Beware of N-Benzoyloxybenzamides
Abstract
1. Introduction
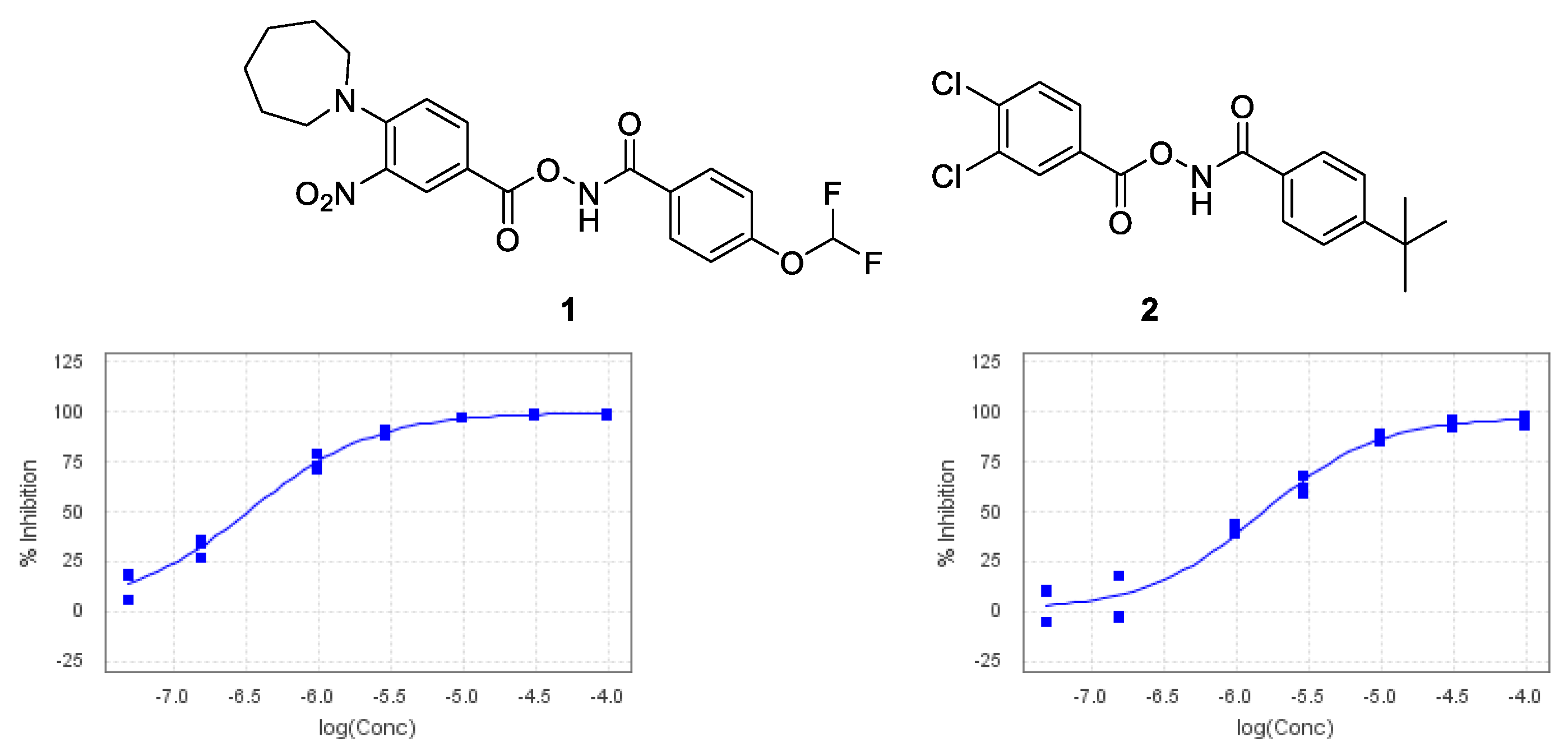
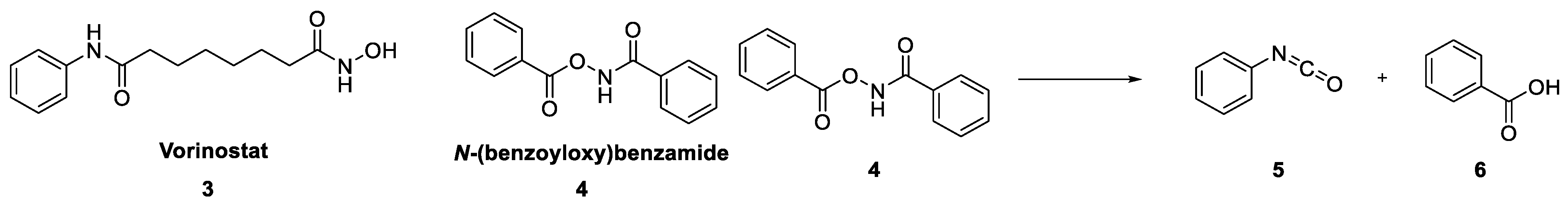
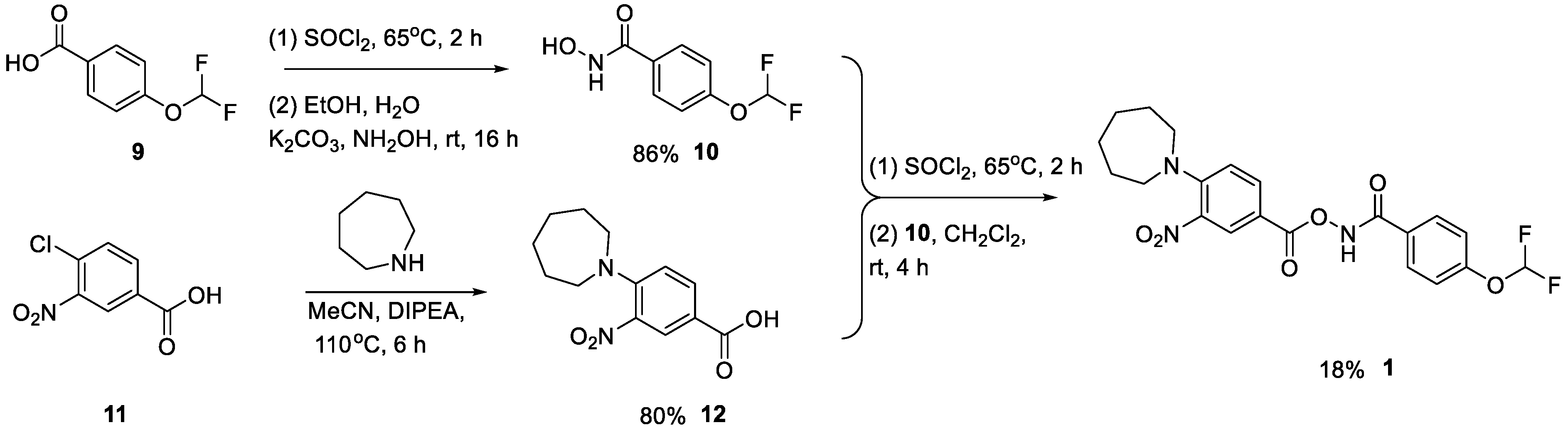
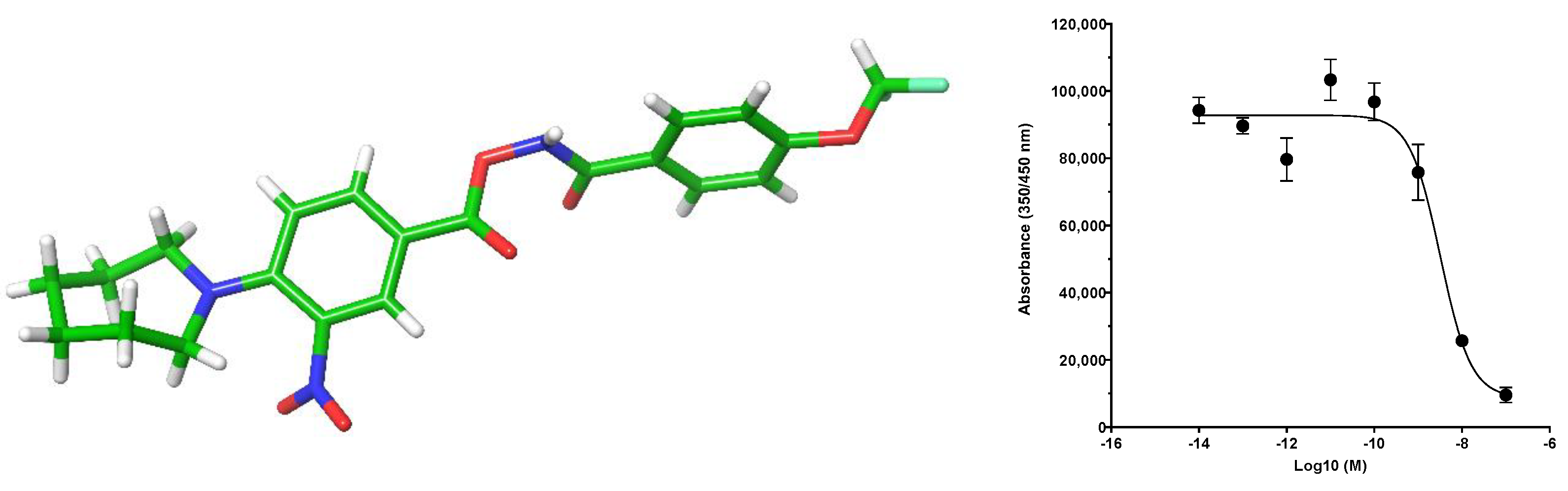
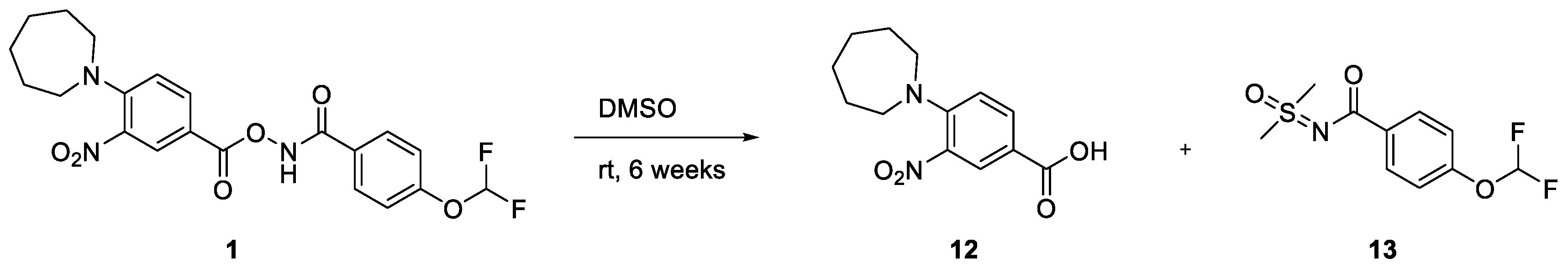
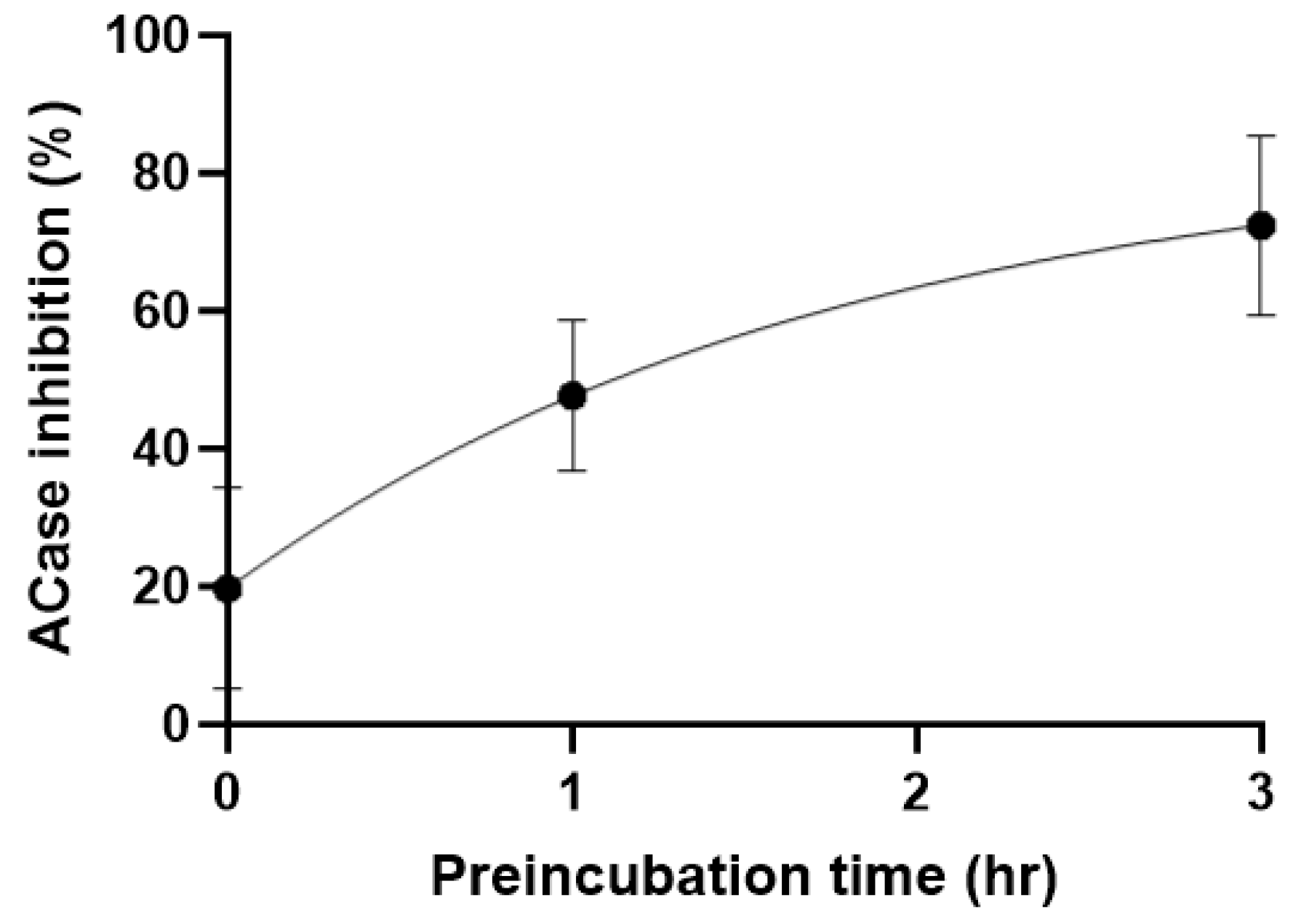
2. Results and Discussion
3. Mechanism of Action
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.S.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Disc. 2011, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Aseeri, M.; Abad, J.L.; Delgado, A.; Fabriàs, G.; Triola, G.; Casas, J.J. High-throughput discovery of novel small-molecule inhibitors of acid Ceramidase. Enzyme. Inhib. Med. Chem. 2023, 38, 343–348. [Google Scholar] [CrossRef]
- Bedia, C.; Camacho, L.; Abad, J.L.; Fabriàs, G.; Levade, T.J. A simple fluorogenic method for determination of acid ceramidase activity and diagnosis of Farber disease. Lipid Res. 2010, 51, 3542–3547. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Obrien, J.S.; Moser, H.W.; Kishimoto, Y. Stimulation of Acid Ceramidase Activity by Saposin D. Arch. Biochem. Biophys. 1994, 311, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Stowell, J.C.; Huot, R.I.; Van Voast, L.J. The Synthesis of N-Hydroxy-N′-phenyloctanediamide and Its Inhibitory Effect on Proliferation of AXC Rat Prostate Cancer Cells. Med. Chem. 1995, 38, 1411–1413. [Google Scholar] [CrossRef]
