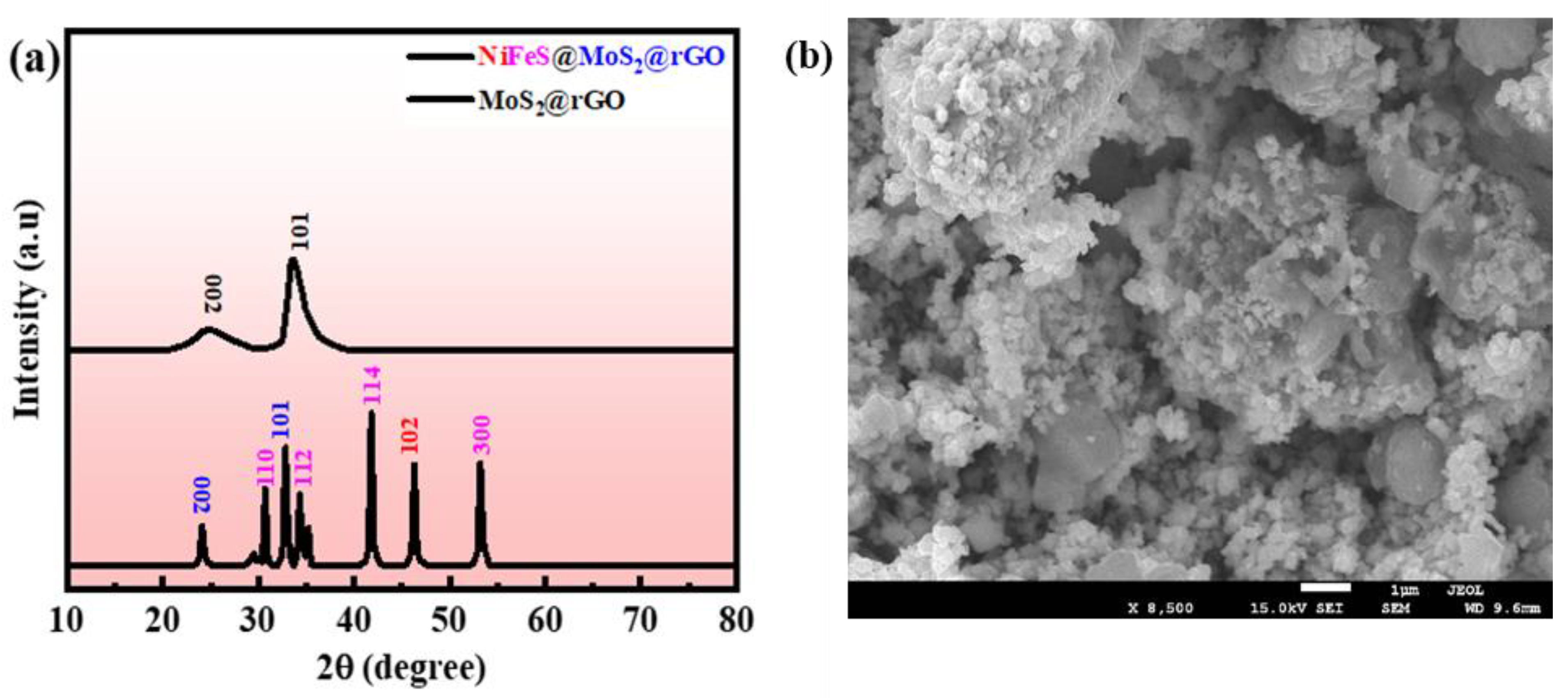
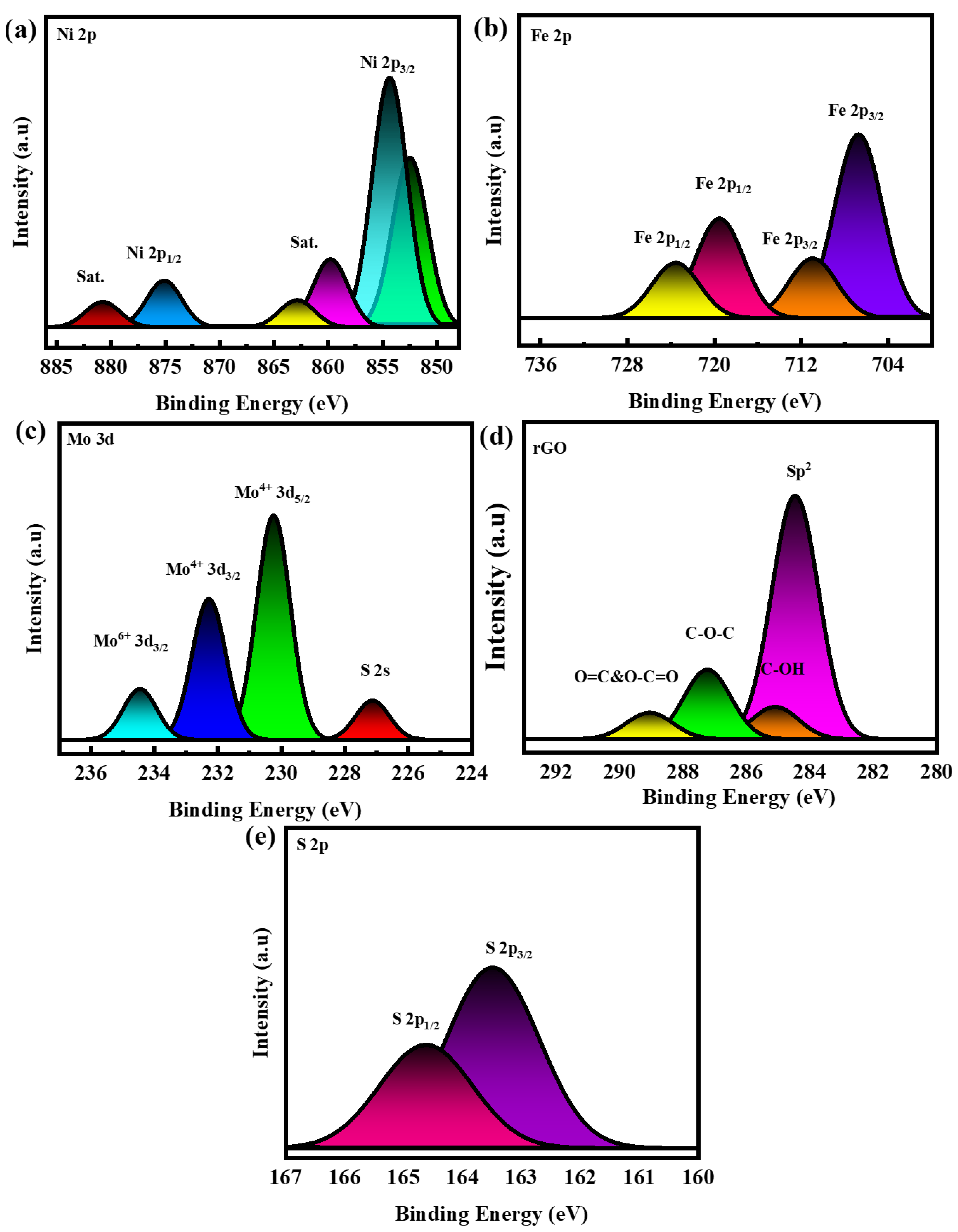
3. Results and Discussion
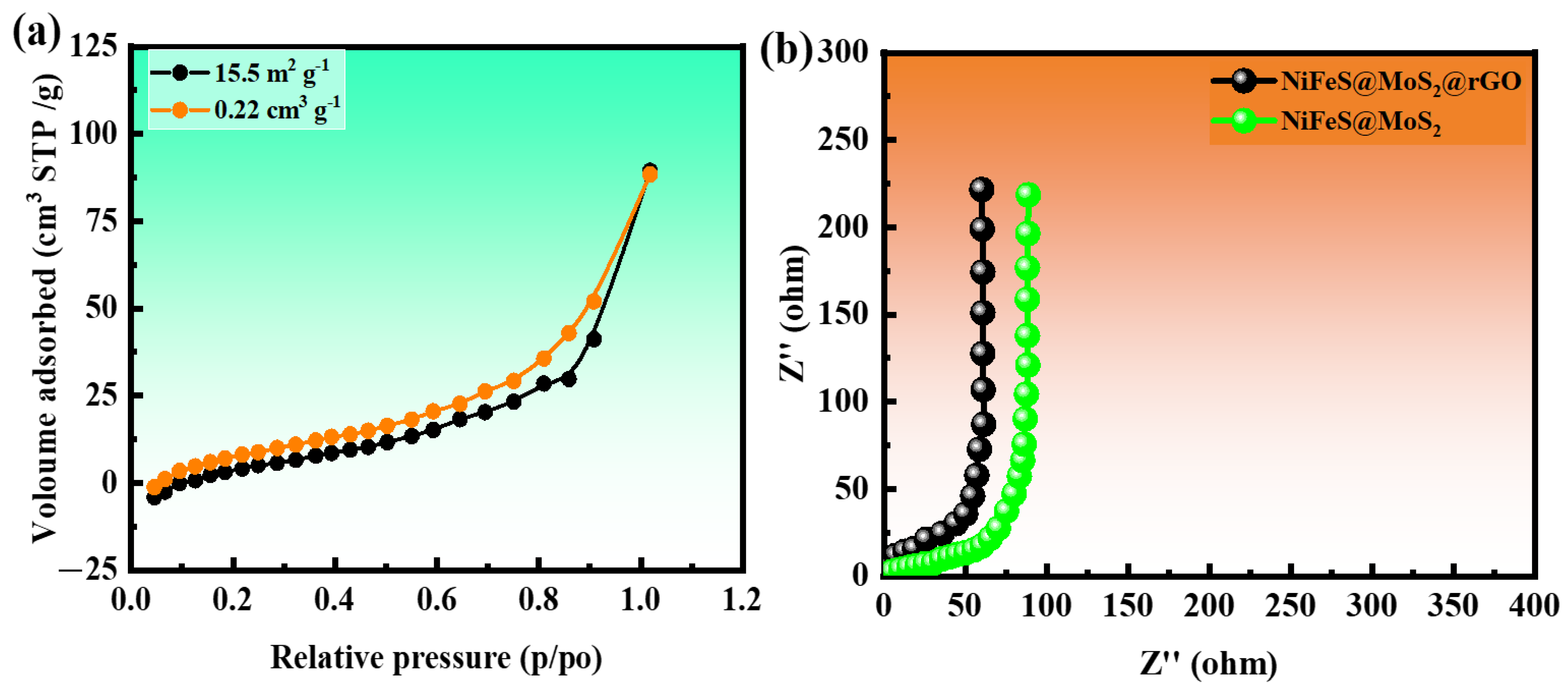
3.1. Brunauer–Emmett–Teller (BET)
3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3. Electrochemical Studies
3.3.1. Cyclic Voltammetry Studies
3.3.2. Galvanostatic Charge–Discharge Studies
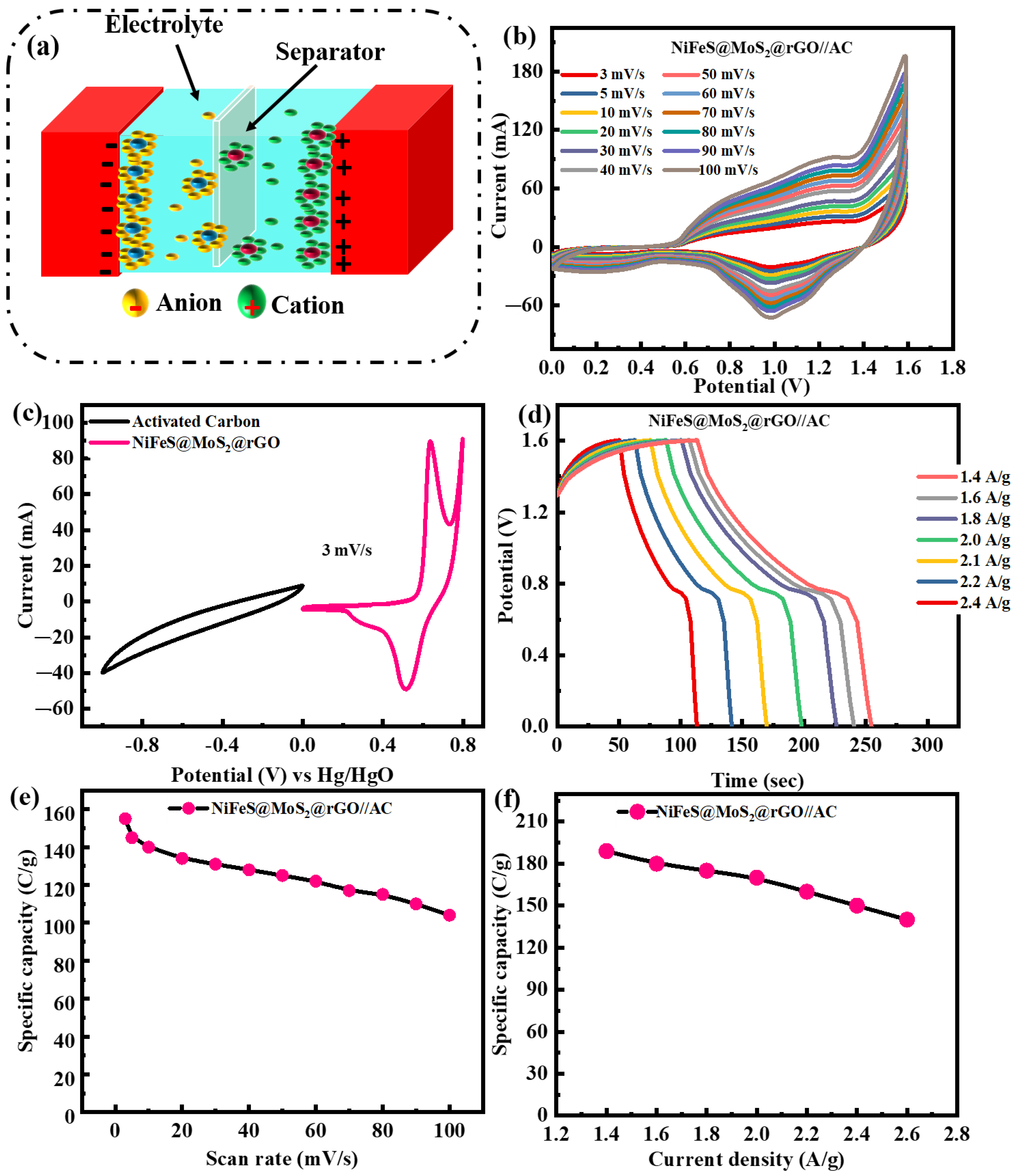
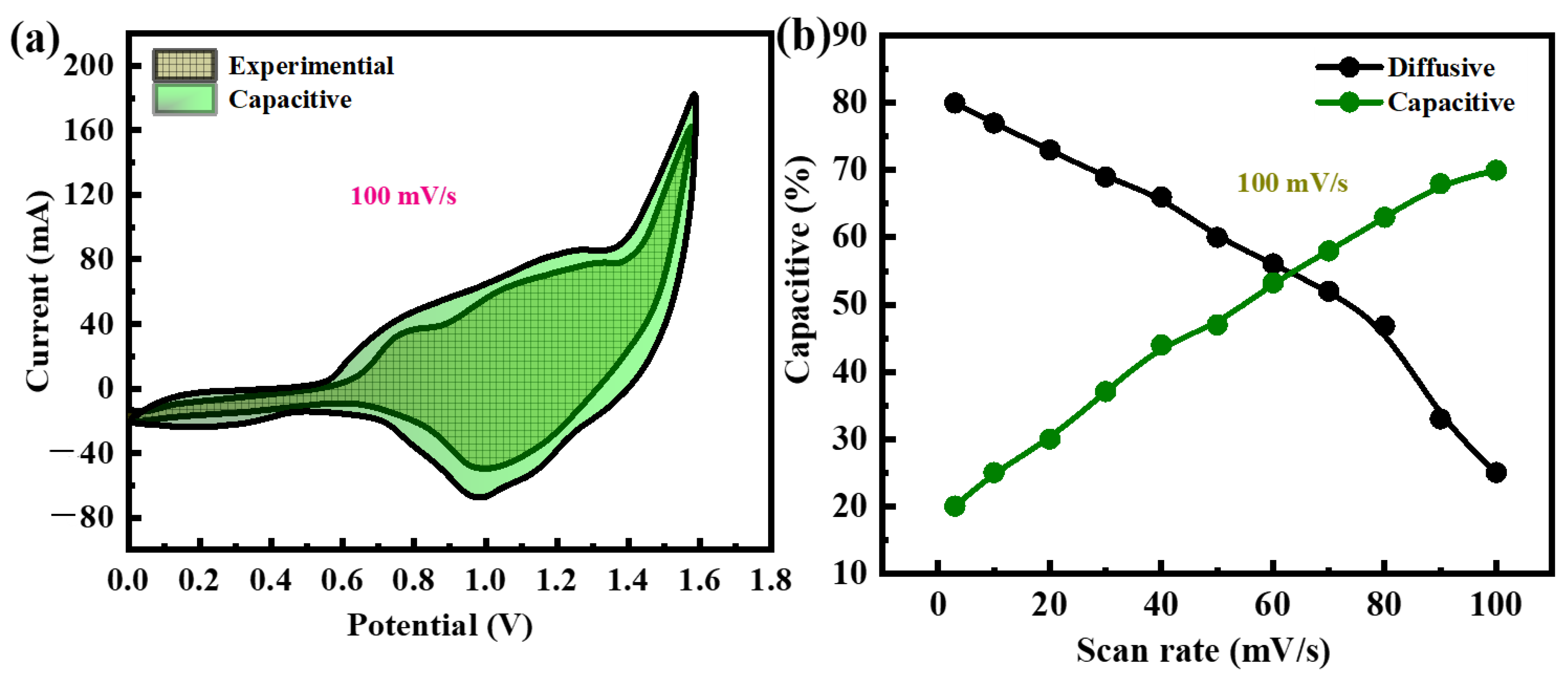
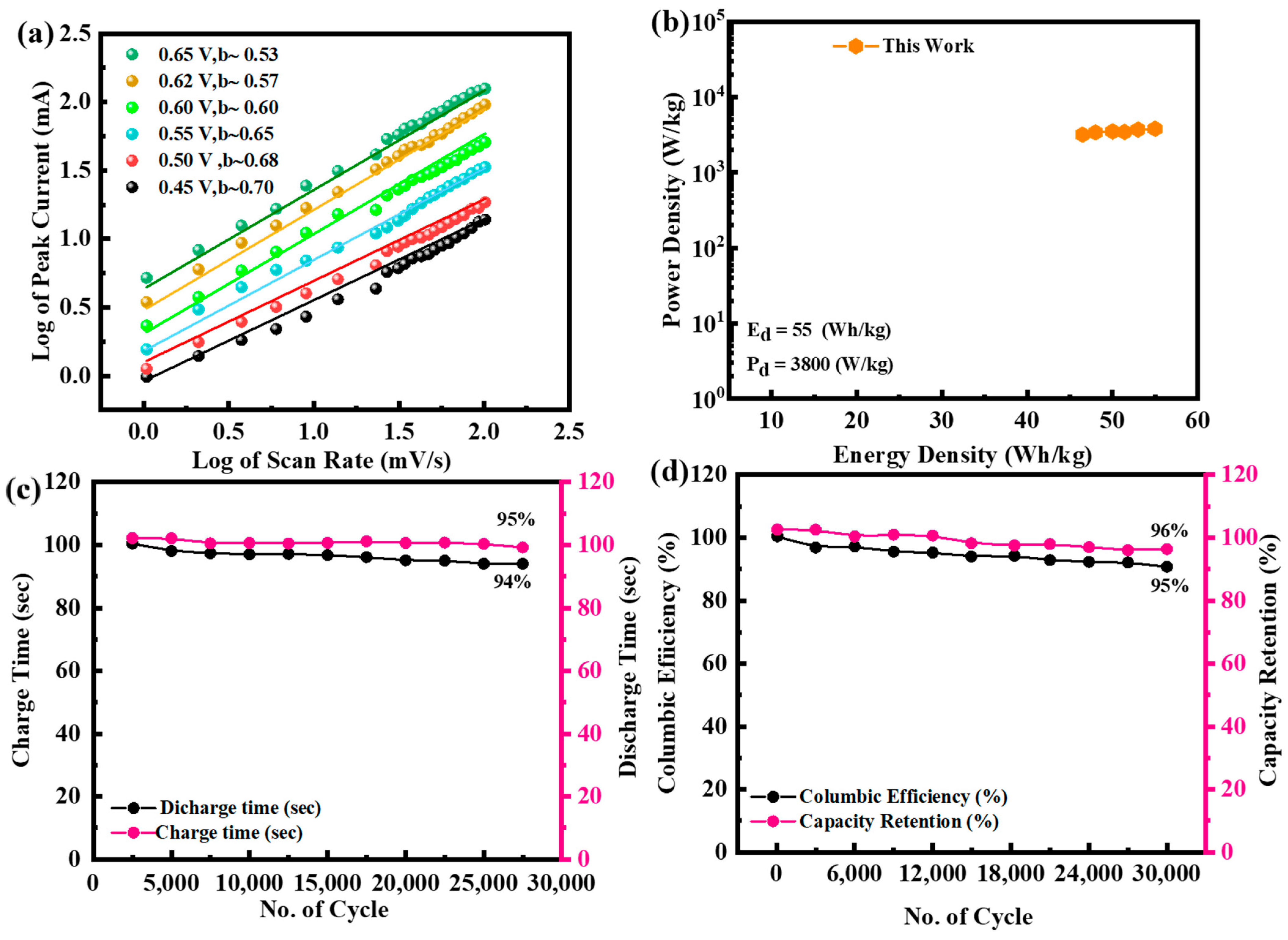
3.4. Electrochemical Performance of NiFeS@MoS2@rGO//AC Supercapattery
4. Conclusions
5. Electrochemical Characterization of Sensors
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Zhan, H.; Feng, G.; Ma, Y.; Zhang, C.; Zhang, Y.; Cao, M. Engineering mesoporous Na4Mn0.9Ni0.1V(PO4)3@NC microspheres cathode towards advanced sodium ion batteries. J. Energy Storage 2024, 97, 112890. [Google Scholar] [CrossRef]
- Yan, R.; Wang, J.; He, S.; Huang, L.; Wang, B.; Zhu, M.; Hu, S. Quasi-solid-state silicon-air batteries with high capacities and wide-temperature adaptabilities. Energy Storage Mater. 2024, 71, 103656. [Google Scholar] [CrossRef]
- Li, Y.; Ni, L.; Luo, J.; Zhu, L.; Zhang, X.; Li, H.; Zada, I.; Yu, J.; Zhu, S.; Lian, K.; et al. Fenton Reaction Doubled Biomass Carbon Activation Efficiency for High-Performance Supercapacitors. Adv. Funct. Mater. 2024, 34, 2403448. [Google Scholar] [CrossRef]
- Gao, L.; Cao, M.; Zhang, C.; Li, J.; Zhu, X.; Guo, X.; Toktarbay, Z. Zinc selenide/cobalt selenide in nitrogen-doped carbon frameworks as anode materials for high-performance sodium-ion hybrid capacitors. Adv. Compos. Hybrid Mater. 2024, 7, 144. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Y.; Hu, Q.; Si, N.; Zhang, C. Electrochemical hydrogen storage materials: State-of-the-art and future perspectives. Energy Fuels 2024, 38, 7579–7613. [Google Scholar] [CrossRef]
