Effect of Ionic Surfactants on Kinetics and Mechanism of the Bi(III) Ion Electroreduction in the Mixed Aqueous–Organic Solutions of Supporting Electrolytes
Abstract
1. Introduction
2. Results and Discussion
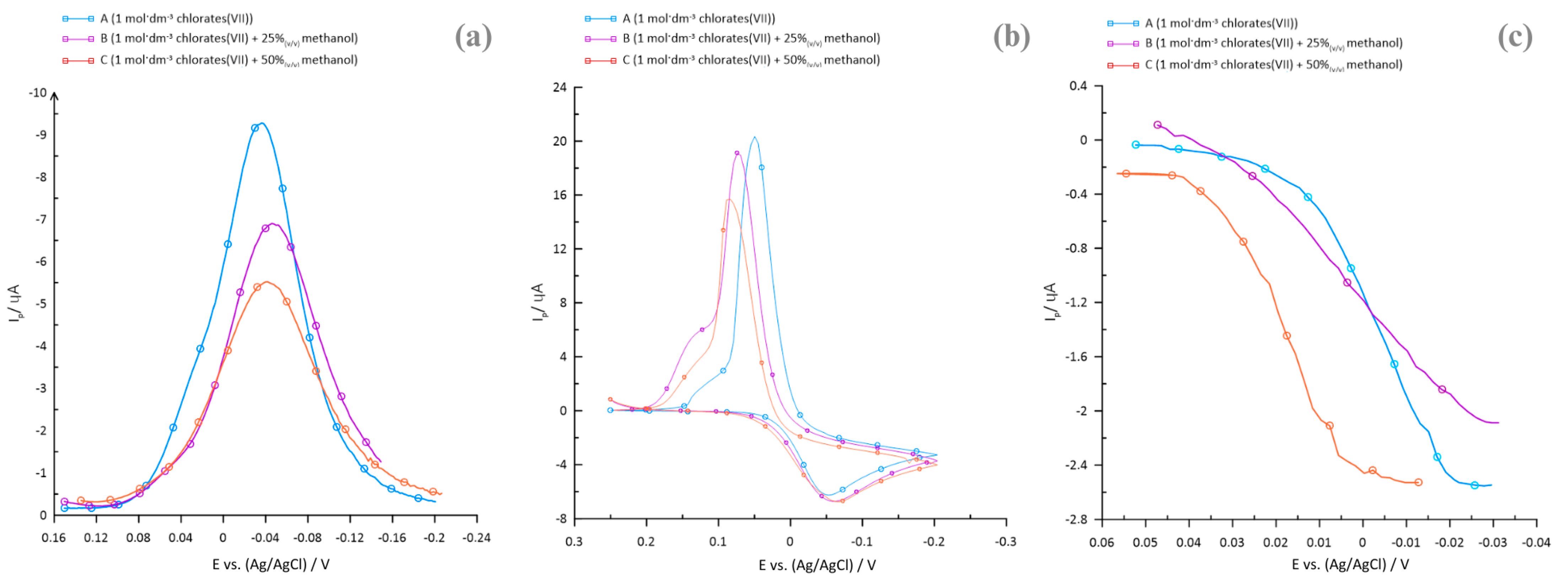
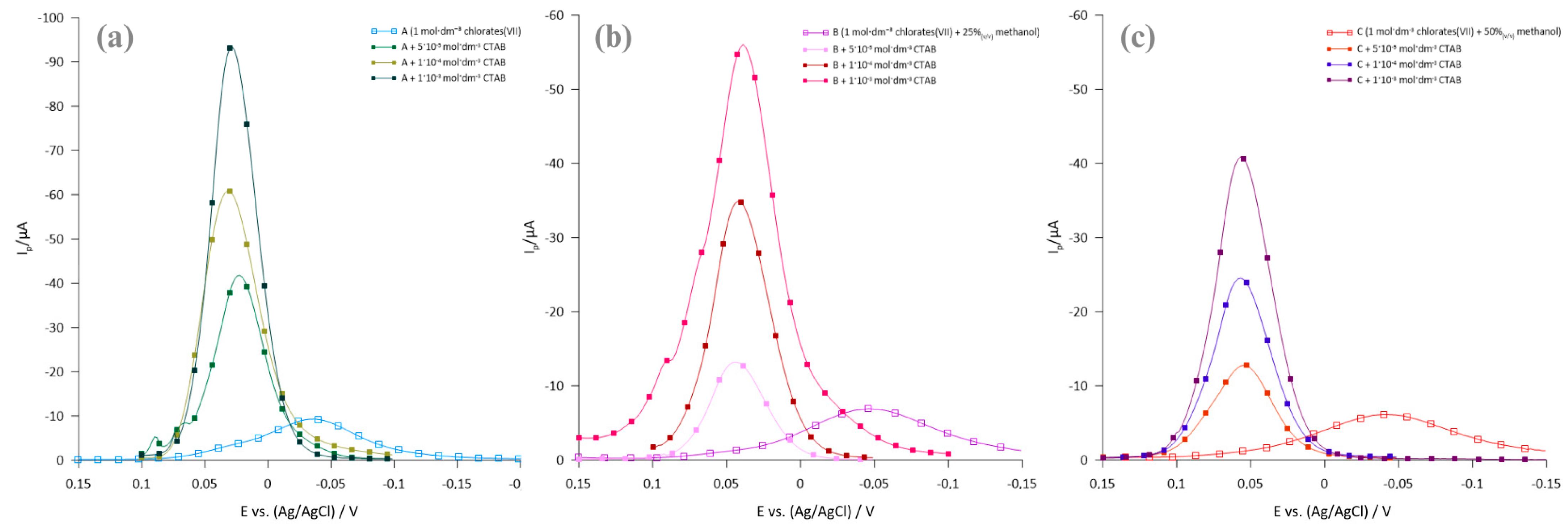
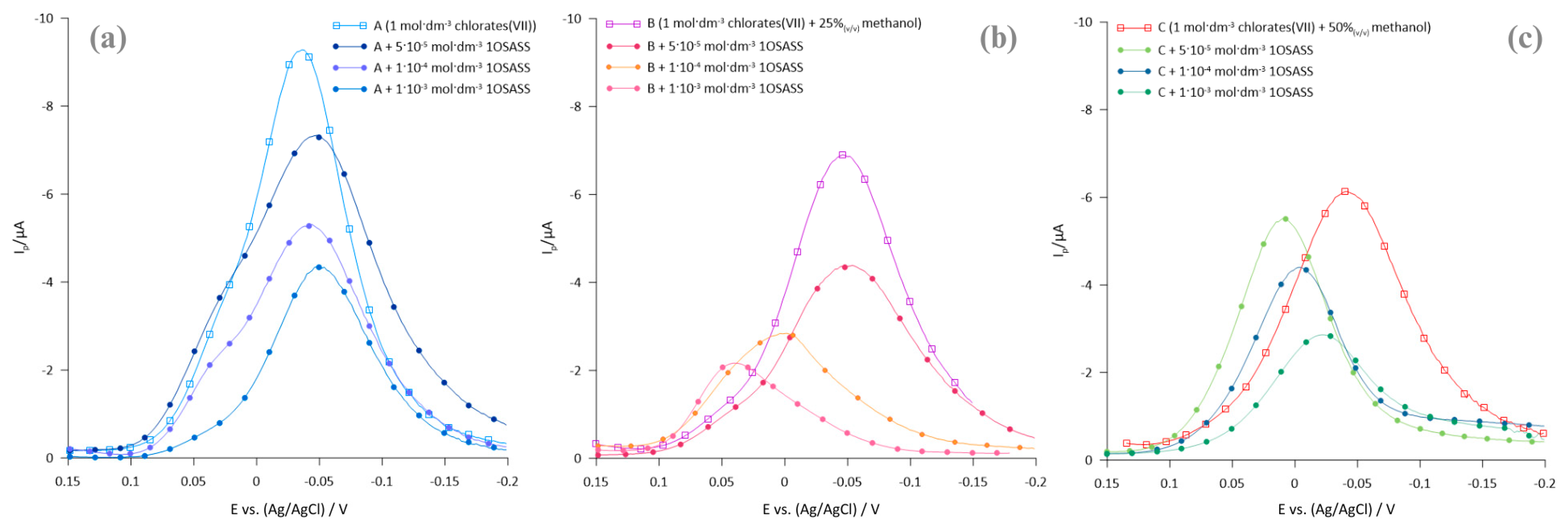
