Applications of Ferric Oxide in Water Splitting by Electrolysis: A Comprehensive Review
Abstract
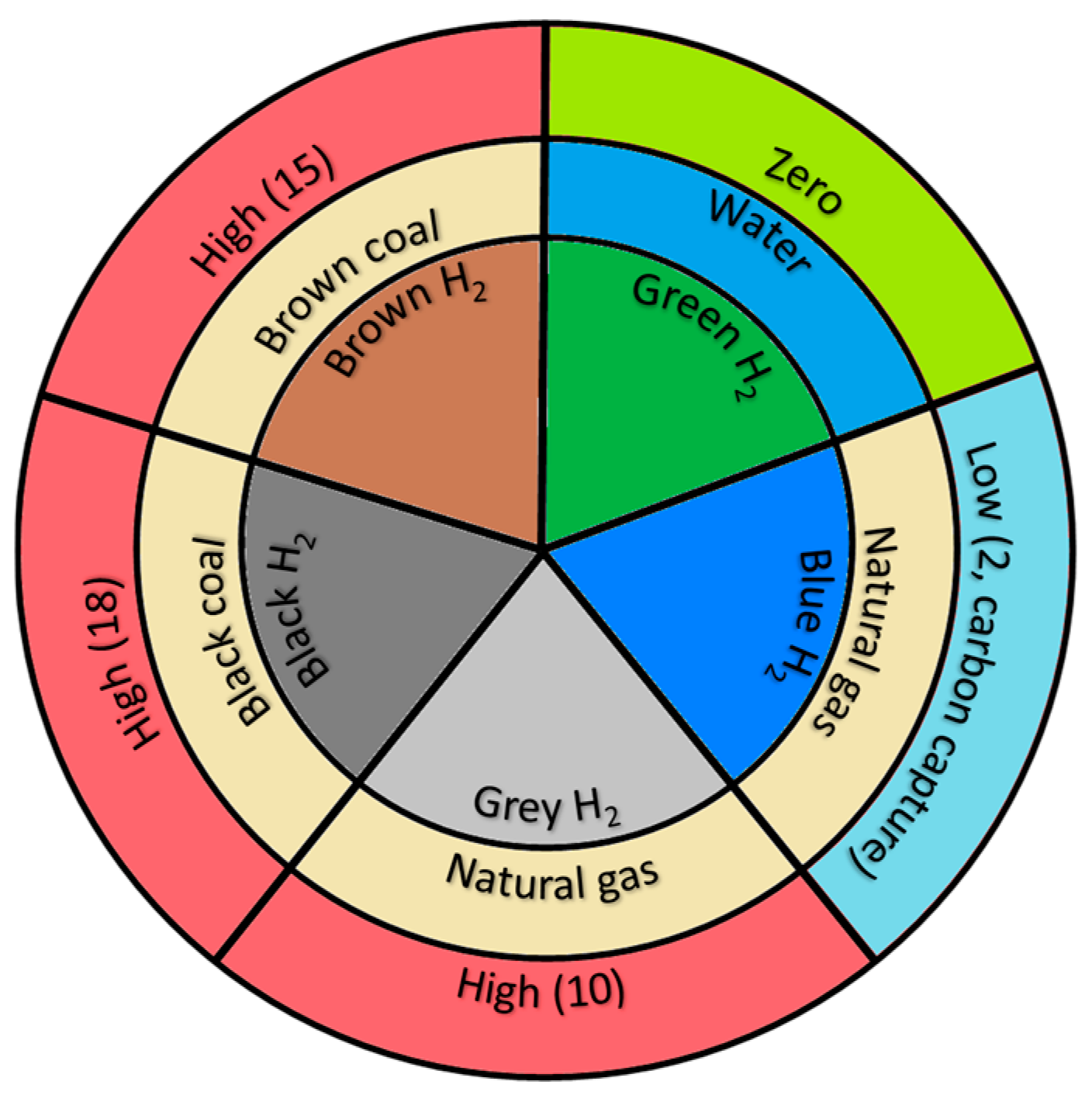
1. Introduction
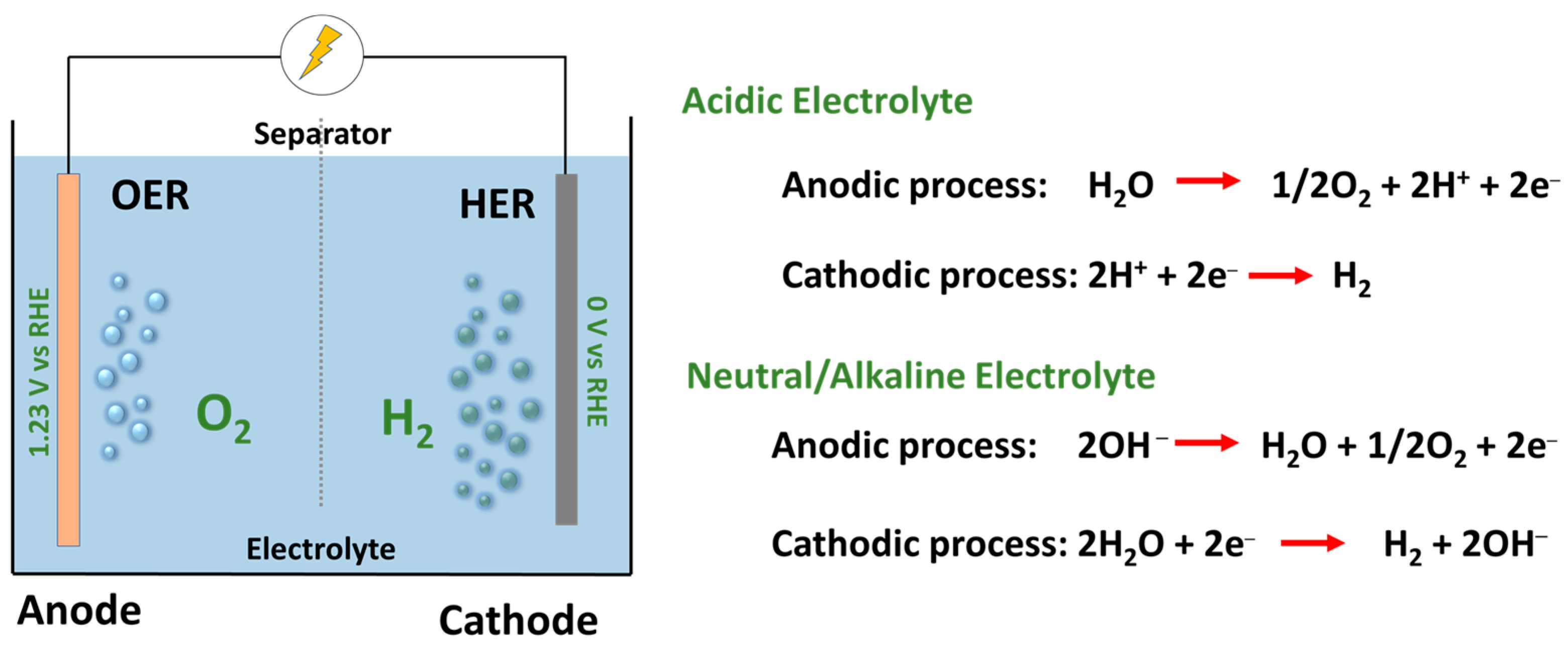
2. Overview of Electrochemical Water Splitting
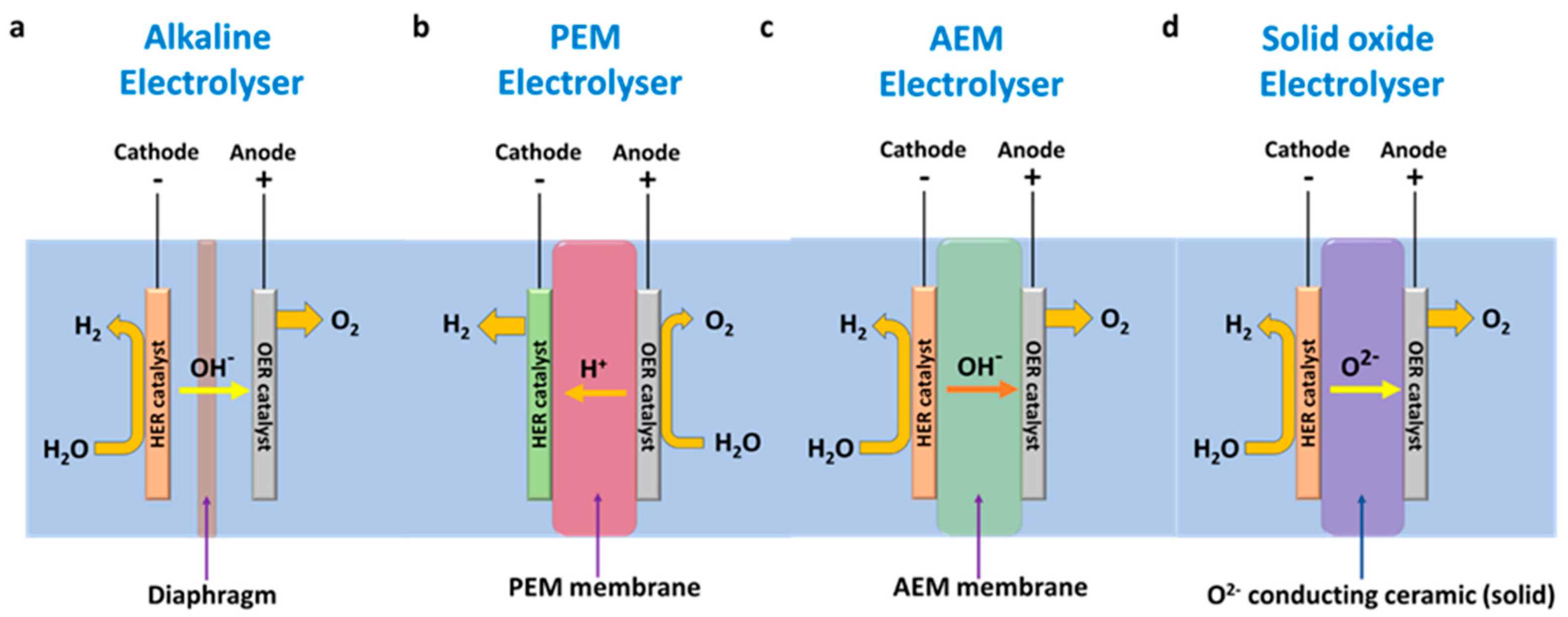
3. Electrolysis Techniques
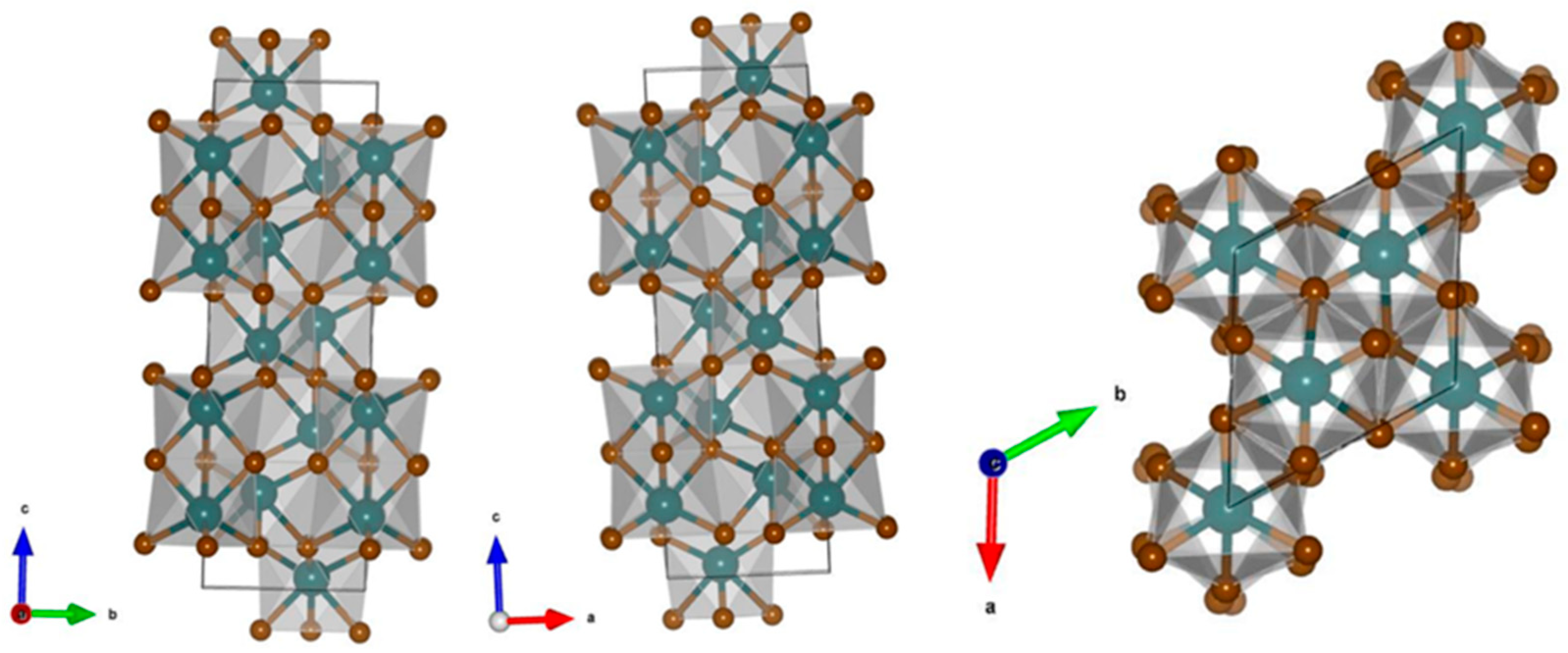
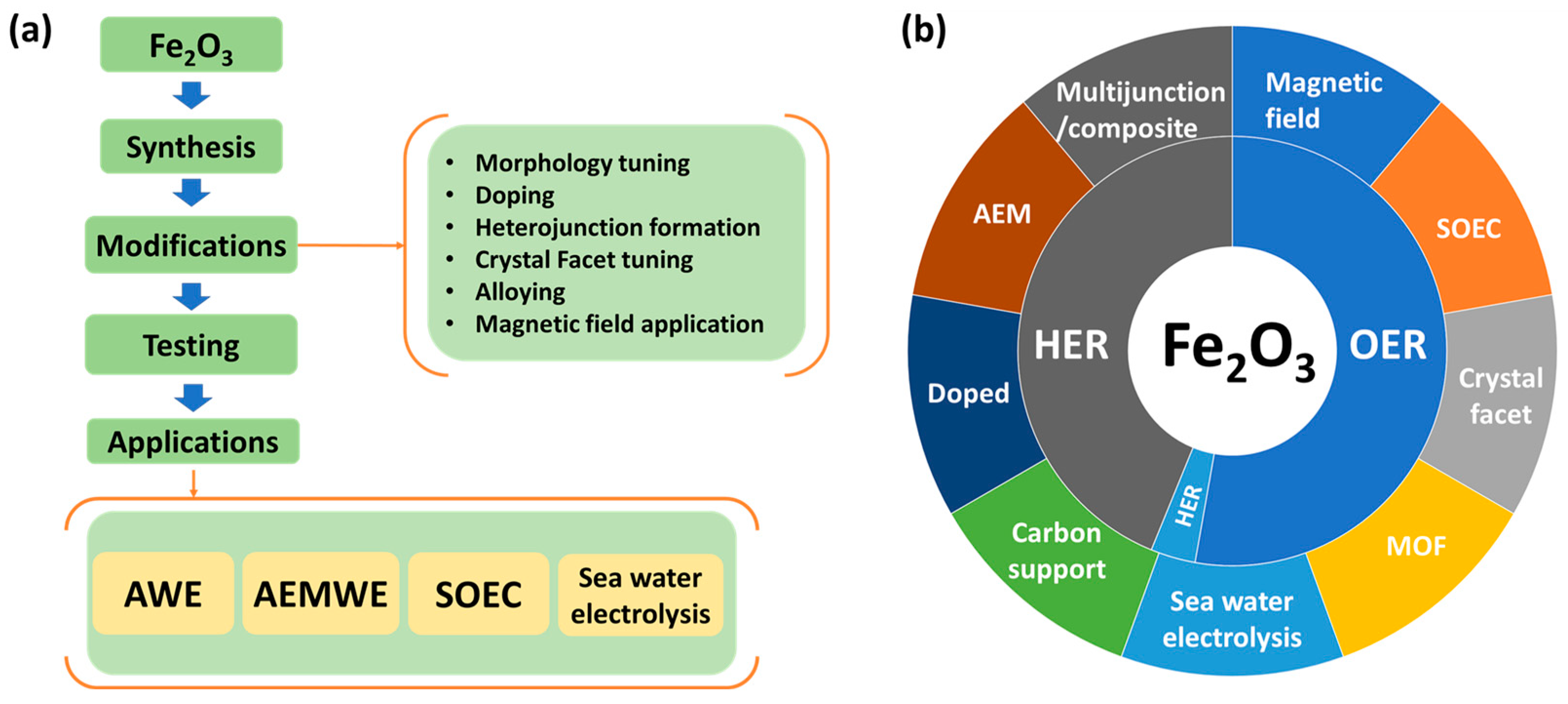
4. Fe2O3
4.1. Water Splitting Applications of Fe2O3
4.2. DFT Studies on the OER Activity of Fe2O3
4.3. Crystal Facet and Morphology Tuning in Fe2O3
4.4. Doped Fe2O3 Catalysts
4.5. Fe2O3 in Heterojunction Systems
4.6. Fe2O3 Supported on Carbon Materials
4.7. Fe2O3 Linked to MOFs
4.8. Sea/Domestic Wastewater Electrolysis
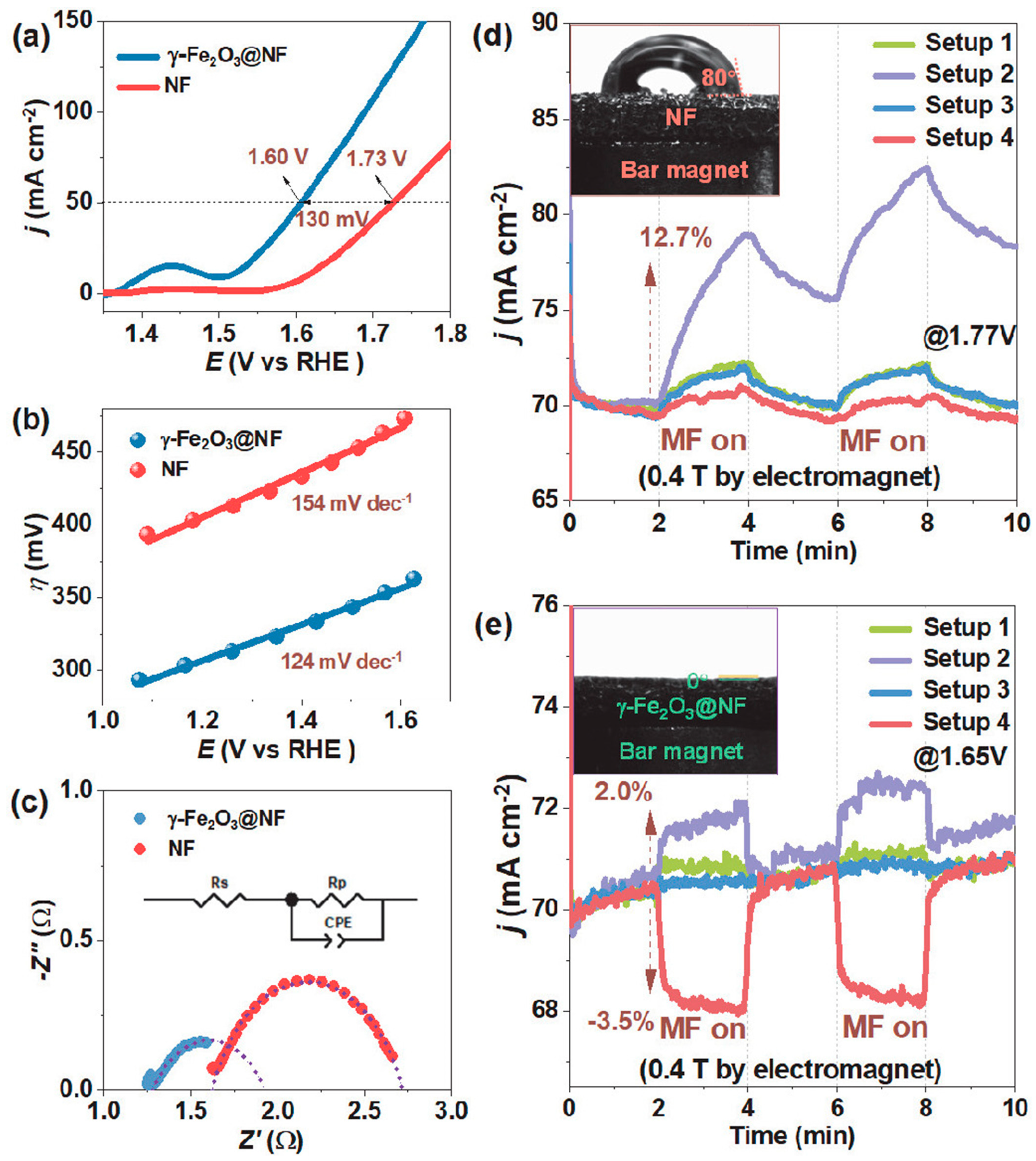
4.9. Influence of Magnetic Fields on Catalysts
4.10. Fe2O3 in AEMWE Systems
4.11. Fe2O3 Supported Ceramics in SOEC
5. Potential Application in Commercial Electrolyzers
6. Discussion
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asafu-Adjaye, J.; Byrne, D.; Alvarez, M. Economic Growth, Fossil Fuel and Non-Fossil Consumption: A Pooled Mean Group Analysis Using Proxies for Capital. Energy Econ. 2016, 60, 345–356. [Google Scholar] [CrossRef]
- Bildirici, M.E.; Bakirtas, T. The Relationship among Oil, Natural Gas and Coal Consumption and Economic Growth in BRICTS (Brazil, Russian, India, China, Turkey and South Africa) Countries. Energy 2014, 65, 134–144. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.-R. Recovery of Greenhouse Gas as Cleaner Fossil Fuel Contributes to Carbon Neutrality. Green Energy Environ. 2023, 8, 351–353. [Google Scholar] [CrossRef]
- Bölük, G.; Mert, M. Fossil & Renewable Energy Consumption, GHGs (Greenhouse Gases) and Economic Growth: Evidence from a Panel of EU (European Union) Countries. Energy 2014, 74, 439–446. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T.N. The Properties of Hydrogen as Fuel Tomorrow in Sustainable Energy System for a Cleaner Planet. Int. J. Hydrog. Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef]
- Oshiro, K.; Fujimori, S. Role of Hydrogen-Based Energy Carriers as an Alternative Option to Reduce Residual Emissions Associated with Mid-Century Decarbonization Goals. Appl. Energy 2022, 313, 118803. [Google Scholar] [CrossRef]
- Haram, M.H.S.M.; Lee, J.W.; Ramasamy, G.; Ngu, E.E.; Thiagarajah, S.P.; Lee, Y.H. Feasibility of Utilising Second Life EV Batteries: Applications, Lifespan, Economics, Environmental Impact, Assessment, and Challenges. Alex. Eng. J. 2021, 60, 4517–4536. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, C.; Zhang, X.; Guo, B. Implementation and Evaluation of a Practical Electrochemical- Thermal Model of Lithium-Ion Batteries for EV Battery Management System. Energy 2021, 221, 119688. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, H.; Lin, F. Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy. Adv. Energy Mater. 2022, 12, 2200383. [Google Scholar] [CrossRef]
- Robiou du Pont, Y.; Jeffery, M.L.; Gütschow, J.; Rogelj, J.; Christoff, P.; Meinshausen, M. Equitable Mitigation to Achieve the Paris Agreement Goals. Nat. Clim Chang. 2017, 7, 38–43. [Google Scholar] [CrossRef]
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of Hydrogen Production Costs in Steam-Methane Reforming Considering Integration with Electrolysis and CO2 Capture. Clean. Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Trasatti, S. Water Electrolysis: Who First? J. Electroanal. Chem. 1999, 476, 90–91. [Google Scholar] [CrossRef]