- Citarella, A.; Moi, D.; Pinzi, L.; Bonanni, D.; Rastelli, G. Hydroxamic Acid Derivatives: From Synthetic Strategies to Medicinal Chemistry Applications. ACS Omega 2021, 6, 21843–21849. [Google Scholar] [CrossRef]
- Izydore, R.A.; Debnath, M.L.; Woodard, T.; Wong, O.T.; Hall, I.H. Hypolipidemic activity of benzohydroxamic acids and dibenzohydroxamic acids in rodents. Res. Commun. Chem. Pathol. Pharmacol. 1990, 70, 307–321. [Google Scholar]
- Hall, I.H.; Barnes, B.J.; Ward, E.S.; Wheaton, J.R.; Izydore, R.A.J. Specific inhibition of Type II inosine monophosphate dehydrogenase activity of Tmolt4 T cell human leukaemia cells by 3-methoxy and di-benzohydroxamic acids, maleic hydrazide and malonic acids. Pharm. Pharmacol. 2001, 53, 749–755. [Google Scholar] [CrossRef]
- El Turk, F.; Fauvet, B.; Ouertatani-Sakouhi, H.; Lugari, A.; Betzi, S.; Roche, P.; Morelli, X.; Lashuel, H.A. An integrative in silico methodology for the identification of modulators of macrophage migration inhibitory factor (MIF) tautomerase activity. Bioorg. Med. Chem. 2010, 18, 5425–5440. [Google Scholar] [CrossRef][Green Version]