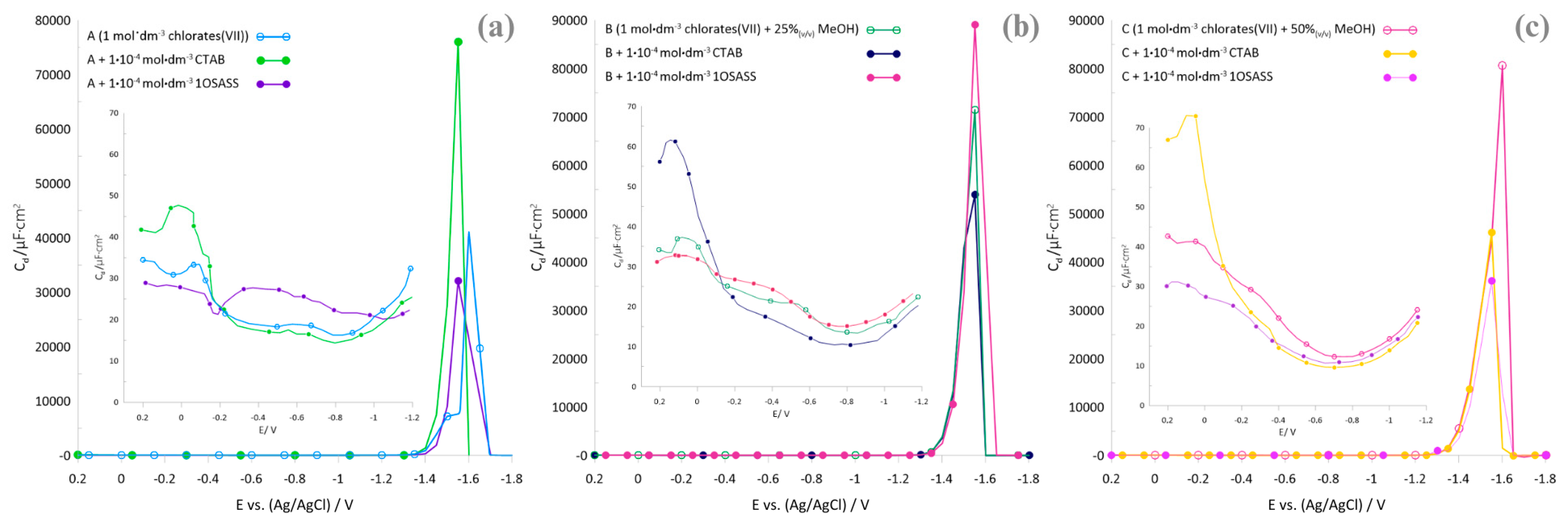
2.1. Polarographic, Voltammetric, and Impedance Measurements
2.2. Kinetics Parameters
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Solutions
- Solution A: 1 mol∙dm−3 aqueous solution of chlorate(VII), without the addition of methanol (A—0%(v/v) MeOH) (chlorate(VII):methanol ratio = 4:0).
