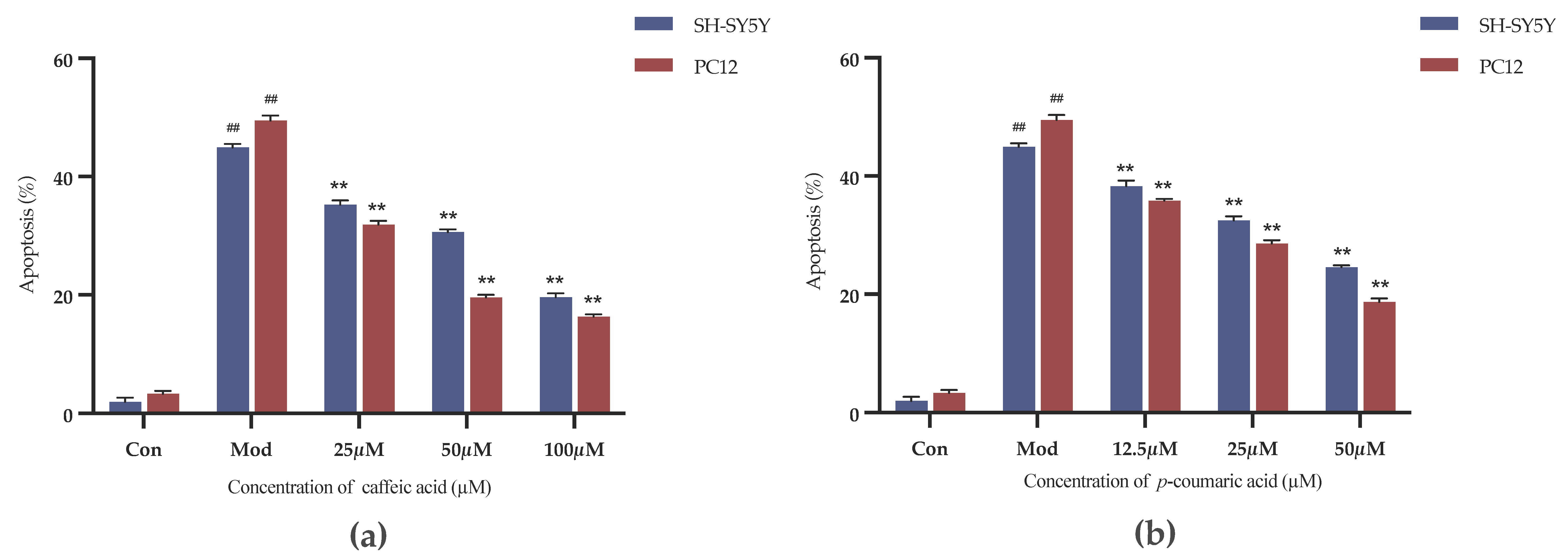Abstract
Levodopa (LD) is the first discovered and the most promising and effective medication for Parkinson’s disease (PD). As the first identified natural source of LD, Vicia faba L. (broad beans), especially its sprouts, has been confirmed to contain many other potential bioactive compounds that could also be therapeutic for PD. In this study, the bioactive components obtained from broad bean sprout extraction (BSE) that could be beneficial for PD treatment were screened, and the related mechanisms were explored. Solvent extraction combined with column chromatography was used to isolate bioactive fractions and monomer compounds, while UPLC-ESI-MS/MS, HRESI-MS and (1H, 13C) NMR were employed for compound identification. Network pharmacology techniques were applied to screen for potential mechanisms. A total of 52 compounds were identified in a 50% MeOH extract of broad bean sprouts. Moreover, twelve compounds were isolated and identified from ethyl acetate and n-butanol portions, including caffeic acid (1), trans-3-indoleacrylic acid (2), p-coumaric acid (3), protocatechualdehyde (4), isovitexin (5), isoquercetin (6), grosvenorine (7), kaempferol-3-O-rutinoside (8), isoschaftoside (9), narcissin (10), kaempferitrin (11) and trigonelline HCl (12). Compounds 2, 4, 7, 8 and 12 were isolated from Vicia faba L. for the first time. The potential mechanisms were determined by analyzing 557 drug targets, 2334 disease targets and 199 intersections between them using a protein–protein interaction (PPI) network, gene ontology (GO) analysis and Kyoto encyclopedia of genes and genomes (KEGG) enrichment. Further in vitro experiments confirmed that caffeic acid (compound 1) and p-coumaric acid (compound 3) have neuroprotective effects in 6-hydroxydopamine-treated SH-SY5Y cells and lipopolysaccharide-treated PC-12 cells through anti-inflammatory and antioxidant mechanisms. In conclusion, this study explored effective components in broad bean sprouts and performed in vitro evaluations.
1. Introduction
PD is a common progressive neurodegenerative disease, especially in middle-aged and elderly individuals. Its main pathological hallmark is the progressive degeneration of dopaminergic neurons in the substantia nigra with the formation of Lewy bodies [1,2], but its pathogenesis has not been clearly confirmed. It has been found that genetics, epigenetics, environmental factors, mitochondrial dysfunction, immune mechanisms, oxidative stress anomalies and inflammatory reactions are related to PD; these have been proven to lead to protein misfolding and aggregation as well as premature neuronal death and result in the occurrence and progression of PD [3,4].
Pharmacologic treatment, with dopamine supplementation being the mainstay, is commonly used to improve symptoms and delay the progression of the disease. LD is the most effective medication for PD, and LD preparations are usually administered along with dopamine receptor (DR) agonists, monoamine oxidase (MAO) inhibitors and catecholamine-O-methyltransferase (COMT) inhibitors in clinical practice [5]. As the disease progresses, individuals commonly require larger or more frequent doses of LD. At the same time, they gradually lose their long-duration responses to dopaminergic medication, and their short-duration responses decrease due to disease-related pathophysiological changes in their brains, which lose the ability to store dopamine [5,6]. Clinical observations found that the long-term use of LD preparations improved functions but resulted in an increase in dyskinesia risk, particularly at high doses [7].
With the rapid aging of society, novel therapeutic approaches and effective medications are urgently needed for PD treatment and to promote public health.
Vicia faba L., an annual plant from the Fabaceae family, is an important natural source of LD [8]. Clinical reports and pharmacodynamic studies have shown that other components in broad beans could also contribute to PD treatment, and eating broad beans can ease the motor symptoms between doses of LD [9]. Studies on bioactive components from different parts of broad beans reported that the contents of flavonoids, phenolic acids and other compounds in broad bean seeds increased after germination, and this increase was verified to be positively correlated with many biological activities [10]. Compared with a dose of LD, a broad bean sprout extract that contained the same dose of LD showed more significant therapeutic effects in a Parkinson’s pharmacodynamic model in our previous research, which suggests that broad bean sprouts may be a good source of bioactive components for treating PD [11]. However, very limited information about the bioactive compounds in broad bean sprouts has been reported.
“Network pharmacology” has been proposed as a new method for screening the mechanisms of action of traditional Chinese medicine formulae [12]. It includes virtual computing, high-throughput data analysis, database retrieval, network visualization technology, and bioinformatic network construction and network topology analyses [13]. ADME-related properties, such as human oral bioavailability (OB), drug-likeness (DL), Caco-2 permeability (Caco-2), the ability to cross the blood–brain barrier (BBB) and Lipinski’s rule of five (Ro5) were initially applied to guide the selection of molecules with appropriate biophysical properties [14,15]. With the recent merging of bioinformatics, the main technology and analytical method of network pharmacology now involves a PPI network, and GO and KEGG enrichment analyses contribute to the investigation of the therapeutic effects and mechanisms of complex natural resources in various diseases [12,16]. With the wide application of network pharmacology, its evaluation methods and criteria have been gradually improved [17,18].
The neuroprotective effects of caffeic acid and p-coumaric acid in PD were studied according to the results of a network pharmacology-based analysis. Cellular models are important for determining the contributions of different factors to PD pathogenesis; among them, the pheochromocytoma cell line PC-12 and the neuroblastoma cell line SH-SY5Y are extensively used [19]. In this study, they were treated with lipopolysaccharide (LPS) and 6-hydroxydopamine (6-OHDA), respectively. LPS is a bacterial endotoxin and the main component of the cell wall of Gram-negative bacteria; it can be released after the destruction of bacterial cells and stimulate an inflammatory response in the body. Therefore, it is often used to construct a typical model of inflammation [20]. 6-hydroxydopamine (6-OHDA) is a hydroxylated derivative of the neurotransmitter dopamine (DA) and can competitively inhibit DA, thereby blocking the mitochondrial respiratory chain of the substantia nigra and increasing oxidative stress [21].
The objective of this study was to screen the bioactive components in broad bean sprouts with potential beneficial effects in PD.
2. Results
2.1. Identification of Compounds from 50% MeOH Extract Through RP-UHPLC-ESI-MS/MS
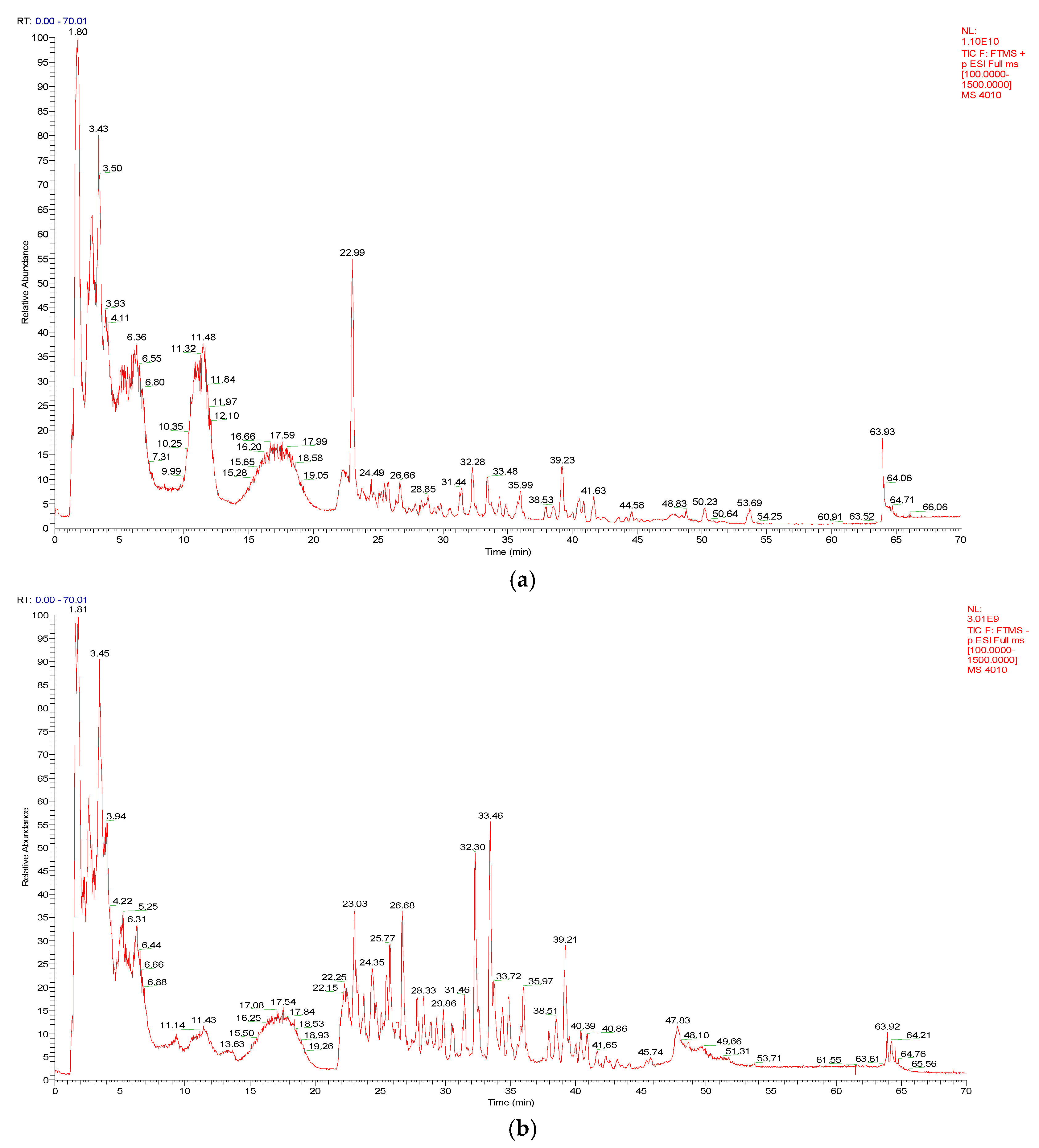
As shown in Figure 1a,b, a total of 52 compounds were identified from a 50% MeOH extract of broad bean sprouts in positive and negative ion modes, including 17 flavonoids, 4 phenolic acids, 9 organic acids, 13 nitrogenous compounds and 9 other compounds. In addition, 37 of these compounds were reported in Vicia faba L. for the first time. The details are shown in Table 1.
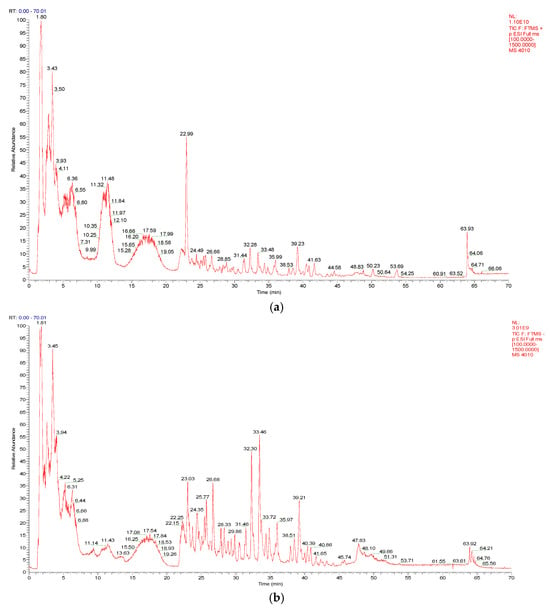
Figure 1.
(a) The total ion chromatogram (TIC) of a 50% MeOH extract of broad bean sprouts obtained in positive ion mode. (b) The total ion chromatogram (TIC) of a 50% MeOH extract of broad bean sprouts obtained in negative ion mode.

Table 1.
Compounds identified in a 50% MeOH extract of broad bean sprouts.
