A Zebrafish Embryo Model to Screen Potential Therapeutic Compounds in Sapindaceae Poisoning
Abstract
1. Introduction
2. Results
2.1. Zebrafish Embryo Acute Toxicity Test
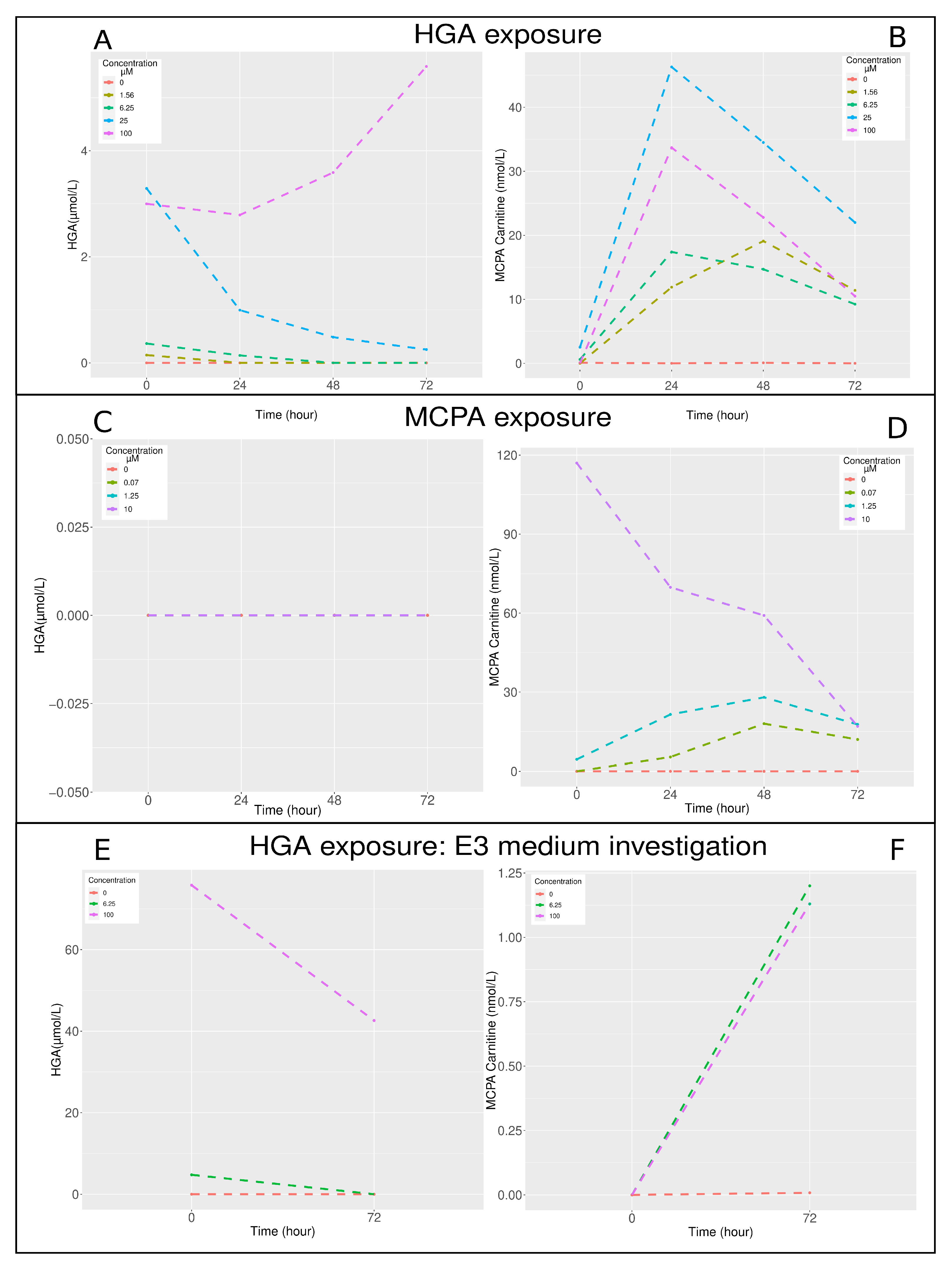
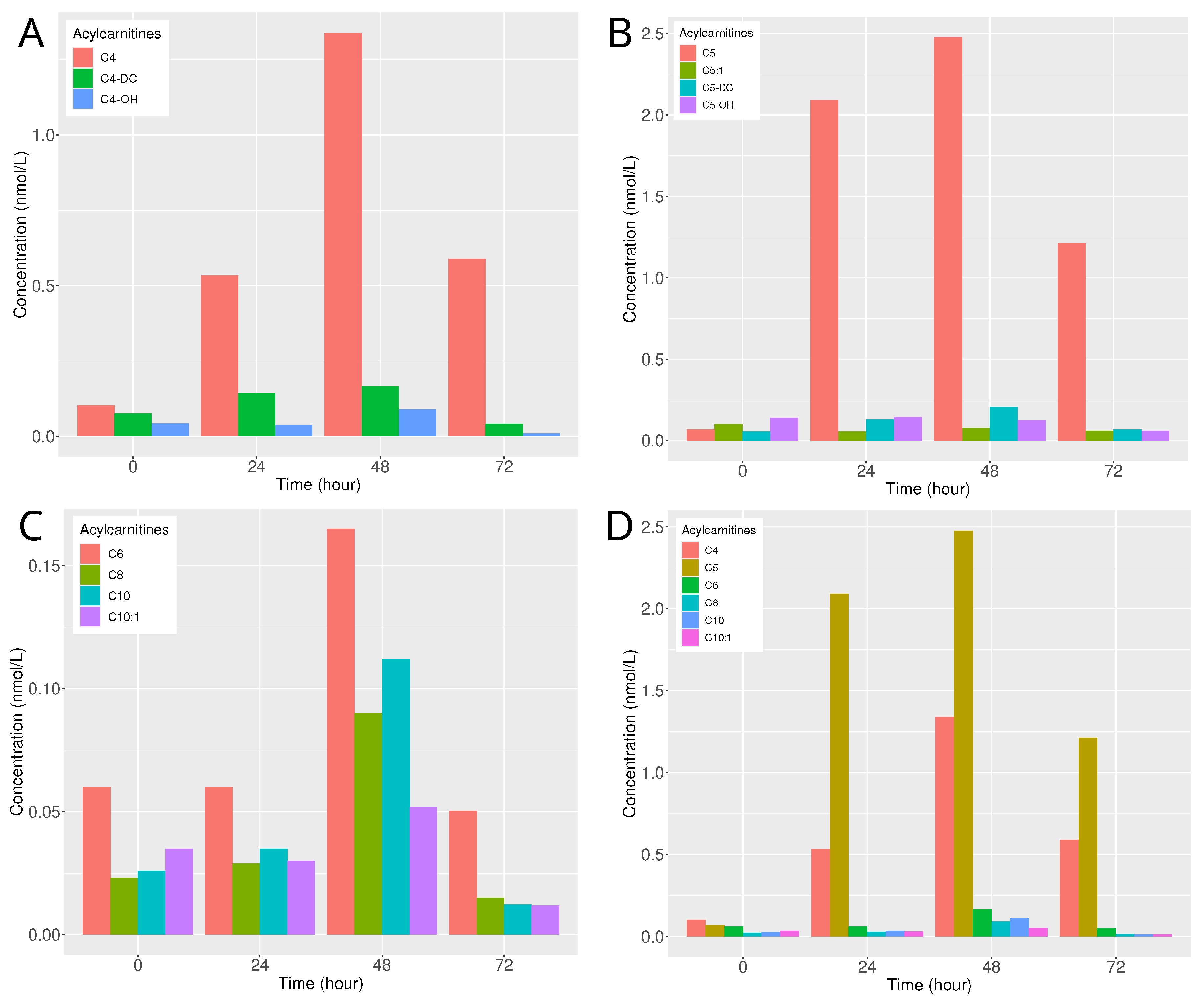
2.2. Toxin, Toxic Metabolite Dosage, and Acylcarnitines Profile
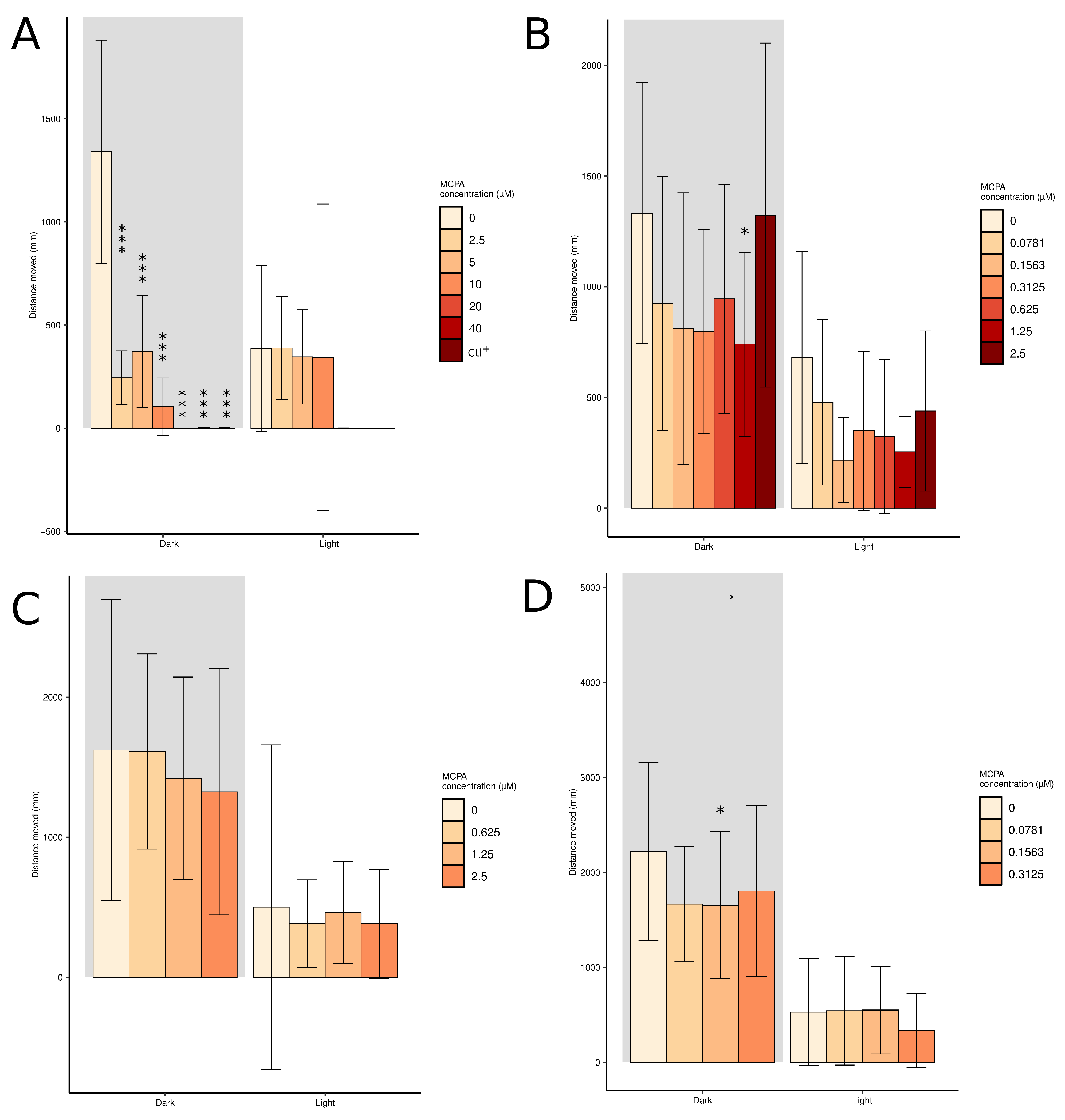
2.3. Short-Term Toxicity Test and Locomotor Behaviour Assessment
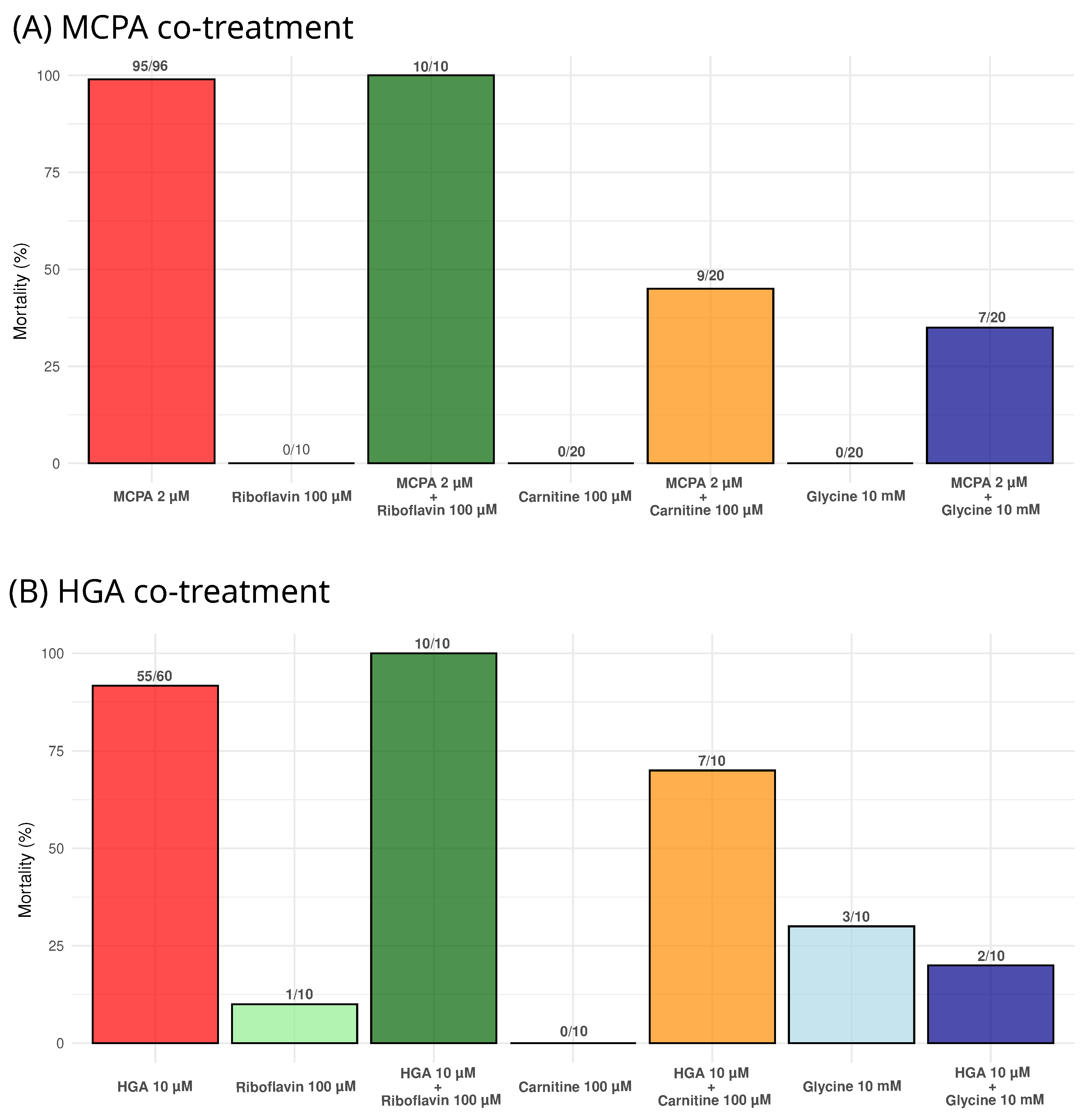
2.4. Impact of Riboflavin, Carnitine, and Glycine on Zebrafish Larval Mortality under MCPA and HGA Exposure
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Zebrafish Husbandry
4.3. Zebrafish Embryo Acute Toxicity Test
4.4. Acylcarnitines Profile
4.5. Short-Term Toxicity Test and Locomotor Behaviour Assessment
4.6. Impact of Riboflavin, Carnitine, and Glycine on Zebrafish Larval Mortality under MCPA and HGA Exposure
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Atypical myopathy |
| C4 | Butyryl-/isobutyrylcarnitine |
| C4-OH | Hydroxy butyryl-/isobutyrylcarnitine |
| C4-DC | Succinyl-/methylmalonylcarnitine |
| C5 | Isovaleryl-/2-methylbutyrylcarnitine |
| C5:1 | Tiglycarnitine |
| C5-OH | Hydroxy isovalerylcarnitine |
| C5-DC | Glutarylcarnitine |
| C6 | Hexanoylcarnitine |
| C8 | Octanoylcarnitine |
| C10 | Decanoylcarnitine |
| DMSO | Dimethyl sulphoxide |
| N EC50 | Median effective concentration |
| HGA | Hypoglycin A |
| LC50 | Median lethal concentration |
| LC-MS | Liquid chromatography–mass spectrometry |
| MCPA | Methylenecyclopropylacetate |
| MCPA-CoA | Methylenecyclopropylacetyl-CoA |
| MCPrG | Methylenecyclopropylglycine |
| MS/MS | Tandem mass spectrometry |
| OECD | Organisation for Economic Co-operation and Development |
| RFT | Range finding test |
| zFET | Zebrafish embryo toxicity |
References
