Tracking Long-Lived Free Radicals in Dandelion Caused by Air Pollution Using Electron Paramagnetic Resonance Spectroscopy
Abstract
1. Introduction
2. Results
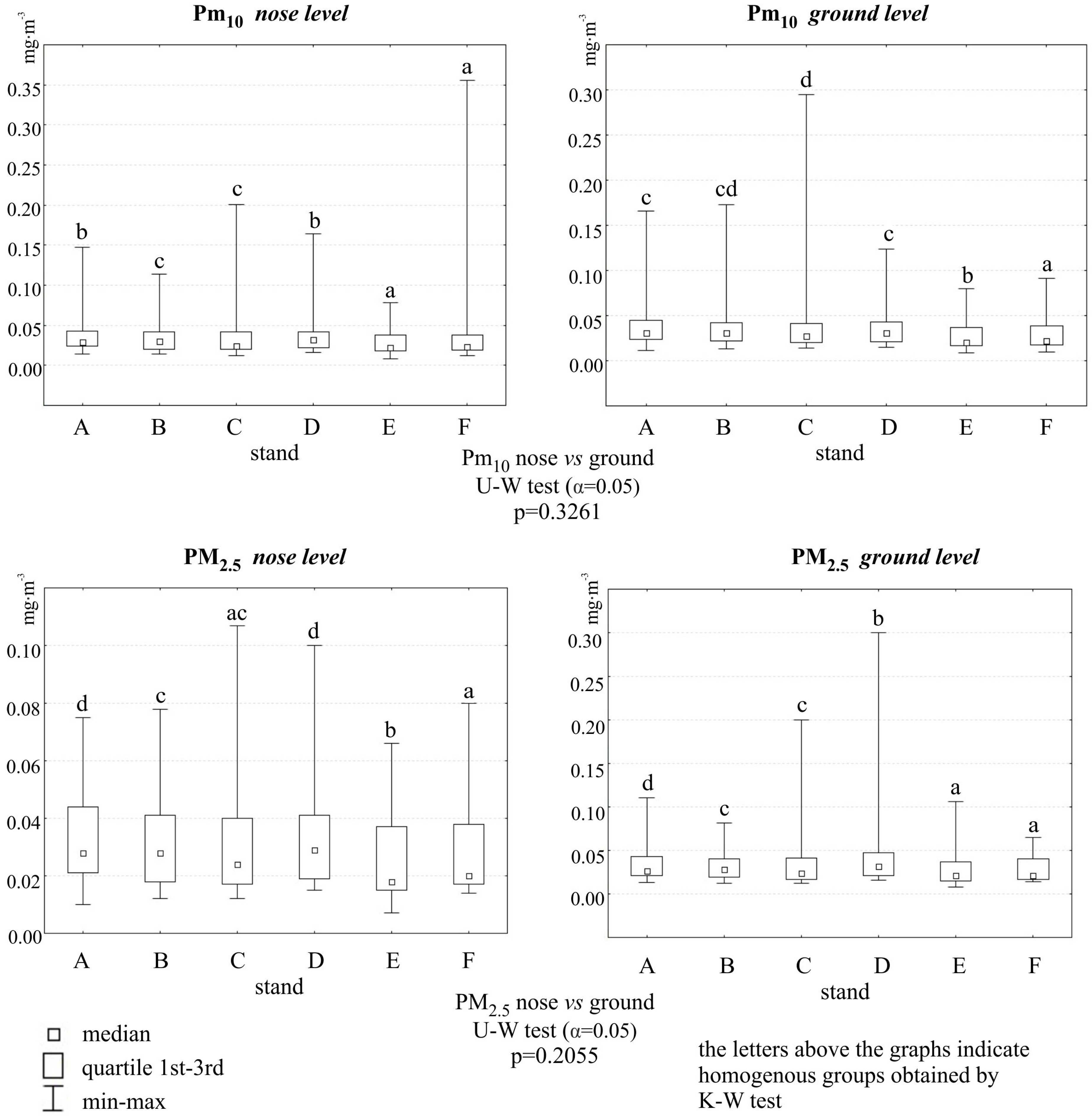
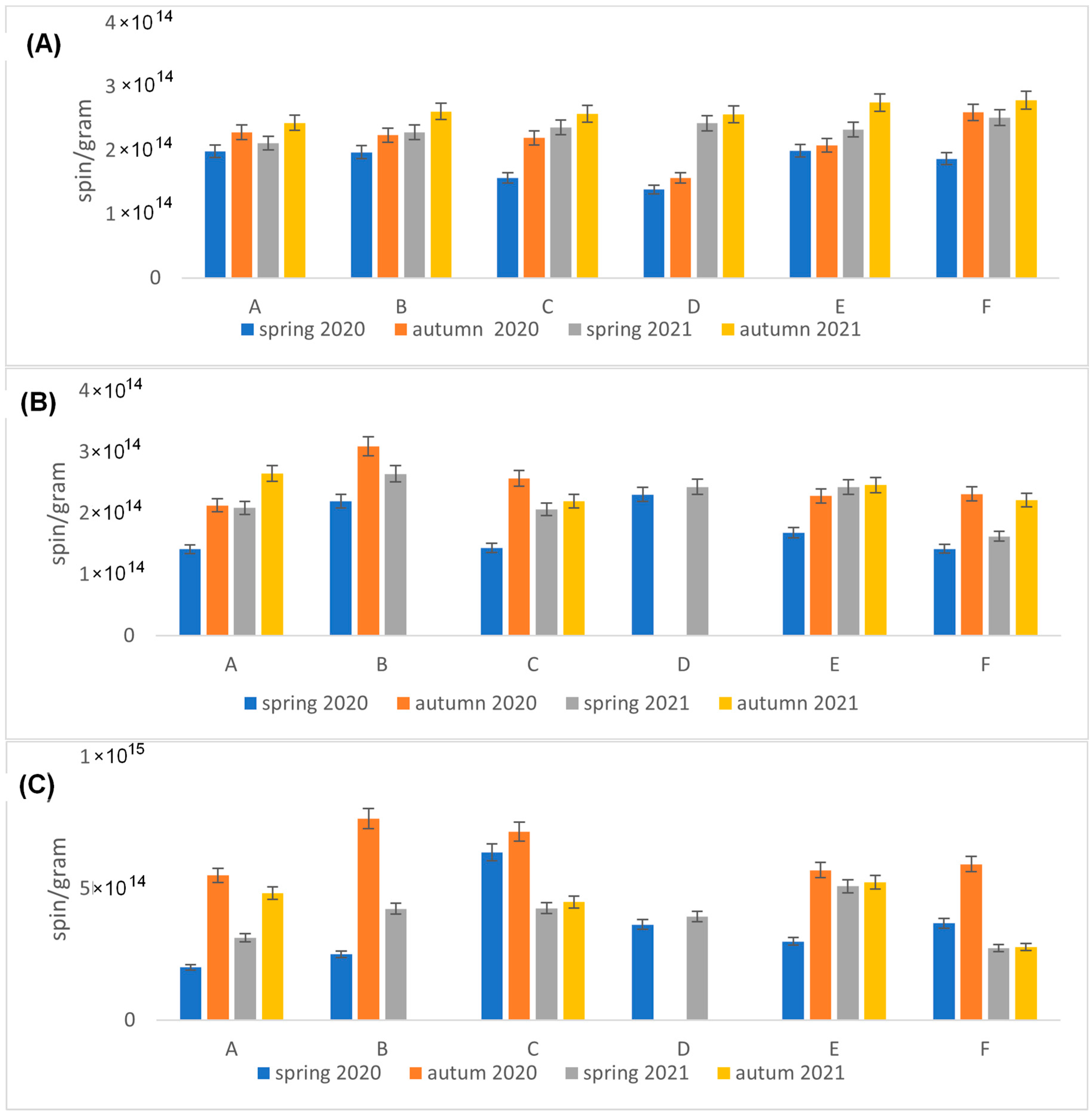
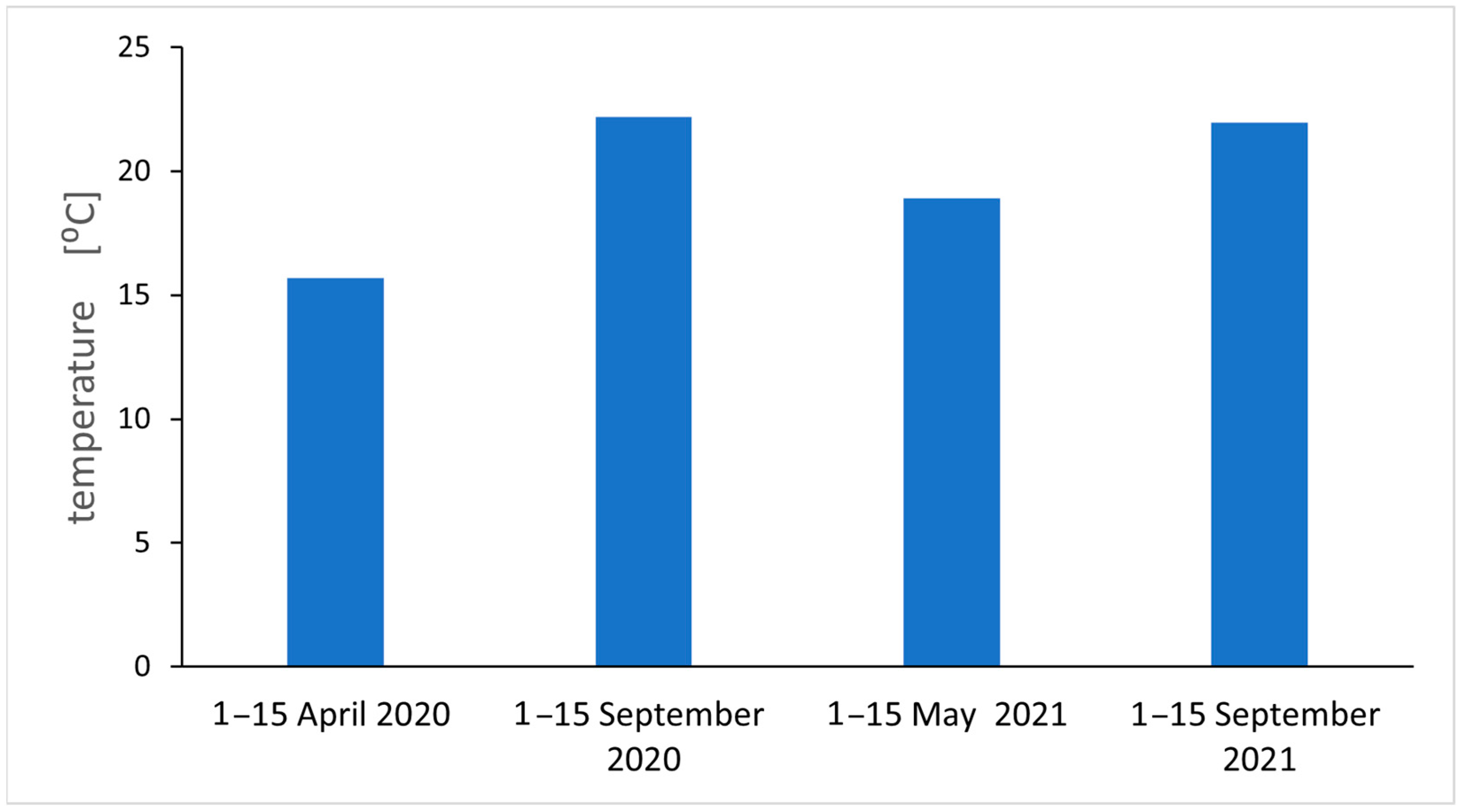
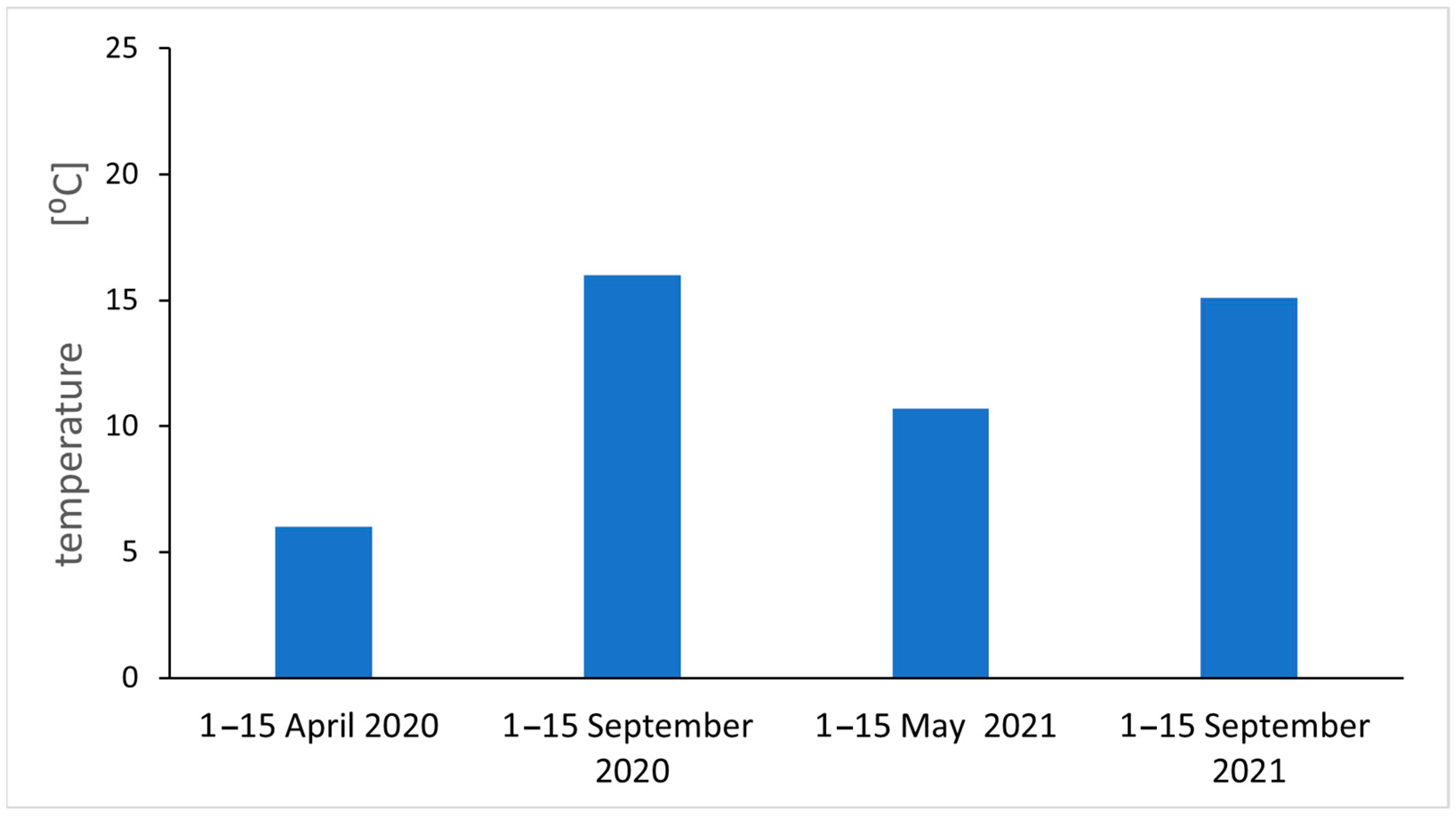
2.1. Particulate Air Pollution
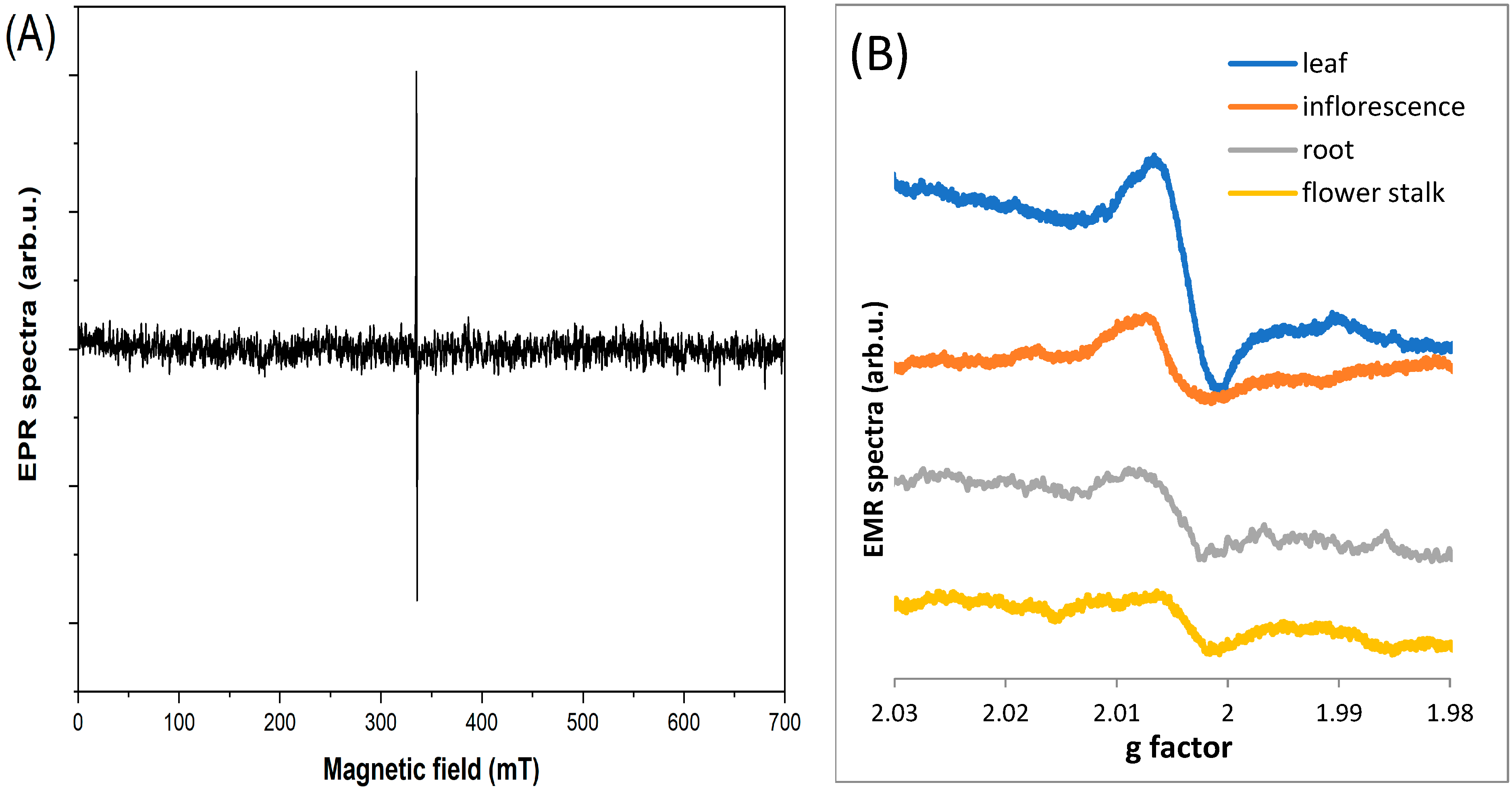
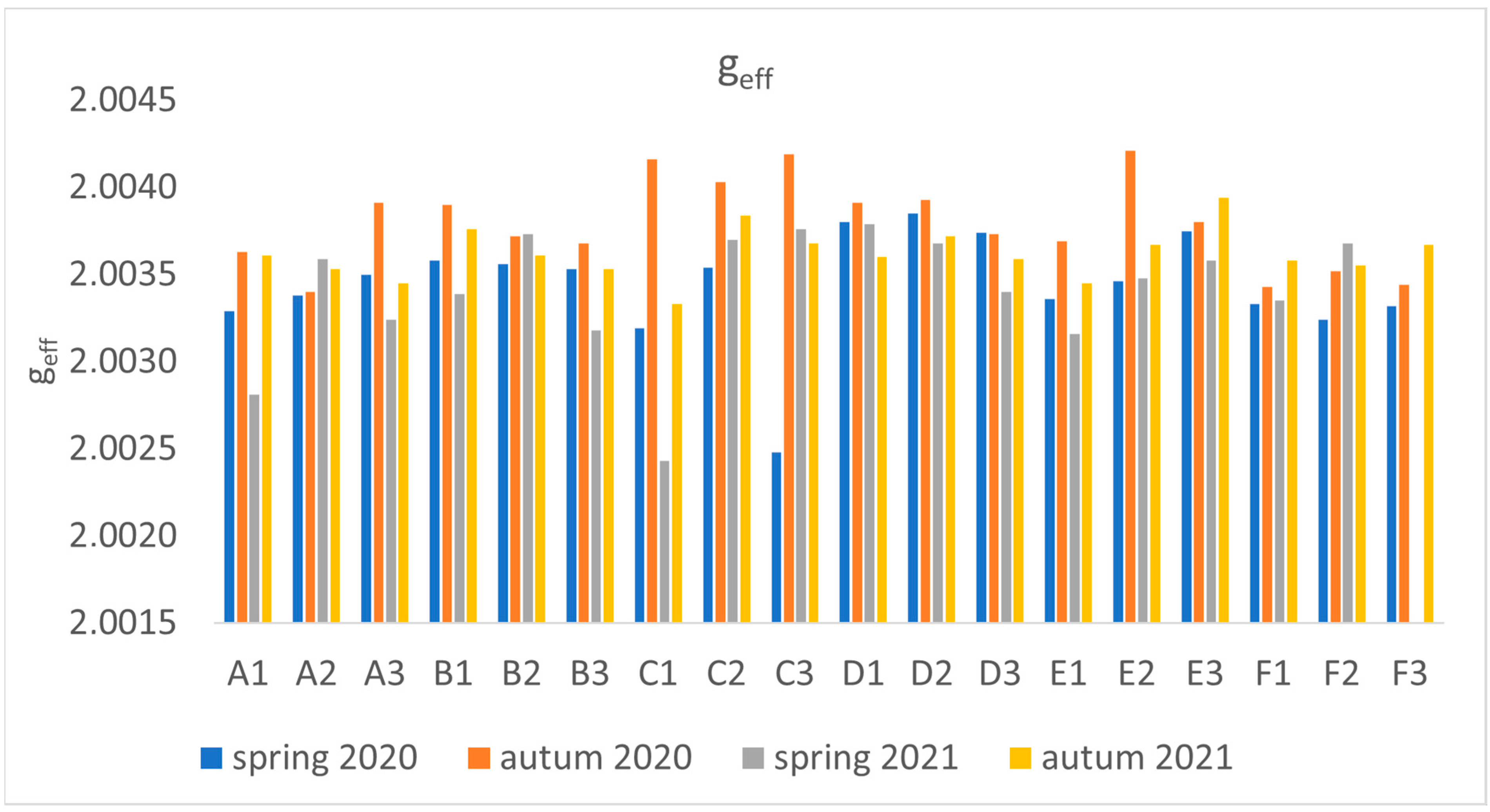
2.2. EPR Analyses of Dandelion
3. Discussion
4. Materials and Methods
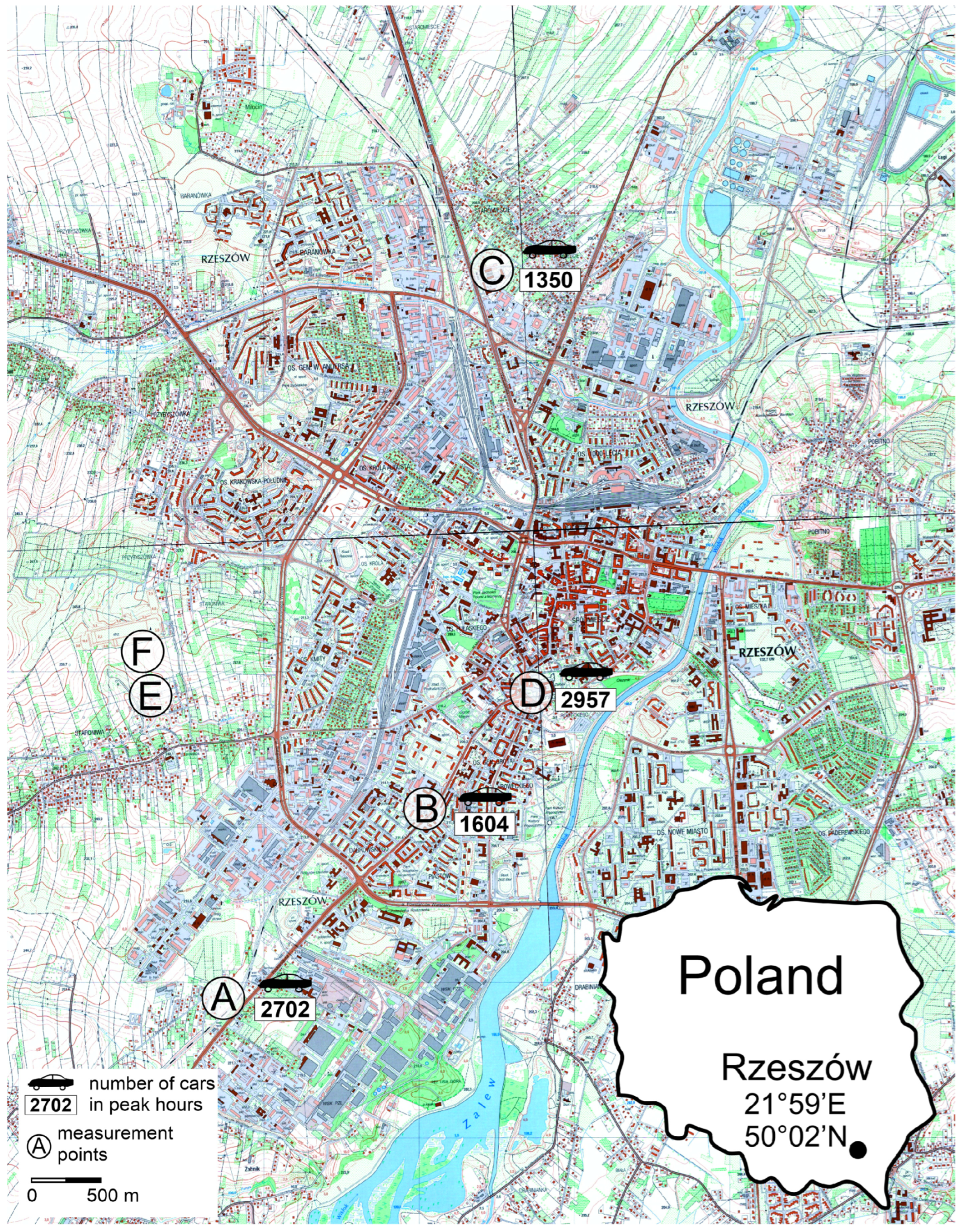
4.1. Study Area
4.2. Materials
4.3. Measurement of Air Pollution
4.4. Electron Paramagnetic Resonance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakrishna, S.; Saravia, J.; Thevenot, P.; Ahlert, T.; Lominiki, S.; Dellinger, B.; Cormier, S.A. Environmentally Persistent Free Radicals Induce Airway Hyperresponsiveness in Neonatal Rat Lungs. Part. Fibre Toxicol. 2011, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- del Río, L.A. ROS and RNS in Plant Physiology: An Overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.K.; Kaushik, P. Free Radicals Mediated Redox Signaling in Plant Stress Tolerance. Life 2023, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Ainur, D.; Chen, Q.; Wang, Y.; Li, H.; Lin, H.; Ma, X.; Xu, X. Pollution Characteristics and Sources of Environmentally Persistent Free Radicals and Oxidation Potential in Fine Particulate Matter Related to City Lockdown (CLD) in Xi’an, China. Environ. Res. 2022, 210, 112899. [Google Scholar] [CrossRef]
