Two Co(II) Isostructural Bifunctional MOFs via Mixed-Ligand Strategy: Syntheses, Crystal Structure, Photocatalytic Degradation of Dyes, and Electrocatalytic Water Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analyses
2.2. Comment on IR, XRD and TGA
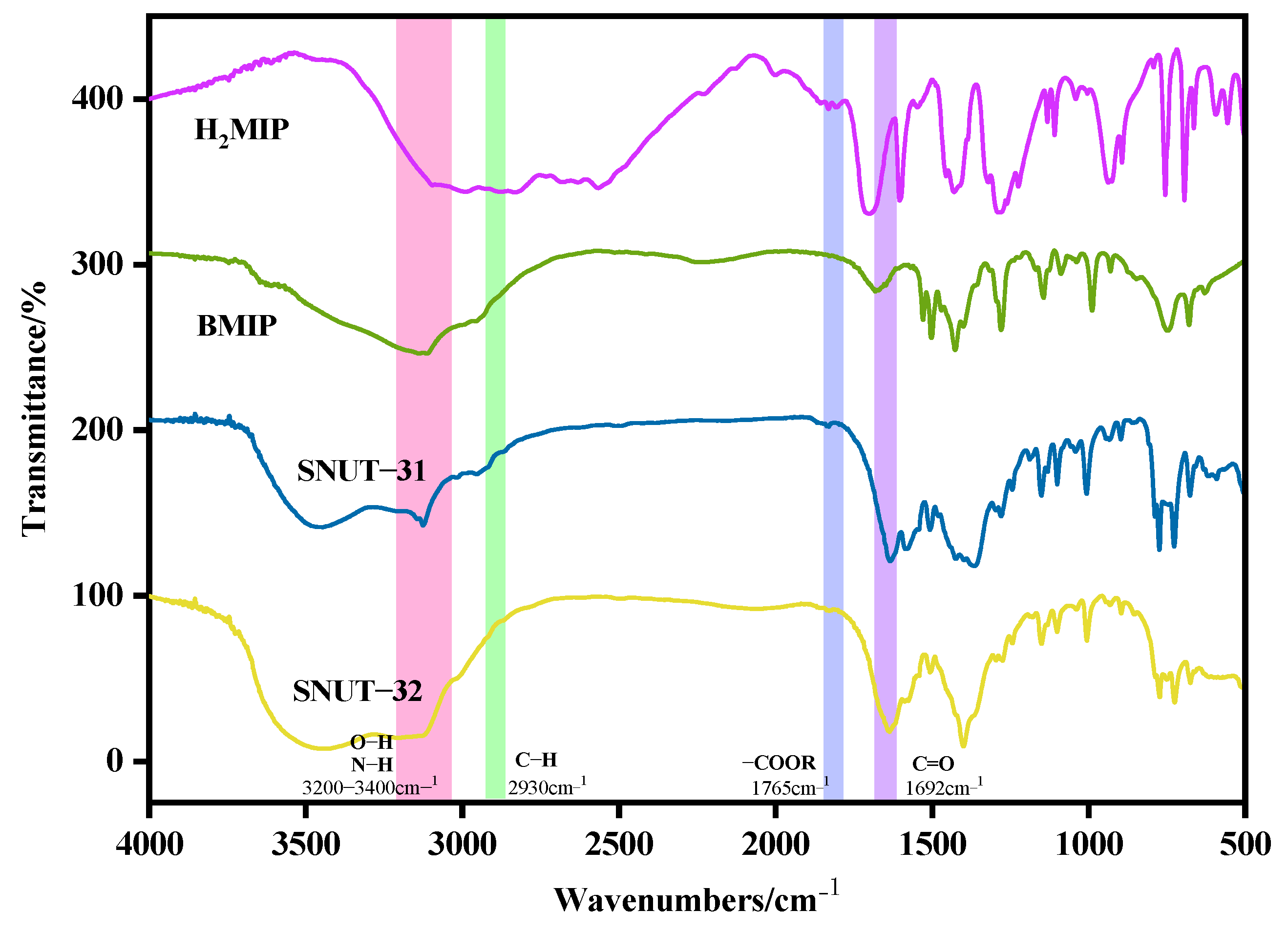
2.2.1. FT-IR Spectra
2.2.2. Powder X-Ray Diffraction
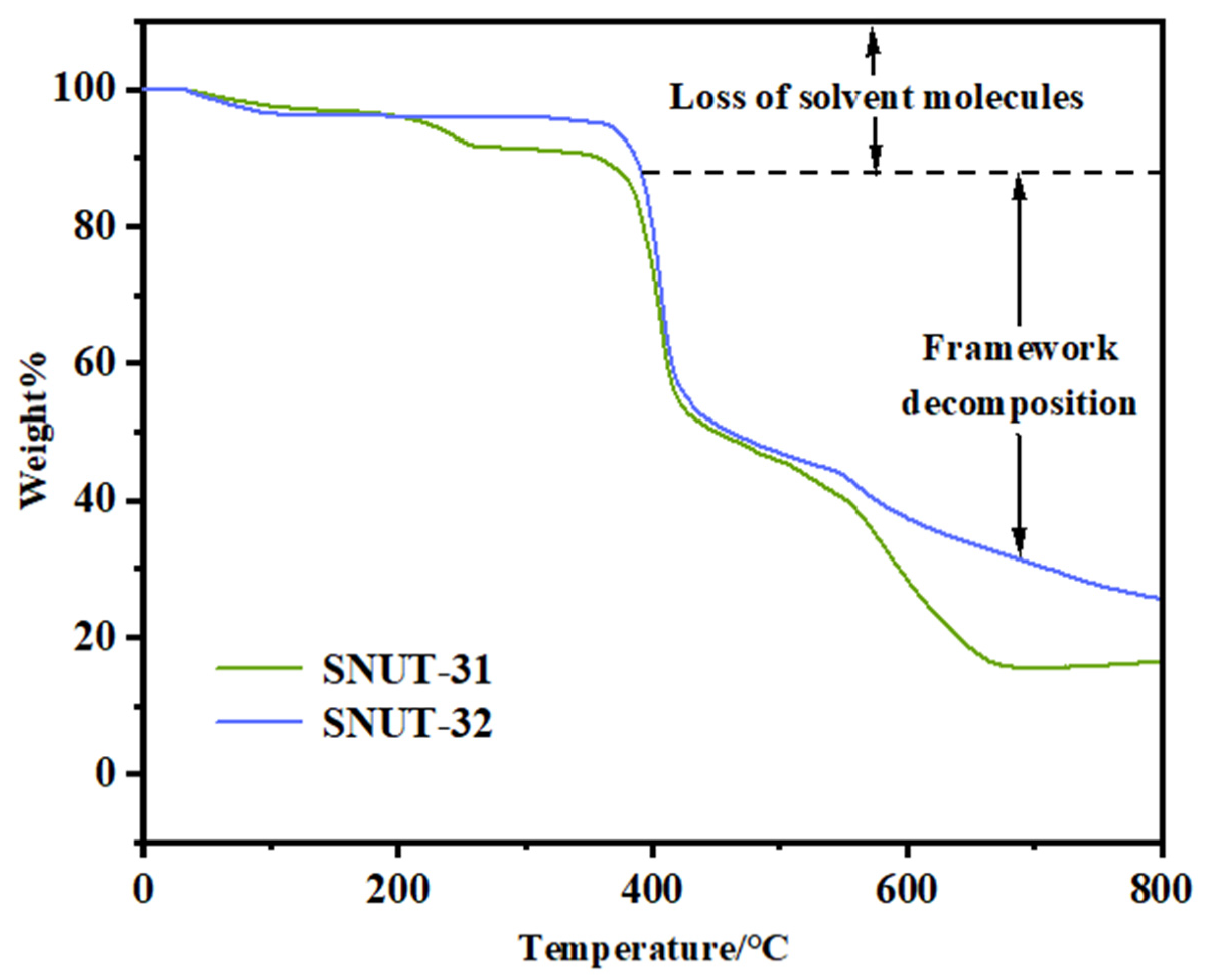
2.2.3. Thermal Analysis
2.3. Gas Adsorption
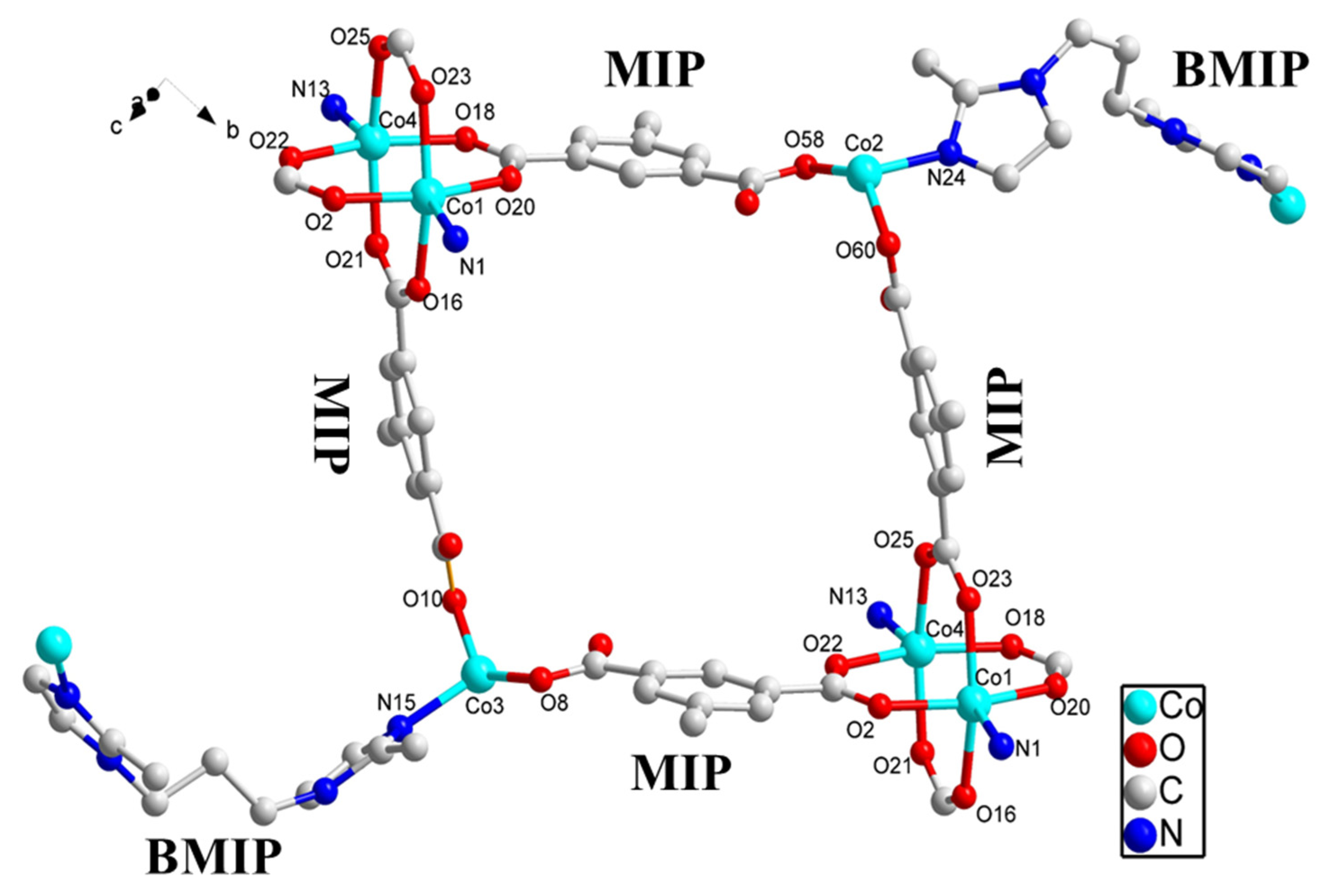
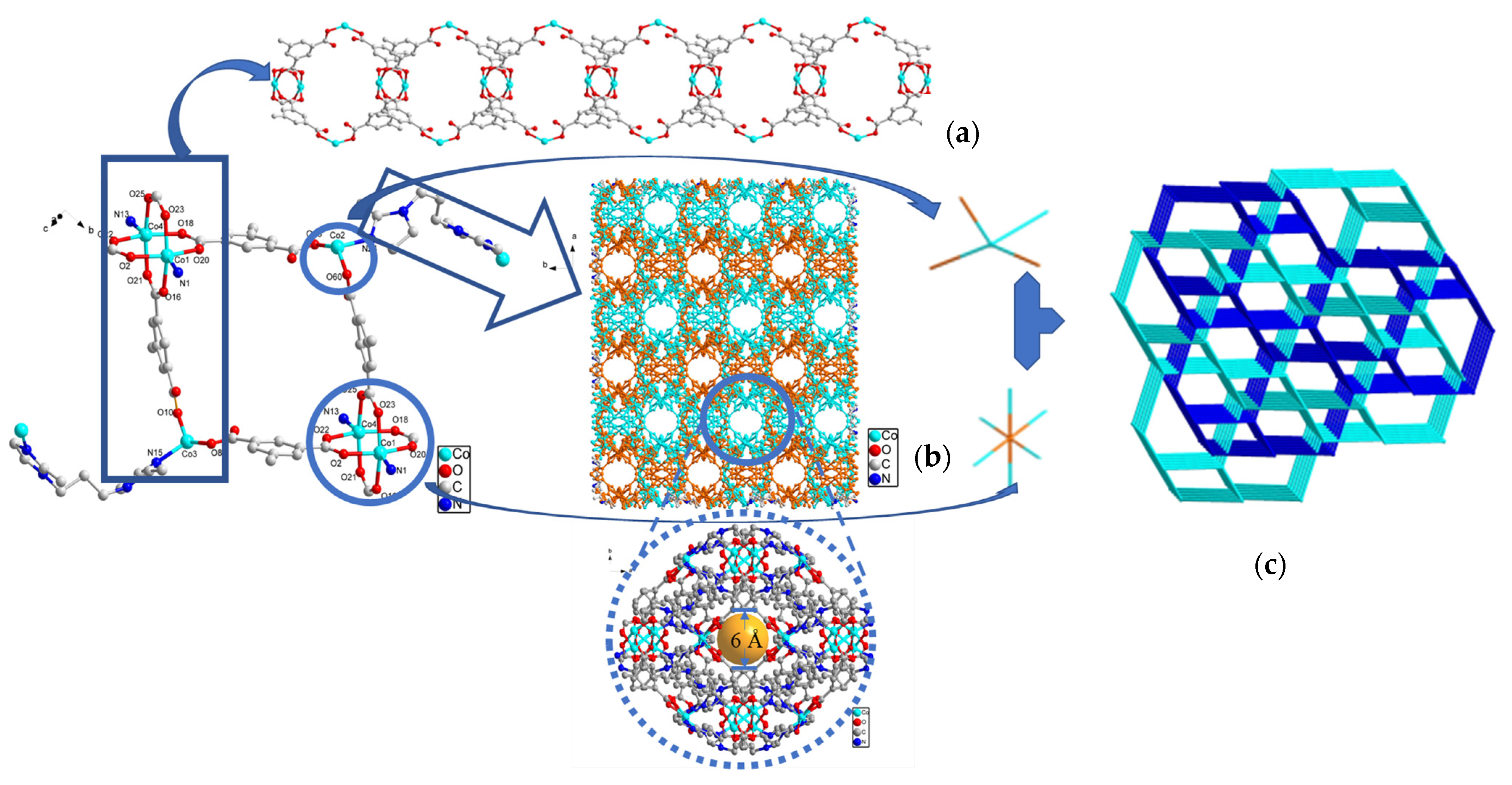
2.4. Description of Crystal Structures
Crystal Structure of {[Co4(MIP)4(BMIP)3]·1/2DMA}n (SNUT-31) and {[Co4(MIP)4(BMIP)3]·(EtOH)2·H2O]}n (SNUT-32)
2.5. Electrochemical Performance
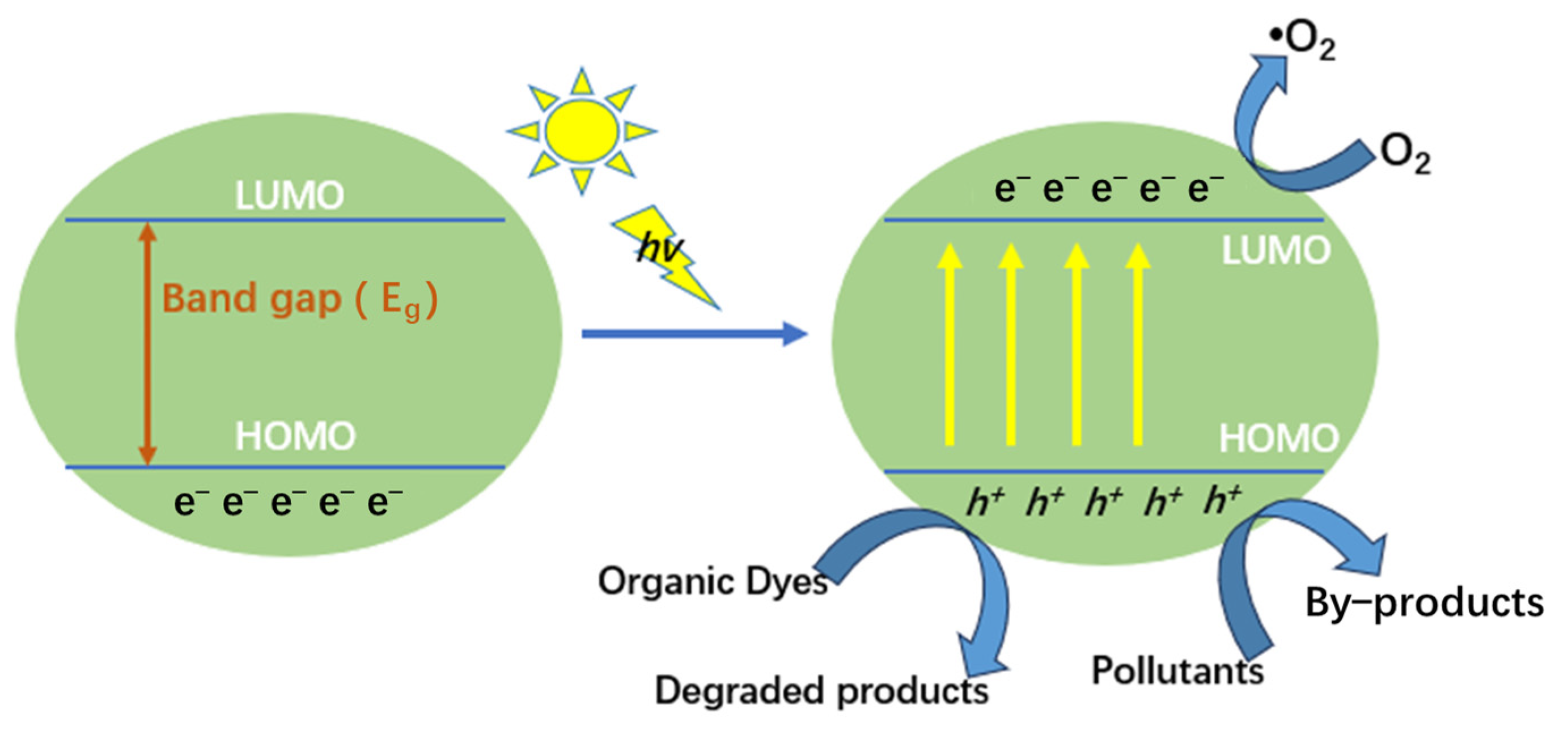
2.6. Photocatalytic Degradation
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of {[Co4(MIP)4(BMIP)3]·1/2DMA}n (SNUT-31)
3.3. Synthesis of {[Co4(MIP)4(BMIP)3]·(EtOH)2·H2O]}n (SNUT-32)
3.4. Electrocatalytic Properties
3.5. Solid-State Uv-Vis Absorption
3.6. Determination of Crystal Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, D.; Deng, T.; He, G.; Chen, A.; Sun, X.; Yang, Y.; Miao, P. Research Progress of Oxygen Evolution Reaction Catalysts for Electrochemical Water Splitting. ChemSusChem 2021, 14, 5359–5383. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Show, K.Y.; Yan, Y.; Ling, M.; Ye, G.; Li, T.; Lee, D.J. Hydrogen production from algal biomass–advances, challenges and prospects. Bioresour. Technol. 2018, 257, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Wei, L.; Shi, J.; Jiang, Q.; Tang, J. A review of enhanced electrocatalytic composites hydrogen/oxygen evolution based on quantum dot. J. Ind. Eng. Chem. 2023, 121, 27–39. [Google Scholar] [CrossRef]