- Swierk, J.R.; Klaus, S.; Trotochaud, L.; Bell, A.T.; Tilley, T.D. Electrochemical Study of the Energetics of the Oxygen Evolution Reaction at Nickel Iron (Oxy)Hydroxide Catalysts. J. Phys. Chem. C 2015, 119, 19022–19029. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y. A Review of the α-Fe2O3 (Hematite) Nanotube Structure: Recent Advances in Synthesis, Characterization, and Applications. Nanoscale 2020, 12, 10912–10932. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Jang, J.-S.; Yun, T.G.; Yoon, K.R.; Kim, I.-D. Three-Dimensional Nanofibrous Air Electrode Assembled with Carbon Nanotubes-Bridged Hollow Fe2O3 Nanoparticles for High-Performance Lithium–Oxygen Batteries. ACS Appl. Mater. Interfaces 2018, 10, 6531–6540. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Review on α-Fe2O3 Based Negative Electrode for High Performance Supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Li, K.; Liu, B.; Gao, L.; Duan, G. Porous α-Fe2O3 Gas Sensor with Instantaneous Attenuated Response toward Triethylamine and Its Reaction Kinetics. Chem. Eng. J. 2022, 427, 131631. [Google Scholar] [CrossRef]
- Meenakshi, S.; Anitta, S.; Sivakumar, A.; Martin Britto Dhas, S.A.; Sekar, C. Shock Waves Exposed α-Fe2O3 Nanoparticles for Electrochemical Sensing of Riboflavin, Uric Acid and Folic Acid. Microchem. J. 2021, 168, 106403. [Google Scholar] [CrossRef]
- Makimizu, Y.; Nguyen, N.T.; Tucek, J.; Ahn, H.-J.; Yoo, J.; Poornajar, M.; Hwang, I.; Kment, S.; Schmuki, P. Activation of α-Fe2O3 for Photoelectrochemical Water Splitting Strongly Enhanced by Low Temperature Annealing in Low Oxygen Containing Ambient. Chem. A Eur. J. 2020, 26, 2685–2692. [Google Scholar] [CrossRef]
- Fawzi Suleiman Khasawneh, O.; Palaniandy, P. Removal of Organic Pollutants from Water by Fe2O3/TiO2 Based Photocatalytic Degradation: A Review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Dash, P.; Raut, S.; Jena, M.; Nayak, B. Harnessing the Biomedical Properties of Ferromagnetic α-Fe2O3 NPs with a Plausible Formation Mechanism. Ceram. Int. 2020, 46, 26190–26204. [Google Scholar] [CrossRef]
- Damizia, M.; Lloreda-Jurado, P.J.; De Filippis, P.; de Caprariis, B.; Chicardi, E.; Sepúlveda, R. Green Hydrogen Production Using Doped Fe2O3 Foams. Int. J. Hydrog. Energy 2024, 51, 834–845. [Google Scholar] [CrossRef]
- Hu, C.; Li, X.; Dong, C.; Li, B.; Zhang, X.; Sun, W.; Ding, Y. Key Modification Strategies for the Rational Design of Hematite to Promote Photoelectrochemical Water Oxidation: A Review of Recent Advances. Mater. Chem. Front. 2023, 7, 5333–5354. [Google Scholar] [CrossRef]
- Zuo, Y.; Mastronardi, V.; Gamberini, A.; Zappia, M.I.; Le, T.-H.-H.; Prato, M.; Dante, S.; Bellani, S.; Manna, L. Stainless Steel Activation for Efficient Alkaline Oxygen Evolution in Advanced Electrolyzers. Adv. Mater. 2024, 36, 2312071. [Google Scholar] [CrossRef]
- Wang, P.C.; Ding, H.P.; Bark, T.; Chen, C.H. Nanosized α-Fe2O3 and Li–Fe Composite Oxide Electrodes for Lithium-Ion Batteries. Electrochim. Acta 2007, 52, 6650–6655. [Google Scholar] [CrossRef]
- Kusano, Y.; Fukuhara, M.; Takada, J.; Doi, A.; Ikeda, Y.; Takano, M. Science in the Art of the Master Bizen Potter. Acc. Chem. Res. 2010, 43, 906–915. [Google Scholar] [CrossRef]
- Han, Y.S.; Yun, S.M.; Kim, D.G. Synthesis of Monodispersed and Spherical SiO2-Coated Fe2O3 Nanoparticle. Bull. Korean Chem. Soc. 2000, 21, 1193–1198. [Google Scholar] [CrossRef]
- Danno, T.; Nakatsuka, D.; Kusano, Y.; Asaoka, H.; Nakanishi, M.; Fujii, T.; Ikeda, Y.; Takada, J. Crystal Structure of β-Fe2O3 and Topotactic Phase Transformation to α-Fe2O3. Cryst. Growth Des. 2013, 13, 770–774. [Google Scholar] [CrossRef]