- Solution B: 1 mol∙dm−3 mixed solution of chlorate(VII) in water with the addition of methanol, where the ratio of chlorate(VII):methanol = 3:1 (B—25%(v/v) MeOH).
- Solution C: 1 mol∙dm−3 mixed solution of chlorate(VII) in water with the addition of methanol, where the ratio of chlorate(VII):methanol = 2:2 (B—50%(v/v) MeOH).
4. Conclusions
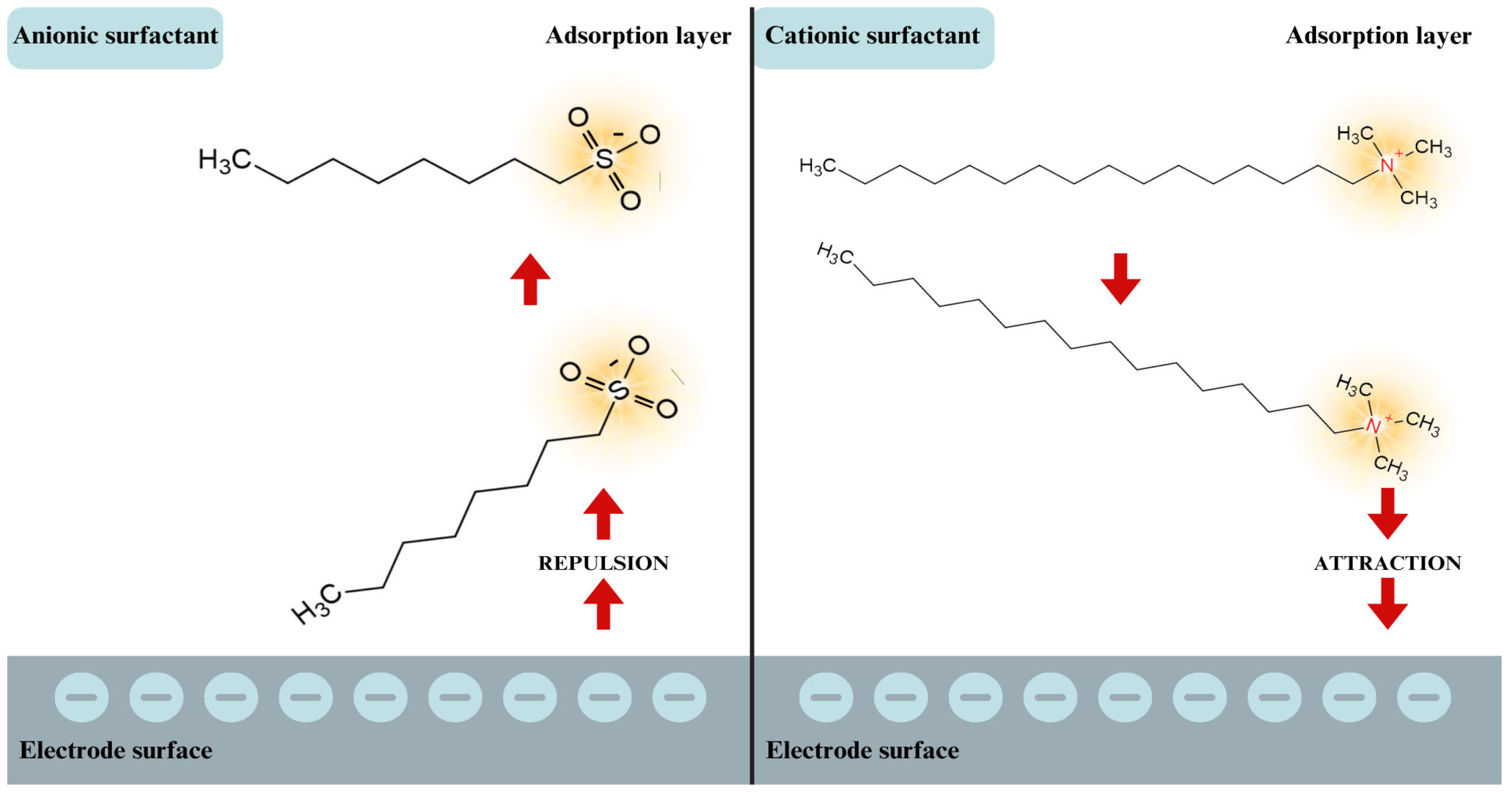
- The kinetics of the electrode process are affected by both the composition of the base electrolyte and the presence of ionic surfactants—hexadecyltrimethylammonium bromide and sodium 1-octane sulfonate.