- Hall, I.H.; Izydore, R.; Hall, E.S.; Miller, M.C., III; Daniels, D.L.; Debnath, M.L.; Woodard, T. The antineoplastic and cytotoxicity of benzohydroxamic acids and related derivatives in murine and human tumor cells. Anti-Cancer Drugs 1992, 3, 273–280. [Google Scholar] [CrossRef]
- Smith, G.F. Designing Drugs to Avoid Toxicity. In Progress in Medicinal Chemistry; Lawton, G., Witty, D.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 50, pp. 1–47. [Google Scholar]
- Hutchison, A.J.; Yuan, J. Preparation of Diaryl Ureas as CB1 Antagonists. WO 2006049941, 11 May 2006. [Google Scholar]
- Okino, N.; He, X.; Gatt, S.; Sandhoff, K.; Ito, M.; Schuchman, E.H.J. The reverse activity of human acid ceramidase. Biol. Chem. 2003, 278, 29948–29953. [Google Scholar] [CrossRef] [PubMed]
- Realini, N.; Solorzano, C.; Pagliuca, C.; Pizzirani, D.; Armirotti, A.; Luciani, R.; Costi, M.P.; Bandiera, T.; Piomelli, D. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Sci. Rep. 2013, 3, 1035. [Google Scholar] [CrossRef] [PubMed]
- Dementiev, A.; Joachimiak, A.; Nguyen, H.; Gorelik, A.; Illes, K.; Shabani, S.; Gelsomino, M.; Ahn, E.