- Sigmund, G.; Santín, C.; Pignitter, M.; Tepe, N.; Doerr, S.H.; Hofmann, T. Environmentally Persistent Free Radicals Are Ubiquitous in Wildfire Charcoals and Remain Stable for Years. Commun. Earth Environ. 2021, 2, 68. [Google Scholar] [CrossRef]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Formation, Characteristics, and Applications of Environmentally Persistent Free Radicals in Biochars: A Review. Bioresour. Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef]
- Oyana, T.J.; Lomnicki, S.M.; Guo, C.; Cormier, S.A. A Scalable Field Study Protocol and Rationale for Passive Ambient Air Sampling: A Spatial Phytosampling for Leaf Data Collection. Environ. Sci. Technol. 2017, 51, 10663–10673. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Rao, G.; Khachatryan, L.; Cormier, S.A.; Lomnicki, S. Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants. Environ. Sci. Technol. 2018, 52, 2468–2481. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, H.; Wang, M.; Mu, Z.; Wang, Y.; Li, Y.; Wang, Y.; Zhang, L.; Zhang, Z. Dominant Fraction of EPFRs from Nonsolvent-Extractable Organic Matter in Fine Particulates over Xi’an, China. Environ. Sci. Technol. 2018, 52, 9646–9655. [Google Scholar] [CrossRef]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally Persistent Free Radicals: Occurrence, Formation Mechanisms and Implications. Environ. Pollut. 2019, 248, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, T.; Tang, Y.-T.; Chen, T.; Khachatryan, L.; Iyer, P.R.; Guo, D.; Chen, A.; Lyu, M.; Li, J.; et al. Environmentally Persistent Free Radicals in PM2.5: A Review. Waste Dispos. Sustain. Energy 2019, 1, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Hegde, V.; Deutsch, W.A. Role of Free Radicals in the Toxicity of Airborne Fine Particulate Matter. Chem. Res. Toxicol. 2001, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, Formation, Environmental Fate and Risks of Environmentally Persistent Free Radicals in Biochars. Environ. Int. 2020, 134, 105172. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Zheng, M.; Jin, R.; Zhu, Q.; Zhao, Y.; Wu, X.; Xu, Y. Highly Elevated Levels and Particle-Size Distributions of Environmentally Persistent Free Radicals in Haze-Associated Atmosphere. Environ. Sci. Technol. 2017, 51, 7936–7944. [Google Scholar] [CrossRef] [PubMed]
- Gehling, W.; Dellinger, B. Environmentally Persistent Free Radicals and Their Lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef]
- Savinov, A.B.; Kurganova, L.N.; Shekunov, Y.I. Lipid Peroxidation Rates in Taraxacum officinale Wigg. and Vicia cracca L. from Biotopes with Different Levels of Soil Pollution with Heavy Metals. Russ. J. Ecol. 2007, 38, 174–180. [Google Scholar] [CrossRef]