- Adsorption of these surfactants on the surface of the R-AgLAFE electrode is determined by their ionic nature.
- Changes in the concentration of methanol and selected surfactants affect the rate of the Bi(III) ion electroreduction but do not change the mechanism of this process.
- CTAB accelerates the multi-step Bi(III) ion electroreduction process, while 1OSASS acts as an inhibitor.
- 1.
- Formation of Active Complex I with the participation of adsorbed surfactant molecules (Surf) and partial dehydration of Bi(III) ions.Bi(H2O)93+ + x(Surf)ads. → [Bi(H2O)(9-a)3+(Surf)x] + aH2O
- 2.
- The transition of the first electron.[Bi(H2O)(9-a)3+(Surf)x] + e− [Bi(H2O)(9-a)2+(Surf)x]
- 3.
- Further dehydration with the formation of Active Complex II.[Bi(H2O)(9-a)2+(Surf)x] + y(Surf)ads. → [Bi(H2O)(9-a-b)2+(Surf)x+y] + bH2O
- 4.
- Second electron transition.[Bi(H2O)(9-a-b)2+(Surf)x+y] + e− → [Bi(H2O)(9-a-b)+(Surf)x+y]
- 5.
- Further dehydration with the formation of Active Complex III.[Bi(H2O)(9-a-b)+(Surf)x+y] + z(Surf)ads. → [Bi(H2O)(9-a-b-c)+(Surf)x+y+z] + cH2O
- 6.
- Transition of the third electron and formation of an amalgam.[Bi+(Surf)x+y+z] + e− → Bi(Hg) + (x + y + z)(Surf)ads.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helmholtz, H. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche. Ann. Phys. 1853, 165, 211–233. [Google Scholar] [CrossRef]
- Marczewska, B. Przyspieszanie procesu Elektroredukcji Jonów Zn(II) na Elektrodzie Rtęciowej przez Niektóre Związki Organiczne, w Wybranych Mieszanych Rozpuszczalnikach Wodno-Organicznych. Habilitation Thesis, University of Warsaw, Warsaw, Poland, 1998. [Google Scholar]
- Sykut, K.; Dalmata, G.; Nowicka, B.; Saba, J.; Toporowicz, B. Study on the ”Cap-Pair” Effect; Annales Universitatis Mariae Curie-Skłodowska; Maria Curie-Skłodowska University: Lublin, Poland, 1979; p. 35. [Google Scholar]
- Gugała-Fekner, D. Adsorption of adenine on mercury electrode in acetate buffer at pH 5 and pH 6 and its effect on electroreduction of zinc ions. Monatshefte Chem.-Chem. Mon. 2018, 149, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Nieszporek, J.; Pańczyk, T.; Nieszporek, K. The Comparison of Catalytic Activity of Carbimazole and Methimazole on Electroreduction of Zinc (II) in Chlorates (VII): Experimental and Molecular Modelling Study. Molecules 2024, 29, 3455. [Google Scholar] [CrossRef] [PubMed]
- Muñiz Álvarez, J.L.; García Calzón, J.A.; López Fonseca, J.M. Electrochemical Reduction of Ge(IV) Catalyzed byo-Catechol at the Dropping Mercury Electrode and at the Hanging Mercury Drop Electrode after Adsorptive Preconcentration. Electroanalysis 1999, 11, 656–659. [Google Scholar] [CrossRef]
- Chandra Sahu, B. Introduction to Corrosion—Basics and Advances; IntechOpen: London, UK, 2023. [Google Scholar]
- Švancara, I.; Sýs, M. Carbon Paste Electrodes Surface-Modified with Surfactants: Principles of Surface Interactions at the Interface between Two Immiscible Liquid Phases. Sensors 2023, 23, 9891. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Zare-Mehrjardi, H.R. Cobalt salophen-modified carbon-paste electrode incorporating a cationic surfactant for simultaneous voltammetric detection of ascorbic acid and dopamine. Sens. Actuators B Chem. 2007, 121, 530–537. [Google Scholar] [CrossRef]