-Y.E.; Nagar, B.; Doan, N.J. Molecular Mechanism of Inhibition of Acid Ceramidase by Carmofur. Med. Chem. 2019, 62, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Pizzirani, D.; Pagliuca, C.; Realini, N.; Branduardi, D.; Bottegoni, G.; Mor, M.; Bertozzi, F.; Scarpelli, R.; Piomelli, D.; Bandiera, T.J. Discovery of a New Class of Highly Potent Inhibitors of Acid Ceramidase: Synthesis and Structure–Activity Relationship (SAR). Med. Chem. 2013, 56, 3518–3530. [Google Scholar] [CrossRef]
- Pizzirani, D.; Bach, A.; Realini, N.; Armirotti, A.; Mengatto, L.; Bauer, I.; Girotto, S.; Pagliuca, C.; De Vivo, M.; Summa, M.; et al. Benzoxazolone Carboxamides: Potent and Systemically Active Inhibitors of Intracellular Acid Ceramidase. Angew. Chem. Int. 2015, 54, 485–489. [Google Scholar] [CrossRef]
- Bach, A.; Pizzirani, D.; Realini, N.; Vozella, V.; Russo, D.; Penna, I.; Melzig, L.; Scarpelli, R.; Piomelli, D.J. Benzoxazolone Carboxamides as Potent Acid Ceramidase Inhibitors: Synthesis and Structure–Activity Relationship (SAR) Studies. Med. Chem. 2015, 58, 9258–9272. [Google Scholar] [CrossRef]
- Di Martino, S.; Tardia, P.; Cilibrasi, V.; Caputo, S.; Mazzonna, M.; Russo, D.; Penna, I.; Realini, N.; Margaroli, N.; Migliore, M.; et al. Lead Optimization of Benzoxazolone Carboxamides as Orally Bioavailable and CNS Penetrant Acid Ceramidase Inhibitors. J. Med. Chem. 2020, 63, 3634–3664. [Google Scholar] [CrossRef]