- An, W.; Gao, Y.; Liu, J.J.C. In Situ Growth of 3D Nano-array Ni0.85Se/NF Structure Supercapattery Electrode with Excellent Cyclic Stability and Superior Energy Density. ChemistrySelect 2017, 2, 7372–7377. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Shanmugam, S.; Vasylechko, L.; Kalai Selvan, R.J.A.A.E.M. Interweaved nickel phosphide sponge as an electrode for flexible supercapattery and water splitting applications. ACS Appl. Energy Mater. 2018, 1, 78–92. [Google Scholar] [CrossRef]
- Akinwolemiwa, B.; Peng, C.; Chen, G. Redox electrolytes in supercapacitors. J. Electrochem. Soc. 2015, 162, A5054. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Zheng, X.; Lin, J.; Liang, H.; Cai, Y.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Interlaced Ni-Co LDH nanosheets wrapped Co9S8 nanotube with hierarchical structure toward high performance supercapacitors. Chem. Eng. J. 2018, 351, 348–355. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Z.; Wang, J.; Pang, S.; Wang, B.; Zhu, M. An overview of silicon-air batteries: Principle, current state and future perspectives. Coord. Chem. Rev. 2024, 517, 216045. [Google Scholar] [CrossRef]
- Yu, X.Y.; Lou, X. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Liu, W.; Niu, H.; Yang, J.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. Ternary transition metal sulfides embedded in graphene nanosheets as both the anode and cathode for high-performance asymmetric supercapacitors. Chem. Mater. 2018, 30, 1055–1068. [Google Scholar] [CrossRef]