- Tuček, J.; Zbořil, R.; Namai, A.; Ohkoshi, S. ε-Fe2O3: An Advanced Nanomaterial Exhibiting Giant Coercive Field, Millimeter-Wave Ferromagnetic Resonance, and Magnetoelectric Coupling. Chem. Mater. 2010, 22, 6483–6505. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Shamsabadipour, A.; Mahtabian, S.; Ahmadi, M.; Rahdar, A.; Díez-Pascual, A.M. Role of Iron Oxide (Fe2O3) Nanocomposites in Advanced Biomedical Applications: A State-of-the-Art Review. Nanomaterials 2022, 12, 3873. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Wu, H.; Yang, T.; Du, Y.; Shen, L.; Ho, G.W. Identification of Facet-Governing Reactivity in Hematite for Oxygen Evolution. Adv. Mater. 2018, 30, 1804341. [Google Scholar] [CrossRef]
- Xiao, M.; Xing, Z.; Jin, Z.; Liu, C.; Ge, J.; Zhu, J.; Wang, Y.; Zhao, X.; Chen, Z. Preferentially Engineering FeN4 Edge Sites onto Graphitic Nanosheets for Highly Active and Durable Oxygen Electrocatalysis in Rechargeable Zn–Air Batteries. Adv. Mater. 2020, 32, 2004900. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Fan, C.; Meng, Q.; Tang, Y.; Qiu, X.; Fu, G.; Ma, T. Dual Single-Atomic Ni-N4 and Fe-N4 Sites Constructing Janus Hollow Graphene for Selective Oxygen Electrocatalysis. Adv. Mater. 2020, 32, 2003134. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-L.; Shao, Q.; Huang, X.; Lang, J.-P. Nanoscale Trimetallic Metal–Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1888–1892. [Google Scholar] [CrossRef]
- Li, M.; Ye, K.-H.; Qiu, W.; Wang, Y.; Ren, H. Heterogeneity between and within Single Hematite Nanorods as Electrocatalysts for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2022, 144, 5247–5252. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Lee, Y.J.; Seo, H. A Versatile Synthesis Strategy and Band Insights of Monoclinic Clinobisvanite BiVO4 Thin Films for Enhanced Photoelectrochemical Water Splitting Activity. Appl. Surf. Sci. 2021, 562, 150078. [Google Scholar] [CrossRef]
- Nguyën, H.C.; Garcés-Pineda, F.A.; de Fez-Febré, M.; Galán-Mascarós, J.R.; López, N. Non-Redox Doping Boosts Oxygen Evolution Electrocatalysis on Hematite. Chem. Sci. 2020, 11, 2464–2471. [Google Scholar] [CrossRef]
- Shah, S.A.; Zhu, G.; Yuan, A.; Ullah, N.; Shen, X.; Khan, H.; Xu, K.; Wang, X.; Yan, X. Loading of Individual Se-Doped Fe2O3-Decorated Ni/NiO Particles on Carbon Cloth: Facile Synthesis and Efficient Electrocatalysis for the Oxygen Evolution Reaction. Dalton Trans. 2020, 49, 15682–15692. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Li, F.-T.; Fan, R.-Y.; Zhao, J.; Dong, B.; Wang, F.-L.; Liu, H.-J.; Yu, J.-F.; Liu, C.-G.; Chai, Y.-M. F, P Double-Doped Fe3O4 with Abundant Defect Sites for Efficient Hydrogen Evolution at High Current Density. J. Mater. Chem. A 2021, 9, 15836–15845. [Google Scholar] [CrossRef]
- Ganje, P.M.; Bandal, H.A.; Kim, H. Free-Standing P-Doped Fe2O3/ZnO Nanotubes as a Bifunctional Electrocatalyst for Electrochemical Water Splitting. Sustain. Energy Fuels 2022, 6, 5579–5590. [Google Scholar] [CrossRef]
- Haschke, S.; Pankin, D.; Petrov, Y.; Bochmann, S.; Manshina, A.; Bachmann, J. Design Rules for Oxygen Evolution Catalysis at Porous Iron Oxide Electrodes: A 1000-Fold Current Density Increase. ChemSusChem 2017, 10, 3644–3651. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, X.; Ren, H.; Chi, J.; Fu, H.; Dong, B.; Liu, C.; Chai, Y. Modulation of Inverse Spinel Fe3O4 by Phosphorus Doping as an Industrially Promising Electrocatalyst for Hydrogen Evolution. Adv. Mater. 2019, 31, 1905107. [Google Scholar] [CrossRef]
- Liu, H.-J.; Zhang, S.; Yang, W.-Y.; Yu, N.; Liu, C.-Y.; Chai, Y.-M.; Dong, B. Directional Reconstruction of Iron Oxides to Active Sites for Superior Water Oxidation. Adv. Funct. Mater. 2023, 33, 2303776. [Google Scholar] [CrossRef]
- Ng, K.-L.; Kok, K.-Y.; Ong, B.-H. Facile Synthesis of Self-Assembled Cobalt Oxide Supported on Iron Oxide as the Novel Electrocatalyst for Enhanced Electrochemical Water Electrolysis. ACS Appl. Nano Mater. 2018, 1, 401–409. [Google Scholar] [CrossRef]