- Bretzel, F.; Benvenuti, S.; Pistelli, L. Metal Contamination in Urban Street Sediment in Pisa (Italy) Can Affect the Production of Antioxidant Metabolites in Taraxacum officinale Weber. Environ. Sci. Pollut. Res. 2014, 21, 2325–2333. [Google Scholar] [CrossRef]
- Makuch-Pietraś, I.; Grabek-Lejko, D.; Górka, A.; Kasprzyk, I. Antioxidant Activities in Relation to the Transport of Heavy Metals from the Soil to Different Parts of Betula pendula (Roth). J. Biol. Eng. 2023, 17, 19. [Google Scholar] [CrossRef]
- Filek, M.; Łabanowska, M.; Kurdziel, M.; Sieprawska, A. Electron Paramagnetic Resonance (EPR) Spectroscopy in Studies of the Protective Effects of 24-Epibrasinoide and Selenium against Zearalenone-Stimulation of the Oxidative Stress in Germinating Grains of Wheat. Toxins 2017, 9, 178. [Google Scholar] [CrossRef]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Deutsch, W.A. The Role of Combustion-Generated Radicals in the Toxicity of PM2.5. Proc. Combust. Inst. 2000, 28, 2675–2681. [Google Scholar] [CrossRef]
- Maskos, Z.; Khachatryan, L.; Cueto, R.; Pryor, W.A.; Dellinger, B. Radicals from the Pyrolysis of Tobacco. Energy Fuels 2005, 19, 791–799. [Google Scholar] [CrossRef]
- Maskos, Z.; Khachatryan, L.; Dellinger, B. Precursors of Radicals in Tobacco Smoke and the Role of Particulate Matter in Forming and Stabilizing Radicals. Energy Fuels 2005, 19, 2466–2473. [Google Scholar] [CrossRef]