- Rui, X.; Tan, H.; Yan, Q. Nanostructured metal sulfides for energy storage. Nanoscale 2014, 6, 9889–9924. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Alshareef, H. One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, H.B.; Wang, Y.; Xu, R.; Lou, X. Formation of 1D hierarchical structures composed of Ni3S2 nanosheets on CNTs backbone for supercapacitors and photocatalytic H2 production. Adv. Energy Mater. 2012, 2, 1497–1502. [Google Scholar] [CrossRef]
- Gou, J.; Xie, S.; Liu, C. Hollow sphere NiS2 as high-performance hybrid supercapacitor electrode materials. In Proceedings of the AIP Conference Proceedings, Atlanta, GA, USA, 17–22 July 2016; AIP Publishing: College Park, Maryland, 2017. [Google Scholar]
- Wang, J.; Chao, D.; Liu, J.; Li, L.; Lai, L.; Lin, J.; Shen, Z. Ni3S2@MoS2 core/shell nanorod arrays on Ni foam for high-performance electrochemical energy storage. Nano Energy 2014, 7, 151–160. [Google Scholar] [CrossRef]
- Lin, J.; Yan, Y.; Zheng, X.; Zhong, Z.; Wang, Y.; Qi, J.; Cao, J.; Fei, W.; Huang, Y.; Feng, J.; et al. Designing and constructing core-shell NiCo2S4@ Ni3S2 on Ni foam by facile one-step strategy as advanced battery-type electrodes for supercapattery. J. Colloid Interface Sci. 2019, 536, 456–462. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Jing, M.; Yang, X.; Song, W.; Ji, X. Mesoporous NiCo2S4 nanoparticles as high-performance electrode materials for supercapacitors. J. Power Sources 2015, 273, 584–590. [Google Scholar] [CrossRef]
- Lin, J.; Zhong, Z.; Wang, H.; Zheng, X.; Wang, Y.; Qi, J.; Cao, J.; Fei, W.; Huang, Y.; Feng, J. Rational constructing free-standing Se doped nickel-cobalt sulfides nanotubes as battery-type electrode for high-performance supercapattery. J. Power Sources 2018, 407, 6–13. [Google Scholar] [CrossRef]