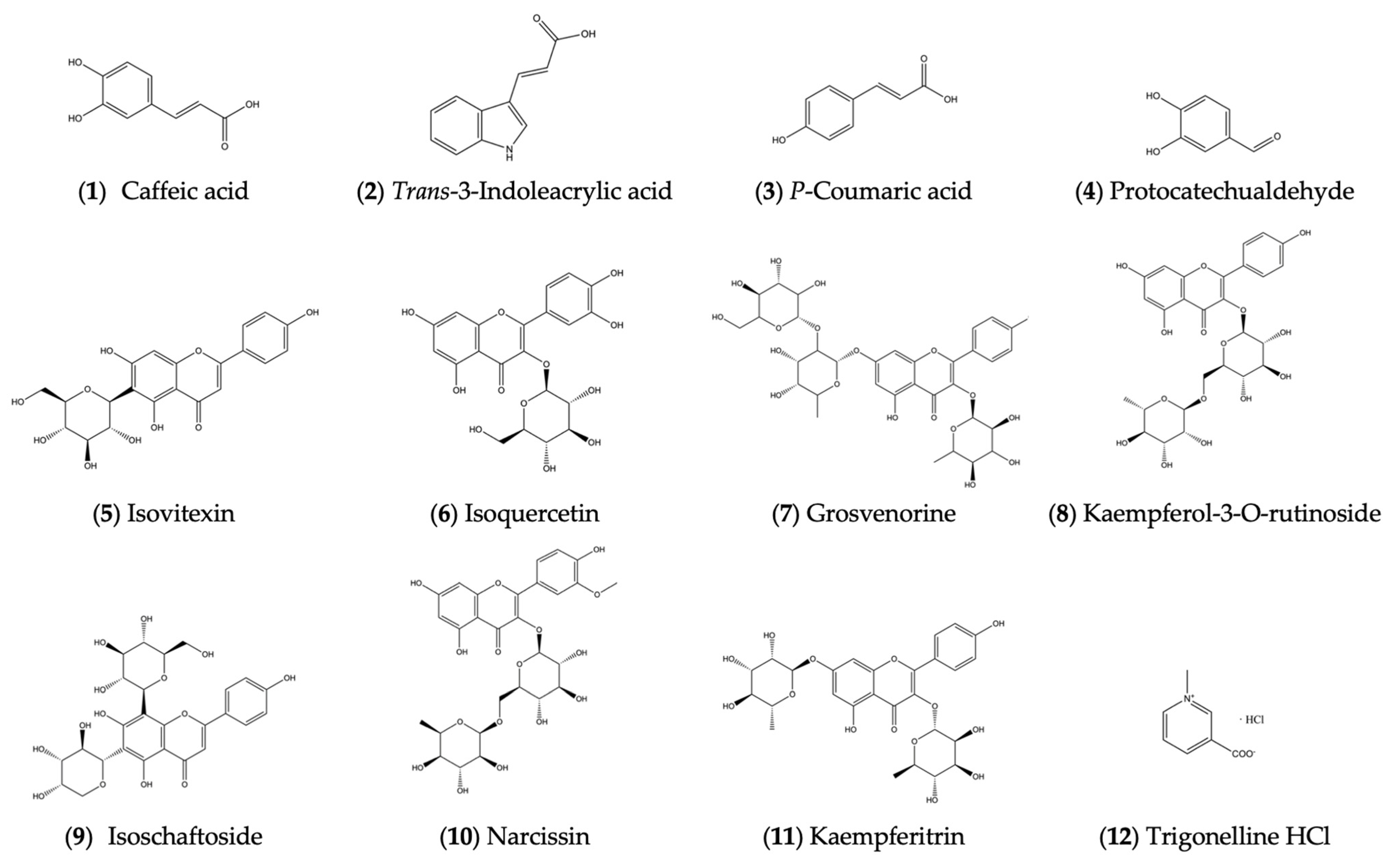
2.2. Identification of Isolated Compounds
The compounds isolated from ethyl acetate and n-butanol portions of broad bean sprouts were identified with HRESI-MS and (1H, 13C) NMR:
Compound 1: Yellow, amorphous powder, C9H8O4, negative-ion HRESI-MS m/z: 179.0359 [M − H]− (Calcd for C9H7O4: 179.0350), positive-ion ESI-MS: 181.0 [M + H]+, negative-ion ESI-MS: 179.0 [M − H]−, 135.1 [M-H-CO2]−. 1H NMR (400 MHz, DMSO-d6) δ 7.41 (d, J = 15.9 Hz, 1H, H-7), 7.02 (d, J = 2.1 Hz, 10H, H-2), 6.96 (dd, J = 8.1, 2.1 Hz, 1H, H-6), 6.75 (d, J = 8.1 Hz, 1H, H-5), 6.16 (d, J = 15.9 Hz, 1H, H-8). 13C NMR (100 MHz, DMSO-d6) δ 168.3 (C-9), 148.6 (C-4), 146.0 (C-3), 145.0 (C-7), 126.1 (C-1), 121.6 (C-6), 116.2 (C-5), 115.5 (C-8), 115.1 (C-2). The above data were basically consistent with the data in the literature [27,64,65], so compound 1 was identified as caffeic acid.
Compound 2: Yellow, amorphous powder, C11H9NO2, negative-ion HRESI-MS m/z: 186.0569 [M − H]− (Calcd for C11H8NO2: 186.0561), positive-ion ESI-MS: 188.1 [M + H]+, 170.1 [M + H-H2O]+, 210.1 [M + Na]+, negative-ion ESI-MS: 186.1 [M − H]−, 142.1 [M − H-CO2]−. 1H NMR (400 MHz, DMSO-d6) δ 11.72 (s, 1H, COOH), 7.90 (d, J = 2.8 Hz, 1H, H-4), 7.87–7.77 (m, 2H, H-10), 7.53–7.38 (m, 1H, H-2, H-7), 7.18 (dtd, J = 17.8, 7.3, 1.3 Hz, 2H, H-5, H-6), 6.31 (d, J = 15.9 Hz, 1H, H-11). 13C NMR (100 MHz, DMSO-d6) δ 169.1 (C-12), 139.0 (C-8), 137.8 (C-10), 131.7 (C-2), 125.5 (C-9), 122.9 (C-6), 121.3 (C-5), 120.2 (C-4), 112.8 (C-11), 112.6 (C-3), 112.1 (C-7). The above data were basically consistent with the data in the literature [66], so compound 2 was identified as trans-3-Indoleacrylic acid.
Compound 3: White, amorphous powder, C9H8O3, negative-ion HRESI-MS m/z: 163.0409 [M − H]− (Calcd for C9H7O3: 163.0401), positive-ion ESI-MS: 165.0 [M + H]+, negative-ion ESI-MS: 163.0 [M − H]−, 119.1 [M − H-CO2]−. 1H NMR (400 MHz, DMSO-d6) δ 7.51 (d, J = 12 Hz, 2H, H-β), 7.47 (d, J = 15.9 Hz, 1H, H-2, H-6), 6.79 (d, J = 12 Hz, 2H, H-3, H-5), 6.28 (d, J = 15.9 Hz, 1H, H-α). 13C NMR (100 MHz, DMSO-d6) δ 168.4 (COOH), 160.0 (C-4), 144.6 (C-β), 130.6 (C-3,5), 125.7 (C-1), 116.2 (C-2,6), 115.8 (C-α). The above data were basically consistent with the data in the literature [65,67], so compound 3 was identified as p-coumaric acid.
Compound 4: Off-white, amorphous powder, C7H6O3, negative-ion HRESI-MS m/z: 137.0251 [M − H]− (Calcd for C7H5O3: 137.0244), positive-ion ESI-MS: 139.0 [M + H]+, negative-ion ESI-MS: 137.0 [M − H]−. 1H NMR (400 MHz, DMSO-d6) δ 9.70 (s, 1H, -CHO), 7.27 (dd, J = 8.1, 2.1 Hz, 1H, H-6), 7.23 (d, J = 2.0 Hz, 1H, H-2), 6.90 (d, J = 8.1 Hz, 1H, H-5). 13C NMR (100 MHz, DMSO-d6) δ 191.5 (C-7), 152.6 (C-4), 146.4 (C-3), 129.3 (C-6), 124.9 (C-1), 116.0 (C-5), 114.8 (C-2). The above data were basically consistent with the data in the literature [68], so compound 4 was identified as protocatechualdehyde.
Compound 5: Yellow, amorphous powder, C21H20O10, positive-ion HRESI-MS m/z: 455.0953 [M + Na]+ (Calcd for C21H20O10Na: 455.0949), positive-ion ESI-MS: 433.1 [M + H]+, negative-ion ESI-MS: 431.1 [M − H]−. 1H NMR (400 MHz, DMSO-d6) δ 13.56 (s, 1H, -OH), 7.93 (d, J = 8.8 Hz, 2H, H-2′, H-6′), 6.93 (d, J = 8.9 Hz, 2H, H-3′, H-5′), 6.79 (s, 1H, H-3), 6.51 (s, 1H, H-8), 4.93–4.80 (brs, -OH), 4.60 (t, J = 10.8 Hz, 2H, H-1), 4.48 (s, 1H, -OH), 4.04 (t, J = 9.1 Hz, 1H, H-2″), 3.69 (d, J = 11.6 Hz, 1H, Ha-6″), 3.40 (d, J = 10.3 Hz, 1H, Hb-6″), 3.24–3.07 (m, 3H, H-3″, H-4″, H-5″). 13C NMR (100 MHz, DMSO-d6) δ 182.4 (C-4), 164.0 (C-2), 163.7 (C-7), 161.6 (C-5), 161.1 (C-4′), 156.7 (C-8a), 128.9 (C-2′,C-6′), 121.6 (C-1′), 116.4 (C-3′,C-5′), 109.4 (C-6), 103.9 (C-4a), 103.3 (C-3), 94.1 (C-8), 82.1 (C-5″), 79.4 (C-3″), 73.5 (C-1″), 71.1 (C-2″), 70.7 (C-4″), 61.9 (C-6″). The above data were basically consistent with the data in the literature [69,70], so compound 5 was identified as isovitexin.
Compound 6: Yellow, amorphous powder, C21H20O12, negative-ion HRESI-MS m/z: 463.0873 [M − H]− (Calcd for C21H19O12: 463.0882), positive-ion ESI-MS: 487.1 [M + Na]+. 1H NMR (400 MHz, DMSO-d6) δ 7.61–7.54 (m, 2H, H-2′, H-6′), 6.89–6.80 (m, 1H, H-5′), 6.40 (d, J = 2.1 Hz, 1H, H-8), 6.19 (d, J = 2.1 Hz, 1H, H-6), 5.50–5.44 (m, 1H, H-1″), 3.58–3.03 (m, 6H, H-2″~H-6″). 13C NMR (100 MHz, DMSO-d6) δ 177.9 (C-4), 164.7 (C-7), 161.7 (C-5), 156.8 (C-2), 156.6 (C-9), 148.9 (C-4′), 145.3 (C-3′), 133.8 (C-3), 122.1 (C-6′), 121.6 (C-1′), 116.6 (C-5′), 115.7 (C-2′), 104.4 (C-10), 101.3 (C-1″), 99.1 (C-6), 94.0 (C-8), 78.0 (C-3″), 77.0 (C-5″), 74.6 (C-2″), 70.4 (C-4″), 61.4 (C-6″). The above data were basically consistent with the data in the literature [29,71,72], so compound 6 was identified as isoquercetin.
Compound 7: Yellow, amorphous powder, C33H40O19, positive-ion HRESI-MS m/z: 763.2052 [M + Na]+ (Calcd for C33H40O19Na: 763.2056), positive-ion ESIMS: 741.2 [M + H]+, 758.2 [M + HH4]+, negative-ion ESIMS: 739.2 [M − H]−. 1H NMR (400 MHz, DMSO-d6) δ 7.86–7.74 (m, 2H, H-2′, H-6′), 6.95–6.87 (m, 2H, H-3′, H-5′), 6.80 (d, J = 2.2 Hz, 1H, H-8), 6.47 (d, J = 2.1 Hz, 1H, H-6), 5.89 (d, J = 1.6 Hz, 1H, 3-Rha H-1), 5.29 (d, J = 1.6 Hz, 1H, 7-Rha H-1), 4.38 (d, J = 7.7 Hz, 1H, Glc H-1), 1.14 (d, J = 6.2 Hz, 3H, Rha H-6), 0.90–0.72 (m, 3H, Rha 6-CH3). 13C NMR (101 MHz, DMSO-d6) δ 178.4 (C-4), 161.8 (C-7), 161.4 (C-5), 160.6 (C-4′), 158.3 (C-9), 156.6 (C-2), 135.0 (C-3), 131.2 (C-2′, 6′), 120.8 (C-1′), 115.9 (C-3′, 5′), 106.3 (C-10), 100.0 (C-6), 95.1 (C-8); Glc: 106.1, 74.4, 77.2, 70.5, 76.7, 61.49; 7-Rha: 97.6, 80.4, 72.5, 71.2, 71.0, 18.3; 3-Rha: 102.4, 71.6, 70.8, 70.5, 70.4, 18.0. The above data were basically consistent with the data in the literature [31], so compound 7 was identified as grosvenorine.
Compound 8: Yellow, amorphous powder, C27H30O15, positive-ion HRESI-MS m/z: 617.1487 [M + Na]+ (Calcd for C27H30O15Na: 617.1477), positive-ion ESI-MS: 595.2 [M + H]+, negative-ion ESI-MS: 593.2 [M − H]−. 1H NMR (400 MHz, DMSO-d6) δ 7.98 (d, J = 8.9 Hz, 2H, H-2′, H-6′), 6.93–6.83 (m, 2H, H-3′, H-5′), 6.41 (d, J = 2.1 Hz, 1H, H-8), 6.20 (d, J = 2.1 Hz, 1H, H-6), 5.08 (dd, J = 9.6, 5.3 Hz, 1H, H-1″), 4.46–4.33 (brs, 1H, H-1‴), 3.69–2.99 (m, 8H, H-2″~ H-5″, H-2‴~H-5‴), 0.98 (d, J = 6.2 Hz, 3H, H-6‴). 13C NMR (100 MHz, DMSO-d6) δ 177.8 (C-4), 164.7 (C-7), 161.7 (C-5), 160.4 (C-4′), 157.3 (C-9), 157.0 (C-2), 133.7 (C-3), 131.3 (C-2′, C-6′), 121.4 (C-1′), 115.6 (C-3′, C-5′), 104.4 (C-10), 101.8 (C-1″), 101.2 (C-1‴), 99.2 (C-6), 94.2 (C-8), 76.8 (C-3″), 76.2 (C-5″), 74.6 (C-2″), 72.3 (C-4‴), 71.1 (C-3‴), 70.8 (C-4″), 70.4 (C-2‴), 68.7 (C-5‴), 67.4 (C-6″), 18.2 (C-6‴). The above data were basically consistent with the data in the literature [72], so compound 8 was identified as kaempferol-3-O-rutinoside.
Compound 9: White, amorphous powder, C26H28O14, negative-ion HRESI-MS m/z: 563.1423 [M − H]− (Calcd for C26H27O14: 563.1406), positive-ion ESI-MS: 565.2 [M + H]+, 587.1 [M + Na]+, negative-ion ESIMS: 563.1 [M − H]−. 1H NMR (400 MHz, DMSO-d6) δ 8.00–7.88 (m, 2H, H-2′, H-6′), 6.98–6.89 (m, 2H, H-3′, H-5′), 6.83 (s, 1H, H-3), 4.89–4.66 (m, 2H, H-1″, H-1‴), 3.91 (dd, J = 11.0, 5.4 Hz, 2H, H-2″, H-2‴), 3.72–3.53 (m, 4H, H-5″a, H-5″b, H-6‴a, H-6‴b), 3.49–3.40 (m, 1H, H-3″), 3.25 (d, J = 4.7 Hz, 3H, H-3‴, H-4‴, H-5‴). 13C NMR (100 MHz, DMSO-d6) δ 182.8 (C-4), 161.7 (C-2), 129.1 (C-2′, C-6′), 121.9 (C-1′), 116.4 (C-3′, C-5′), 103.1 (C-3), 79.3 (C-3‴), 56.5 (C-6‴). The above data were basically consistent with the data in the literature [25,47], so compound 9 was identified as isoschaftoside.