- Farooq, A.; Khalil, S.; Basha, B.; Habib, A.; Al-Buriahi, M.S.; Warsi, M.F.; Yousaf, S.; Shahid, M. Electrochemical Investigation of C-Doped CoFe2O4/Fe2O3 Nanostructures for Efficient Electrochemical Water Splitting. Int. J. Hydrog. Energy 2024, 51, 1318–1332. [Google Scholar] [CrossRef]
- Tong, H.; Zheng, X.; Qi, M.; Li, D.; Zhu, J.; Jiang, D. Synergistically Coupled CoMo/Fe2O3 Electrocatalyst for Highly Efficient and Stable Overall Water Splitting. J. Colloid Interface Sci. 2024, 676, 837–846. [Google Scholar] [CrossRef]
- Alothman, A.A.; Shah, J.H.; Aljadoa, K.A.; Soykan, G.; Yalçin, Ş.; Abid, A.G.; Allakhverdiev, S.I. Fabrication of Heterojunction Electrode Based on Fe2O3@CuO-400 Nanocomposite Constructed for Hydrogen Production. Int. J. Hydrog. Energy 2024, 61, 1004–1014. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Deng, L.; Li, W.; Ren, Z.; Yang, M.; Yang, X.; Zhu, Y. Synthesis and Characterization of an IrO2–Fe2O3 Electrocatalyst for the Hydrogen Evolution Reaction in Acidic Water Electrolysis. RSC Adv. 2017, 7, 20252–20258. [Google Scholar] [CrossRef]
- Mosallaei, H.; Hadadzadeh, H.; Foelske, A.; Sauer, M.; Rudbari, H.A.; Blacque, O. [Ru(Tmphen)3]2[Fe(CN)6] and [Ru(Phen)3][Fe(CN)5(NO)] Complexes and Formation of a Heterostructured RuO2–Fe2O3 Nanocomposite as an Efficient Alkaline HER and OER Electrocatalyst. Dalton Trans. 2022, 51, 6314–6331. [Google Scholar] [CrossRef] [PubMed]
- Mosallaei, H.; Hadadzadeh, H.; Ensafi, A.A.; Mousaabadi, K.Z.; Weil, M.; Foelske, A.; Sauer, M. Evaluation of HER and OER Electrocatalytic Activity over RuO2–Fe2O3 Nanocomposite Deposited on HrGO Nanosheets. Int. J. Hydrog. Energy 2023, 48, 1813–1830. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, X.; Ding, G.; Guo, Z.; Xue, Y.; Li, G.; Yu, R. Constructing Oxygen Vacancy-Enriched Fe2O3@NiO Heterojunctions for Highly Efficient Electrocatalytic Alkaline Water Splitting. CrystEngComm 2021, 24, 199–207. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, R.; Li, D.; Yang, M.; Tong, Y.; Qiu, Z.; Jing, B.; Yang, T.; Hu, R.; Yang, Y.; et al. Tungsten Oxide Decorating-Regulated Iron-Nickel Oxide Heterostructure as Electrocatalyst for Oxygen Evolution Reaction. J. Alloys Compd. 2023, 968, 171910. [Google Scholar] [CrossRef]
- Kong, A.; Zhang, H.; Sun, Y.; Lv, Y.; Liu, M.; Li, H.; Wang, Y.; Fu, Y.; Zhang, H.; Li, W.; et al. Surface Reconstruction Enables Outstanding Performance of Fe2O3/Ni(OH)2 Nanosheet Arrays for Ultrahigh Current Density Oxygen Evolution Reaction. Appl. Surf. Sci. 2023, 613, 156023. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, X.; Yao, J.; Zhao, J.; Xu, Y.; Yan, Q.; Ye, K.; Zhu, K.; Cao, D.; Wang, G. Ultrathin Fe2O3 Shell-Encapsulated NiFe Alloy Nanoparticles Embedded in Tubular Carbon Matrix for Enhanced Oxygen Evolution Reaction. J. Alloys Compd. 2023, 935, 167922. [Google Scholar] [CrossRef]
- Kim, J.; Heo, J.N.; Do, J.Y.; Chava, R.K.; Kang, M. Electrochemical Synergies of Heterostructured Fe2O3-MnO Catalyst for Oxygen Evolution Reaction in Alkaline Water Splitting. Nanomaterials 2019, 9, 1486. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wu, H.; Xiao, F.; Cao, E.; Du, S.; Wu, Y.; Ren, Z. Self-Assembly-Induced Mosslike Fe2O3 and FeP on Electro-Oxidized Carbon Paper for Low-Voltage-Driven Hydrogen Production Plus Hydrazine Degradation. ACS Sustain. Chem. Eng. 2018, 6, 15727–15736. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, J.; Batool, S.; Zafar, M.N.; Hanif, A.; Zahidullah; Nazar, M.F.; Ul-Hamid, A.; Jabeen, U.; Dahshan, A.; et al. Design and Fabrication of Fe2O3/FeP Heterostructure for Oxygen Evolution Reaction Electrocatalysis. J. Alloys Compd. 2022, 894, 162409. [Google Scholar] [CrossRef]