- Arangio, A.M.; Tong, H.; Socorro, J.; Pöschl, U.; Shiraiwa, M. Quantification of Environmentally Persistent Free Radicals and Reactive Oxygen Species in Atmospheric Aerosol Particles. Atmos. Chem. Phys. 2016, 16, 13105–13119. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Boman, J.; Shehadeh, Z.F.; Al-Malawi, D.; Allah, R.; Hemeda, O.M.; Morsy, M.M. Spectroscopic Investigation of PM2.5 Collected at Industrial, Residential and Traffic Sites in Taif, Saudi Arabia. J. Aerosol Sci. 2015, 79, 97–108. [Google Scholar] [CrossRef]
- Cruz, A.L.N.D.; Cook, R.L.; Lomnicki, S.M.; Dellinger, B. Effect of Low Temperature Thermal Treatment on Soils Contaminated with Pentachlorophenol and Environmentally Persistent Free Radicals. Environ. Sci. Technol. 2012, 46, 5971–5978. [Google Scholar] [CrossRef]
- Environmental Protection Agency Project Officer K. Blackburn. Recommendations for and Documentation of Biological Values for Use in Risk Assessment; Office of Health and Environmental Assessment: Washington, DC, USA, 1988. [Google Scholar]
- Pryor, W.A.; Prier, D.G.; Church, D.F. Electron-Spin Resonance Study of Mainstream and Sidestream Cigarette Smoke: Nature of the Free Radicals in Gas-Phase Smoke and in Cigarette Tar. Environ. Health Perspect. 1983, 47, 345. [Google Scholar] [CrossRef]