Compound 10: Yellow, amorphous powder, C28H32O16, negative-ion HRESI-MS m/z: 623.1632 [M − H]− (Calcd for C28H31O16: 623.1618), positive-ion ESI-MS: 647.2 [M + Na]+. 1H NMR (400 MHz, DMSO-d6) δ 7.85 (d, J = 2.1 Hz, 1H, H-2′), 7.51 (dd, J = 8.4, 2.1 Hz, 1H, H-6′), 6.91 (d, J = 8.4 Hz, 1H, H-5′), 6.42 (d, J = 2.1 Hz, 1H, H-8), 6.20 (d, J = 2.1 Hz, 1H, H-6), 5.40 (d, J = 5.7 Hz, 1H, H-1″), 4.40 (s, 1H, H-1‴), 3.83 (s, 3H, OCH3), 0.97 (d, J = 6.1 Hz, 3H, H-6‴). 13C NMR (100 MHz, DMSO-d6) δ 177.8 (C-4), 156.9 (C-9), 133.5 (C-3), 121.5 (C-1′), 115.7 (C-5′), 101.4 (C-1″), 72.2 (C-4‴), 71.0 (C-3‴), 70.8 (C-2‴), 56.1 (C-5‴), 18.2 (C-6‴). The above data were basically consistent with the data in the literature [73], so compound 10 was identified as narcissin.
Compound 11: White, amorphous powder, C27H30O14, negative-ion HRESI-MS m/z: 577.1563 [M − H]− (Calcd for C27H29O14: 577.1563), positive-ion ESI-MS: 579.2 [M + H]+, 601.2 [M + Na]+, negative-ion ESIMS: 577.2 [M − H]−. 1H NMR (400 MHz, DMSO-d6) δ 7.79 (d, J = 8.6 Hz, 2H, H-2′, H-6′), 6.91 (d, J = 8.5 Hz, 2H, H-3′, H-5′), 6.79 (d, J = 2.1 Hz, 1H, H-8), 6.46 (d, J = 2.1 Hz, 1H, H-6), 5.55 (d, J = 1.8 Hz, 1H, H-1‴), 5.30 (d, J = 1.5 Hz, 1H, H-1″), 1.13 (d, J = 6.1 Hz, 3H, H-6‴), 0.80 (d, J = 5.1 Hz, 3H, H-6″). The above data were basically consistent with the data in the literature [71], so compound 11 was identified as kaempferitrin.
Compound 12: White, amorphous powder, C7H7NO2, positive-ion HRESI-MS m/z: 138.0542 [M + H]+ (Calcd for C7H8NO2: 138.0550). 1H NMR (400 MHz, DMSO-d6) δ 14.67 (s, 1H, HCl), 9.50 (d, J = 1.5 Hz, 1H, COOH), 9.18 (d, J = 6.1 Hz, 1H, H-2), 8.93 (dt, J = 8.1, 1.5 Hz, 2H, H-4, H-6), 8.23 (dd, J = 8.1, 6.1 Hz, 1H, H-5), 4.42 (s, 3H, N-CH3). 13C NMR (100 MHz, DMSO-d6) δ 163.5 (C-7), 148.9 (C-6), 147.3 (C-2), 145.3 (C-4), 131.2 (C-3), 128.2 (C-5), 48.6 (CH3). The above data were basically consistent with the data in the literature [74], so compound 12 was identified as trigonelline HCl.
The structures of the above 12 compounds are shown in Figure 2.
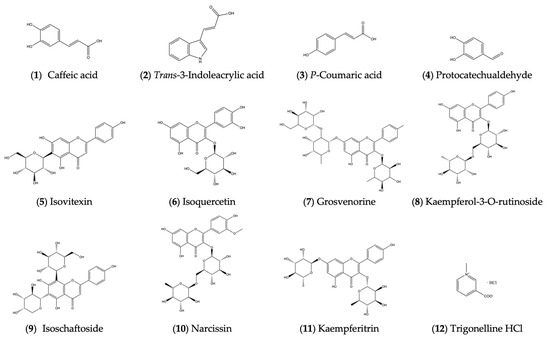
Figure 2.
Structures of compounds isolated from ethyl acetate and n-butanol portions.
2.3. Screening of Potential Bioactive Compounds and Related Mechanisms Through Network Pharmacology-Based Analysis
2.3.1. Bioactive Compositions of Broad Bean Sprouts and Target Prediction
A total of 557 potential targets of broad bean sprouts were screened based on gastrointestinal absorption and Lipinski’s rule of five.
2.3.2. Prediction of PD Targets and the Intersections
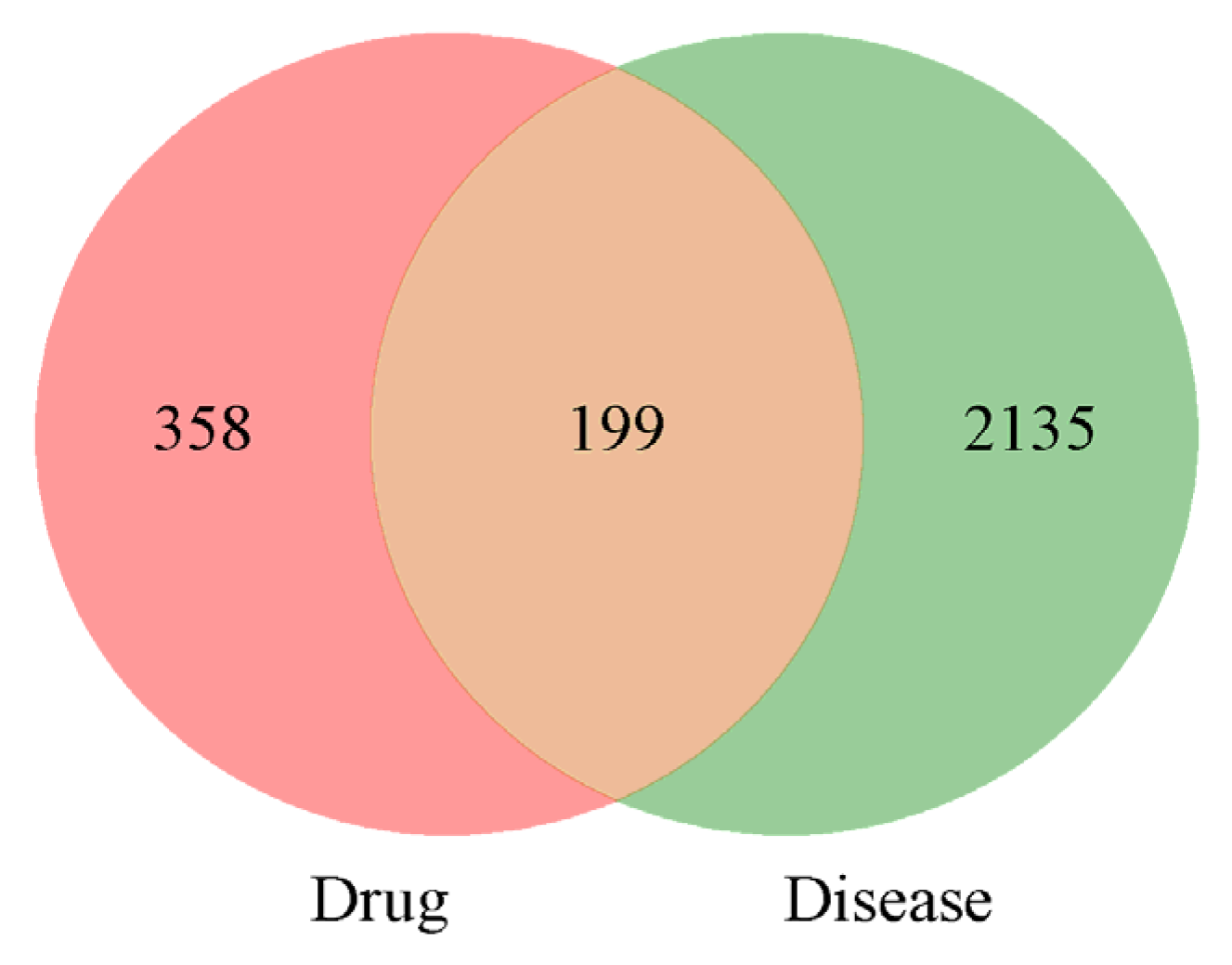
A total of 2334 potential targets of PD were screened, and then, 199 intersections of broad bean sprouts and PD were obtained. The details are shown in Figure 3.
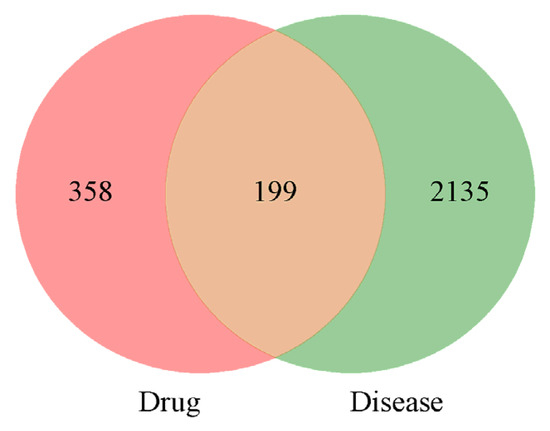
Figure 3.
Venn diagram of bioactive ingredients and disease targets.
2.3.3. Screening of Core Components
According to Figure 4, there were 237 nodes and 739 edges in the network. By analyzing the network, it could be concluded that the average node degree was 6.2, and there were 19 components whose node degrees were more than twice the average: hydroxygenkwanin (BSE1), luteolin (BSE2), kaempferol (BSE3), genkwanin (BSE4), calycosin (BSE5), biochanin A (BSE6), formononetin (BSE7), liquiritigenin (BSE8), curdione (BSE9), artemisinic acid (BSE10), demethoxyyangonin (BSE11), ferulic acid (BSE14), ligustilide (BSE15), azelaic acid (BSE16), caffeic acid (BSE18), 4-methylumbelliferone (BSE19), p-coumaric acid (BSE20), α-linolenic acid (BSE33), tryptophan (BSE34) and naringenin (BSE37). Among them, caffeic acid (BSE18) and p-coumaric acid (BSE20) were isolated from the ethyl acetate portion and are named compounds 1 and 3 in this study.
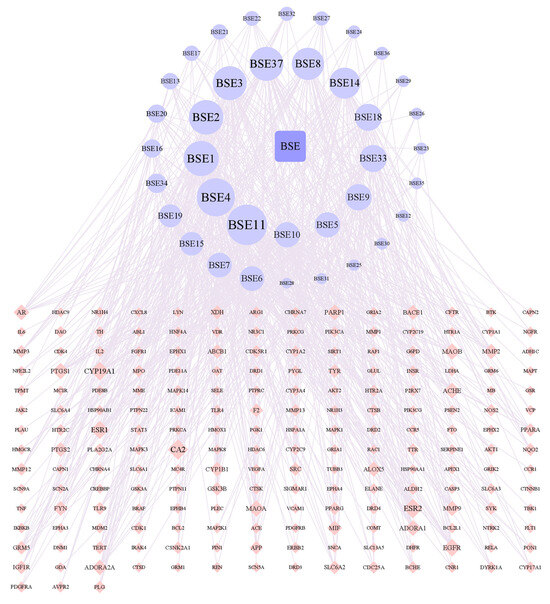
Figure 4.
Network of interactions between drug component targets.