- Liang, X.Q.; Wang, S.; Feng, J.Y.; Xu, Z.; Guo, Z.Y.; Luo, H. Structural transformation of metal–organic frameworks and identification of electrocatalytically active species during the oxygen evolution reaction under neutral conditions. Inorg. Chem. Front. 2023, 10, 2961–2977. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and-hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Xu, Z.; Wang, X. Recent Development of Molybdenum Sulfides as Advanced Electrocatalysts for Hydrogen Evolution Reaction. ACS Catal. 2015, 4, 1693–1705. [Google Scholar] [CrossRef]
- Chen, W.F.; Muckerman, J.T.; Fujita, E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Xiao, P.; Chen, W.; Wang, X. A Review of Phosphide-Based Materials for Electrocatalytic Hydrogen Evolution. Adv. Energy Mater. 2015, 5, 1500985. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro and Nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X.; Li, C.M. Template-assisted synthesis of CoP nanotubes to efficiently catalyze hydrogen-evolving reaction. J. Mater. Chem. A 2014, 2, 14812–14816. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Arellano, M.J.; Ye, G.; Kim, N.D.; Samuel, E.L.G.; Peng, Z.; Zhu, Z.; Qin, F.; Bao, J.; et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668–8670. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.R.; Hong, Q.L.; Li, Q.H.; Du, Y.; Zhang, H.X.; Chen, S.; Zhou, T.; Zhang, J. Cobalt Boron Imidazolate Framework Derived Cobalt Nanoparticles Encapsulated in B/N Codoped Nanocarbon as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2018, 180, 1136. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Dong, J.; Qiu, M.; Kong, J.; Zhang, Y.; Li, Y.; Xu, G.; Zhang, J.; Ye, J. Surface step decoration of isolated atom as electron pumping: Atomic-level insights into visible-light hydrogen evolution. Nano Energy 2018, 45, 109–117. [Google Scholar] [CrossRef]
- Liao, W.M.; Zhang, J.H.; Wang, Z.; Yin, S.Y.; Pan, M.; Wang, H.P.; Su, C.Y. Post-synthetic exchange (PSE) of UiO-67 frameworks with Ru/Rh half-sandwich units for visible-light-driven H2 evolution and CO2 reduction. J. Mater. Chem. A 2018, 6, 11337–11345. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; He, X.; Xu, Z.; Wang, Y.; Jia, L. Synthesis of AgBr@MOFs Nanocomposite and Its Photocatalytic Activity for Dye Degradation. Polyhedron 2019, 165, 31–37. [Google Scholar] [CrossRef]
- Gholizadeh Khasevani, S.; Gholami, M.R. Evaluation of the Reaction Mechanism for Photocatalytic Degradation of Organic Pollutants with MIL-88A/BiOI Structure under Visible Light Irradiation. Res. Chem. Intermed. 2019, 45, 1341–1356. [Google Scholar] [CrossRef]
- Zhang, R.; Du, B.; Li, Q.; Cao, Z.; Feng, G.; Wang, X. a-Fe2O3 Nanoclusters Confined into UiO-66 for Efficient VisibleLight Photodegradation Performance. Appl. Surf. Sci. 2019, 466, 956–963. [Google Scholar] [CrossRef]