- Khan, N.A.; Rashid, N.; Ahmad, I.; Zahidullah; Zairov, R.; ur Rehman, H.; Nazar, M.F.; Jabeen, U. An Efficient Fe2O3/FeS Heterostructures Water Oxidation Catalyst. Int. J. Hydrog. Energy 2022, 47, 22340–22347. [Google Scholar] [CrossRef]
- Guo, W.H.; Zhang, Q.; Fu, H.C.; Yang, Y.X.; Chen, X.H.; Luo, H.Q.; Li, N.B. Semi-Sacrificial Template Growth of FeS/Fe2O3 Heterogeneous Nanosheets on Iron Foam for Efficient Oxygen Evolution Reaction. J. Alloys Compd. 2022, 909, 164670. [Google Scholar] [CrossRef]
- Sohail, M.; Ayyob, M.; Wang, A.; Sun, Z.; El Maati, L.A.; Altuijri, R.; Zairov, R.; Ahmad, I. An Efficient Fe2Se3/Fe2O3 Heterostructure Electrocatalyst for Oxygen Evolution Reaction. Int. J. Hydrog. Energy 2024, 52, 1290–1297. [Google Scholar] [CrossRef]
- Haschke, S.; Wu, Y.; Bashouti, M.; Christiansen, S.; Bachmann, J. Engineering Nanoporous Iron(III) Oxide into an Effective Water Oxidation Electrode. ChemCatChem 2015, 7, 2455–2459. [Google Scholar] [CrossRef]
- Yuan, W.; Zhao, M.; Yuan, J.; Li, C.M. Ni Foam Supported Three-Dimensional Vertically Aligned and Networked Layered CoO Nanosheet/Graphene Hybrid Array as a High-Performance Oxygen Evolution Electrode. J. Power Sources 2016, 319, 159–167. [Google Scholar] [CrossRef]
- Cheng, H.; Su, Y.-Z.; Kuang, P.-Y.; Chen, G.-F.; Liu, Z.-Q. Hierarchical NiCo2O4 Nanosheet-Decorated Carbon Nanotubes towards Highly Efficient Electrocatalyst for Water Oxidation. J. Mater. Chem. A 2015, 3, 19314–19321. [Google Scholar] [CrossRef]
- Kargar, A.; Yavuz, S.; Kim, T.K.; Liu, C.-H.; Kuru, C.; Rustomji, C.S.; Jin, S.; Bandaru, P.R. Solution-Processed CoFe2O4 Nanoparticles on 3D Carbon Fiber Papers for Durable Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 17851–17856. [Google Scholar] [CrossRef]
- Bandal, H.A.; Jadhav, A.R.; Chaugule, A.A.; Chung, W.-J.; Kim, H. Fe2O3 Hollow Nanorods/CNT Composites as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Electrochim. Acta 2016, 222, 1316–1325. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, H.; Zhang, X.; Liu, R.; Liu, S.; Wang, G.; Zhang, Y.; Zhao, H. Fe/Fe2O3 Nanoparticles Anchored on Fe-N-Doped Carbon Nanosheets as Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc-Air Batteries. Nano Res. 2016, 9, 2123–2137. [Google Scholar] [CrossRef]
- Alduhaish, O.; Ubaidullah, M.; Al-Enizi, A.M.; Alhokbany, N.; Alshehri, S.M.; Ahmed, J. Facile Synthesis of Mesoporous α-Fe2O3@g-C3N4-NCs for Efficient Bifunctional Electro-Catalytic Activity (OER/ORR). Sci Rep 2019, 9, 14139. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Sun, Y.; Chen, Y.; Chen, H.; Han, S.; Lin, H. Fe2O3 Nanocatalysts on N-Doped Carbon Nanomaterial for Highly Efficient Electrochemical Hydrogen Evolution in Alkaline. J. Power Sources 2019, 426, 74–83. [Google Scholar] [CrossRef]
- Shah, S.A.; Shen, X.; Yuan, A.; Ji, Z.; Yue, X.; Zhu, G.; Zhou, H.; Xu, K.; Zhu, J.; Chen, Y. One Step In-Situ Synthesis of Ni3S2/Fe2O3/N-Doped Carbon Composites on Ni Foam as an Efficient Electrocatalyst for Overall Water Splitting. Appl. Surf. Sci. 2020, 527, 146918. [Google Scholar] [CrossRef]
- Mousavi, D.S.; Shahrokhian, S.; Iraji zad, A. Sugar-Cubic Fe2O3/Nitrogen-Doped Graphene Nanocomposite as High-Performance Anode Material for Oxygen Evolution Reaction. J. Alloys Compd. 2022, 910, 164852. [Google Scholar] [CrossRef]
- Kang, S.; Lee, K.; Ryu, J.H.; Ali, G.; Akbar, M.; Chung, K.Y.; Chung, C.-Y.; Han, H.; Kim, K.M. Transition Metal Compounds on Functionalized Multiwall Carbon Nanotubes for the Efficient Oxygen Evolution Reaction. ACS Appl. Nano Mater. 2023, 6, 4319–4327. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. (David) Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef]