- Gehling, W.; Khachatryan, L.; Dellinger, B. Hydroxyl Radical Generation from Environmentally Persistent Free Radicals (EPFRs) in PM2.5. Environ. Sci. Technol. 2014, 48, 4266–4272. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Ahn, J. Leaves Are a Source of Biogenic Persistent Free Radicals. Environ. Sci. Technol. Lett. 2023, 10, 662–667. [Google Scholar] [CrossRef]
- Kang, M.-J.; Shin, S.-R.; Kim, K.-S. Antioxidative and Free Radical Scavenging Activity of Water Extract from Dandelion (Taraxacum officinale). Korean J. Food Preserv. 2002, 9, 253–259. [Google Scholar]
- Jung, K.; Richter, J.; Kabrodt, K.; Lücke, I.M.; Schellenberg, I.; Herrling, T. The Antioxidative Power AP—A New Quantitative Time Dependent (2D) Parameter for the Determination of the Antioxidant Capacity and Reactivity of Different Plants. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Chemerys, I.; Myslyuk, O.; Chemerys, V. Effect of Vehicle Emissions on the Morphological and Physiological Changes of Taraxacum officinale Web. Ukr. J. Ecol. 2020, 10, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Keane, B.; Collier, M.H.; Shann, J.R.; Rogstad, S.H. Metal Content of Dandelion (Taraxacum officinale) Leaves in Relation to Soil Contamination and Airborne Particulate Matter. Sci. Total Environ. 