- Nieszporek, J. Nicotinamide as a Catalyst for Zn2+ Electroreduction in Acetate Buffer. Electrocatalysis 2020, 11, 8. [Google Scholar] [CrossRef]
- Kaliszczak, W.; Nosal-Wiercińska, A. Influence of mixed 6- thioguanine-nonionic surfactant adsorption layers on kinetics and mechanism of bi(III) ion electroreduction. Electrocatalysis 2019, 10, 621–627. [Google Scholar] [CrossRef]
- Nieszporek, J.; Gugała-Fekner, D.; Sieńko, D.; Saba, J.; Nieszporek, K. Kinetics and mechanism of Zn(II) ion electroreduction in the presence of vetranal. Collect. Czechoslov. Chem. Commun. 2008, 73, 616–626. [Google Scholar] [CrossRef]
- Mirceski, V.; Skrzypek, S.; Stojanov, L. Square-wave voltammetry. ChemTexts 2018, 4, 17. [Google Scholar] [CrossRef]
- Nosal-Wiercińska, A. The catalytic activity of cysteine and cystine on the electroreduction of Bi(III) ions. J. Electroanal. Chem. 2011, 662, 298–305. [Google Scholar] [CrossRef]
- Nosal-Wiercińska, A.; Martyna, M.; Skrzypek, S.; Szabelska, A.; Wiśniewska, M. Electroreduction of Bi(III) ions in the aspect of expanding the “cap-pair” effect: The role of the nanosized active complexes. Appl. Nanosci. 2022, 12, 947–955. [Google Scholar] [CrossRef]
- Taraszewska, J. Adsorption of methanol on the mercury electrode from solutions of H2O-CH3OH-NaClO4. J. Electroanal. Chem. Interfacial Electrochem. 1974, 49, 443–451. [Google Scholar] [CrossRef]
- Avranas, A.; Papadopoulos, N.; Papoutsi, D.; Sotiropoulos, S. Adsorption of the neutral macromonomeric surfactant Tween-80 at the mercury/electrolyte solution interface as a function of electrode potential and time. Langmuir 2000, 16, 6043–6053. [Google Scholar] [CrossRef]
- Avranas, A.; Retter, U.; Malasidou, E. The Adsorption and Condensed Film Formation of Cetyltrimethylammonium Bromide at the Mercury/Electrolyte Interface. J. Colloid Interface Sci. 2002, 248, 347–354. [Google Scholar] [CrossRef]
- Koniari, A.; Avranas, A. Adsorption of cationic surfactants on covered hanging mercury drop electrode surface of the variable area. J. Colloid Interface Sci. 2012, 382, 82–89. [Google Scholar] [CrossRef]
- Shatla, A.S.; Bawol, P.P.; Baltruschat, H. Adsorption of Iodide and Bromide on Au(111) Electrodes from Aprotic Electrolytes: Role of the Solvent. ChemElectroChem 2020, 7, 4782. [Google Scholar] [CrossRef]
- Nosal-Wiercińska, A.; Kaliszczak, W.W.; Grochowski, M.; Wiśniewska, M.; Klepka, T. Effects of mixed adsorption layers of 6-mercaptopurine—Triton X-100 and 6-mercaptopurine—Tween 80 on the double layer parameters at the mercury/chlorates(VII) interface. J. Mol. Liq. 2018, 253, 143–148. [Google Scholar] [CrossRef]
- Lovrić, M.; Branica, M. Reduction of Bi(III) from highly concentrated perchloric acid. Ind. J. Chem. 1990, 29A, 435–438. [Google Scholar]