- Nagaraju, G.; Sekhar, S.C.; Ramulu, B.; Yu, J. An integrated approach toward renewable energy storage using rechargeable Ag@Ni0.67Co0.33S-based hybrid supercapacitors. Small 2019, 15, 1805418. [Google Scholar] [CrossRef]
- Boukhalfa, S.; Evanoff, K.; Yushin, G.J.E.; Science, E. Atomic layer deposition of vanadium oxide on carbon nanotubes for high-power supercapacitor electrodes. Energy Environ. Sci. 2012, 5, 6872–6879. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Liu, Y.-J.; Gan, T.; Liu, Y.-M.; Wang, L.-L.; Fan, Y. Synthesis and electrochemical performances of layered tungsten sulfide-graphene nanocomposite as a sensing platform for catechol, resorcinol and hydroquinone. Electrochim. Acta 2013, 107, 379–387. [Google Scholar] [CrossRef]
- Kang, B.K.; Woo, M.H.; Lee, J.; Song, Y.H.; Wang, Z.; Guo, Y.; Yamauchi, Y.; Kim, J.H.; Lim, B.; Yoon, D. Mesoporous Ni–Fe oxide multi-composite hollow nanocages for efficient electrocatalytic water oxidation reactions. J. Mater. Chem. A 2017, 5, 4320–4324. [Google Scholar] [CrossRef]
- Tang, Q.; Bairi, P.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Zeng, H.; Ji, Q.; Shrestha, L. Quasi 2D mesoporous carbon microbelts derived from fullerene crystals as an electrode material for electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 44458–44465. [Google Scholar] [CrossRef]
- Hu, W.; Chen, R.; Xie, W.; Zou, L.; Qin, N.; Bao, D. CoNi2S4 nanosheet arrays supported on nickel foams with ultrahigh capacitance for aqueous asymmetric supercapacitor applications. ACS Appl. Mater. Interfaces 2014, 6, 19318–19326. [Google Scholar] [CrossRef]
- Masikhwa, T.M.; Barzegar, F.; Dangbegnon, J.K.; Bello, A.; Madito, M.J.; Momodu, D.; Manyala, N. Asymmetric supercapacitor based on VS 2 nanosheets and activated carbon materials. RSC Adv. 2016, 6, 38990–39000. [Google Scholar] [CrossRef]