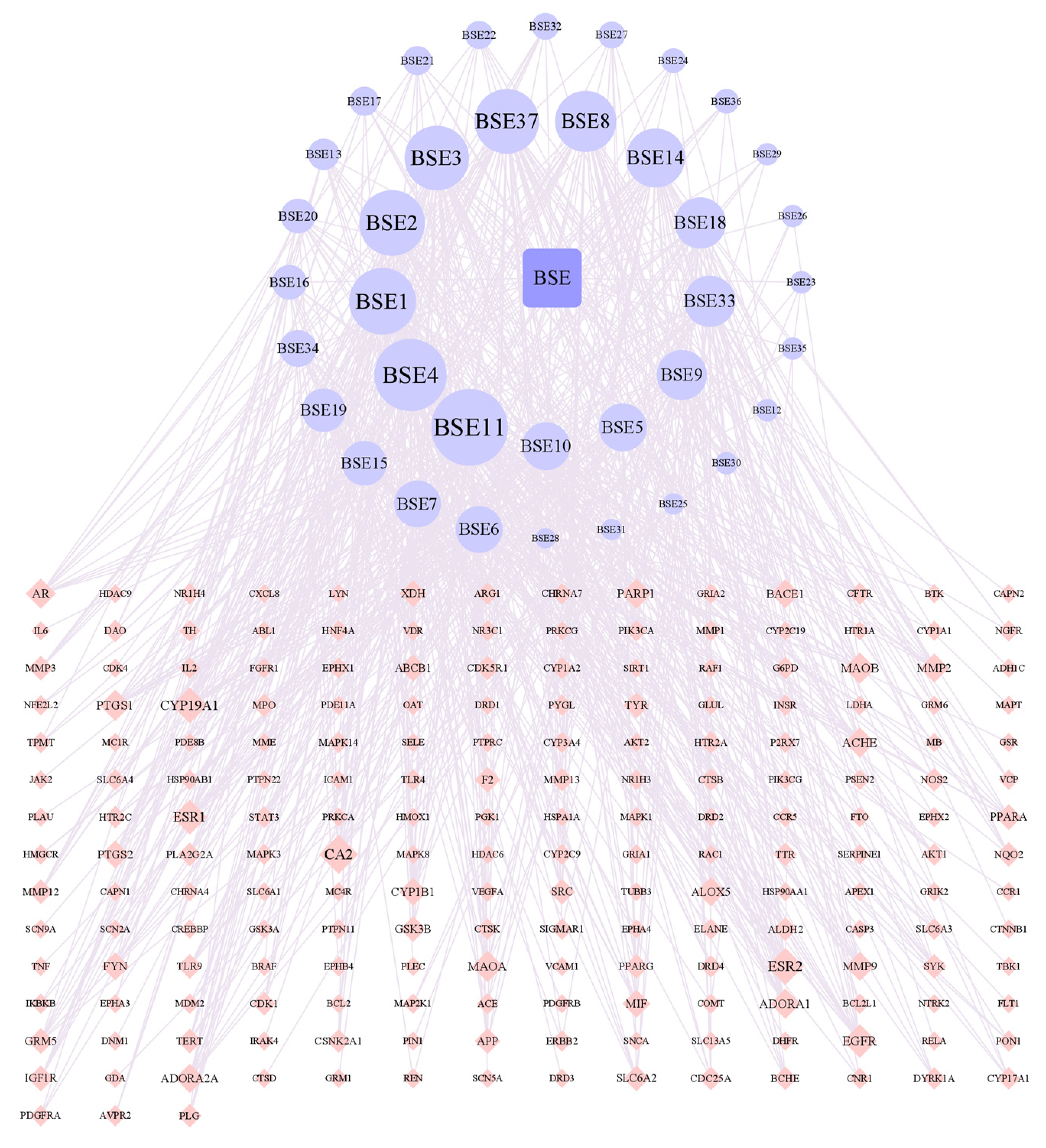
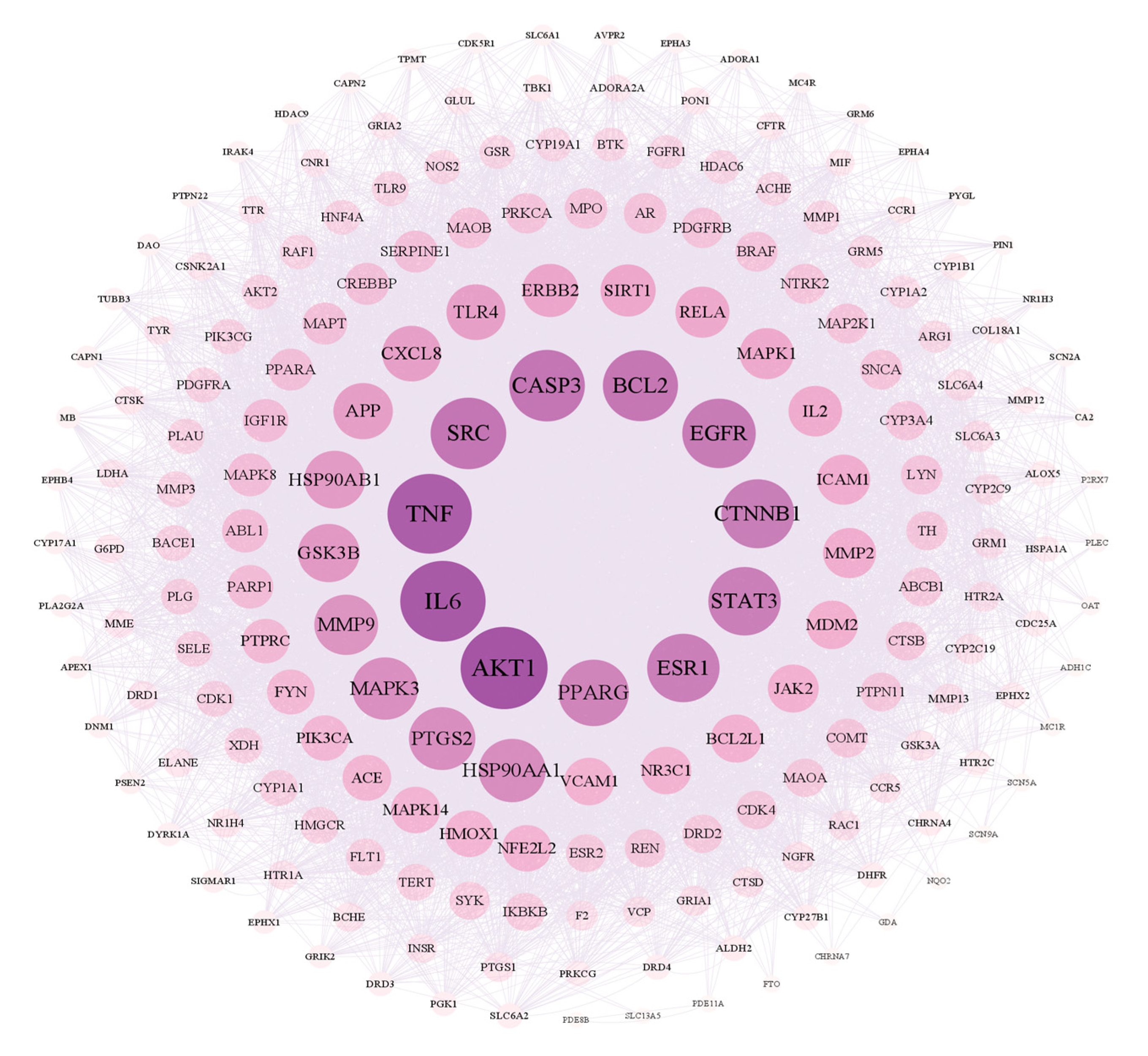
2.3.4. The PPI Network for Broad Bean Sprouts and Common PD Targets
As shown in Figure 5, there are 199 nodes and 7449 edges in the network. After analyzing the network, it could be concluded that the average node degree was 74.80, and there were 20 targets whose node degrees were more than twice the average: AKT1, IL6, TNF, CASP3, SRC, BCL2, EGFR, CTNNB1, STAT3, ESR1, PPARG, HSP90AA1, PTGS2, MAPK3, MMP9, GSK3B, HSP90AB1, APP, TLR4 and CXCL8. The following were regarded as the top three targets: AKT1 (serine/threonine-protein kinase 1), which plays a key role in cell growth and survival, with the highest degree value of 260; IL6 (interleukin 6), representing significant anti-inflammatory effects, with a degree value of 254; and TNF (tumor necrosis factor), with a degree value of 250.
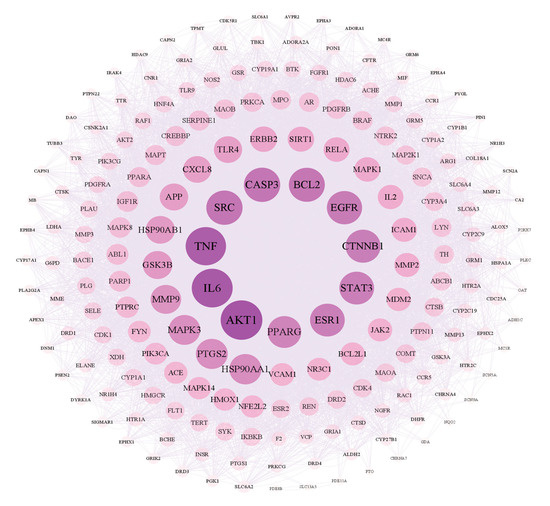
Figure 5.
Network of interactions between PPI targets.
2.3.5. GO Enrichment Analysis and KEGG Pathway Enrichment Analysis
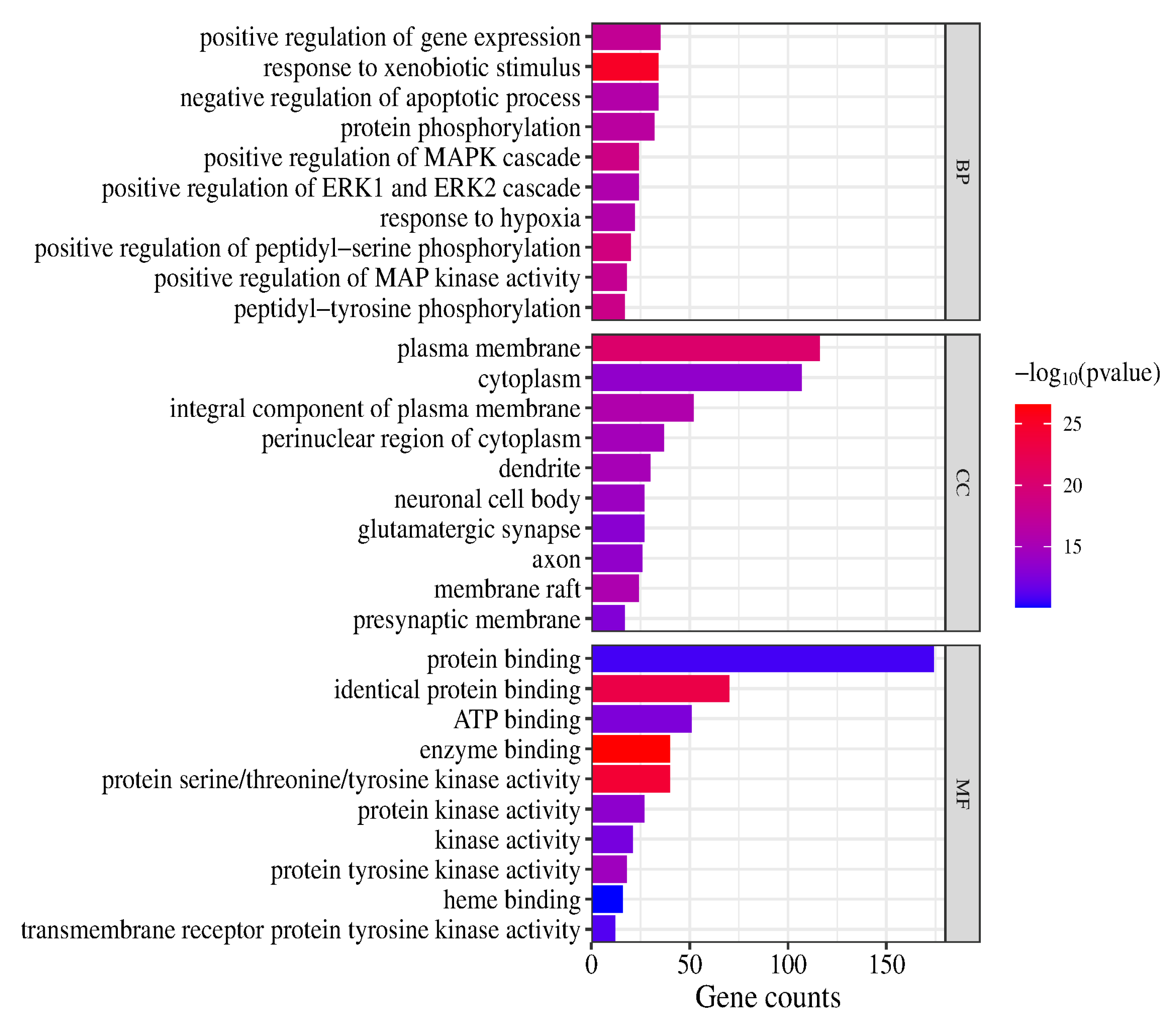
In the GO and KEGG pathway enrichment analyses, 907 biological process (BP) terms, 121 cellular component (CC) terms and 210 molecular function (MF) terms were obtained (p < 0.05). The highly enriched BP terms were positive regulation of gene expression, response to xenobiotic stimulus, negative regulation of apoptotic process, protein phosphorylation, positive regulation of MAPK cascade, positive regulation of ERK1 and ERK2 cascade, response to hypoxia, positive regulation of peptidyl-serine phosphorylation, positive regulation of MAP kinase activity and peptidyl-tyrosine phosphorylation. The highly enriched CC terms were plasma membrane, cytoplasm, integral component of plasma membrane, perinuclear region of cytoplasm, dendrite, neuronal cell body, glutamatergic synapse, axon, membrane raft and presynaptic membrane. The highly enriched MF terms were protein binding, identical protein binding, ATP binding, enzyme binding, protein serine/threonine/tyrosine kinase activity, protein kinase activity, kinase activity, protein tyrosine kinase activity, heme binding and transmembrane receptor protein tyrosine kinase activity. (The top 10 terms are presented in a column chart in Figure 6.)
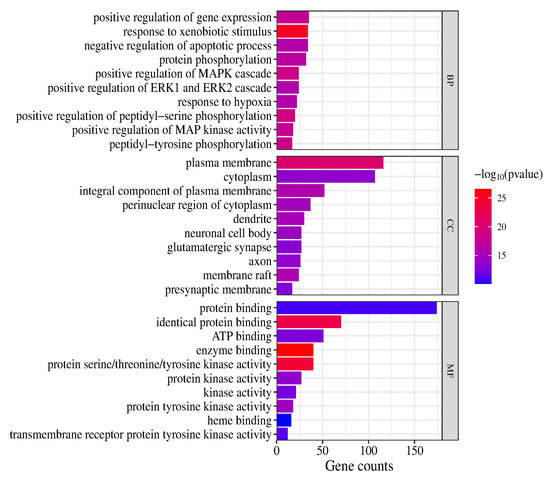
Figure 6.
GO enrichment analysis (top 10).
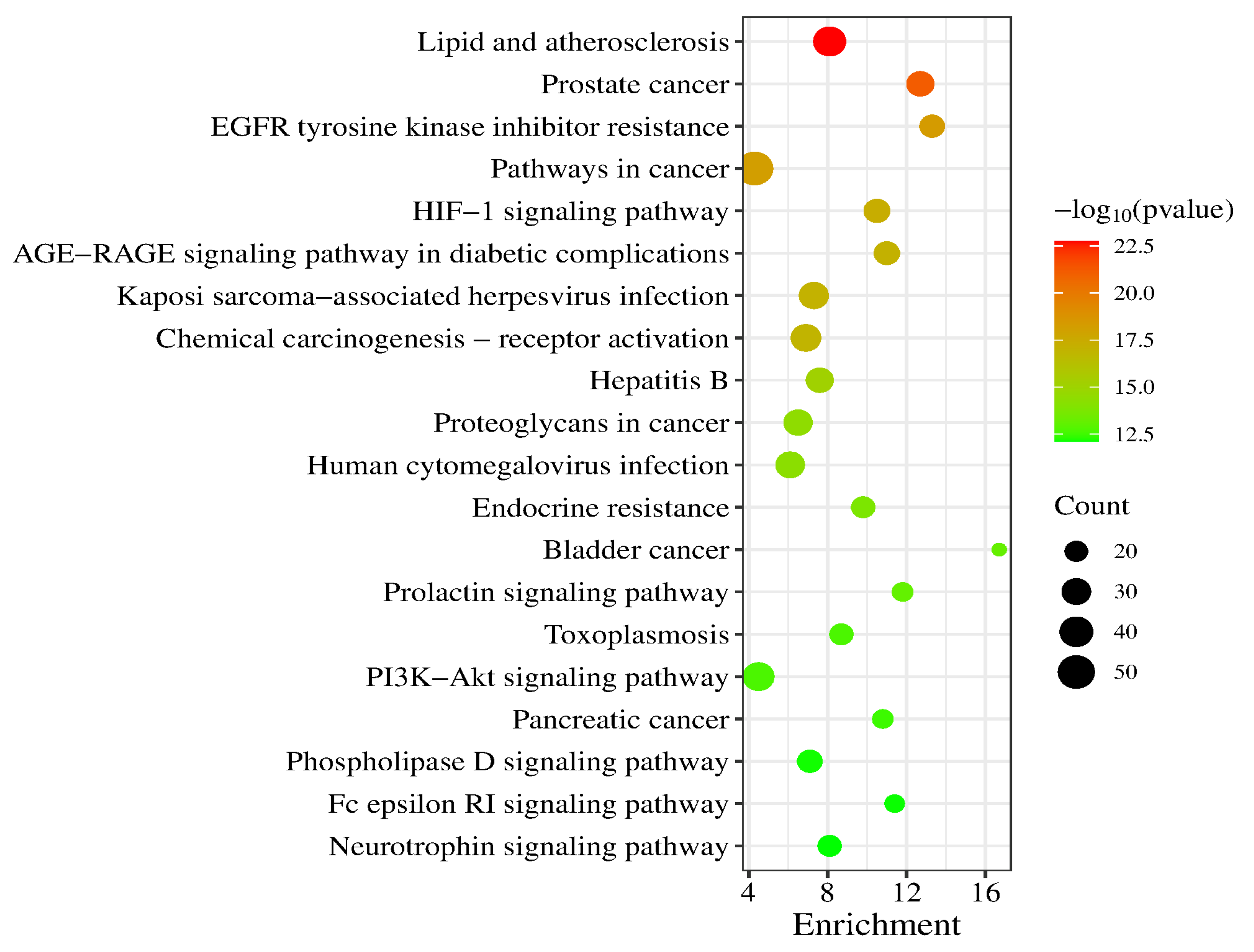
A total of 186 signaling pathways were obtained after KEGG pathway analysis (p < 0.05). The top 20 signaling pathways were Lipid and atherosclerosis, Prostate cancer, EGFR tyrosine kinase inhibitor resistance, Pathways in cancer, HIF-1 signaling pathway, AGE-RAGE signaling pathway in diabetic complications, Kaposi sarcoma-associated herpesvirus infection, Chemical carcinogenesis-receptor activation, Hepatitis B, Proteoglycans in cancer, Human cytomegalovirus infection, Endocrine resistance, Bladder cancer, Prolactin signaling pathway, Toxoplasmosis, PI3K-Akt signaling pathway, Pancreatic cancer, Phospholipase D signaling pathway, Fc epsilon RI signaling pathway and Neurotrophin signaling pathway (a bubble chart of the top 20 is shown in Figure 7).