- Shi, C.C.; Zhao, Z.X.; Zhao, L. Porphyrin-based Fe/La metal-organic frameworks as photocatalysts for dye photodegradation: Syntheses and mechanism investigation. Inorg. Chem. Commun. 2023, 154, 110920. [Google Scholar] [CrossRef]
- Qi, H.L.; Liu, X.; Wu, Y.Q. A robust Co(II) organic framework for photocatalytic dye degradation and treatment efect on the neonatal sepsis. J. Iran. Chem. Soc. 2021, 18, 1703–1712. [Google Scholar] [CrossRef]
- Guo, A.; Wang, X.; Liu, H. Efficient photocatalytic degradation of water contaminants via Ag decorated porphyrin-based organic framework materials. Surf. Interfaces 2023, 38, 102843. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Tran, K.T.; Tan, L.V. Microwave-assisted solvothermal synthesis of bimetallic metal-organic framework for efficient photodegradation of organic dyes. Mater. Chem. Phys. 2021, 272, 125040. [Google Scholar] [CrossRef]
- Li, B.; Dong, J.P.; Zhou, Z.; Wang, R.; Wang, L.Y.; Zang, S.Q. Robust lanthanide metal–organic frameworks with “all-in-one” multifunction: Efficient gas adsorption and separation, tunable light emission and luminescence sensing. J. Mater. Chem. 2021, C9, 3429–3439. [Google Scholar] [CrossRef]
- Abazari, R.; Yazdani, E.; Nadafan, M.; Kirillov, A.M.; Gao, J.; Slawin, A.M.Z.; Carpenter-Warren, C.L. Third-order nonlinear optical behavior of an amide-tricarboxylate zinc(II) metal-organic framework with two-fold 3D+3D interpenetration. Inorg. Chem. 2021, 60, 9700–9708. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhao, Y.; Zhang, X.D.; Kang, Y.S.; Lu, Q.Y.; Azam, M.; Saud, A.; Sun, W.Y. Metal-organic frameworks with 1,4-di(1H-imidazole-4-yl)benzene and varied carboxylate ligands for selectively sensing Fe(III) ions and ketone molecules. Dalton Trans. 2017, 46, 13943–13951. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Chen, S.; Kirchon, A.; Li, B.; Li, Y.; Zhou, H.C. Metal-organic framework containing planar metal-binding sites: Efficiently and cost-effectively enhancing the kinetic separation of C2H2/C2H4. J. Am. Chem. Soc. 2019, 141, 3807–3811. [Google Scholar] [CrossRef]
- Cui, H.; Xie, Y.; Ye, Y.; Shi, Y.; Liang, B.; Chen, B. An ultra microporous metal-organic framework with record high selectivity for inverse CO2/C2H2 separation. Bull. Chem. Soc. Jpn. 2021, 94, 2698–2701. [Google Scholar] [CrossRef]
- Ye, Y.; Xian, S.; Cui, H.; Tan, K.; Gong, L.; Liang, B.; Pham, T.; Pandey, H.; Krishna, R.; Lan, P.; et al. Metal-organic framework based hydrogen-bonding nano trap for efficient acetylene storage and separation. J. Am. Chem. Soc. 2022, 144, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Wang, T.T.; Tuo, M.Q.; Pan, H.B.; Ge, J.; Huang, P.P.; Gao, J.H.; Jiang, M. Solvothermal synthesis of four coordination polymers based on a dual-ligand strategy for sensitive detection of Hg2+ ions and photocatalytic degradation. Polyhedron 2024, 252, 116884–116895. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.L.; Feng, D.; Xie, L.H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.; Zhou, H.C. Highly stable Zr(IV)-Based metal-organic frameworks for the detection and removal of antibiotics and organic explosives in water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Ye, Y.; Liu, X.; Cui, H.; Li, Z.; Zhang, Y.; Liang, B.; Li, H.; Chen, B. A robust mixed-lanthanide Poly MOF membrane for ratiometric temperature sensing. Angew. Chem. Int. Ed. Engl. 2020, 59, 21752–21757. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Q.; Liu, C.Y.; Zeng, X.Y.; Chen, J.; Lü, J.; Lin, R.G.; Cao, R.; Lin, Z.J.; Su, J.W. MOF-808: A metal-organic framework with intrinsic peroxidase-like catalytic activity at neutral pH for colorimetric biosensing. Inorg. Chem. 2018, 57, 9096–9104. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Weichselbaum, R.R.; Lin, W.B. Chlorin-based nanoscale metal-organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J. Am. Chem. Soc. 2016, 138, 12502–12510. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Gao, J.; Yao, J.; Zhang, Q. Recent progress in metal organic frameworks based hydrogels and aerogels and their applications. Coord. Chem. Rev. 2019, 398, 213016. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Yu, S.; Wu, Y.; Xue, Q.; Zhu, J.J.; Zhou, Y. A novel multi-walled carbon nanotube-coupled CoNi MOF composite enhances the oxygen evolution reaction through synergistic effects. J. Mater. Chem. A 2022, 10, 4936–4943. [Google Scholar] [CrossRef]