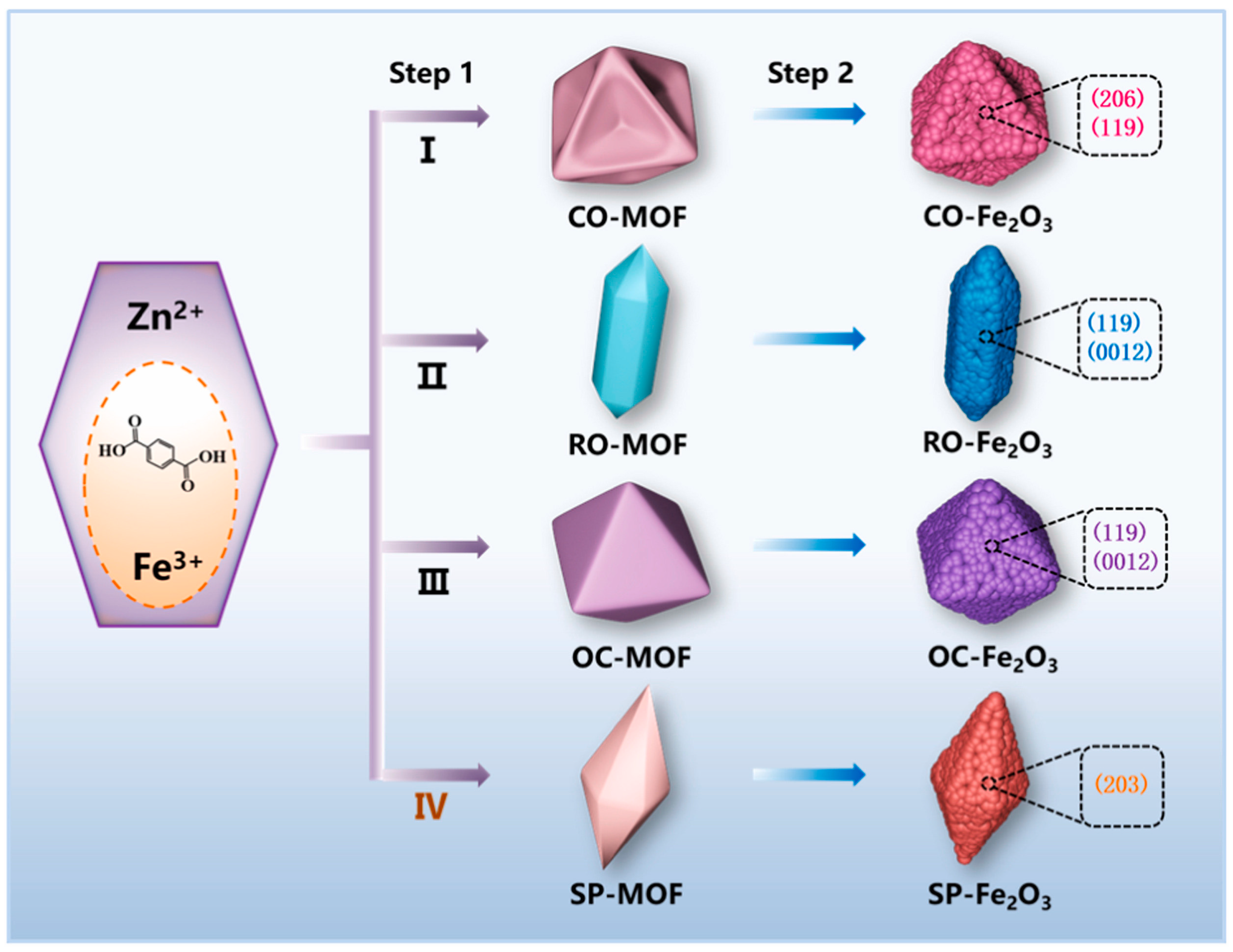
- Niu, Y.; Yuan, Y.; Zhang, Q.; Chang, F.; Yang, L.; Chen, Z.; Bai, Z. Morphology-Controlled Synthesis of Metal-Organic Frameworks Derived Lattice Plane-Altered Iron Oxide for Efficient Trifunctional Electrocatalysts. Nano Energy 2021, 82, 105699. [Google Scholar] [CrossRef]
- Song, H.J.; Yoon, H.; Ju, B.; Lee, D.-Y.; Kim, D.-W. Electrocatalytic Selective Oxygen Evolution of Carbon-Coated Na2Co1–xFexP2O7 Nanoparticles for Alkaline Seawater Electrolysis. ACS Catal. 2020, 10, 702–709. [Google Scholar] [CrossRef]
- Miao, J.; Lang, Z.; Zhang, X.; Kong, W.; Peng, O.; Yang, Y.; Wang, S.; Cheng, J.; He, T.; Amini, A.; et al. Polyoxometalate-Derived Hexagonal Molybdenum Nitrides (MXenes) Supported by Boron, Nitrogen Codoped Carbon Nanotubes for Efficient Electrochemical Hydrogen Evolution from Seawater. Adv. Funct. Mater. 2019, 29, 1805893. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Cao, Z.; Zhu, P.; Cao, Y.; Xu, X.; Xu, C.; Yin, Z. Heterogeneous Bimetallic Sulfides Based Seawater Electrolysis towards Stable Industrial-Level Large Current Density. Appl. Catal. B Environ. 2021, 291, 120071. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Zhu, D.; Liu, D.; Liu, Y.; Yang, S. Fe2O3/NiO Interface for the Electrochemical Oxygen Evolution in Seawater and Domestic Sewage. ACS Appl. Mater. Interfaces 2021, 13, 37152–37161. [Google Scholar] [CrossRef]
- Cui, T.; Zhai, X.; Guo, L.; Chi, J.-Q.; Zhang, Y.; Zhu, J.; Sun, X.; Wang, L. Controllable Synthesis of a Self-Assembled Ultralow Ru, Ni-Doped Fe2O3 Lily as a Bifunctional Electrocatalyst for Large-Current-Density Alkaline Seawater Electrolysis. Chin. J. Catal. 2022, 43, 2202–2211. [Google Scholar] [CrossRef]
- Cui, Z.; Yan, Z.; Yin, J.; Wang, W.; Yue, M.-E.; Li, Z. Engineering P-Fe2O3-CoP Nanosheets for Overall Freshwater and Seawater Splitting. J. Colloid Interface Sci. 2023, 652, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Koza, J.A.; Uhlemann, M.; Gebert, A.; Schultz, L. Desorption of Hydrogen from the Electrode Surface under Influence of an External Magnetic Field. Electrochem. Commun. 2008, 10, 1330–1333. [Google Scholar] [CrossRef]
- Koza, J.A.; Mühlenhoff, S.; Żabiński, P.; Nikrityuk, P.A.; Eckert, K.; Uhlemann, M.; Gebert, A.; Weier, T.; Schultz, L.; Odenbach, S. Hydrogen Evolution under the Influence of a Magnetic Field. Electrochim. Acta 2011, 56, 2665–2675. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, Y.; Xia, S.; Yu, J.; Ding, B. Direct Magnetic Reinforcement of Electrocatalytic ORR/OER with Electromagnetic Induction of Magnetic Catalysts. Adv. Mater. 2021, 33, 2007525. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Elouarzaki, K.; Xu, Z.J. Electrochemistry in Magnetic Fields. Angew. Chem. Int. Ed. 2022, 61, e202203564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, C.; Wu, J.; Liu, H.; Zhang, B.; Jiang, Z.; Li, S.; Xu, P. Recent Advances in Magnetic Field-Enhanced Electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 10303–10316. [Google Scholar] [CrossRef]
- Dastafkan, K.; Meyer, Q.; Chen, X.; Zhao, C. Efficient Oxygen Evolution and Gas Bubble Release Achieved by a Low Gas Bubble Adhesive Iron–Nickel Vanadate Electrocatalyst. Small 2020, 16, 2002412. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, H.; Luo, L. Gas Bubbles in Electrochemical Gas Evolution Reactions. Langmuir 2019, 35, 5392–5408. [Google Scholar] [CrossRef]
- Li, X.; Hao, C.; Du, Y.; Lu, Y.; Fan, Y.; Wang, M.; Wang, N.; Meng, R.; Wang, X.; Xu, Z.J.; et al. Harnessing Magnetic Fields to Accelerate Oxygen Evolution Reaction. Chin. J. Catal. 2023, 55, 191–199. [Google Scholar] [CrossRef]