- Li, X.; Aftab, S.; Liu, H.; Vikraman, D.; Hussain, S.; Kang, J.; Al-Kahtani, A.A. Enhancing perovskite solar cells and X-ray photodetectors with hybrid MoSe2@CNT composites: A path to improved efficiency and sensitivity. J. Power Sources 2024, 624, 235588. [Google Scholar] [CrossRef]
- Manikandan, R.; Raj, C.J.; Rajesh, M.; Kim, B.C.; Nagaraju, G.; Lee, W.; Yu, K. Rationally designed spider web-like trivanadium heptaoxide nanowires on carbon cloth as a new class of pseudocapacitive electrode for symmetric supercapacitors with high energy density and ultra-long cyclic stability. J. Mater. Chem. A 2018, 6, 11390–11404. [Google Scholar] [CrossRef]
- Fatima, S.; Sajid, I.H.; Khan, M.F.; Rizwan, S. Synthesis and characterization of erbium decorated V2CTx for water splitting properties. Int. J. Hydrogen Energy 2024, 55, 110–117. [Google Scholar] [CrossRef]
- Manikandan, A.; Hema, E.; Durka, M.; Amutha Selvi, M.; Alagesan, T.; Arul Antony, S.; Polymers, O. Mn2+ doped NiS (MnxNi1−xS:X= 0.0, 0.3 and 0.5) nanocrystals: Structural, morphological, opto-magnetic and photocatalytic properties. J. Inorg. Organomet. Polym. Mater. 2015, 25, 804–815. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Veerasubramani, G.K.; Kim, S. Mechanically delaminated few layered MoS2 nanosheets based high performance wire type solid-state symmetric supercapacitors. J. Power Sources 2016, 321, 112–119. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S.; Chemistry, E. Ultrathin graphene-like 2D porous carbon nanosheets and its excellent capacitance retention for supercapacitor. J. Ind. Eng. Chem. 2018, 68, 257–266. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Rao, W.; Sang, T.; Song, B.; Wong, C. Tunable electromagnetic properties and enhanced microwave absorption ability of flaky graphite/cobalt zinc ferrite composites. J. Alloys Compd. 2016, 662, 409–414. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, D.-H. Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J. Colloid Interface Sci. 2004, 273, 165–169. [Google Scholar] [CrossRef]
- Yu, X.; Park, H. Sulfur-incorporated, porous graphene films for high performance flexible electrochemical capacitors. Carbon 2014, 77, 59–65. [Google Scholar] [CrossRef]
- Sajjad, M.; Amin, M.; Javed, M.S.; Imran, M.; Hu, W.; Mao, Z.; Lu, W. Recent trends in transition metal diselenides (XSe2:X= Ni, Mn, Co) and their composites for high energy faradic supercapacitors. J. Energy Storage 2021, 43, 103176. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Lee, S.-H.; Ryu, K.-S. Porous thin layered nanosheets assembled ZnCo2O4 grown on Ni-foam as an efficient electrode material for hybrid supercapacitor applications. Int. J. Hydrogen Energy 2017, 42, 3122–3129. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Sun, J.; Xu, C. High-performance hybrid supercapacitor based on the porous copper cobaltite/cupric oxide nanosheets as a battery-type positive electrode material. Int. J. Hydrogen Energy 2021, 46, 28144–28155. [Google Scholar] [CrossRef]
- Farbod, F.; Mazloum-Ardakani, M.; Naderi, H.R.; Mohammadian-Sarcheshmeh, H. Synthesis of a porous interconnected nitrogen-doped graphene aerogel matrix incorporated with ytterbium oxide nanoparticles and its application in superior symmetric supercapacitors. Electrochim. Acta 2019, 306, 480–488. [Google Scholar] [CrossRef]