- Taherinia, D.; Hatami, H.; Valadi, F.M. Trimetallic Co-Ni-Mn metal-organic framework as an efficient electrocatalyst for alkaline oxygen evolution reaction. J. Electroanal. Chem. 2022, 922, 116720. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xu, Z.; Li, L.; Lin, S. Formation of NiFe-MOF nanosheets on Fe foam to achieve advanced electrocatalytic oxygen evolution. Dalton Trans. 2022, 51, 5053–5060. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jing, Y.; Du, C.F.; Wang, J. Tuning the reversible chemisorption of hydroxyl ions to promote the electrocatalysis on ultrathin metal-organic framework nanosheets. J. Energy Chem. 2022, 65, 71–77. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, K.; Cheng, Z.; Chen, H.; Tan, G.; Feng, X.; Wang, L.; Mu, S. Altered electronic structure of trimetallic FeNiCo-MOF nanosheets for efficient oxygen evolution. Chem. Commun. 2023, 59, 4750–4753. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Hwang, S.K.; Shinde, P.A.; Raju, G.S.R.; Ranjith, K.S.; Huh, Y.S.; Han, Y.K. Co-metal–organic framework derived CoSe2@MoSe2 core–shell structure on carbon cloth as an efficient bifunctional catalyst for overall water splitting. Chem. Eng. J. 2022, 429, 132379. [Google Scholar] [CrossRef]




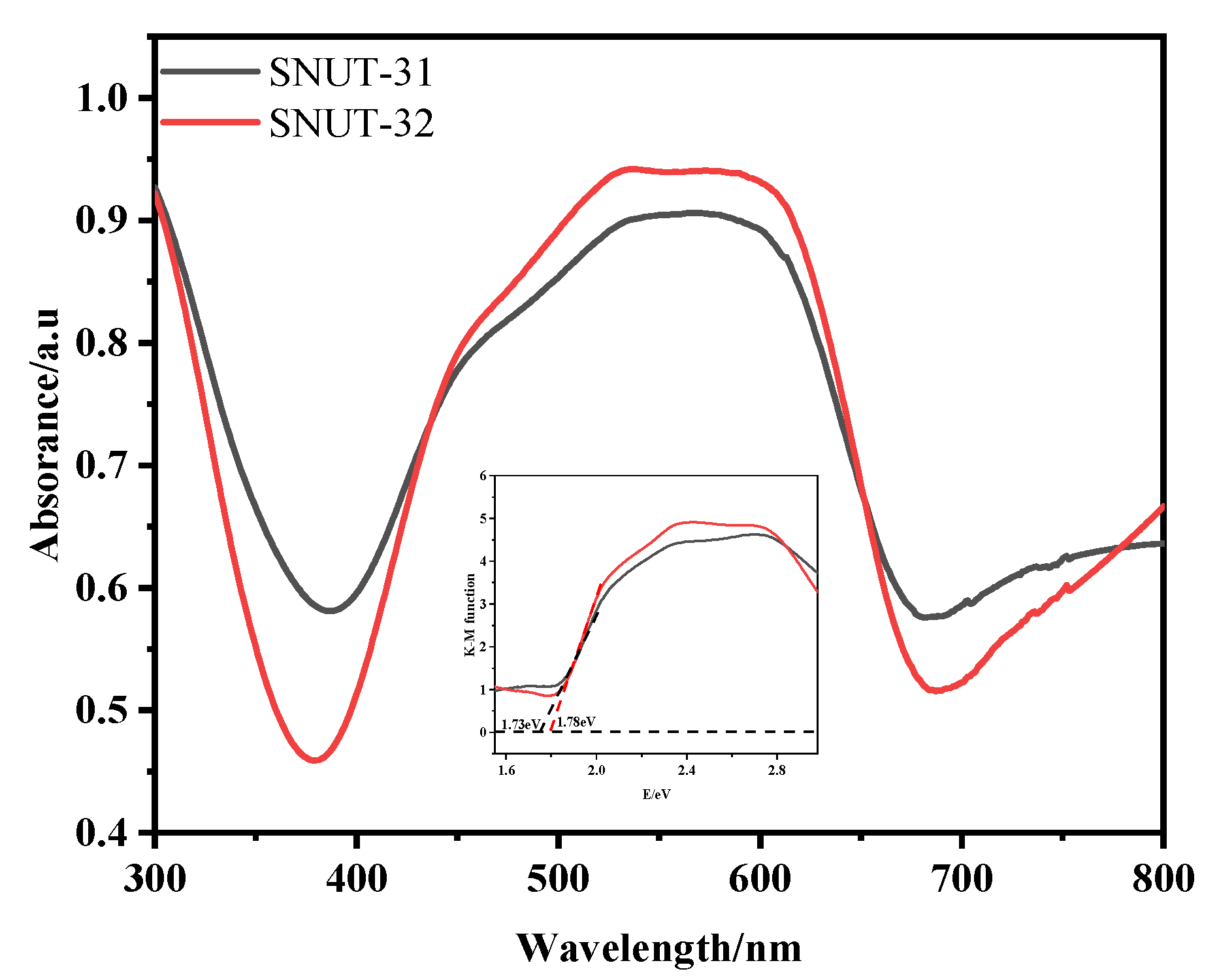
| MOF | Current Density (mA cm−2) | Overpoten Tial (mV) | Tafel Slope (mV dec–1) | Ref. |
|---|---|---|---|---|
| Fe-MOF/FF | 100 | 371 | 114.2 | [43] |
| Ni-MOF/FF | 100 | 383 | 160.7 | [43] |
| NiFe-MOF/FF | 100 | 240 | 73.4 | [43] |