- Saravanan, P.; Hsu, J.-H.; Sivaprahasam, D.; Kamat, S.V. Structural and Magnetic Properties of γ-Fe2O3 Nanostructured Compacts Processed by Spark Plasma Sintering. J. Magn. Magn. Mater. 2013, 346, 175–177. [Google Scholar] [CrossRef]
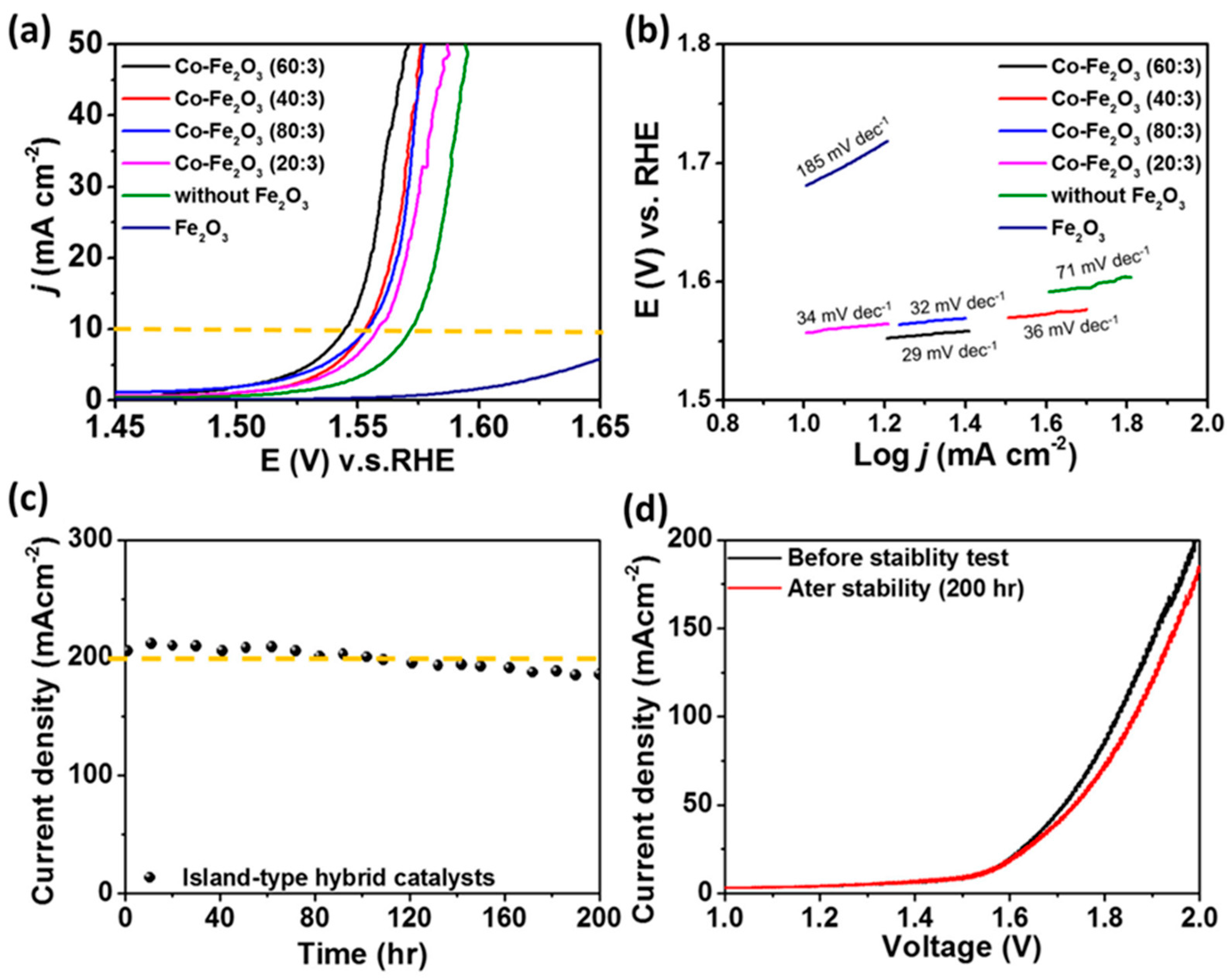
- Chen, H.-Y.; Chen, G.-C.; Liao, K.-W.; Wei, W.-H.; Huang, H.-C.; Wang, C.-H. Island-Type Hybrid Catalysts Applied for Anion Exchange Membrane Water Electrolysis. Catalysts 2022, 12, 102. [Google Scholar] [CrossRef]
- Lahrichi, A.; El Issmaeli, Y.; Kalanur, S.S.; Pollet, B.G. Advancements, Strategies, and Prospects of Solid Oxide Electrolysis Cells (SOECs): Towards Enhanced Performance and Large-Scale Sustainable Hydrogen Production. J. Energy Chem. 2024, 94, 688–715. [Google Scholar] [CrossRef]
- Choi, H.-J.; Na, Y.-H.; Kwak, M.; Kim, T.W.; Seo, D.-W.; Woo, S.-K.; Kim, S.-D. Development of Solid Oxide Cells by Co-Sintering of GDC Diffusion Barriers with LSCF Air Electrode. Ceram. Int. 2017, 43, 13653–13660. [Google Scholar] [CrossRef]
- Shimura, K.; Nishino, H.; Kakinuma, K.; Brito, M.E.; Uchida, H. Effect of Samaria-Doped Ceria (SDC) Interlayer on the Performance of La0.6Sr0.4Co0.2Fe0.8O3-δ/SDC Composite Oxygen Electrode for Reversible Solid Oxide Fuel Cells. Electrochim. Acta 2017, 225, 114–120. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Kong, L.B.; Chan, S.H.; Hing, P.; Kilner, J.A. Iron Oxide as an Effective Sintering Aid and a Grain Boundary Scavenger for Ceria-Based Electrolytes. Solid State Ion. 2004, 167, 203–207. [Google Scholar] [CrossRef]
- Avila-Paredes, H.J.; Kim, S. The Effect of Segregated Transition Metal Ions on the Grain Boundary Resistivity of Gadolinium Doped Ceria: Alteration of the Space Charge Potential. Solid State Ion. 2006, 177, 3075–3080. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Leng, Y.J.; He, Z.M. Sintering, Microstructure and Grain Growth of Fe-Doped Ce0.9Gd0.1O2−δ Ceramics Derived from Oxalate Coprecipitation. J. Cryst. Growth 2005, 274, 603–611. [Google Scholar] [CrossRef]
- Qu, Y.; Yu, J.; Tian, N.; Shen, H. Improved Performance of a Samarium-Doped Ceria Interlayer of Intermediate Temperature Solid Oxide Electrolysis Cells by Doping the Transition Metal Oxide Fe2O3. RSC Adv. 2021, 11, 30911–30917. [Google Scholar] [CrossRef]


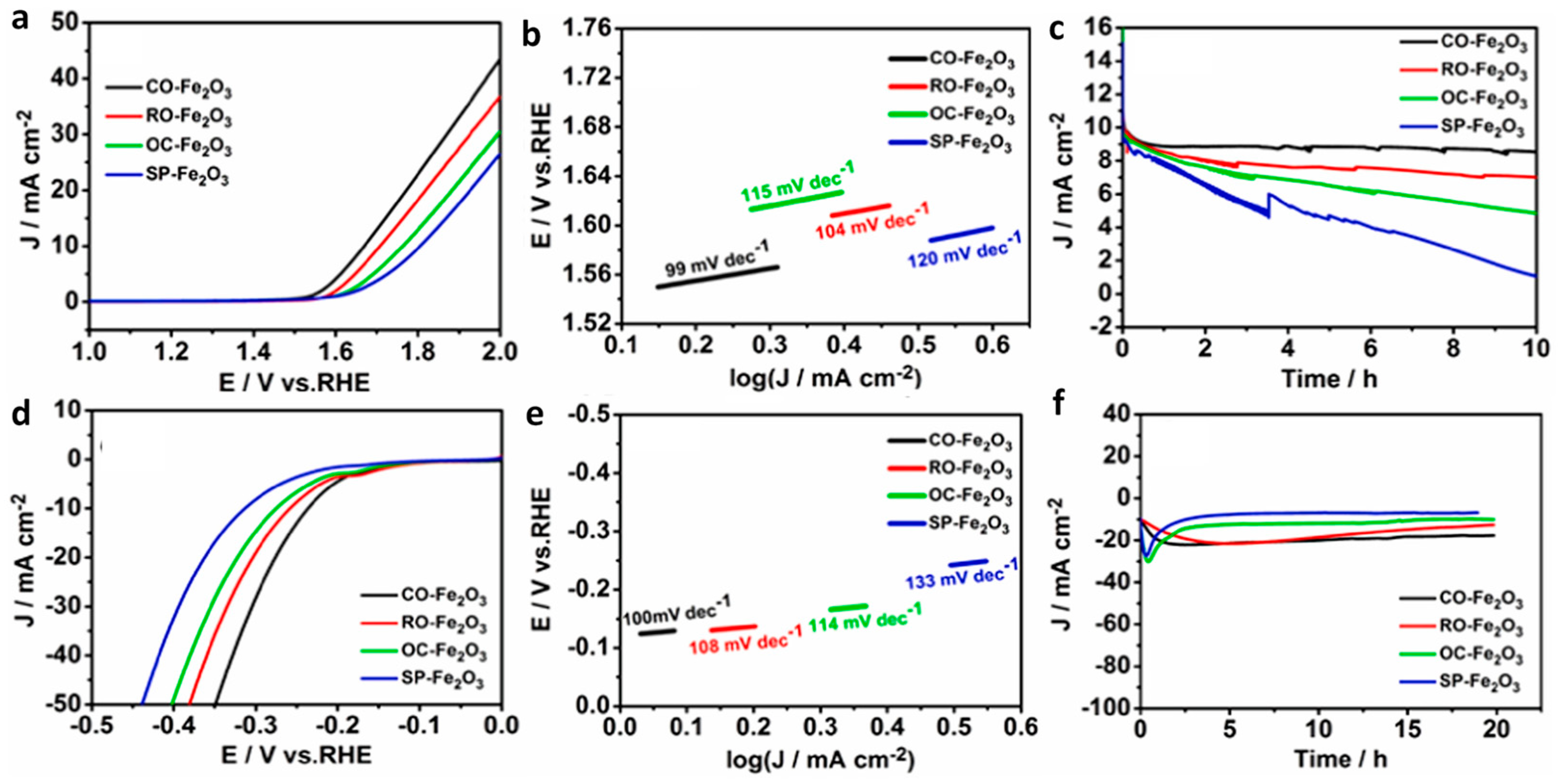
| Catalyst/Electrode | Synthesis Method | Electrolyte | Stability | Overpotential mV vs. SHE | Tafel Slope (mV dec−1) | Ref |
|---|---|---|---|---|---|---|
| Ni and Zn-doped Fe2O3 | Combustion method | 0.1 M KOH | - | OER: 350 (for 10 mA cm−2) | OER: 26 | [40] |
| Se-Fe2O3@Ni/NiO | Thermal method | 1 M KOH | 21 h | OER: 205 (for 10 mA cm−2) | OER: 36 | [41] |
| P-doped Fe2O3/ZnO | Chemical bath deposition/hydrothermal/CVD | 1 M KOH | 24 h | OER: 250 HER: 139 (for 10 mA cm−2) | OER:40 HER: 66 | [42] |
| Zn and S co-doped Fe2O3 | Hydrothermal/CVD | 1 M KOH | 100 h | OER: 350 (for 500 mA cm−2) | OER: 47.5 | [43] |
| Co3O4/Fe3O4 | Co-precipitation method | 0.1 M KOH | 8 h | OER: - HER: - | OER: 62 HER: 102 | [44] |
| C-doped CoFe2O4/Fe2O3 | Calcination approach | 1 M KOH | 5.5 h | OER: 260 HER: 236 (for 20 mA cm−2) | OER: 183 HER: 146 | [45] |
| CoMo/Fe2O3 | Hydrothermal/Electrodeposition methods | 1 M KOH | 100 h | OER: 266 HER: 71 (for 10 mA cm−2) | OER: 54 HER: 85 | [46] |
| Fe2O3@CuO | Hydrothermal | 1 M KOH | 25 h | OER: 230 HER: 130 (for 10 mA cm−2) | OER: 54 HER: 77 | [47] |
| IrO2–Fe2O3 | Thermal decomposition | 0.5 M H2SO4 | 600 cycles | HER: 78 (for 10 mA cm−2) | HER: 36.2 | [48] |
| RuO2–Fe2O3 | Synthesis thermal treatment | 1 M KOH | - | OER: 292 (for 500 mA cm−2) | OER: 56.08 HER: −43 | [49] |
| RuO2/Fe2O3 | Synthesis | 1 M KOH | 18 h | OER: 386 HER: −239 (for 10 mA cm−2) | OER: 67 HER: 97 | [50] |