- Imran, M.; Muhammad, Z.; Muzafar, N.; Afzal, A.M.; Iqbal, M.W.; Mumtaz, S.; Munnaf, S.A.; Albaqami, M.D.; Ahmad, Z. Enhanced the electrochemical performance of CoMgS nanocomposite electrode with the doping of ZnO for supercapacitor-battery hybrid device and photochemical activity. J. Appl. Electrochem. 2024, 54, 1501–1515. [Google Scholar] [CrossRef]
- Dhaiveegan, P.; Hsu, Y.-K.; Tsai, Y.-H.; Hsieh, C.-K.; Lin, J.-Y. Pulse-reversal deposition of Ni3S2 thin films on carbon fiber cloths for supercapacitors. Surf. Coatings Technol. 2018, 350, 1003–1009. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, L.; Tang, J.; Chai, J.; Xu, J.; Cao, L.; Yang, M.; Yang, M.; Kong, W.; Wang, S. Efficient coupling of a hierarchical V2O5@Ni3S2 hybrid nanoarray for pseudocapacitors and hydrogen production. J. Mater. Chem. A 2017, 5, 17954–17962. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, J.; Wu, J.; Xu, R.; Lai, M.; Gong, C.; Chen, X.; Zhou, P. Excellent electrochemical performance hierarchical Co3O4@Ni3S2 core/shell nanowire arrays for asymmetric supercapacitors. Electrochim. Acta 2016, 207, 87–96. [Google Scholar] [CrossRef]
- Dunn, R.; Griggs, S.A.; Olson, J.; Beasley, M.; Gorman, B.S. A meta-analytic validation of the Dunn and Dunn model of learning-style preferences. J. Educ. Res. 1995, 88, 353–362. [Google Scholar]
- Yüksek, Ö.; Anılan, T.; Saka, F. Problems and possible solutions to environmental impacts of small hydropower plants in Turkey. Turk. J. Electromechanics Energy 2020, 5. [Google Scholar]
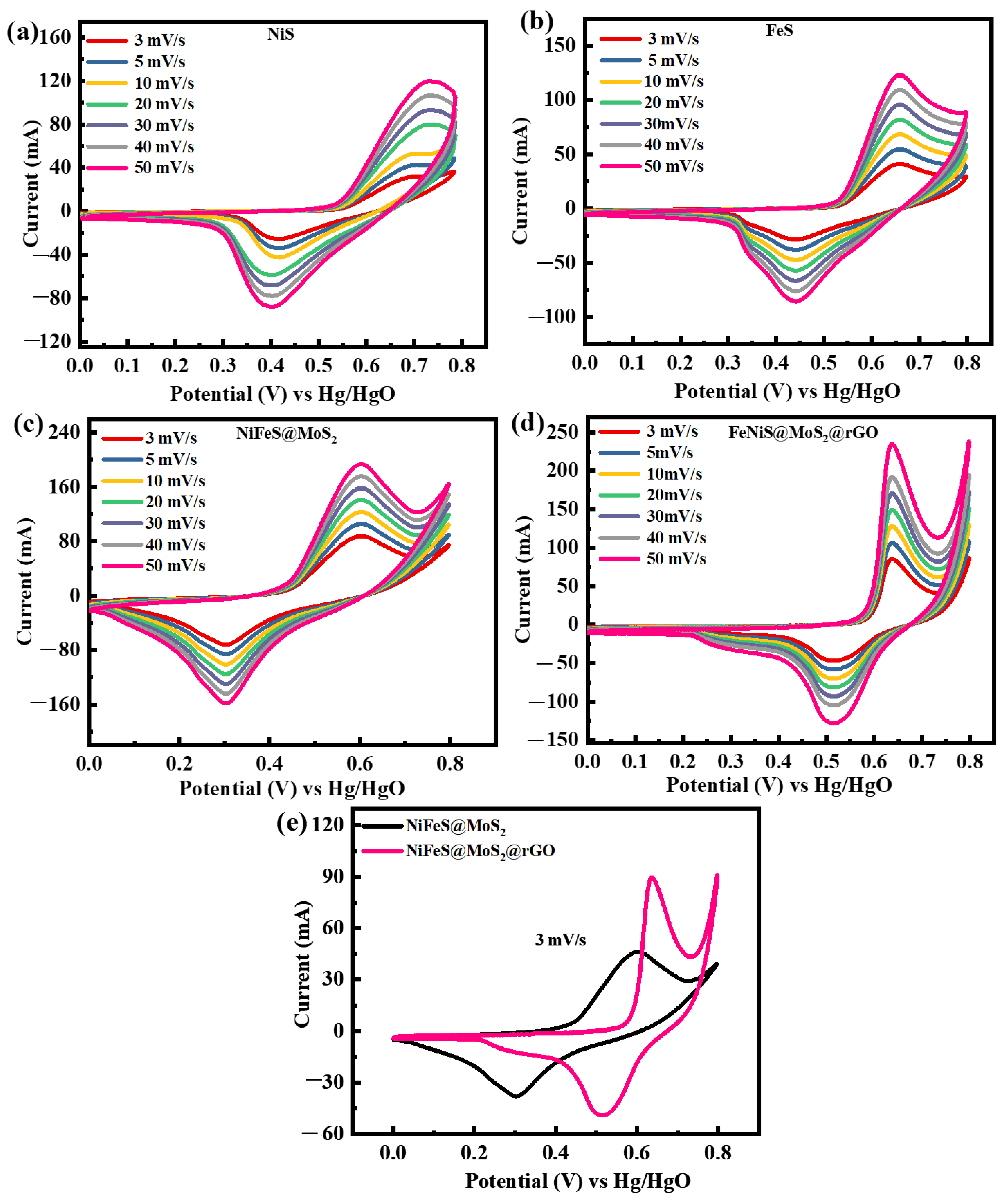
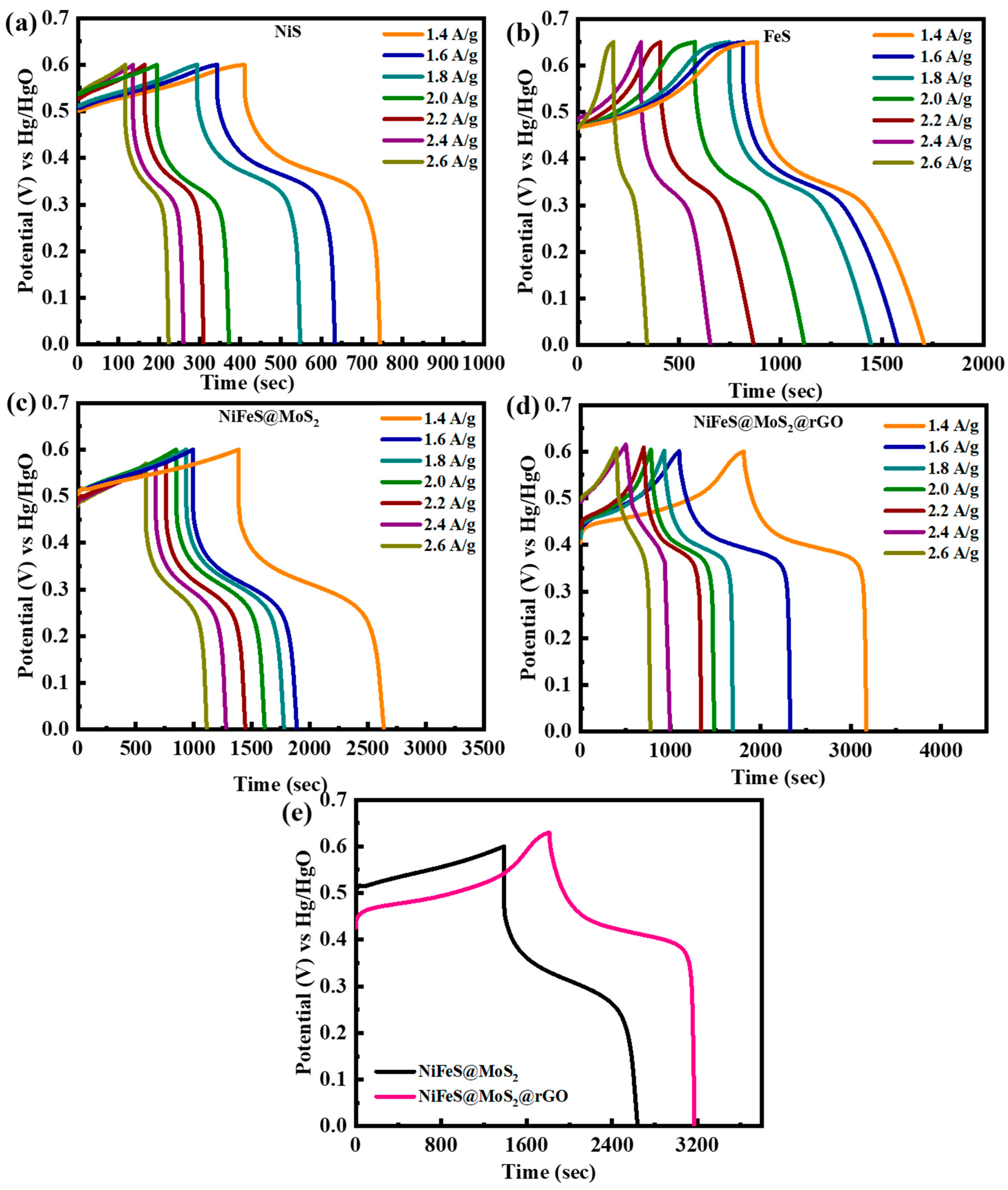
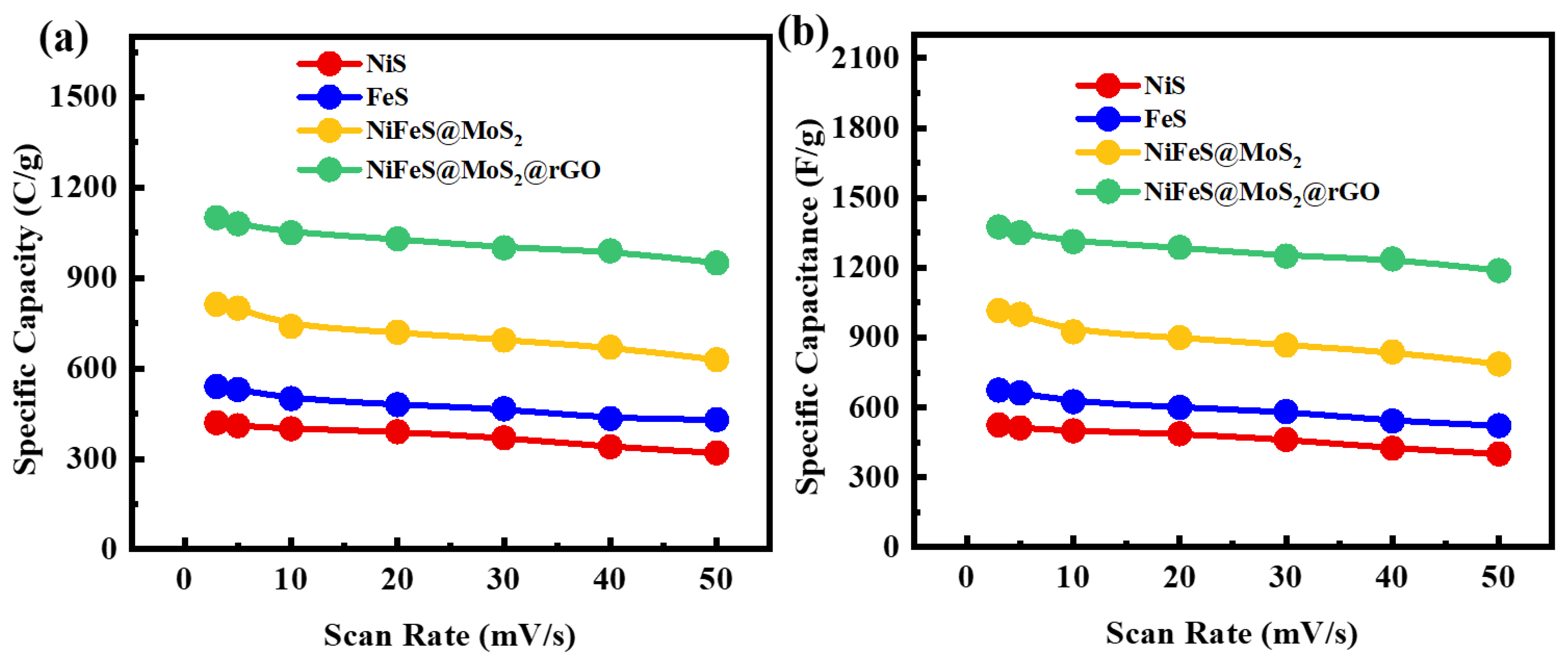
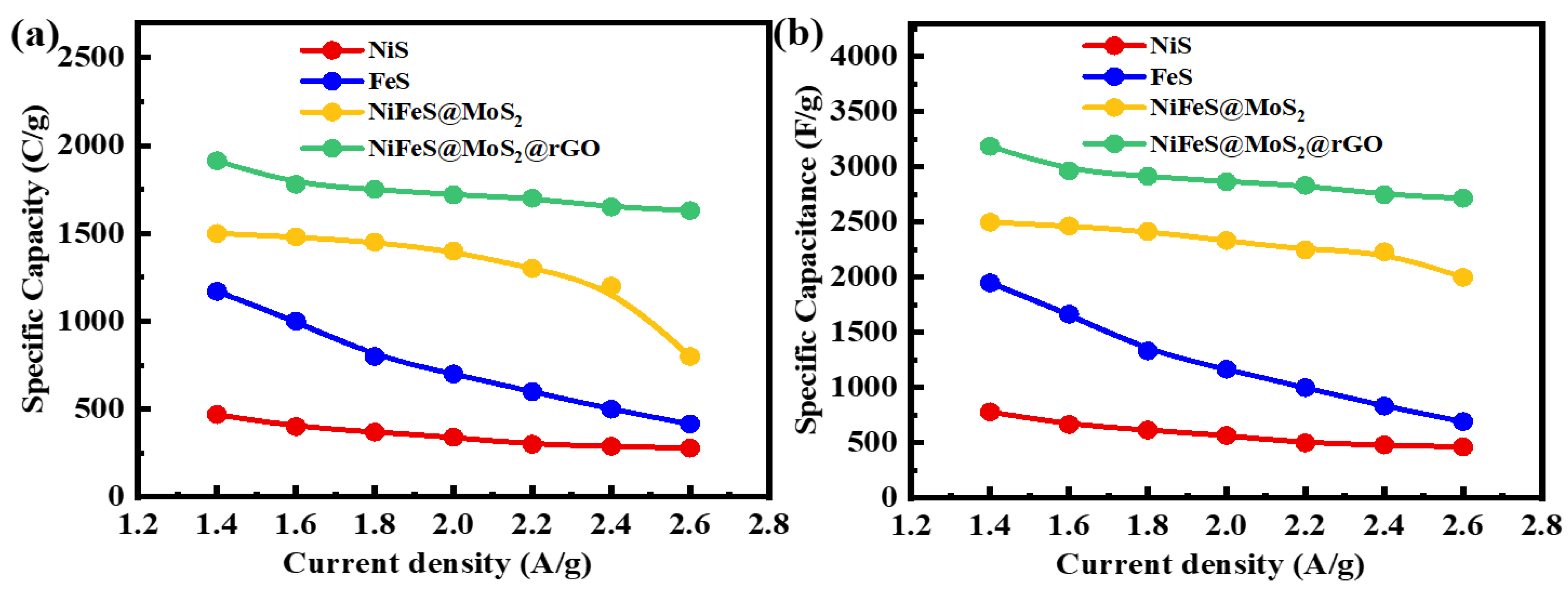

| Sr.No | Samples | Methods | Electrolyte | Specific Capacitance | Cycle Life | Ref. |
|---|---|---|---|---|---|---|