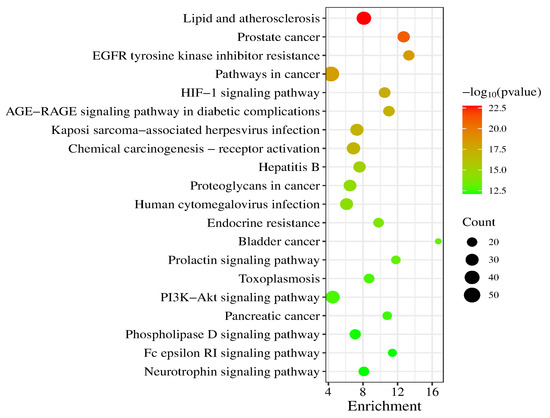
Figure 7.
KEGG enrichment analysis (top 20).
2.4. Experimental Validation of Neuroprotective Effects of Caffeic Acid and P-Coumaric Acid
PC-12 and SH-SY5Y cells were treated with LPS (200 µg/mL) and 6-OHDA (50 µM), respectively, and the cells’ viability and apoptosis were determined. The reactive oxygen species (ROS) levels and release of cellular IL-6 and TNF-α were also detected to further explore the mechanisms.
2.4.1. Results of Drug Administration Concentrations Investigation
The MTT method was used to determine the effects of the caffeic acid and p-coumaric acid concentrations on the viability of PC-12 and SH-SY5Y cells. The optical densities (ODs) for the blank control group (Con) and intervention groups were detected. The inhibition rates are shown in Table 2.
Inhibition rate (%) = (ODCon − ODIntervention group)/ODCon × 100%

Table 2.
Cell growth inhibition rates (%) under treatment with different concentrations of the drug ().
The results indicate that caffeic acid and p-coumaric acid at concentrations ranging from 3.125 to 100 µM did not impact cell viability over the corresponding treatment durations, and the inhibition rate did not increase compared to that in the negative control group (p > 0.05). It is worth noting that the inhibition rate observed with p-coumaric acid at 100 µM was clearly higher than that in the other groups. Therefore, caffeic acid at 100, 50 and 25 µM and p-coumaric acid at 50, 25 and 12.5 µM were employed for the intervention groups (high, medium and low doses) in the following experiments.
2.4.2. Results of Cell Viability Evaluation
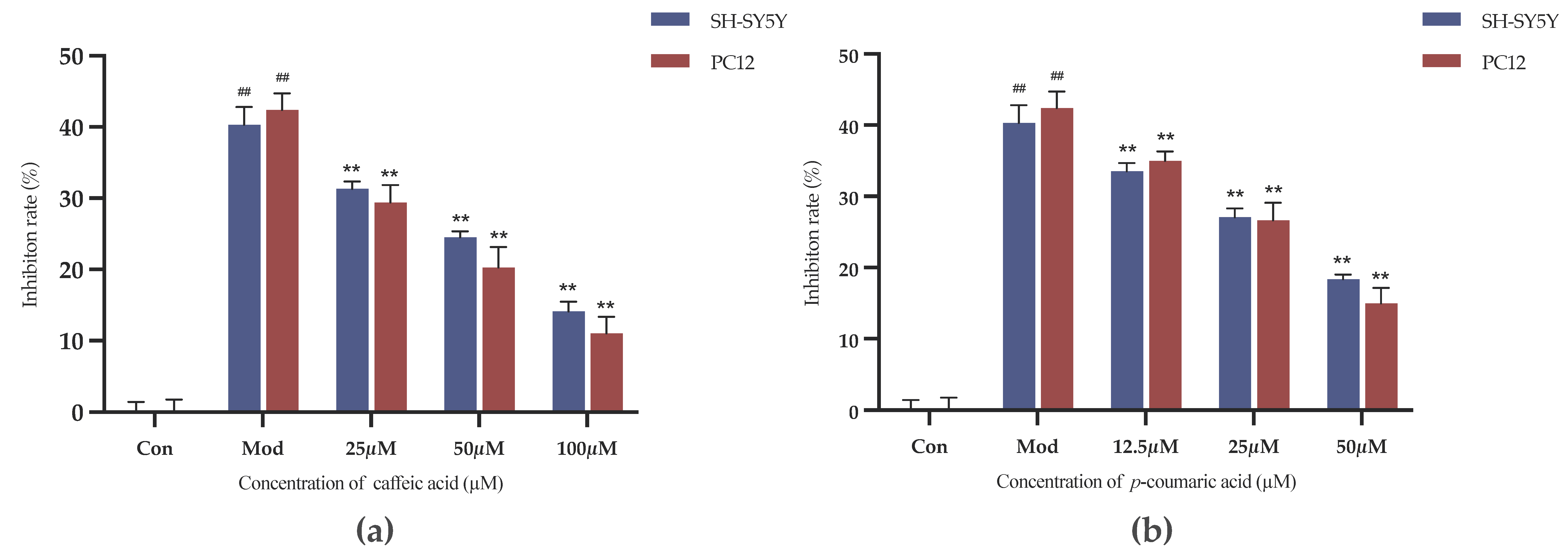
As shown in Figure 8, the inhibition rates were decreased in the intervention groups compared to the model control group (Mod). Caffeic acid and p-coumaric acid could significantly alleviate the inhibitory effects of LPS and 6-OHDA on the viability of PC-12 and SH-SY5Y cells at the chosen concentrations (p < 0.01), indicating that they might have anti-inflammatory and antioxidant effects and thereby exert neuroprotective effects on PC-12 and SH-SY5Y cells.
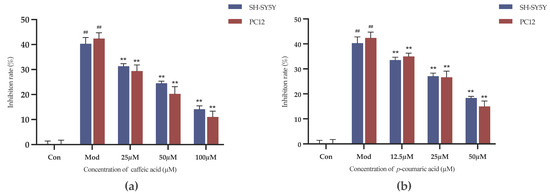
Figure 8.
(a) Cell growth inhibition rates (%) under treatment with different concentrations of caffeic acid. (b) Cell growth inhibition rates (%) under treatment with different concentrations of p-coumaric acid. Different from control: ## p < 0.01; different from model: ** p < 0.01.
2.4.3. Results of Cell Apoptosis Assessment
The Annexin-V FITC/PI Staining was applied to evaluate the cell apoptosis. As shown in Figure 9, caffeic acid and p-coumaric acid both had significant inhibitory effects on the apoptosis of 6-OHDA-treated SH-SY5Y cells and LPS-treated PC-12 cells over the selected concentration ranges (p < 0.01).
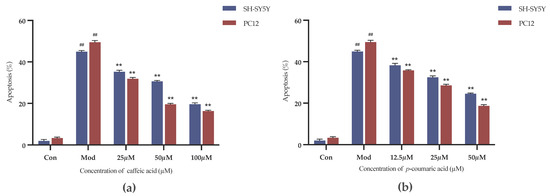
Figure 9.
(a) Cell apoptosis rates (%) under treatment with different concentrations of caffeic acid. (b) Cell apoptosis rates (%) under treatment with different concentrations of p-coumaric acid. Different from control: ## p < 0.01; different from model: ** p < 0.01.
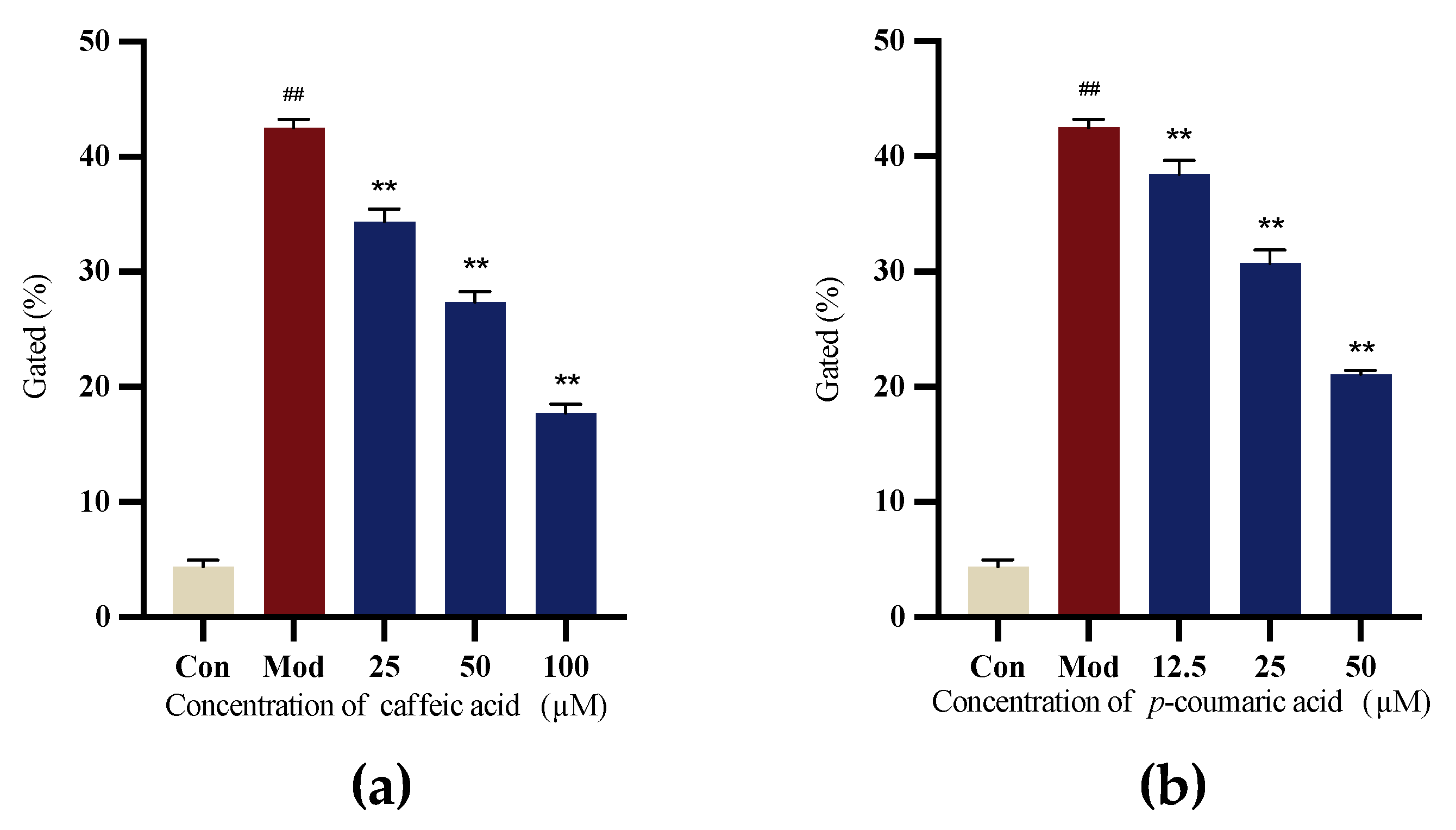
2.4.4. Intracellular ROS Levels
6-OHDA-induced SH-SY5Y cells were applied to evaluate the antioxidant capacity. The results indicated that caffeic acid and p-coumaric acid exhibited antioxidant properties, the Mod (LPS) group showed the highest ROS level, and the intervention groups showed a significant reduction in intracellular ROS levels (Figure 10).
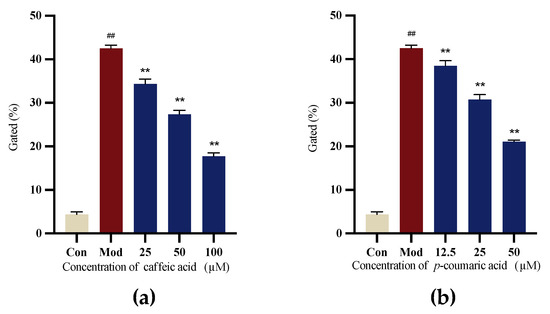
Figure 10.
(a) Gated (%) signals for 6-OHDA-treated SH-SY5Y cells treated with different concentrations of caffeic acid. (b) Gated (%) signals for to 6-OHDA-treated SH-SY5Y cells treated with different concentrations of p-coumaric acid. Different from control: ## p < 0.01; different from model: ** p < 0.01.
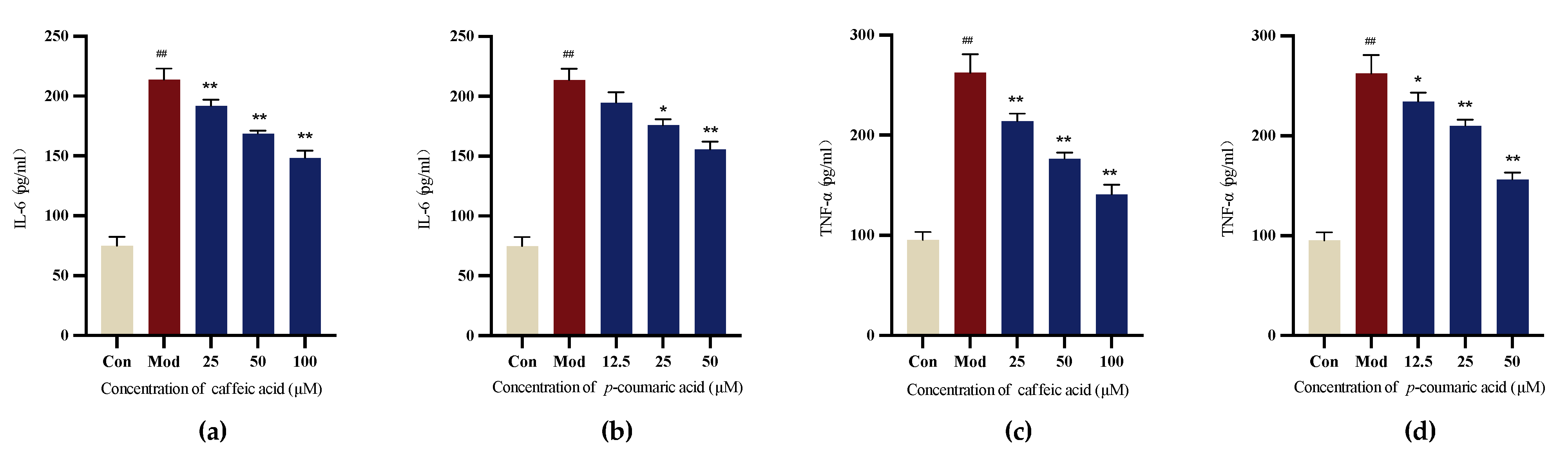
2.4.5. Detection of Cellular Inflammatory Factors Using Enzyme-Linked Immunosorbent Assay (ELISA)
The results demonstrate that caffeic acid and p-coumaric acid could reduce the release of cellular IL-6 and TNF-α compared to that in the Mod (LPS) group for LPS-treated PC-12 cells, indicating their ability to modulate immune responses (Figure 11).