2001, 281, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Degórska, A. An Assessment of Urban Habitat Contamination with Selected Heavy Metals within the City of Katowice Using the Common Dandelion (Taraxacum officinale Web.) as a Bioindicator. Environ. Socio-Econ. Stud. 2013, 1, 29–40. [Google Scholar] [CrossRef]
- Ianovici, N. Taraxacum officinale (Asteraceae) in the Urban Environment: Seasonal Fluctuations of Plant Traits and Their Relationship with Meteorological Factors. Acta Agrobot. 2016, 69, 1677. [Google Scholar] [CrossRef]
- Raport Częściowy nr 4. Badania Natężenia Ruchu Drogowego; Politechnika Rzeszowska: Rzeszów, Poland, 2023.
- Rochaix, J.-D. Regulation of Photosynthetic Electron Transport. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 375–383. [Google Scholar] [CrossRef]
- Giacomino, A.; Malandrino, M.; Colombo, M.L.; Miaglia, S.; Maimone, P.; Blancato, S.; Conca, E.; Abollino, O. Metal Content in Dandelion (Taraxacum officinale) Leaves: Influence of Vehicular Traffic and Safety upon Consumption as Food. J. Chem. 2016, 2016, 9842987. [Google Scholar] [CrossRef]

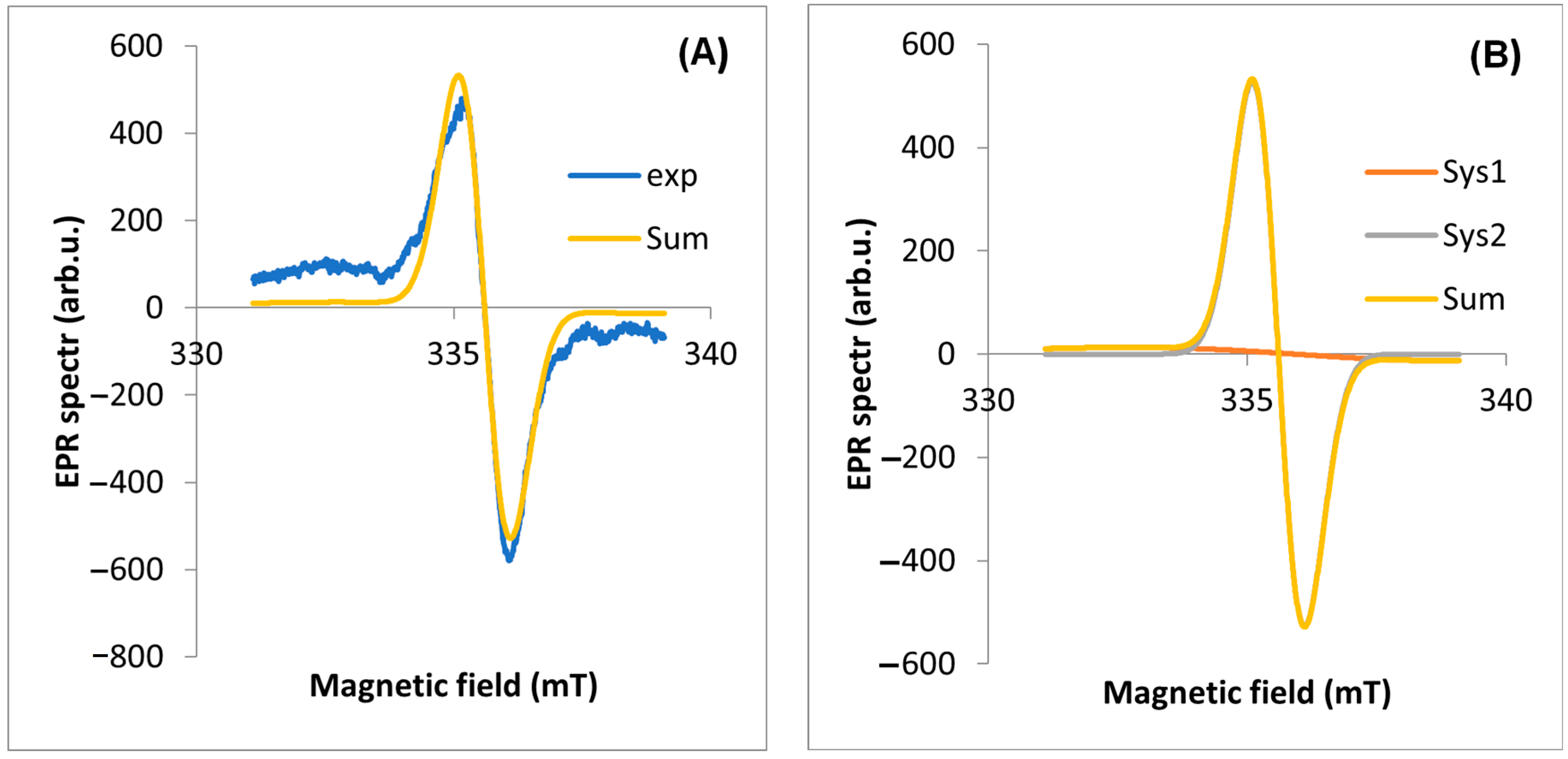
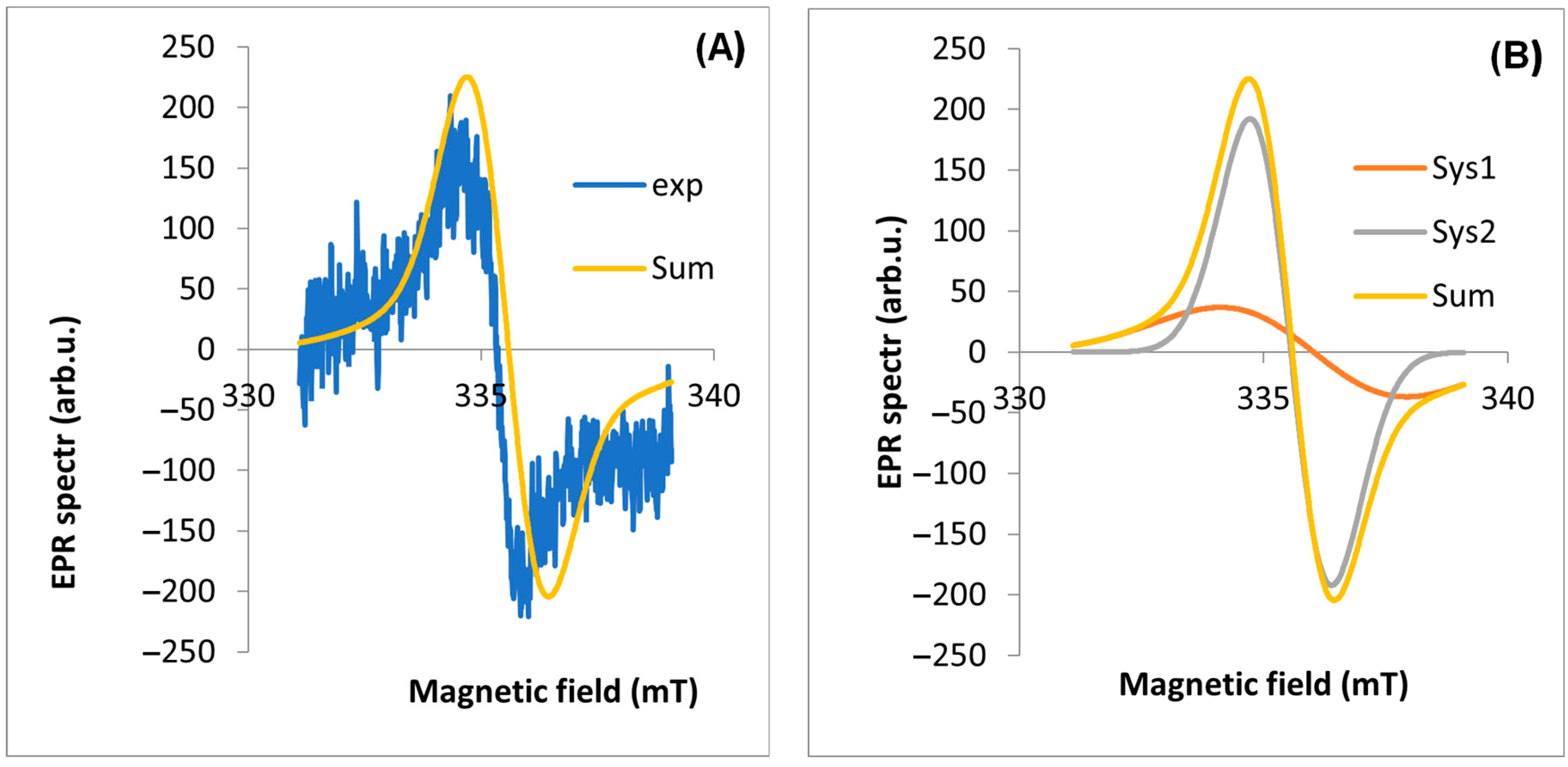
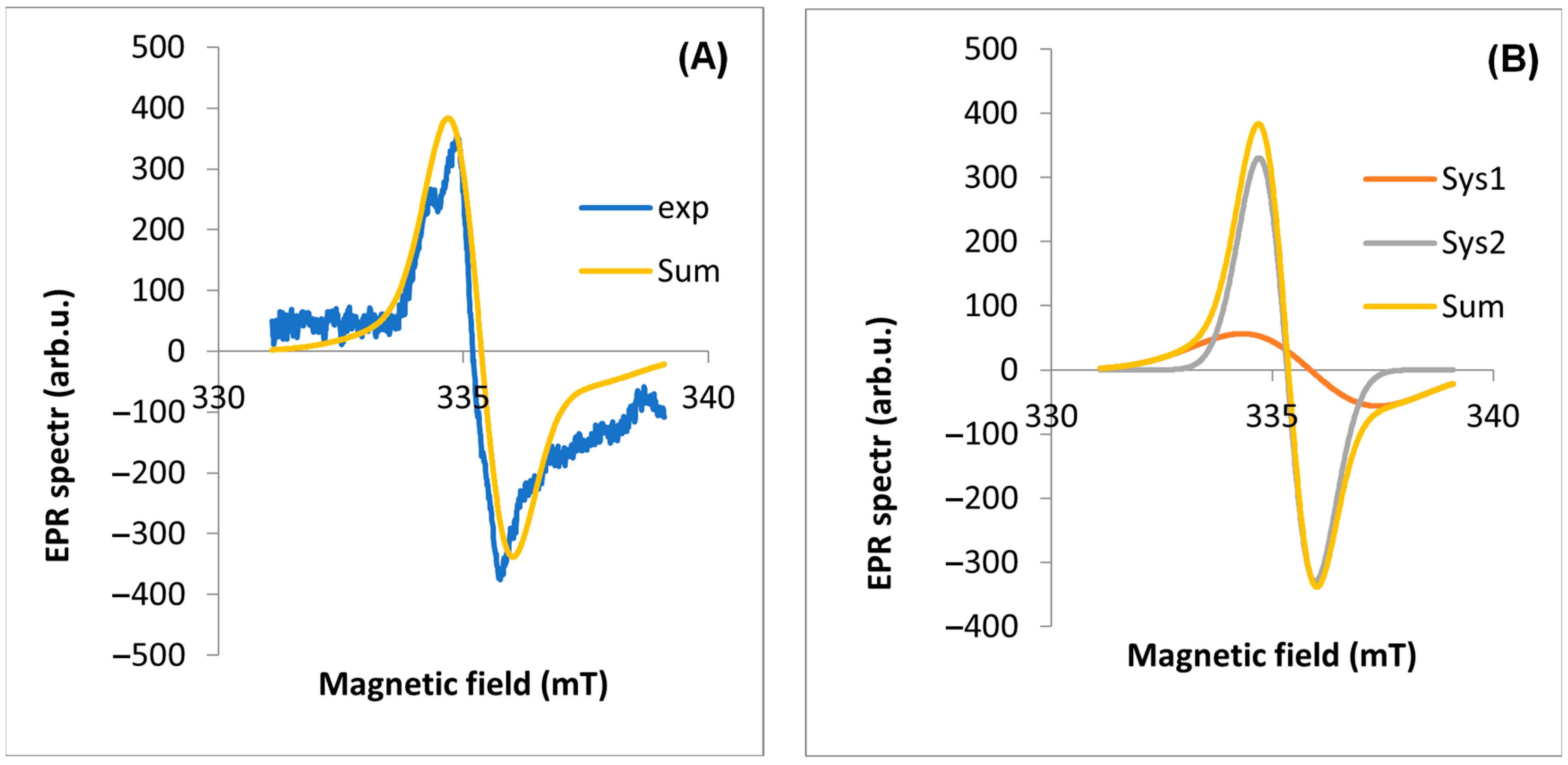
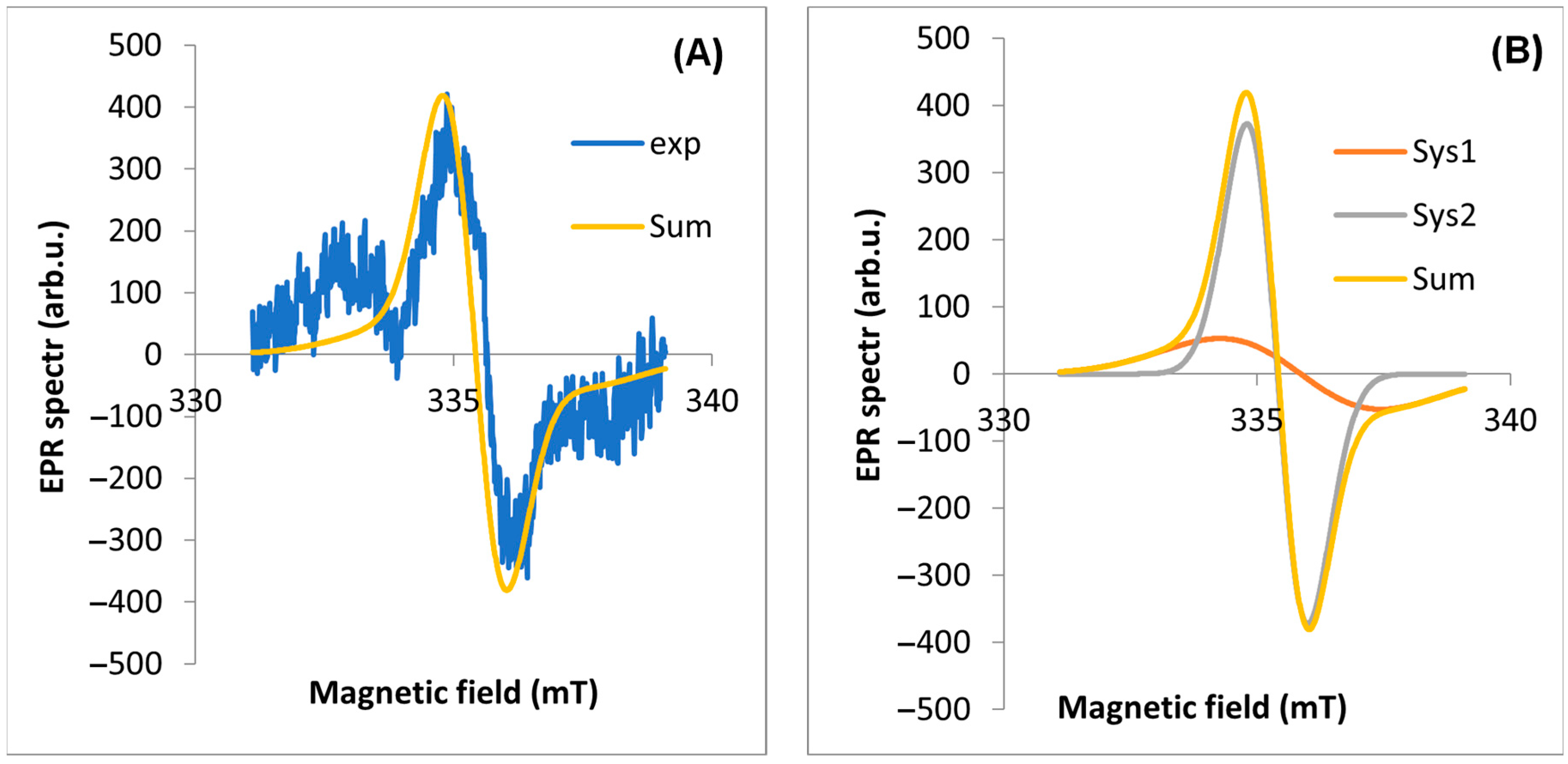

| EPR Line Components | Inflorescence | Stalk | Root | Leaf | |
|---|---|---|---|---|---|
| Experimental line | Intensity [arb. unit] | 0.223 | 0.112 | 0.124 | 1.332 |
| Experimental line | geff | 2.0040 | 2.0038 | 2.0039 | 2.0038 |