| 1 | Nanoporous net-like Ni3S2 thin film | Electrodeposition | 1 moL.−1 KOH | 7.25 F·cm−2 at 5 mA·cm−2 | 77%/5000 0.05 mA·cm−2 | [44] |
| 2 | V2O2/Ni3S2 nanoflake | Hydrothermal | 2 moL.−1 KOH | 4.2 F·cm−2 at 5 mA·cm−2 | 85%/2500 10 mA·cm−2 | [45] |
| 3 | Hierarchical Co3@NiS2 core/shell nanowire arrays | Hydrothermal | 3 moL.−1 KOH | 1710 F·g−1 at 1 A·g−1 | 85%/1000 4 A·g−1 | [46] |
| Sr.No | Sample | Specific Capacity (CV) | Specific Capacity (GCD) |
|---|---|---|---|
| 1 | NiS | 65 | 70 |
| 2 | FeS | 72 | 80 |
| 3 | NiFeS@MoS2 | 100 | 110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmeen, A.; Afzal, A.M.; Alqarni, A.S.; Iqbal, M.W.; Mumtaz, S. Design and Optimization of MoS2@rGO@NiFeS Nanocomposites for Hybrid Supercapattery Performance and Sensitive Electrochemical Detection. Molecules 2024, 29, 5195. https://doi.org/10.3390/molecules29215195
Yasmeen A, Afzal AM, Alqarni AS, Iqbal MW, Mumtaz S. Design and Optimization of MoS2@rGO@NiFeS Nanocomposites for Hybrid Supercapattery Performance and Sensitive Electrochemical Detection. Molecules. 2024; 29(21):5195. https://doi.org/10.3390/molecules29215195
Chicago/Turabian StyleYasmeen, Aneeqa, Amir Muhammad Afzal, Areej S. Alqarni, Muhammad Waqas Iqbal, and Sohail Mumtaz. 2024. "Design and Optimization of MoS2@rGO@NiFeS Nanocomposites for Hybrid Supercapattery Performance and Sensitive Electrochemical Detection" Molecules 29, no. 21: 5195. https://doi.org/10.3390/molecules29215195
APA StyleYasmeen, A., Afzal, A. M., Alqarni, A. S., Iqbal, M. W., & Mumtaz, S. (2024). Design and Optimization of MoS2@rGO@NiFeS Nanocomposites for Hybrid Supercapattery Performance and Sensitive Electrochemical Detection. Molecules, 29(21), 5195. https://doi.org/10.3390/molecules29215195