- Huybrechts, B.; Callebaut, A. Pyrrolizidine alkaloids in food and feed on the Belgian market. Food Addit. Contam. Part A 2015, 32, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Fowden, L.; Pratt, H.M. Cyclopropylamino acids of the genus Acer: Distribution and biosynthesis. Phytochemistry 1973, 12, 1677–1681. [Google Scholar] [CrossRef]
- Sherratt, H. Hypoglycin, the famous toxin of the unripe Jamaican ackee fruit. Trends Pharmacol. Sci. 1986, 7, 186–191. [Google Scholar] [CrossRef]
- Spencer, P.S.; Palmer, V.S. The enigma of litchi toxicity: An emerging health concern in southern Asia. Lancet Glob. Health 2017, 5, e383–e384. [Google Scholar] [CrossRef] [PubMed]
- Valberg, S.J.; Sponseller, B.T.; Hegeman, A.D.; Earing, J.; Bender, J.B.; Martinson, K.L.; Patterson, S.E.; Sweetman, L. Seasonal pasture myopathy/atypical myopathy in North America associated with ingestion of hypoglycin A within seeds of the box elder tree. EQuine Vet. J. 2013, 45, 419–426. [Google Scholar] [CrossRef]
- Unger, L.; Nicholson, A.; Jewitt, E.; Gerber, V.; Hegeman, A.; Sweetman, L.; Valberg, S. Hypoglycin A Concentrations in Seeds of Acer Pseudoplatanus Trees Growing on Atypical Myopathy-Affected and Control Pastures. J. Vet. Intern. Med. 2014, 28, 1289–1293. [Google Scholar] [CrossRef]
- Votion, D.M.; van Galen, G.; Sweetman, L.; Boemer, F.; de Tullio, P.; Dopagne, C.; Lefère, L.; Mouithys-Mickalad, A.; Patarin, F.; Rouxhet, S.; et al. Identification of methylenecyclopropyl acetic acid in serum of European horses with atypical myopathy. Equine Vet. J. 2014, 46, 146–149. [Google Scholar] [CrossRef]
- Westermann, C.; van Leeuwen, R.; van Raamsdonk, L.; Mol, H. Hypoglycin A Concentrations in Maple Tree Species in the Netherlands and the Occurrence of Atypical Myopathy in Horses. J. Vet. Intern. Med. 2016, 30, 880–884. [Google Scholar] [CrossRef]