| A | g | 2.0025 | 2.0015 | 2.0025 | 2.0021 |
| A | lwpp [mT] | 3.0804 | 3.8110 | 3.1860 | 6.3861 |
| A | weight | 1 | 1 | 1 | 6.58 |
| B | g | 2.0056 | 2.0043 | 2.0052 | 2.0040 |
| B | lwpp [mT] | 1.2740 | 1.6700 | 1.2000 | 1.0082 |
| B | weight | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefaniuk, I.; Cieniek, B.; Ćwik, A.; Kluska, K.; Kasprzyk, I. Tracking Long-Lived Free Radicals in Dandelion Caused by Air Pollution Using Electron Paramagnetic Resonance Spectroscopy. Molecules 2024, 29, 5173. https://doi.org/10.3390/molecules29215173
Stefaniuk I, Cieniek B, Ćwik A, Kluska K, Kasprzyk I. Tracking Long-Lived Free Radicals in Dandelion Caused by Air Pollution Using Electron Paramagnetic Resonance Spectroscopy. Molecules. 2024; 29(21):5173. https://doi.org/10.3390/molecules29215173
Chicago/Turabian StyleStefaniuk, Ireneusz, Bogumił Cieniek, Agata Ćwik, Katarzyna Kluska, and Idalia Kasprzyk. 2024. "Tracking Long-Lived Free Radicals in Dandelion Caused by Air Pollution Using Electron Paramagnetic Resonance Spectroscopy" Molecules 29, no. 21: 5173. https://doi.org/10.3390/molecules29215173
APA StyleStefaniuk, I., Cieniek, B., Ćwik, A., Kluska, K., & Kasprzyk, I. (2024). Tracking Long-Lived Free Radicals in Dandelion Caused by Air Pollution Using Electron Paramagnetic Resonance Spectroscopy. Molecules, 29(21), 5173. https://doi.org/10.3390/molecules29215173