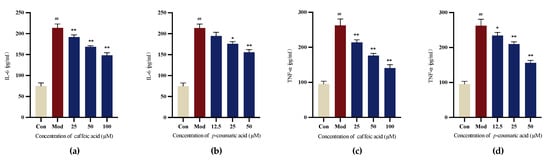
Figure 11.
(a) Cellular IL-6 levels under treatment with different concentrations of caffeic acid. (b) Cellular IL-6 levels under treatment with different concentrations of p-coumaric acid. (c) Cellular TNF-α levels under treatment with different concentrations of caffeic acid. (d) Cellular TNF-α levels under treatment with different concentrations of p-coumaric acid. Different from control: ## p < 0.01; different from model: ** p < 0.01, * p < 0.05.
3. Discussion
The identification of the compounds from 50% MeOH extracts of broad bean sprouts might be helpful for discovering differences in components between the seeds and sprouts, which might lead to the discovery of new anti-PD drugs.
The network pharmacology results suggest that there are some potential PD-relevant bioactive components in broad bean sprouts, including hydroxygenkwanin, luteolin, kaempferol, genkwanin, calycosin, biochanin A, formononetin, liquiritigenin, curdione, artemisinic acid, demethoxyyangonin, ferulic acid, ligustilide, azelaic acid, caffeic acid, 4-methylumbelliferone, p-coumaric acid, α-linolenic acid, tryptophan and naringenin, which warrant further exploration. They also indicate that the potential bioactive components in broad bean sprouts might be effective for PD treatment based on their antioxidant, anticancer, anti-inflammatory and nerve-protecting properties, among others. The related pathways include EGFR tyrosine kinase inhibitor resistance and HIF-1 signaling pathway. The phenolic compounds and flavonoids among them might represent further potential candidates according to several studies [10,75].
The 12 isolated compounds widely exist in a variety of natural plants, and compounds 2, 4, 7, 8 and 12 were isolated from Vicia faba L. for the first time. Caffeic acid (compound 1) is a natural product that has been discovered in many legume plants [76]. Studies have verified caffeic acid to be a promising compound for treating neurodegenerative disorders and various types of organ damage [77,78]. Furthermore, it could improve motor activities and lessen the inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration [79]. Trans-3-indoleacrylic acid (compound 2) has been isolated from red algae [66]. P-Coumaric acid (compound 3) is a common phenolic acid identified in many plants, including fava beans, and it has proven antioxidant activity [76,80]. Protocatechualdehyde (compound 4) was identified as a potential component that ameliorated streptozotocin-induced diabetic cardiomyopathy in mice through inhibiting the activation of the NLRP3 inflammasome [81]; this may also contribute to PD treatment through its anti-inflammatory activity. Isovitexin (compound 5) has been reported to be present in Asian rice and have multiple bioactivities, such as antibacterial, α-glucosidase-inhibiting, blood pressure-regulating and antioxidant activities [82]. Isoquercetin (compound 6) is a natural product in Camellia sinensis and Geranium carolinianum, and has been used in studies on kidney cancer and renal cell carcinoma [83]. Grosvenorine (compound 7) has been isolated from Siraitia Grosvenorii Leaf, a herb used for pharyngitis, pharyngeal pain and cough [84]. Kaempferol-3-O-rutinoside (compound 8), a natural product from Camellia sinensis, Camellia reticulata and other organisms, was reported to inhibit inflammation and have cardioprotective effects [85,86,87]. Isoschaftoside (compound 9) has been obtained from Camellia sinensis, Glycine max and other organisms. According to the literature, it could inhibit lipopolysaccharide-induced inflammation in microglia through the regulation of HIF-1α-mediated metabolic reprogramming [88]. Narcissin (compound 10) was found in Hypericum ascyron, Halimodendron halodendron and other organisms. Its potential therapeutic effects in hypertension, cancer and Alzheimer’s disease have been researched [89]. Kaempferitrin (compound 11) is a natural product found in Camellia sinensis, Annulohypoxylon bovei and other organisms. In addition to being a potential insulin mimetic, kaempferitrin has antioxidant, anti-inflammatory and anti-convulsant activities [90,91]. Trigonelline HCl (compound 12), a nicotinic acid derivative mainly found in Trigonella foenum-graecum L. (fenugreek), has been proven to be a promising inhibitor of type I collagen fibrillation [92]. It has also been reported to be an NRF2 inhibitor, thereby being able to reduce the expression of HO-1 and ATF3, suggesting that it might play a crucial role in the cytoprotective system and have potential anti-inflammatory activity [93].
It is interesting to note that caffeic acid (compound 1) and p-coumaric acid (compound 3), which had quite high degree values in the core components, might be the more promising compounds for PD. Through experimental validation, the in vitro neuroprotective effects of caffeic acid and p-coumaric acid were demonstrated. These findings highlight their significant potential to simultaneously support cell growth, inhibit cell apoptosis, reduce oxidative stress and modulate immune responses. Additionally, the potential clinical application of broad bean sprouts for PD is worthy of further investigation.
4. Materials and Methods
4.1. Plant Material
Broad bean sprouts (greenhouse plantings) were purchased from Nanjing, China. The seeds were soaked in warm water at 20 °C for 24 h and placed in a seedling tray with a sponge with air permeability and water storage in a dark room. The room temperature was maintained at 20~25 °C. Germination was carried out for 2 days, and water was sprayed regularly once a day. When the sprouts had become exposed by 0.5~1 cm, the germinant beans were placed in the seedling tray one by one and moved into a shed covered by a shade net to maintain a weak light condition. The room temperature was kept at 20~25 °C. Water was sprayed 4~5 times a day. When the broad bean sprouts reached a height of 30 cm, they were harvested in time.
Fresh broad bean sprouts (10 kg) were squeezed into juice, which was boiled and filtered. The filtered juice was concentrated through rotary evaporation, and the initial BSE was obtained.
4.2. Chemicals and Reagents
Analytical grade ethanol (EtOH), petroleum ether (60–90 °C), methylene chloride (DCM), n-BuOH, acetic acid (HAc) and ethyl acetate (EtOAc) were purchased from China National Medicines Corporation Ltd. (Beijing, China). HPLC-grade methyl alcohol and acetonitrile (ACN) were purchased from Tedia Company, Inc. (Fairfield, OH, USA). Silica gel (300–400 mesh) and thin-layer chromatography (TLC) plates (silica gel GF254, 200 × 200 mm) were obtained from Qingdao Haiyang Chemical Co., Ltd. (Qingdao, China). HPLC-grade formic acid was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Polyamide (100–200 mesh) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China).
4.3. Analysis Using RP-UHPLC-ESI-MS/MS
RP-UHPLC-ESI-MS/MS was performed using a Q Exactive Plus Orbitrap from Thermo Fisher. A Waters ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.8 μm) column was used for analytical UHPLC with a flow rate of 0.3 mL/min. The injection volume was 10 μL. The column temperature was set at 35 °C, and the total running time was 70 min.
The mobile phase consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The gradient elution program was as follows: 0~10 min, 0%~30% B; 10~25 min, 30%~40% B; 25~30 min, 40%~50% B; 30~40 min, 50%~70% B; 40~45 min, 70%~100% B; 45~60 min, held at 100% B; 60.5 min, 0% B.
BSE (200 mg) was extracted with 10 mL of 50% MeOH and filtered through a 0.22 μm syringe filter.
4.4. Extraction, Isolation and Identification
BSE (100 g) was extracted with 10 times (v/w) the amount of EtOH and then extracted sequentially with petroleum ether, EtOAc and n-BuOH at room temperature. Each extraction was carried out three times (400, 300 and 300 mL).
The EtOAc fraction (685 mg) was stirred evenly with silica gel and then loaded on the top of the silica gel in a silica gel column. With the elution of DCM/MeOH (40/1, v/v), fraction a, compound 1 (11.03 mg) and fraction b were obtained. Fraction a was further separated through preparative thin-layer chromatography with DCM/MeOH (40/1, v/v); then, compounds 2 (5.29 mg), 3 (6.17 mg) and 4 (4.35 mg) were obtained.
The n-BuOH fraction (16.81 g) was stirred evenly with silica gel and then loaded on the top of the silica gel in a silica gel column. With the elution of DCM/MeOH (10/1~3/1, v/v), fractions 1~5 were collected. Fraction 3 was separated using a silica gel column with DCM/MeOH (10/1, v/v) to obtain fraction 3.1 and fraction 3.2. Fractions 2 and 3.1 were combined to produce a new fraction, which was separated through preparative thin-layer chromatography with DCM/MeOH/HAc (40/4/1, v/v); then, compounds 5 (6.10 mg), 6 (9.98 mg) and 7 (5.18 mg) were obtained. Fractions 3.2 and 4 were combined and separated using a polyamide column, employing a mixture elution with H2O and MeOH; this produced compounds 8 (5.47 mg), 9 (6.98 mg), 10 (4.84 mg) and 11 (25.15 mg). Fraction 5 was separated using a polyamide column, employing a mixture elution with H2O and MeOH, and compound 12 (22.71 mg) was obtained.
1H-NMR and 13C-NMR spectra were recorded in DMSO-d6 on Bruker 400 MHz or 100 MHz instruments. High-resolution electrospray ionization (ESI)-MS (positive and negative mode) was performed on an Agilent 6200 series/6500 series Q-TOF10.1 (48.0).
4.5. Network Pharmacology-Based Analysis
4.5.1. Screening Bioactive Compositions and Prediction of Potential Drug Targets
The main chemical ingredients of broad bean sprouts were identified from the UHPLC-ESI-MS/MS results in this study and related literature. All the components were verified using SWISS ADME according to the following criteria: gastrointestinal absorption was ‘High’ and at least two criteria of Lipinski’s rule of five (Lipinski, ghose, veber, egan, muegge) were met (‘yes’).
The SMILES or structures of the obtained compounds were searched for in the PubChem database (https://pubchem.ncbi.nlm.nih.gov (accessed on 1 March 2023)) and entered into the Swiss Target Prediction tool (http://www.swisstargetpred iction.ch (accessed on 6 March 2023)); then, the potential targets (Probability ≥ 0) of the components could be elucidated.
4.5.2. Acquisition of PD Target Genes and Analysis of the Intersection
The keywords ‘Parkinson’s disease’ and ‘Parkinsonism’ were, respectively, put into the GeneCards (https://www.genecards.org/ (accessed on 6 March 2023)) and Online Mendelian Inheritance in Man (OMIM, https://omim.org/ (accessed on 6 March 2023)) databases for all the target genes. After removing all the duplicates, all the target genes were entered into the UniProt database (https://www.uniprot.org/ (accessed on 6 March 2023)) to obtain the UniProt IDs. A Venn diagram was used to visualize the intersecting targets of broad bean sprouts and PD.
4.5.3. Core Component Screening and Construction of Component Target Network
Cytoscape 3.9.1 was used to draw network diagrams. ‘Network analysis’ in the software was used to calculate the degree.
4.5.4. Construction of PPI Network of Common Targets of Broad Bean Sprouts and PD
The common targets were entered into STRING11.0 (https://string-db.org/ (accessed on 6 March 2023)) to obtain the PPI information; Homo Sapiens was set as the limited organism, and the minimum required interaction score was set to 0.4. Cytoscape 3.9.1 was used to draw a PPI network diagram of the intersecting targets. The node size and color were set according to the degree in the diagram.
4.5.5. GO Analysis and KEGG Pathway Enrichment Analysis
The common targets were entered into the David database (https://david.ncifcrf.gov/ (accessed on 6 March 2023)) for GO and KEGG enrichment analysis (Homo Sapiens, p < 0.05); then, cellular component, molecular function, and biological process items on massive genetic information along with drug–disease signaling pathways could be identified. The top 10 GO terms and top 20 KEGG pathways with the smallest p values were selected and uploaded to the bioinformatics mapping website (http://www.bioinformatics.com.cn/ (accessed on 6 March 2023)) to draw column graphs and bubble graphs.
4.6. In Vitro Evaluations of Caffeic Acid and P-Coumaric Acid
4.6.1. Cell Culture
Cells and reagents were provided by KeyGEN BioTECH (Nanjing, China). PC-12 cells were cultured in a medium prepared by mixing Minimum Essential Medium (MEM) and 10% (v/v) F12 Medium supplemented with 10% FBS. SH-SY5Y cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS). The cells were cultured at 37 °C in a humidified 5% CO2 incubator. Each group was tested in triplicate.
4.6.2. Investigation of Drug Administration Concentrations
Cells were seeded in 96-well plates at a density of 5 × 104 cells/mL. After 24 h, the culture medium was removed and equal volumes (100 μL) of medium with increasing concentrations (3.125, 6.25, 12.5, 25, 50 and 100 µM) of caffeic acid and p-coumaric acid were added. The cells in the blank control group (Con) were incubated with serum-free MEM.
An MTT assay was applied to evaluate the viability of the cells. Cells were incubated with 20 μL of MTT solution (5 mg/mL) for 4 h. DMSO was then used to dissolve crystals to halt the reaction. Then, the absorbance of the cells at 490 nm was detected using a microplate reader (TECAN, Männedorf, Switzerland). Data are expressed as percentages of the absorbance of the untreated cells. Finally, appropriate concentrations (low, medium and high doses) of caffeic acid and p-coumaric acid were selected for further examinations.
4.6.3. Evaluation of Cell Viability Using MTT Assay
SH-SY5Y and PC-12 cells were both separated into five groups: a blank control group (Con), a model control group (Mod) and intervention groups (low, medium and high doses). The cells were seeded in 6-well plates at a density of 3 × 105 cells/mL. After 24 h, the culture medium was removed, and equal volumes of medium with selected concentrations of caffeic acid and p-coumaric acid were added. After 24 h, SH-SY5Y cells were treated with medium containing 50 µM 6-OHDA, and PC-12 cells were treated with medium containing 200 µg/mL LPS for further incubation (24 h). LPS and 6-OHDA were acquired from MCE (USA).
The MTT assay was performed as described in Section 4.6.2.
4.6.4. Assessing Cell Apoptosis Through Annexin-V FITC/PI Staining
The cells were prepared and groups were established as described in Section 4.6.3. The cells were assayed using an Annexin V-FITC and PI Detection Kit (KeyGEN BioTECH, Nanjing, China) based on the manufacturer’s instructions. The percentage of apoptotic cells was detected using a flow cytometer (BECKMAN COULTER, California, Brea, CA, USA).