- Costa, R.; Pereira, C.M.; Silva, F. Electric double layer studies at the interface of mercury–binary ionic liquid mixtures with a common anion. RSC Adv. 2013, 3, 11697. [Google Scholar] [CrossRef]
- Krishnan, C.V.; Garnett, M. New insights into the double layer structure from impedance measurements: Implications for biological systems. Electrochim. Acta 2006, 51, 1541–1549. [Google Scholar] [CrossRef]
- Kravtsov, V.I. Kinetics and Mechanism of Electrode Reactions of Metal Complexes in Aqueous Electrolyte Solution. Russ. Chem. Rev. 1976, 45, 4. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications, Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J., White, R.E., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; Volume 32, pp. 143–248. [Google Scholar]
- Nosal-Wiercińska, A. Intermolecular Interactions in Systems Containing Bi(III)—ClO−4–H2O–Selected Amino Acids in the Aspect of Catalysis of Bi(III) Electroreduction. Electroanalysis 2014, 26, 1013–1023. [Google Scholar] [CrossRef]
- Marczewska, B. Mechanism of the Acceleration Effect of Thiourea on the Electrochemical Reduction of Zinc(II) Ions in Binary Mixtures on Mercury Electrode. Electroanalysis 1998, 10, 50–53. [Google Scholar] [CrossRef]
- Marczewska, B. The influence of aminobenzoic acid on Zn(II) electroreduction on a mercury electrode in water-methanol. Anorgonische Phys. Chem. 1997, 128, 1223–1235. [Google Scholar] [CrossRef]
- Marczewska, B. The Acceleration of Zn(II) Electroreduction on Mercury Electrode by 1,5-Diaminonaphthalene and N, N′-Diphenylthiourea, Catalytically Passive in the Aqueous Solution, in Binary Mixtures. Electroanalysis 1999, 11, 1101. [Google Scholar] [CrossRef]
- Ayala, R.; Martínez, J.M.; Pappalardo, R.R.; Refson, K.; Marcos, E.S. Effect of Basicity on the Hydrolysis of the Bi(III) Aqua Ion in Solution: An Ab Initio Molecular Dynamics Study. J. Phys. Chem. A 2018, 122, 1905–1915. [Google Scholar] [CrossRef]
- Kolsi, L.; Yli-Kauhaluoma, J.; Moreira, V. Catalytic, Tunable, One-Step Bismuth(III) Triflate Reaction with Alcohols: Dehydration Versus Dimerization. ACS Omega 2018, 3, 8836–8842. [Google Scholar] [CrossRef]
- Barek, J. Voltammetric and amperometric applications of silver amalgam electrodes for monitoring of reducible organic compounds. TrAC Trends Anal. Chem. 2023, 11, 117416. [Google Scholar] [CrossRef]
- Nazmutdinov, R.R.; Rusanova, M.Y.; VanderPorten, D.; Tsirlina, G.A.; Fawcett, W.R. Toward the reactivity prediction: Outersphere electroreduction of transition-metal ammine complexes. J. Phys. Chem. C 2009, 113, 2881–2890. [Google Scholar] [CrossRef]
- Nosal-Wiercińska, A.; Martyna, M.; Szabelska, A.; Gołębiowska, B. Catalysis of Indium Ion Electroreduction in the Presence of Acetazolamide in Chlorates(VII) Solutions with Varied Water Activity. ChemPhysChem 2024, 25, e202300789. [Google Scholar] [CrossRef]
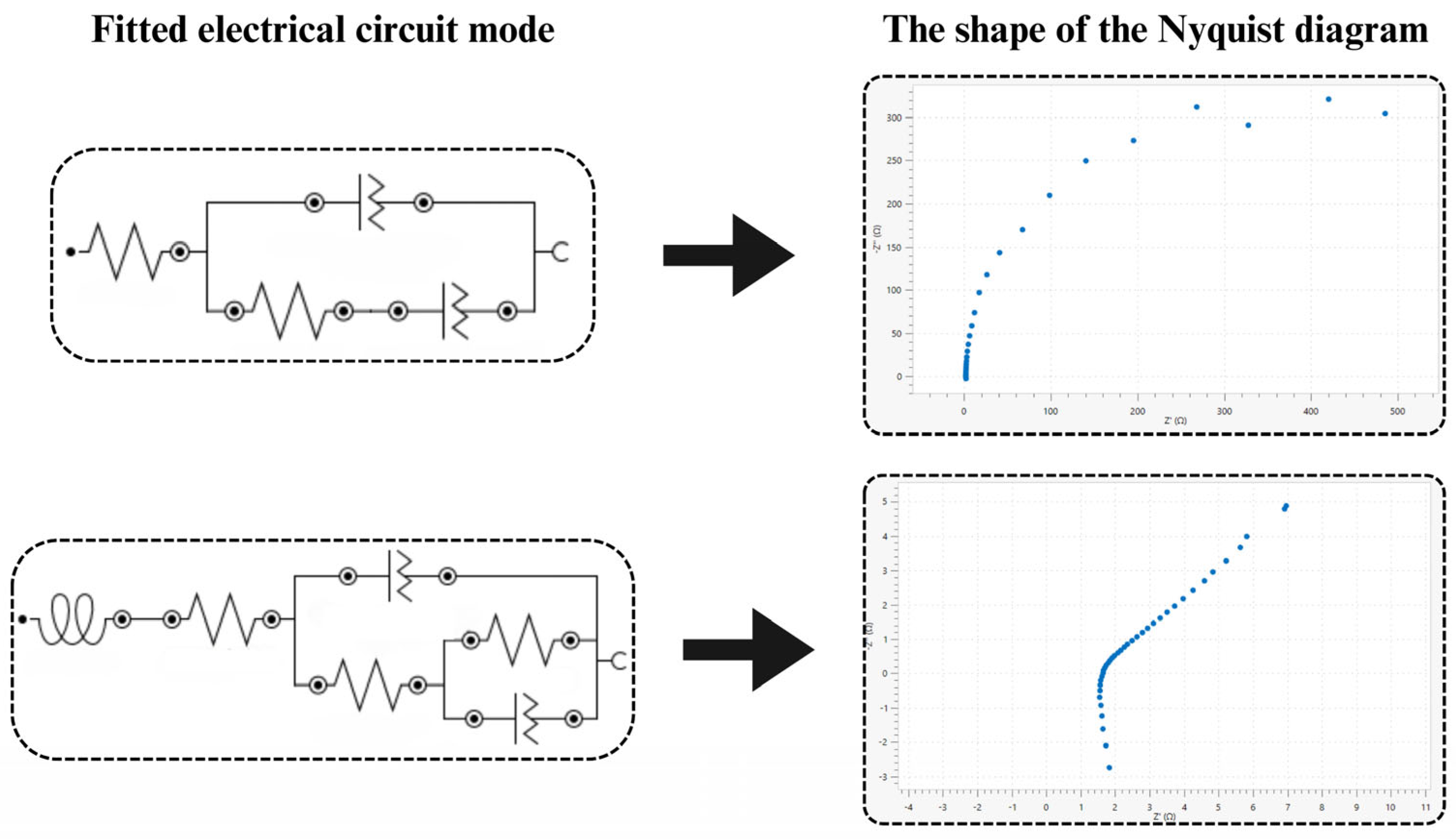
| CCTAB/mol∙dm−3 | Ra/Ω·cm2 | C1OSASS/mol∙dm−3 | Ra/Ω·cm2 |
|---|---|---|---|
| Solution A (1 mol∙dm−3 chlorate(VII) ions) | |||
| 0 | 121 | 0 | 121 |
| 5 × 10−5 | 1.56 | 5 × 10−5 | 123.03 |