| Fe2O3@NiO | Hydrothermal | 1 M KOH | 20 h | OER: 224 HER: 187 (for 10 mA cm−2) | OER: 20 HER: 53.8 | [51] |
| WO3/Fe2O3-NiO | Chemical etching reaction and decomposition | 1 M KOH | 100 h | OER: 211 (for 100 mA cm−2) | OER: 39.5 | [52] |
| Fe2O3/Ni(OH)2 | Electrodeposition | 1 M KOH | 18 h | OER: 291 (for 10 mA cm−2) | OER: 53.7 | [53] |
| Ni1Fe2@Fe2O3@C | High-temperature calcination | 1 M KOH | 30 h | OER: 271 (for 10 mA cm−2) | OER: 78 | [54] |
| Fe2O3-MnO | Sol-gel method | 1 M KOH | 1000 cycles | OER: 370 (for 10 mA cm−2) | OER: 66 | [55] |
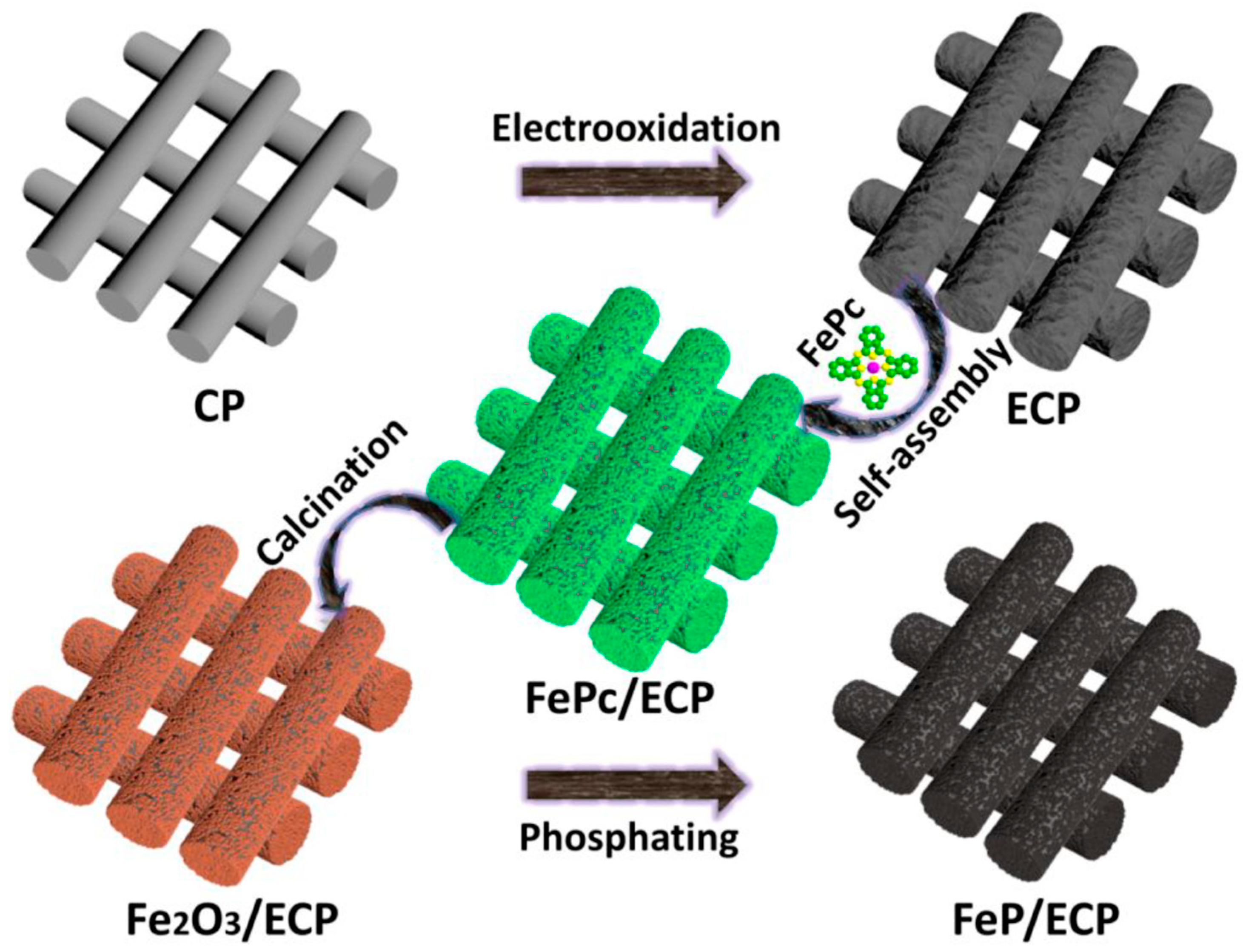
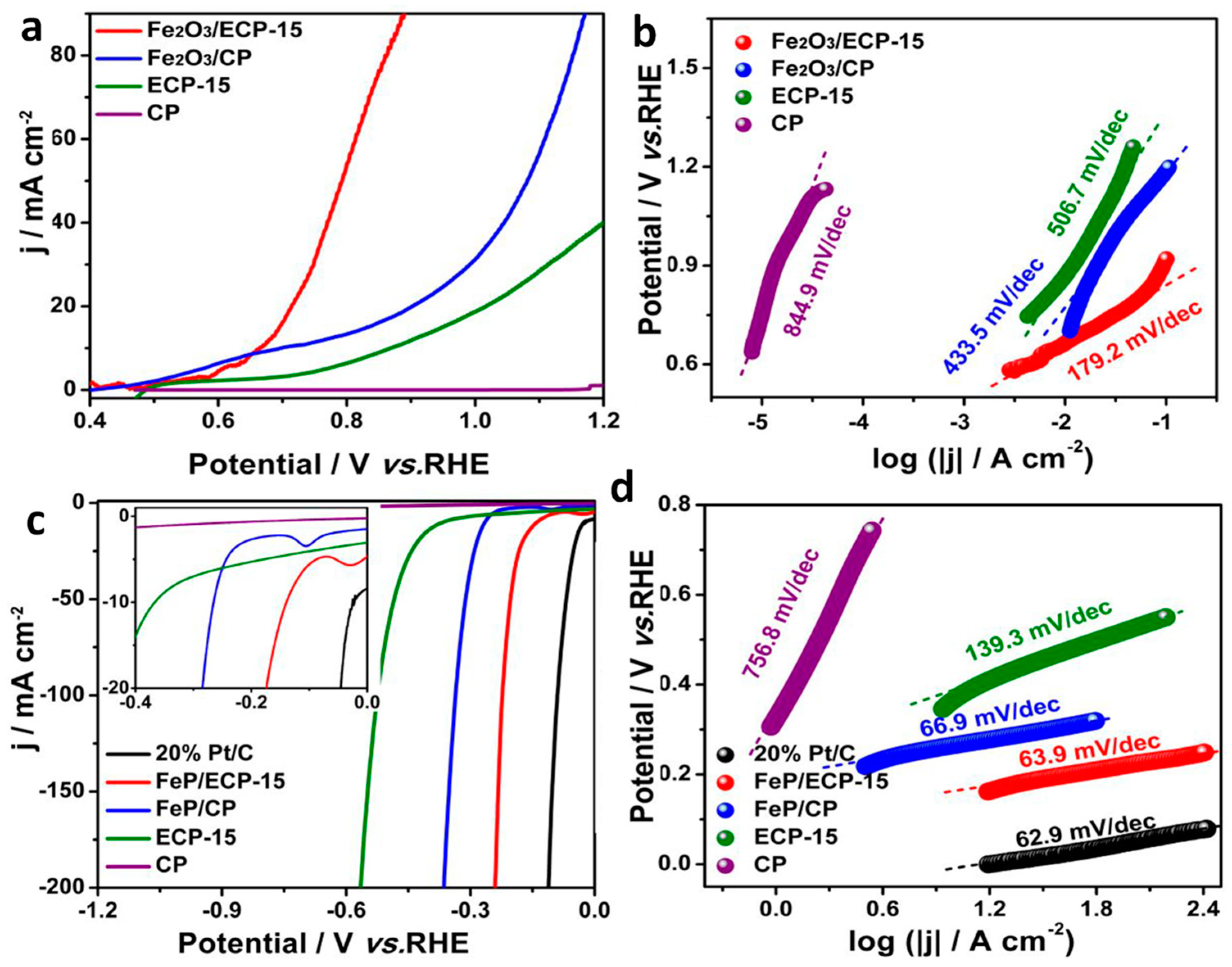
| Fe2O3ǁǁFeP | Electrochemical oxidation/solution self-assembly/pyrolysis | 1 M KOH | 20 h | Hydrazine overpotential: 0.61 V HER: 138 (for 10 mA cm−2) | OER: 179.2 HER: 63.9 | [56] |
| Fe2O3/FeP | Hydrothermal | 1 M KOH | 12 h | OER: 264 (for 10 mA cm−2) | OER: 47 | [57] |
| Fe2O3/FeS | Hydrothermal | 1 M KOH | 10 h | OER: 264 (for 40 mA cm−2) | OER: 90 | [58] |
| FeS/Fe2O3 | Chemical etching/solvothermal | 1 M KOH | 10 h | OER: 266.5 (for 10 mA cm−2) | OER: 51.17 | [59] |
| Fe2Se3/Fe2O3 | Synthesis | 1 M KOH | 24 h | OER: 160 (for 20 mA cm−2) | OER: 30.02 | [60] |
| Fe2O3/CNT | Co-precipitation method | 1 M KOH | 12h | OER: 383 (for 10 mA cm−2) | OER: 62 | [61] |
| Fe/Fe2O3-Fe-N-doped C | Pyrolysis | 0.1 M KOH | 10h | OER: 0.69 V vs Ag/AgCl (for 10 mA cm−2) | OER:77.5 | [62] |
| Fe2O3/g-C3N4 | Thermal method | 0.5 M KOH | 10 min | OER: 425 (for 10 mA cm−2) | OER: 280 | [63] |
| Fe2O3-C | Pyrolysis | 1M KOH | 48 h | HER: 245 (for 10 mA cm−2) | HER: 76.6 | [64] |
| Ni3S2/Fe2O3/N-doped carbon | Thermal process/CVD | 1 M KOH | 200 h | OER: 188 (52 mA cm−2) HER: 78 (10 mA cm−2) | OER: 64.3 HER: 115.8 | [65] |
| Fe2O3/N-graphene | Co-deposition (hydrothermal and electrodeposition) | 1 M KOH | 22 h | OER: 313 (for 100 mA cm−2) | OER: 81 | [66] |
| Fe2O3/MWCNT | Pulsed laser ablation | 1M KOH | 10 h | OER: 310 (for 10 mA cm−2) | OER: 20.35 | [67] |
| MOF-Fe2O3 | Solvothermal | 1 M KOH | 20 h | OER: 439 HER: 230 (for 10 mA cm−2) | OER: 99 HER: 100 | [68] |
| Fe2O3/NiO | Chemical bath deposition | 1 M KOH | 50 h | OER: 182 (for 10 mA cm−2) | OER: 45 | |
| Sea water | OER: 291 (for 1 A cm−2) | [69] | ||||
| Domestic sewage | OER: 329 (for 1 A cm−2) | |||||
| P-Fe2O3-CoP | Hydrothermal-gas-phase phosphorization process | Freshwater | 12 h | OER: 250 HER: 219 (for 10 mA cm−2) | OER: 42 HER: 79 | [70] |
| Seawater | OER: 270 HER: 152 (for 10 mA cm−2) | OER: 59 HER: 95 | ||||
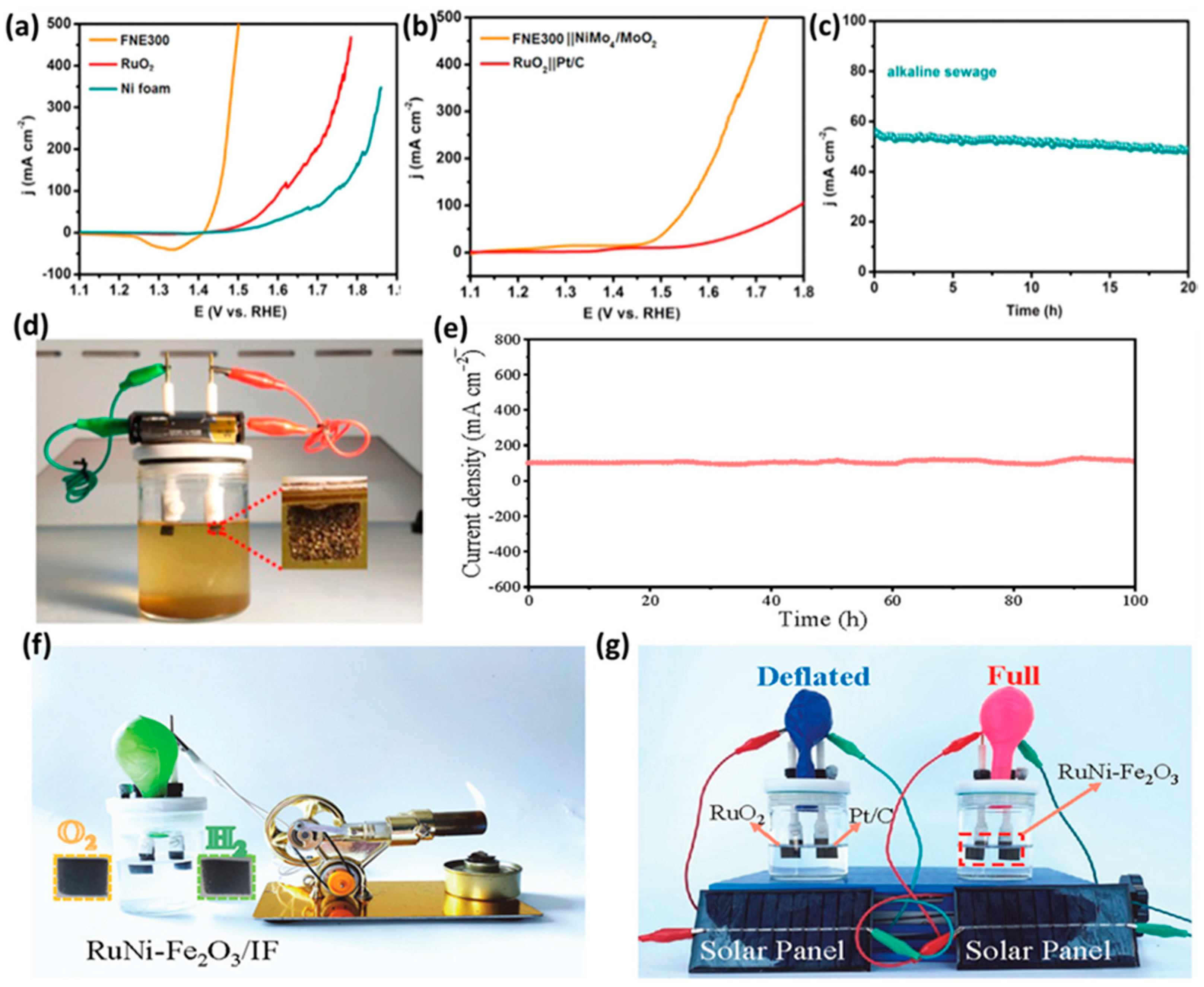
| RuNi-Fe2O3/IF | Hydrothermal | 1 M KOH | 100 h | OER: 329 HER: 75 (for 100 mA cm−2) | OER: 60.85 HER: 85.08 | [71] |
| 1 M KOH + seawater | OER: 424 HER: 298 (for 100 mA cm−2) | OER: 69.58 HER: 114.31 | ||||
| Co-Fe2O3 (AEM) | Microwave-assisted hydrothermal | 1.0 M KOH | 500 h | OER: 310 (for 10 mA cm−2) | OER: 29 | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollet, B.G.; Kalanur, S.S. Applications of Ferric Oxide in Water Splitting by Electrolysis: A Comprehensive Review. Molecules 2024, 29, 4990. https://doi.org/10.3390/molecules29214990
Pollet BG, Kalanur SS. Applications of Ferric Oxide in Water Splitting by Electrolysis: A Comprehensive Review. Molecules. 2024; 29(21):4990. https://doi.org/10.3390/molecules29214990
Chicago/Turabian StylePollet, Bruno G., and Shankara S. Kalanur. 2024. "Applications of Ferric Oxide in Water Splitting by Electrolysis: A Comprehensive Review" Molecules 29, no. 21: 4990. https://doi.org/10.3390/molecules29214990
APA StylePollet, B. G., & Kalanur, S. S. (2024). Applications of Ferric Oxide in Water Splitting by Electrolysis: A Comprehensive Review. Molecules, 29(21), 4990. https://doi.org/10.3390/molecules29214990