- Caputo, S.; Di Martino, S.; Cilibrasi, V.; Tardia, P.; Mazzonna, M.; Russo, D.; Penna, I.; Summa, M.; Bertozzi, S.M.; Realini, N.; et al. Design, Synthesis, and Biological Evaluation of a Series of Oxazolone Carboxamides as a Novel Class of Acid Ceramidase Inhibitors. J. Med. Chem. 2020, 63, 15821–15851. [Google Scholar] [CrossRef]
- Diamanti, E.; Bottegoni, G.; Goldoni, L.; Realini, N.; Pagliuca, C.; Bertozzi, F.; Piomelli, D.; Pizzirani, D. Pyrazole-Based Acid Ceramidase Inhibitors: Design, Synthesis, and Structure–Activity Relationships. Synthesis 2016, 48, 2739–2756. [Google Scholar]
- Ortega, J.A.; Arencibia, J.M.; La Sala, G.; Borgogno, M.; Bauer, I.; Bono, L.; Braccia, C.; Armirotti, A.; Girotto, S.; Ganesan, A.; et al. Pharmacophore Identification and Scaffold Exploration to Discover Novel, Potent, and Chemically Stable Inhibitors of Acid Ceramidase in Melanoma Cells. Med. Chem. 2017, 60, 5800–5815. [Google Scholar] [CrossRef]
- Qi, T.; Fang, N.; Huang, W.; Chen, J.; Luo, Y.; Xia, Y. Iron(II)-Catalyzed Nitrene Transfer Reaction of Sulfoxides with N-Acyloxyamides. Org. Lett. 2022, 24, 5674–5678. [Google Scholar] [CrossRef] [PubMed]