- Boemer, F.; Detilleux, J.; Cello, C.; Amory, H.; Marcillaud-Pitel, C.; Richard, E.; van Galen, G.; van Loon, G.; Lefère, L.; Votion, D.M. Acylcarnitines profile best predicts survival in horses with atypical myopathy. PLoS ONE 2017, 12, e0182761. [Google Scholar] [CrossRef]
- Sander, J.; Terhardt, M.; Janzen, N.; Renaud, B.; Kruse, C.J.; François, A.C.; Wouters, C.P.; Boemer, F.; Votion, D.M. Tissue Specific Distribution and Activation of Sapindaceae Toxins in Horses Suffering from Atypical Myopathy. Animals 2023, 13, 2410. [Google Scholar] [CrossRef]
- van Galen, G.; Saegerman, C.; Marcillaud Pitel, C.; Patarin, F.; Amory, H.; Baily, J.D.; Cassart, D.; Gerber, V.; Hahn, C.; Harris, P.; et al. European outbreaks of atypical myopathy in grazing horses (2006–2009): Determination of indicators for risk and prognostic factors. Equine Vet. J. 2012, 44, 621–625. [Google Scholar] [CrossRef] [PubMed]
- van Galen, G.; Marcillaud Pitel, C.; Saegerman, C.; Patarin, F.; Amory, H.; Baily, J.D.; Cassart, D.; Gerber, V.; Hahn, C.; Harris, P.; et al. European outbreaks of atypical myopathy in grazing equids (2006–2009): Spatiotemporal distribution, history and clinical features. Equine Vet. J. 2012, 44, 614–620. [Google Scholar] [CrossRef] [PubMed]
- González-Medina, S.; Ireland, J.L.; Piercy, R.J.; Newton, J.R.; Votion, D.M. Equine atypical myopathy in the UK: Epidemiological characteristics of cases reported from 2011 to 2015 and factors associated with survival. Equine Vet. J. 2017, 49, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Osmundsen, H.; Sherratt, H. A Novel mechanism for inhibition of β-oxidation by methylenecyclopropylacetyl-CoA, a metabolite of hypoglycin. FEBS Lett. 1975, 55, 38–41. [Google Scholar] [CrossRef]
- Chen, K.; Anderson, R.C.; McCowen, M.C.; Harris, P.N. Pharmacologic action of hypoglycin A and B. J. Pharmacol. Exp. Ther. 1957, 121, 272–285. [Google Scholar]