4.6.5. Intracellular ROS Levels
SH-SY5Y cells were separated into five groups: Con, Mod and intervention groups (low, medium and high doses). The cells were seeded in 6-well plates at a density of 3 × 105 cells/mL. After 24 h, the culture medium was removed and equal volumes of medium with selected concentrations of caffeic acid and p-coumaric acid were added. After 24 h, SH-SY5Y cells were treated with medium containing 50 µM 6-OHDA for further incubation (24 h).
The cell membrane-permeable fluorescein analog 2′,7′-dichlorofluorescin diacetate (DCFH-DA) was applied to detect the intracellular ROS. Cells were washed twice with PBS and incubated in PBS containing 10 μM DCFH-DA for 20 min at 37 °C and then washed twice with serum-free MEM. The intracellular ROS were measured using a flow cytometer (BECKMAN COULTER, California, USA) with Ex = 488 nm and Em = 525 nm.
4.6.6. Enzyme-Linked Immunosorbent Assay (ELISA)
PC-12 cells were separated into five groups: Con, Mod and intervention groups (low, medium and high doses). The cells were seeded in 6-well plates at a density of 3 × 105 cells/mL. After 24 h, the culture medium was removed, and equal volumes of medium with selected concentrations of caffeic acid and p-coumaric acid were added. After 24 h, PC-12 cells were treated with medium containing 200 µg/mL LPS for further incubation (24 h).
Following the ELISA kit manufacturer’s instructions, we detected the contents of IL-6 (KeyGEN BioTECH, Nanjing, China) and TNF-α (KeyGEN BioTECH, Nanjing, China) in the supernatant of PC-12 cells.
4.6.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 10. The significance of differences in variables between groups was assessed using one-way analysis of variance (one-way ANOVA). The data are expressed as the means ± standard deviations, and p < 0.05 was considered statistically significant; * p < 0.05 and ** p < 0.01.
Author Contributions
Conceptualization, investigation, data curation and writing—original draft preparation, D.H.; investigation and data curation, G.Q. and X.L.; writing—review and editing, J.C., K.Z. and L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
Authors Danni Hu, Guanglei Qing, Xuecheng Liu were employed by the company Nanjing Core Tech Biomedical Co., Ltd. Author Lingyun He are employed by the company Nanjing Core Tech Biomedical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Parkinson’s Disease and Parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef]
- Ramón, C. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef]
- AlDakheel, A.; Kalia, L.V.; Lang, A.E. Pathogenesis-Targeted, Disease-Modifying Therapies in Parkinson Disease. Neurotherapeutics 2014, 11, 6–23. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548. [Google Scholar] [CrossRef]
- Chou, K.L.; Stacy, M.; Simuni, T.; Miyasaki, J.; Oertel, W.H.; Sethi, K.; Fernandez, H.H.; Stocchi, F. The Spectrum of “off” in Parkinson’s Disease: What Have We Learned over 40 Years. Park. Relat. Disord. 2018, 51, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Turcano, P.; Mielke, M.M.; Bower, J.H.; Parisi, J.E.; Cutsforth-Gregory, J.K.; Ahlskog, J.E.; Savica, R. Levodopa-Induced Dyskinesia in Parkinson Disease: A Population-Based Cohort Study. Neurology 2018, 91, e2238–e2243. [Google Scholar] [CrossRef]
- Rabey, J.M.; Vered, Y.; Shabtai, H.; Graff, E.; Harsat, A.; Korczyn, A.D. Broad Bean (Vicia faba) Consumption and Parkinson’s Disease. Adv. Neurol. 1993, 60, 681–684. [Google Scholar] [PubMed]
- Apaydin, H.; Ertan, S.; Özekmekçi, S. Broad Bean (Vicia faba) A Natural Source ofL-Dopa Prolongs “on” Periods in Patients with Parkinson’s Disease Who Have “On-off” Fluctuations. Mov. Disord. 2000, 15, 164–166. [Google Scholar] [CrossRef]
- Mekky, R.H.; Thabet, M.M.; Rodríguez-Pérez, C.; Elnaggar, D.M.; Mahrous, E.A.; Segura-Carretero, A.; Abdel-Sattar, E. Essam Abdel-Sattar Comparative Metabolite Profiling and Antioxidant Potentials of Seeds and Sprouts of Three Egyptian Cultivars of Vicia faba L. Food Res. Int. 2020, 136, 109537. [Google Scholar] [CrossRef]
- Liu, X.C.; Jin, H.J.; Chen, B.H.; He, L.Y.; Liu, T.S. Protective Effect of Broad Bean Seedling Extract on Parkinson’s Disease. Sci. Technol. Food Ind. 2022, 43, 379–386. (In Chinese) [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese Medicine Network Pharmacology: Theory, Methodology and Application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Mäkelä, S.; Aittokallio, T. Network Pharmacology Applications to Map the Unexplored Target Space and Therapeutic Potential of Natural Products. Nat. Prod. Rep. 2015, 32, 1249–1266. [Google Scholar] [CrossRef]
- Ru, J.L.; Li, P.; Wang, J.A.; Zhou, W.; Li, B.H.; Huang, C.; Li, P.D.; Guo, Z.H.; Tao, W.Y.; Yang, Y.F.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Rule of Five Violations among the FDA-Approved Small Molecule Protein Kinase Inhibitors. Pharmacol. Res. 2023, 191, 106774. [Google Scholar] [CrossRef]
- Zhao, L. Network Pharmacology, a Promising Approach to Reveal the Pharmacology Mechanism of Chinese Medicine Formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wang, X.; Zhang, D.M.; Hu, Y.J.; Li, S. Traditional Chinese medicine network pharmacology: Development in new era under guidance of network pharmacology evaluation method guidance. China J. Chin. Mater. Medica. 2022, 47, 7–17. [Google Scholar] [CrossRef]
- Li, S. Network pharmacology evaluation method guidance-Draft. World J. Tradit. Chin. Med. 2021, 7, 146–154. [Google Scholar] [CrossRef]
- Björn, H.F.; Theodora, S.; Elisabeth, D. Cellular Models for Parkinson’s Disease. J. Neurochem. 2016, 139 (Suppl. S1), 120–130. [Google Scholar] [CrossRef]
- Ramirez, A.I.; De Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Litvan, I.; Halliday, G.; Hallett, M.; Goetz, C.G.; Rocca, W.; Duyckaerts, C.; Ben-Shlomo, Y.; Dickson, D.W.; Lang, A.E.; Chesselet, M.-F.; et al. The Etiopathogenesis of Parkinson Disease and Suggestions for Future Research. Part I. J. Neuropathol. Exp. Neurol. 2007, 66, 251–257. [Google Scholar] [CrossRef]
- Valente, I.M.; Cabrita, A.R.J.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Maia, M.R.G. Unravelling the Phytonutrients and Antioxidant Properties of European Vicia faba L. Seeds. Food Res. Int. 2019, 116, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.Y.; Wang, M.X.; Luo, K.K.; Hu, T.B.; Li, H.M.; Huo, S.H.; Hao, L.Y.; Si, N.; Gao, W.Y.; Liu, Y.Y.; et al. Evaluation of efficacy of Dihuang Baoyuan Granules for diabetes mellitus and composition analysis and in vivo distribution based on UHPLC-LTQ-Orbitrap MS. China J. Chin. Mater. Medica 2024, 49, 2783–2797. (In Chinese) [Google Scholar] [CrossRef]
- Xie, C.; Veitch, N.C.; Houghton, P.J.; Simmonds, M.S.J. Flavone C-Glycosides from Viola Yedoensis MAKINO. Chem. Pharm. Bull. 2003, 51, 1204–1207. [Google Scholar] [CrossRef]
- Hooper, A.M.; Tsanuo, M.K.; Chamberlain, K.; Tittcomb, K.; Scholes, J.; Hassanali, A.; Khan, Z.R.; Pickett, J.A. Isoschaftoside, a C-Glycosylflavonoid from Desmodium Uncinatum Root Exudate, Is an Allelochemical against the Development of Striga. Phytochemistry 2010, 71, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, W.; Li, Y.; Jilany Khan, G.; Chen, Y.; Shen, T.; Bao, N.; Hua, J.; Xue, Z.; Zhai, K.; et al. Identification of Phytochemicals and Antioxidant Activity of Premna Microphylla Turcz. Stem through UPLC-LTQ-Orbitrap-MS. Food Chem. 2022, 373, 131482. [Google Scholar] [CrossRef] [PubMed]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant Activity and Polyphenol Composition of Sugarcane Molasses Extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; del Mar Contreras, M.; Arráez-Román, D.; Fernández-Gutiérrez, A.; Segura-Carretero, A. UHPLC-ESI-QTOF-MS-Based Metabolic Profiling of Vicia faba L. (Fabaceae) Seeds A Key Strategy for Characterization in Foodomics. Electrophoresis 2014, 35, 1571–1581. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, Y.F.; Liu, Z.Y.; Song, L.M.; Liu, Y.H.; Zhang, S.L.; Chu, H.B. Study on the Characteristic Spectra and Multi indicator Component Screening and Content Determination of Kidney and Pharynx Formula Substance Standards Based on UHPLC-MS/MS Method. Mod. Tradit. Chin. Med. Mater. Medica-World Sci. Technol. 2024, 26, 704–720. (In Chinese) [Google Scholar]
- Dou, L.L.; Duan, L.; Guo, L.; Liu, L.L.; Zhang, Y.D.; Li, P.; Liu, E.H. An UHPLC-MS/MS Method for Simultaneous Determination of Quercetin 3-O-Rutinoside, Kaempferol 3-O-Rutinoside, Isorhamnetin 3-O-Rutinoside, Bilobalide and Ligustrazine in Rat Plasma, and Its Application to Pharmacokinetic Study of Xingxiong Injection. Chin. J. Nat. Med. 2017, 15, 710–720. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, S.H.; He, Y.J.; Mo, C.M.; Wu, C.Q.; Zhang, R.Y.; Zheng, X.Y.; Tang, Q. Systematic Characterization of Flavonoids from Siraitia Grosvenorii Leaf Extract Using an Integrated Strategy of High-Speed Counter-Current Chromatography Combined with Ultra High Performance Liquid Chromatography and Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. J. Sep. Sci. 2020, 43, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xing, S.; Luu, T.; Fan, M.; Li, X. The Gastrointestinal Tract Metabolism and Pharmacological Activities of Grosvenorine, a Major and Characteristic Flavonoid in the Fruits of Siraitia Grosvenorii. Chem. Biodivers. 2015, 12, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, D.; Wu, D.; Liu, W.; Liu, X. TiO2 Nanotube Immobilised 5-Lipoxygenase-Mediated Screening and Isolation of Anti-Inflammatory Active Compounds from the Leaves of Lonicera Japonica Thunb. J. Enzym. Inhib. Med. Chem. 2022, 37, 2540–2550. [Google Scholar] [CrossRef]
- Bi, W.; Zhao, G.; Zhou, Y.; Xia, X.; Wang, J.; Wang, G.; Lu, S.; He, W.; Bi, T.; Li, J. Metabonomics Analysis of Flavonoids in Seeds and Sprouts of Two Chinese Soybean Cultivars. Sci. Rep. 2022, 12, 5541. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Warad, I.; Fernández-Gutiérrez, A.; Segura-Carretero, A. UHPLC/MS 2 -Based Approach for the Comprehensive Metabolite Profiling of Bean (Vicia faba L.) by-Products: A Promising Source of Bioactive Constituents. Food Res. Int. 2017, 93, 87–96. [Google Scholar] [CrossRef]
- Pérez-Vásquez, A.; Díaz-Rojas, M.; Castillejos-Ramírez, E.V.; Pérez-Esquivel, A.; Montaño-Cruz, Y.; Rivero-Cruz, I.; Torres-Colín, R.; González-Andrade, M.; Rodríguez-Sotres, R.; Gutiérrez-González, J.A.; et al. Protein Tyrosine Phosphatase 1B Inhibitory Activity of Compounds from Justicia Spicigera (Acanthaceae). Phytochemistry 2022, 203, 113410. [Google Scholar] [CrossRef]
- Ji, Y.-L.; Feng, X.; Chang, Y.-Q.; Zheng, Y.-G.; Hou, F.-J.; Zhang, D.; Guo, L. Chemical Characterization of Different Parts of Forsythia Suspensa and α-Glucosidase and Pancreatic Lipase Inhibitors Screening Based on UPLC-QTOF-MS/MS and Plant Metabolomics Analysis. Arab. J. Chem. 2024, 17, 105723. [Google Scholar] [CrossRef]
- Sharaf, M.; El-Ansari, M.A.; Saleh, N.A.M. Flavonoids of Four Cleome and Three Capparis Species. Biochem. Syst. Ecol. 1997, 25, 161–166. [Google Scholar] [CrossRef]
- Wang, H.; Hou, X.M.; Li, B.Q.; Yang, Y.; Li, Q.; Si, Y.C. Study on Active Components of Cuscuta Chinensis Promoting Neural Stem Cells Proliferation: Bioassay-Guided Fractionation. Molecules 2021, 26, 6634. [Google Scholar] [CrossRef]
- Walewska, E.; Werner, J.; Strzelecka, H. Investigations of Phenolic and Flavonoid Glycosides in Pyrus Leaves. Part 1 [Folium Pyri]. Herba Pol. 1979, 25, 99–105. [Google Scholar]
- Xiao, H.B.; Krucker, M.; Albert, K.; Liang, X.M. Determination and Identification of Isoflavonoids in Radix Astragali by Matrix Solid-Phase Dispersion Extraction and High-Performance Liquid Chromatography with Photodiode Array and Mass Spectrometric Detection. J. Chromatogr. A 2004, 1032, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.M.; Maia, M.R.G.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Profiling of Phenolic Compounds and Antioxidant Properties of European Varieties and Cultivars of Vicia faba L. Pods. Phytochem. 2018, 152, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous Determination of 78 Compounds of Rhodiola Rosea Extract by Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Biochem. Res. Int. 2021, 2021, 9957490. [Google Scholar] [CrossRef]