| Ag@Co-MOF | 10 | 344 | 107 | [44] |
| CoNi MOF-mCNTs | 10 | 306 | 42 | [39] |
| FeNiCo-MOF | 10 | 239 | 42.4 | [45] |
| CoNiMn-MOF | 20 | 220 | 66 | [40] |
| CC/MOF-CoSe2@MoSe2 | 20 | 211.4 | 96.61 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, S.; Tuo, M.; Sheng, Y.; Guo, X.; Lu, J.; Wang, D. Two Co(II) Isostructural Bifunctional MOFs via Mixed-Ligand Strategy: Syntheses, Crystal Structure, Photocatalytic Degradation of Dyes, and Electrocatalytic Water Oxidation. Molecules 2024, 29, 4989. https://doi.org/10.3390/molecules29214989
Yue S, Tuo M, Sheng Y, Guo X, Lu J, Wang D. Two Co(II) Isostructural Bifunctional MOFs via Mixed-Ligand Strategy: Syntheses, Crystal Structure, Photocatalytic Degradation of Dyes, and Electrocatalytic Water Oxidation. Molecules. 2024; 29(21):4989. https://doi.org/10.3390/molecules29214989
Chicago/Turabian StyleYue, Siyu, Mengqi Tuo, Yemeng Sheng, Xinyu Guo, Jiufu Lu, and Dong Wang. 2024. "Two Co(II) Isostructural Bifunctional MOFs via Mixed-Ligand Strategy: Syntheses, Crystal Structure, Photocatalytic Degradation of Dyes, and Electrocatalytic Water Oxidation" Molecules 29, no. 21: 4989. https://doi.org/10.3390/molecules29214989
APA StyleYue, S., Tuo, M., Sheng, Y., Guo, X., Lu, J., & Wang, D. (2024). Two Co(II) Isostructural Bifunctional MOFs via Mixed-Ligand Strategy: Syntheses, Crystal Structure, Photocatalytic Degradation of Dyes, and Electrocatalytic Water Oxidation. Molecules, 29(21), 4989. https://doi.org/10.3390/molecules29214989