- Melde, K.; Jackson, S.; Bartlett, K.; Sherratt, H.; Ghisla, S. Metabolic consequences of methylenecyclopropylglycine poisoning in rats. Biochem. J. 1991, 274, 395–400. [Google Scholar] [CrossRef]
- Qiu, Y.; Perry, R.J.; Camporez, J.P.; Zhang, X.M.; Kahn, M.; Cline, G.; I. Shulman, G.; Vatner, D. In Vivo Studies on the Mechanism of Methylenecyclopropylacetic acid and Methylenecyclopropylglycine-Induced Hypoglycemia. Biochem. J. 2018, 475, BCJ20180063. [Google Scholar] [CrossRef]
- Delguste, C.; Baise, E.; Schwarzwald, C.C.; Sandersen, C.F.; Cassart, D.; Rollin, F.; Amory, H. Myopathies atypiques chez les chevaux au pré: Une série de cas en Belgique. Ann. Med. Vet. 2002, 146, 231–243. [Google Scholar] [CrossRef]
- Kruse, C.J.; Stern, D.; Mouithys-Mickalad, A.; Niesten, A.; Art, T.; Lemieux, H.; Votion, D.M. In Vitro Assays for the Assessment of Impaired Mitochondrial Bioenergetics in Equine Atypical Myopathy. Life 2021, 11, 719. [Google Scholar] [CrossRef]
- Wouters, C. Metabolomic/Lipidomic Investigations in Equine Atypical Myopathy and In Vitro Screening of Non-Pharmaceutical Compounds. Ph.D. Thesis, Univerity of Liège, Liège, Belgium, 2021. [Google Scholar]
- Tang, B.; More, V. Recent Advances in Drug Discovery Toxicology. Int. J. Toxicol. 2023, 42, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, D.; Xu, J.; Hu, J.; Wang, Y. Complex in vitro model: A transformative model in drug development and precision medicine. Clin. Transl. Sci. 2024, 17, e13695. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Lin, X.; Liu, Z.; Duan, X.; Yuan, Y.; Zhang, J.; Liang, Q.; Ji, X.; Sun, N.; Yu, H.; et al. Worm Generator: A System for High-Throughput in Vivo Screening. Nano Lett. 2023, 23, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.; Otterstrom, J.; Hoade, Y.; Bjedov, I.; Stead, E.; Whelan, M.; Gestri, G.; Paran, Y.; Payne, E. A versatile, automated and high-throughput drug screening platform for zebrafish embryos. Biol. Open 2021, 10, bio058513. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Simonich, M.T.; Tanguay, R.L. Developmental Toxicity Assessment Using Zebrafish-Based High-Throughput Screening. In Zebrafish: Methods and Protocols; Amatruda, J.F., Houart, C., Kawakami, K., Poss, K.D., Eds.; Springer: New York, NY, USA, 2024; pp. 71–82. [Google Scholar] [CrossRef]
- Truong, L.; Harper, S.L.; Tanguay, R.L. Evaluation of Embryotoxicity Using the Zebrafish Model. In Drug Safety Evaluation: Methods and Protocols; Gautier, J.C., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 271–279. [Google Scholar] [CrossRef]
- McGrath, P.; Li, C.Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 3417. [Google Scholar] [CrossRef]
- Silva Brito, R.; Canedo, A.; Farias, D.; Rocha, T.L. Transgenic zebrafish (Danio rerio) as an emerging model system in ecotoxicology and toxicology: Historical review, recent advances, and trends. Sci. Total Environ. 2022, 848, 157665. [Google Scholar] [CrossRef]
- Lu, P.H.; Lee, H.Y.; Liou, Y.L.; Tung, S.F.; Kuo, K.L.; Chen, Y.H. Nephroprotective Role of Zhibai Dihuang Wan in Aristolochic Acid-Intoxicated Zebrafish. BioMed Res. Int. 2020, 2020, 5204348. [Google Scholar] [CrossRef]
- Tsai, J.N.; Wang, Y.H.; Lin, P.J.; Chang, C.F.; Sun, C.Y.; Chen, Y.H. Nephroprotective effects of coriander (Coriandrum sativum) leaves aqueous extracts in aristolochic acid-intoxicated zebrafish embryos. Environ. Toxicol. 2024, 39, 4014–4021. [Google Scholar] [CrossRef]
- Bochnia, M.; Sander, J.; Ziegler, J.; Terhardt, M.; Sander, S.; Janzen, N.; Cavalleri, J.M.V.; Zuraw, A.; Wensch-Dorendorf, M.; Zeyner, A. Detection of MCPG metabolites in horses with atypical myopathy. PLoS ONE 2019, 14, e0211698. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Warren, K.S.; Yellen, G.; Fishman, M.C. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc. Natl. Acad. Sci. USA 1997, 94, 4554–4559. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Incardona, J.P.; Pelkki, K.; Shepardson, S.; Hodson, P.V. AhR2-mediated, CYP1A-independent cardiovascular toxicity in zebrafish (Danio rerio) embryos exposed to retene. Aquat. Toxicol. 2011, 101, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Meng, Y.; Huang, Y.; Liu, Z.; Zhong, K.; Ma, J.; Zhang, W.; Li, Y.; Lu, H. Development toxicity and cardiotoxicity in zebrafish from exposure to iprodione. Chemosphere 2021, 263, 127860. [Google Scholar] [CrossRef] [PubMed]
- Votion, D.M.; Linden, A.; Saegerman, C.; Engels, P.; Erpicum, M.; Thiry, E.; Delguste, C.; Rouxhet, S.; Demoulin, V.; Navet, R.; et al. History and Clinical Features of Atypical Myopathy in Horses in Belgium (2000–2005). J. Vet. Intern. Med. 2007, 21, 1380–1391. [Google Scholar] [CrossRef]
- Lu, H.; Shagirova, A.; Goggi, J.; Yeo, H.; Roy, S. Reissner fibre-induced urotensin signalling from cerebrospinal fluid-contacting neurons prevents scoliosis of the vertebrate spine. Biol. Open 2020, 9, bio052027. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tanaka, K. Selective inactivation of various acyl-CoA dehydrogenases by (methylenecyclopropyl)acetyl-CoA. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1990, 1038, 216–221. [Google Scholar] [CrossRef]
- Lai, M.T.; Li, D.; Oh, E.; Liu, H.W. Inactivation of medium-chain acyl-CoA dehydrogenase by a metabolite of hypoglycin: Characterization of the major turnover product and evidence suggesting an alternative flavin modification pathway. J. Am. Chem. Soc. 1993, 115, 1619–1628. [Google Scholar] [CrossRef]