| 1 × 10−4 | 1.08 | 1 × 10−4 | 128.67 |
| 1 × 10−3 | 0.39 | 1 × 10−3 | 132.45 |
| Solution B (1 mol∙dm−3 chlorate(VII) ions + 25%(v/v) MeOH) | |||
| 0 | 172.40 | 0 | 172.40 |
| 5 × 10−5 | 3.03 | 5 × 10−5 | 176.74 |
| 1 × 10−4 | 1.96 | 1 × 10−4 | 183.32 |
| 1 × 10−3 | 0.91 | 1 × 10−3 | 198.14 |
| Solution C (1 mol∙dm−3 chlorate(VII) ions+ 50%(v/v) MeOH) | |||
| 0 | 196.47 | 0 | 196.47 |
| 5 × 10−5 | 4.68 | 5 × 10−5 | 202.90 |
| 1 × 10−4 | 2.41 | 1 × 10−4 | 208.39 |
| 1 × 10−3 | - * | 1 × 10−3 | 211.43 |
| CCTAB/mol∙dm−3 | ∆E/V/V/mV·s−1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 50 | 100 | 200 | 500 | 1000 | |
| Solution A (1 mol∙dm−3 chlorate(VII) ions) | ||||||||
| 0 | 0.074 | 0.072 | 0.073 | 0.088 | 0.070 | 0.074 | 0.089 | 0.099 |
| 5 × 10−5 | 0.069 | 0.065 | 0.064 | 0.059 | 0.079 | 0.073 | 0.099 | 0.090 |
| 1 × 10−4 | 0.039 | 0.040 | 0.044 | 0.060 | 0.075 | 0.070 | 0.083 | 0.079 |
| 1 × 10−3 | 0.029 | 0.025 | 0.027 | 0.055 | 0.055 | 0.060 | 0.073 | 0.077 |
| Solution B (1 mol∙dm−3 chlorate(VII) + 25%(v/v) MeOH) | ||||||||
| 0 | 0.084 | 0.089 | 0.090 | 0.132 | 0.171 | 0.181 | 0.230 | 0.259 |
| 5 × 10−5 | 0.078 | 0.076 | 0.078 | 0.088 | 0.078 | 0.083 | 0.101 | 0.140 |
| 1 × 10−4 | 0.043 | 0.044 | 0.045 | 0.090 | 0.070 | 0.079 | 0.093 | 0.139 |
| 1 × 10−3 | 0.033 | 0.032 | 0.032 | 0.095 | 0.095 | 0.089 | 0.094 | 0.130 |
| Solution C (1 mol∙dm−3 chlorate(VII)+ 50%(v/v) MeOH) | ||||||||
| 0 | 0.088 | 0.090 | 0.098 | 0.151 | 0.180 | 0.191 | 0.240 | 0.264 |
| 5 × 10−5 | 0.079 | 0.078 | 0.079 | 0.093 | 0.120 | 0.130 | 0.191 | 0.201 |
| 1 × 10−4 | 0.051 | 0.053 | 0.052 | 0.090 | 0.110 | 0.120 | 0.180 | 0.190 |
| 1 × 10−3 * | - | - | - | - | - | - | - | - |
| C1OSASS [mol∙dm−3] | ∆E/V/v/mV·s−1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 50 | 100 | 200 | 500 | 1000 | |
| Solution A (1 mol∙dm−3 chlorate(VII) ions) | ||||||||
| 0 | 0.074 | 0.072 | 0.073 | 0.088 | 0.070 | 0.074 | 0.089 | 0.099 |
| 5 × 10−5 | 0.083 | 0.083 | 0.081 | 0.100 | 0.122 | 0.156 | 0.180 | 0.208 |
| 1 × 10−4 | 0.085 | 0.087 | 0.090 | 0.112 | 0.127 | 0.158 | 0.183 | 0.220 |
| 1 × 10−3 | 0.089 | 0.090 | 0.093 | 0.122 | 0.132 | 0.162 | 0.187 | 0.213 |
| Solution B (1 mol∙dm−3 chlorate(VII) ions + 25%(v/v) MeOH) | ||||||||
| 0 | 0.084 | 0.089 | 0.090 | 0.132 | 0.171 | 0.181 | 0.230 | 0.259 |
| 5 × 10−5 | 0.097 | 0.093 | 0.094 | 0.137 | 0.178 | 0.185 | 0.235 | 0.260 |
| 1 × 10−4 | 0.100 | 0.100 | 0.104 | 0.139 | 0.207 | 0.208 | 0.239 | 0.269 |
| 1 × 10−3 | 0.105 | 0.107 | 0.106 | 0.143 | 0.210 | 0.214 | 0.243 | 0.272 |
| Solution C (1 mol∙dm−3 chlorate(VII) ions + 50%(v/v) MeOH) | ||||||||
| 0 | 0.088 | 0.090 | 0.098 | 0.151 | 0.180 | 0.191 | 0.240 | 0.264 |
| 5 × 10−5 | 0.100 | 0.107 | 0.104 | 0.154 | 0.179 | 0.196 | 0.239 | 0.265 |
| 1 × 10−4 | 0.109 | 0.103 | 0.107 | 0.159 | 0.210 | 0.217 | 0.247 | 0.273 |
| 1 × 10−3 * | 0.122 | 0.123 | 0.121 | 0.163 | 0.215 | 0.219 | 0.257 | 0.277 |
| Csurf./mol·dm−3/Surfactnt | Ef0/V | E1/2r/V | Csurf./mol·dm−3 /Surfactant | Ef0/V | E1/2r/V |
|---|---|---|---|---|---|