- Bendif, H.; Miara, M.D.; Peron, G.; Sut, S.; Dall’Acqua, S.; Flamini, G.; Maggi, F. NMR, HS-SPME-GC/MS, and HPLC/MSn Analyses of Phytoconstituents and Aroma Profile of Rosmarinus Eriocalyx. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- de Rijke, E.; Out, P.; Niessen, W.M.A.; Ariese, F.; Gooijer, C.; Brinkman, U.A. Analytical Separation and Detection Methods for Flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- Gruz, J.; Novák, O.; Strnad, M. Rapid Analysis of Phenolic Acids in Beverages by UPLC–MS/MS. Food Chem. 2008, 111, 789–794. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS Profiling of Artemisia Herba-Alba and Evaluation of Its Bioactive Properties. Food Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef]
- Cheng, J.; Di, L.Q.; Li, J.S.; Zhao, X.L.; Zhou, W. Simultaneous determination of ten effective components in Tongsaimai Pellets by UPLC-MS/MS. Chin. Tradit. Herb. Drugs 2014, 45, 659–664. (In Chinese) [Google Scholar]
- Rubab, F.; Ijaz, H.; Hussain, S.; Munir, A.; Stuppner, S.; Jakschitz, T.; Bonn, G.K.; Ishtiaq, S. Gastroprotective Effects of Caragana Ambigua Stocks on Ethanol-Induced Gastric Ulcer in Rats Supported by LC-MS/MS Characterization of Formononetin and Biochanin A. J. Sci. Food Agric. 2022, 102, 7030–7038. [Google Scholar] [CrossRef]
- Yang, X.-M.; Wang, Y.-F.; Li, Y.-Y.; Ma, H.-L. Thermal Stability of Ginkgolic Acids from Ginkgo Biloba and the Effects of Ginkgol C17:1 on the Apoptosis and Migration of SMMC7721 Cells. Fitoterapia 2014, 98, 66–76. [Google Scholar] [CrossRef]
- Selemani, M.A.; Kazingizi, L.F.; Manzombe, E.; Bishi, L.Y.; Mureya, C.; Gwata, T.T.; Rwere, F. Phytochemical Characterization and in Vitro Antibacterial Activity of Xeroderris Stuhlmannii (Taub.) Mendonca & E.P. Sousa Bark Extracts. S. Afr. J. Bot. 2021, 142, 344–351. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.Z.; Wei, X.S.; Zuo, H.; Ma, L.; Qamar, M.T.U.; Li, M.; Han, S.J.; Hu, L.H.; Wang, L.Q. LC-MS/MS-Based Metabolomics Approach Revealed Novel Phytocompounds from Sugarcane Rind with Promising Pharmacological Value. J. Sci. Food Agric. 2022, 102, 6632–6642. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Hullar, M.A.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Le Marchand, L.; et al. Associations of Plasma Trimethylamine N-Oxide, Choline, Carnitine, and Betaine with Inflammatory and Cardiometabolic Risk Biomarkers and the Fecal Microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Liu, M.; Dai, G.L.; Li, C.Y.; Wu, T.; Zou, G.D.; Ju, W.Z.; Xu, M.J. Simultaneous Determination of Nicotinamide and N1 -Methylnicotinamide in Human Serum by LC-MS/MS to Associate Their Serum Concentrations with Obesity. Biomed. Chromatogr. 2022, 36, 5261. [Google Scholar] [CrossRef]
- Wang, D.W.; Luo, X.D.; Jiang, B. Chemical constituents in twigs and leaves of Melodinus fusiformis. Chin. Tradit. Herb. Drugs 2012, 43, 653–657. (In Chinese) [Google Scholar]
- Thorsteinsdottir, U.A.; Runolfsdottir, H.L.; Eiriksson, F.F.; Agustsdottir, I.M.; Edvardsson, V.O.; Palsson, R.; Thorsteinsdottir, M. Optimization and Validation of a UPLC-MS/MS Assay for Simultaneous Quantification of 2,8-Dihydroxyadenine, Adenine, Allopurinol, Oxypurinol and Febuxostat in Human Plasma. J. Chromatogr. B 2024, 1235, 124041. [Google Scholar] [CrossRef]
- Pavón-Pérez, J.; Oviedo, C.A.; Elso-Freudenberg, M.; Henríquez-Aedo, K.; Aranda, M.; Pavón-Pérez, J.; Oviedo, C.A.; Elso-Freudenberg, M.; Henríquez-Aedo, K.; Aranda, M. LC-MS/MS METHOD FOR L-DOPA QUANTIFICATION IN DIFFERENT TISSUES OF VICIA FABA. J. Chil. Chem. Soc. 2019, 64, 4651–4653. [Google Scholar] [CrossRef]
- Han, L.; Liu, E.; Kojo, A.; Zhao, J.; Li, W.; Zhang, Y.; Wang, T.; Gao, X. Qualitative and Quantitative Analysis of Eclipta Prostrata L. by LC/MS. Sci. World J. 2015, 2015, e980890. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Ji, J.; Li, N.; Yu, Y.; Duan, G.; Zhang, X. Fast Determination of Curcumol, Curdione and Germacrone in Three Species of Curcuma Rhizomes by Microwave-Assisted Extraction Followed by Headspace Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2006, 1117, 115–120. [Google Scholar] [CrossRef]
- Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid Glycosides from Rhodiola Rosea. Chem. Pharm. Bull. 2003, 51, 467–470. [Google Scholar] [CrossRef]
- Xu, M.Y.; Xu, Z.L.; Xu, Q.Z.; Zhang, H.Y.; Liu, M.Y.; Geng, F.; Zhang, N. UPLC-MS/MS Method for the Determination of 14 Compounds in Rat Plasma and Its Application in a Pharmacokinetic Study of Orally Administered Xiaoyao Powder. Molecules 2018, 23, 2514. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Molares, I.; Pérez, O.; Rivero-Cruz, I.; Acevedo, L.; Enriquez-Mendoza, I.; Bye, R.; Franzblau, S.; Timmermann, B. Antimycobacterial Compounds from Piper Sanctum. J. Nat. Prod. 2004, 67, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Fiori, J.; Naldi, M.; Gotti, R. HS–SPME–GC–MS for the Quantitation and Chiral Characterization of Camphor and Menthol in Creams. Chroma 2010, 72, 941–947. [Google Scholar] [CrossRef]
- Arciszewska, Ż.; Gama, S.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Gołebiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic Acid/Eu(III) Complexes: Solution Equilibrium Studies, Structure Characterization and Biological Activity. Int. J. Mol. Sci. 2022, 23, 888. [Google Scholar] [CrossRef] [PubMed]
- Kola, A.; Vigni, G.; Baratto, M.C.; Valenain, D. A Combined NMR and UV-Vis Approach to Evaluate Radical Scavenging Activity of Rosmarinic Acid and Other Polyphenols. Molecules 2023, 28, 6629. [Google Scholar] [CrossRef]
- Davyt, D.; Entz, W.; Fernandez, R.; Mariezcurrena, R.; Mombrú, A.M.; Saldaña, J.; Domínguez, L.; Coll, J.; Manta, E. A New Indole Derivative from the Red Alga Chondria Atropurpurea. Isolation, Structure Determination, and Anthelmintic Activity. J. Nat. Prod. 1998, 61, 1560–1563. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Devadasu, C.; Babu, P.S. Isolation, Characterization, and RP-HPLC Estimation of P-Coumaric Acid from Methanolic Extract of Durva Grass (Cynodon Dactylon Linn.) (Pers.). Int. J. Anal. Chem. 2015, 2015, 201386. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.L.; Gong, L.M.; Huang, F.B.; Cao, M.R.; Liu, Y.B.; Yuan, H.W.; Li, B.; Jian, Y.Q.; Peng, C.Y.; Zhou, S.P.; et al. A Rapid and Accurate 1HNMR Method for the Identification and Quantification of Major Constituents in Qishen Yiqi Dripping Pills. J. AOAC Int. 2021, 104, 506–514. [Google Scholar] [CrossRef]
- Nam, T.-G.; Lee, S.M.; Park, J.-H.; Kim, D.-O.; Baek, N.-i.; Eom, S.H. Flavonoid Analysis of Buckwheat Sprouts. Food Chem. 2015, 170, 97–101. [Google Scholar] [CrossRef]
- Ajayi, O.S.; Aderogba, M.A.; Obuotor, E.M.; Majinda, R.R.T. Acetylcholinesterase Inhibitor from Anthocleista Vogelii Leaf Extracts. J. Ethnopharmacol. 2019, 231, 503–506. [Google Scholar] [CrossRef]
- Regasini, L.O.; Vellosa, J.C.R.; Silva, D.H.S.; Furlan, M.; de Oliveira, O.M.M.; Khalil, N.M.; Brunetti, I.L.; Young, M.C.M.; Barreiro, E.J.; Bolzani, V.S. Flavonols from Pterogyne Nitens and Their Evaluation as Myeloperoxidase Inhibitors. Phytochemistry 2008, 69, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Chen, Y.Y.1; Sun, J.Q.; Li, D.P.; Lu, F.L.; Yan, X.J. Chemical Constituents of n-Butanol Extract from the Leaves of Semiliquidambar cathayensis. J. Chin. Med. Mater. 2023, 46, 1946–1950. (In Chinese) [Google Scholar] [CrossRef]
- Znati, M.; Jannet, H.B.; Cazaux, S.; Souchard, J.P.; Skhiri, F.H.; Bouajila, J. Antioxidant, 5-Lipoxygenase Inhibitory and Cytotoxic Activities of Compounds Isolated from the Ferula Lutea Flowers. Molecules 2014, 19, 16595. [Google Scholar] [CrossRef]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Unraveling the Active Hypoglycemic Agent Trigonelline in Balanites Aegyptiaca Date Fruit Using Metabolite Fingerprinting by NMR. J. Pharm. Biomed. Anal. 2015, 115, 383–387. [Google Scholar] [CrossRef]
- Fuentes-Herrera, P.B.; Herrera-Cabrera, B.E.; Martínez-Ayala, A.L.; Zamilpa, A.; Delgado-Alvarado, A. Content and Yield of L-DOPA and Bioactive Compounds of Broad Bean Plants: Antioxidant and Anti-Inflammatory Activity In Vitro. Plants 2023, 12, 3918. [Google Scholar] [CrossRef]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef]
- Barros Silva, R.; Santos, N.A.G.; Martins, N.M.; Ferreira, D.A.S.; Barbosa, F., Jr.; Souza, V.C.O.; Kinoshita, A.; Baffa, O.; Del-Bel, E.; Santos, A.C. Caffeic Acid Phenethyl Ester Protects against the Dopaminergic Neuronal Loss Induced by 6-Hydroxydopamine in Rats. Neuroscience 2013, 233, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Fontanilla, C.V.; Ma, Z.; Wei, X.; Klotsche, J.; Zhao, L.; Wisniowski, P.; Dodel, R.C.; Farlow, M.R.; Oertel, W.H.; Du, Y. Caffeic Acid Phenethyl Ester Prevents 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurodegeneration. Neuroscience 2011, 188, 135–141. [Google Scholar] [CrossRef]
- Zaitone, S.A.; Ahmed, E.; Elsherbiny, N.M.; Mehanna, E.T.; El-Kherbetawy, M.K.; ElSayed, M.H.; Alshareef, D.M.; Moustafa, Y.M. Caffeic Acid Improves Locomotor Activity and Lessens Inflammatory Burden in a Mouse Model of Rotenone-Induced Nigral Neurodegeneration: Relevance to Parkinson’s Disease Therapy. Pharmacol. Rep. 2019, 71, 32–41. [Google Scholar] [CrossRef]
- Kiliç, I.; Yesiloglu, Y. Spectroscopic Studies on the Antioxidant Activity of P-Coumaric Acid. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Ding, P.; Ye, Z.; Liu, B.; Zhang, L.Y. Effect of Protocatechualdehyde on Streptozotocin Induced Diabetic Cardiomyopathy in Mice. Mod. Food Sci. Technol. 2023, 1–7. (In Chinese) [Google Scholar] [CrossRef]
- Mu, J.F.; Song, J.X.; Li, R.; Xue, T.Y.; Wang, D.X.; Yu, J.H. Isovitexin Prevents DSS-Induced Colitis through Inhibiting Inflammation and Preserving Intestinal Barrier Integrity through Activating AhR. Chem. Biol. Interact. 2023, 382, 110583. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, C.; Placido, P.D.; Bruzzese, D.; Pagliuca, M.; Ungaro, P.; Bosso, D.; Ribera, D.; Iaccarino, S.; Scafuri, L.; Liotti, A.; et al. Isoquercetin as an Adjunct Therapy in Patients With Kidney Cancer Receiving First-Line Sunitinib (QUASAR): Results of a Phase I Trial. Front. Pharmacol. 2018, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, L.M.; Sui, F.; Wang, Z.M.; Huo, H.R.; Dai, L.; Jiang, T.L. Chemistry and Pharmacology of Siraitia Grosvenorii: A Review. Chin. J. Nat. Med. 2014, 12, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Dai, D.K.; Zheng, B.Z.-Y.; Duan, R.; Chan, G.K.-L.; Dong, T.T.-X.; Qin, Q.-W.; Tsim, K.W.-K. The Binding of Kaempferol-3-O-Rutinoside to Vascular Endothelial Growth Factor Potentiates Anti-Inflammatory Efficiencies in Lipopolysaccharide-Treated Mouse Macrophage RAW264.7 Cells. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 80, 153400. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Kang, C.W.; Kim, G.D. Kaempferol-3-O-β-rutinoside Suppresses the Inflammatory Responses in Lipopolysaccharide-stimulated RAW264.7 Cells via the NF-κB and MAPK Pathways. Int. J. Mol. Med. 2019, 44, 2321–2328. [Google Scholar] [CrossRef]
- Hua, F.; Li, J.Y.; Zhang, M.; Zhou, P.; Wang, L.; Ling, T.J.; Bao, G.H. Kaempferol-3-O-Rutinoside Exerts Cardioprotective Effects through NF-κB/NLRP3/Caspase-1 Pathway in Ventricular Remodeling after Acute Myocardial Infarction. J. Food Biochem. 2022, 46, e14305. [Google Scholar] [CrossRef]
- Guan, S.; Sun, L.; Wang, X.; Huang, X.; Luo, T. Isoschaftoside Inhibits Lipopolysaccharide-Induced Inflammation in Microglia through Regulation of HIF-1α-Mediated Metabolic Reprogramming. Evid. Based Complement. Altern. Med. 2022, 2022, 5227335. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, C.; Hou, L.; Xu, K.; Zhang, Y.; Wang, X.; Li, J.; Liu, K.; Xia, Q. Narcissin Induces Developmental Toxicity and Cardiotoxicity in Zebrafish Embryos via Nrf2/HO-1 and Calcium Signaling Pathways. J. Appl. Toxicol. 2024, 44, 344–354. [Google Scholar] [CrossRef]
- Patel, D.K. Pharmacological Activities and Therapeutic Potential of Kaempferitrin in Medicine for the Treatment of Human Disorders: A Review of Medicinal Importance and Health Benefits. Cardiovasc. Hematol. Disord. Drug Targets 2021, 21, 104–114. [Google Scholar] [CrossRef]
- Li, J.C.; Yang, J.K.; Xian, Q.W.; Su, H.W.; Ni, Y.F.; Wang, L. Kaempferitrin Attenuates Unilateral Ureteral Obstruction-Induced Renal Inflammation and Fibrosis in Mice by Inhibiting NOX4-Mediated Tubular Ferroptosis. Phytother. Res. PTR 2024, 38, 2656–2668. [Google Scholar] [CrossRef] [PubMed]
- Rasheeda, K.; Fathima, N.N. Trigonelline Hydrochloride: A Promising Inhibitor for Type I Collagen Fibrillation. Colloids Surf. B Biointerfaces 2018, 170, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Roy, S.; Dutta, A.; Jana, K.; Ukil, A. Leishmania Donovani Targets Host Transcription Factor NRF2 To Activate Antioxidant Enzyme HO-1 and Transcriptional Repressor ATF3 for Establishing Infection. Infect. Immun. 2021, 89, e0076420. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).