- Westermann, C.M.; Dorland, L.; Votion, D.M.; de Sain-van der Velden, M.G.M.; Wijnberg, I.D.; Wanders, R.J.A.; Spliet, W.G.M.; Testerink, N.; Berger, R.; Ruiter, J.P.N.; et al. Acquired multiple Acyl-CoA dehydrogenase deficiency in 10 horses with atypical myopathy. Neuromuscul. Disord. 2008, 18, 355–364. [Google Scholar] [CrossRef]
- Sander, J.; Terhardt, M.; Janzen, N. Study on the Metabolic Effects of Repeated Consumption of Canned Ackee. J. Agric. Food Chem. 2020, 68, 14603–14609. [Google Scholar] [CrossRef] [PubMed]
- Böhme, S.; Stärk, H.J.; Reemtsma, T.; Kühnel, D. Effect propagation after silver nanoparticle exposure in zebrafish (Danio rerio) embryos: A correlation to internal concentration and distribution patterns. Environ. Sci. Nano 2015, 2, 603–614. [Google Scholar] [CrossRef]
- Pelka, K.E.; Henn, K.; Keck, A.; Sapel, B.; Braunbeck, T. Size does matter—Determination of the critical molecular size for the uptake of chemicals across the chorion of zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2017, 185, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Louis, K.M.; Yang, S.P.; Pedersen, J.A.; Hamers, R.J.; Peterson, R.E.; Heideman, W. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology 2012, 6, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Creton, R. The calcium pump of the endoplasmic reticulum plays a role in midline signaling during early zebrafish development. Dev. Brain Res. 2004, 151, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Henn, K.; Braunbeck, T. Dechorionation as a tool to improve the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Comp. Biochem. Physiol. Part Toxicol. Pharmacol. 2011, 153, 91–98. [Google Scholar] [CrossRef]
- Kim, K.T.; Tanguay, R.L. The role of chorion on toxicity of silver nanoparticles in the embryonic zebrafish assay. Environ. Anal. Health Toxicol. 2014, 29, e2014021. [Google Scholar] [CrossRef]