- Floresta, G.; Patamia, V.; Mazzeo, P.P.; Lombardo, G.M.; Pistarà, V.; Bacchi, A.; Rescifina, A.; Punzo, F.J. Structural, morphological, and modeling studies of N-(benzoyloxy)benzamide as a specific inhibitor of Type II inosine monophosphate dehydrogenase. Mol. Struct. 2024, 1303, 137588. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubitt, J.; Davies, M.; Riseley, R.; Evans, G.; Gardiner, S.E.; Kariuki, B.M.; Ward, S.E.; Lloyd-Evans, E.; Waller-Evans, H.; Jones, D.H. Beware of N-Benzoyloxybenzamides. Molecules 2024, 29, 5143. https://doi.org/10.3390/molecules29215143
Cubitt J, Davies M, Riseley R, Evans G, Gardiner SE, Kariuki BM, Ward SE, Lloyd-Evans E, Waller-Evans H, Jones DH. Beware of N-Benzoyloxybenzamides. Molecules. 2024; 29(21):5143. https://doi.org/10.3390/molecules29215143
Chicago/Turabian StyleCubitt, Jonathan, Mari Davies, Ross Riseley, Gabrielle Evans, Sian E. Gardiner, Benson M. Kariuki, Simon E. Ward, Emyr Lloyd-Evans, Helen Waller-Evans, and D. Heulyn Jones. 2024. "Beware of N-Benzoyloxybenzamides" Molecules 29, no. 21: 5143. https://doi.org/10.3390/molecules29215143
APA StyleCubitt, J., Davies, M., Riseley, R., Evans, G., Gardiner, S. E., Kariuki, B. M., Ward, S. E., Lloyd-Evans, E., Waller-Evans, H., & Jones, D. H. (2024). Beware of N-Benzoyloxybenzamides. Molecules, 29(21), 5143. https://doi.org/10.3390/molecules29215143