| Solution A (1 mol∙dm−3 chlorate(VII) ions) | |||||
| 0 | 0.016 | 0.010 | 0 | 0.016 | 0.0100 |
| 5 × 10−5 CTAB | 0.013 | 0.014 | 5 × 10−5 1OSASS | 0.020 | 0.0035 |
| 1 × 10−4 CTAB | 0.015 | 0.010 | 1 × 10−4 1OSASS | 0.016 | 0.0083 |
| 1 × 10−3 CTAB | 0.016 | 0.011 | 1 × 10−3 1OSASS | 0.018 | 0.0081 |
| Solution B (1 mol∙dm−3 chlorate(VII) ions + 25%(v/v) MeOH) | |||||
| 0 | 0.031 | 0.014 | 0 | 0.031 | 0.014 |
| 5 × 10−5 CTAB | 0.016 | 0.013 | 5 × 10−5 1OSASS | 0.30 | 0.013 |
| 1 × 10−4 CTAB | 0.025 | 0.020 | 1 × 10−4 1OSASS | 0.028 | 0.014 |
| 1 × 10−3 CTAB | 0.025 | 0.021 | 1 × 10−3 1OSASS | 0.027 | 0.018 |
| Solution C (1 mol∙dm−3 chlorate(VII) ions + 50%(v/v) MeOH) | |||||
| 0 | 0.037 | 0.230 | 0 | 0.037 | 0.230 |
| 5 × 10−5 CTAB | 0.040 | 0.039 | 5 × 10−5 1OSASS | 0.044 | 0.042 |
| 1 × 10−4 CTAB | 0.042 | 0.035 | 1 × 10−4 1OSASS | 0.042 | 0.038 |
| 1 × 10−3 CTAB * | - | - | 1 × 10−3 1OSASS | 0.041 | 0.032 |
| Csurf./mol·dm−3/Surfactnt | α | 104 ks/cm·s−1 | Csurf./mol·dm−3/Surfactat | α | 104 ks/cm·s−1 |
|---|---|---|---|---|---|
| Solution A (1 mol∙dm−3 chlorates(VII)) | |||||
| 0 | 0.33 | 1.30 | 0 | 0.33 | 1.30 |
| 5 × 10−5 CTAB | 0.44 | 32.40 | 5 × 10−5 1OSASS | 0.025 | 1.00 |
| 1 × 10−4 CTAB | 0.57 | 54.41 | 1 × 10−4 1OSASS | 0.023 | 0.95 |
| 1 × 10−3 CTAB | 0.68 | 76.50 | 1 × 10−3 1OSASS | 0.020 | 0.84 |
| Solution B (1 mol∙dm−3 chlorate(VII) + 25%(v/v) MeOH) | |||||
| 0 | 0.32 | 0.70 | 0 | 0.32 | 0.70 |
| 5 × 10−5 CTAB | 0.41 | 7.07 | 5 × 10−5 1OSASS | 0.22 | 0.80 |
| 1 × 10−4 CTAB | 0.55 | 34.70 | 1 × 10−4 1OSASS | 0.20 | 0.40 |
| 1 × 10−3 CTAB | 0.60 | 49.10 | 1 × 10−3 1OSASS | 0.17 | 0.18 |
| Solution C (1 mol∙dm−3 chlorate(VII + 50%(v/v) MeOH) | |||||
| 0 | 0.31 | 0.46 | 0 | 0.31 | 0.46 |
| 5 × 10−5 CTAB | 0.380 | 4.06 | 5 × 10−5 1OSASS | 0.200 | 0.68 |
| 1 × 10−4 CTAB | 0.490 | 12.88 | 1 × 10−4 1OSASS | 0.180 | 0.21 |
| 1 × 10−3 CTAB * | - | - | 1 × 10−3 1OSASS | 0.160 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlak, A.; Nosal-Wiercińska, A. Effect of Ionic Surfactants on Kinetics and Mechanism of the Bi(III) Ion Electroreduction in the Mixed Aqueous–Organic Solutions of Supporting Electrolytes. Molecules 2024, 29, 4986. https://doi.org/10.3390/molecules29214986
Pawlak A, Nosal-Wiercińska A. Effect of Ionic Surfactants on Kinetics and Mechanism of the Bi(III) Ion Electroreduction in the Mixed Aqueous–Organic Solutions of Supporting Electrolytes. Molecules. 2024; 29(21):4986. https://doi.org/10.3390/molecules29214986
Chicago/Turabian StylePawlak, Alicja, and Agnieszka Nosal-Wiercińska. 2024. "Effect of Ionic Surfactants on Kinetics and Mechanism of the Bi(III) Ion Electroreduction in the Mixed Aqueous–Organic Solutions of Supporting Electrolytes" Molecules 29, no. 21: 4986. https://doi.org/10.3390/molecules29214986
APA StylePawlak, A., & Nosal-Wiercińska, A. (2024). Effect of Ionic Surfactants on Kinetics and Mechanism of the Bi(III) Ion Electroreduction in the Mixed Aqueous–Organic Solutions of Supporting Electrolytes. Molecules, 29(21), 4986. https://doi.org/10.3390/molecules29214986