- Lamprea, M.R.; Cardenas, F.P.; Setem, J.; Morato, S. Thigmotactic responses in an open-field. Braz. J. Med. Biol. Res. 2008, 41, 135–140. [Google Scholar] [CrossRef]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Votion, D.M. Analysing hypoglycin A, methylenecyclopropylacetic acid conjugates and acylcarnitines in blood to confirm the diagnosis and improve our understanding of atypical myopathy. Equine Vet. Educ. 2018, 30, 29–30. [Google Scholar] [CrossRef]
- Fabius, L.S.; Westermann, C.M. Evidence-based therapy for atypical myopathy in horses. Equine Vet. Educ. 2018, 30, 616–622. [Google Scholar] [CrossRef]
- Brooks, S.E.H.; Audretsch, J.J. The protective effects of feeding on the hepatic ultrastructure of rats treated with hypoglycin. J. Pathol. 1971, 104, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Deberg, M.; Schoos, R.; Baise, E.; Amory, H.; Gault, G.; Carlier, J.; Gaillard, Y.; Marcillaud-Pitel, C.; Votion, D. Quantification of hypoglycin A in serum using aTRAQ® assay. J. Chromatogr. B 2015, 997, 75–80. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, R.; Brooks, J.; Hunter, D.; Padnos, B.; Irons, T.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J. Zebrafish (Danio rerio) embryo-larvae locomotor activity data analysis: Evaluating anxiolytic effects of the antidepressant compound citalopram. Data Brief 2019, 27, 104812. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio; RStudio Team: Boston, MA, USA, 2015. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Renaud, B.; Kruse, C.J.; François, A.C.; Cesarini, C.; van Loon, G.; Palmers, K.; Boemer, F.; Luis, G.; Gustin, P.; Votion, D.M. Large-scale study of blood markers in equine atypical myopathy reveals subclinical poisoning and advances in diagnostic and prognostic criteria. Environ. Toxicol. Pharmacol. 2024, 110, 104515. [Google Scholar] [CrossRef]

| Supplier Name 1 | Chemical Structure 2 | Supplier (Cas Number) | Purity |
|---|---|---|---|
| (S)-Hypoglycin A | 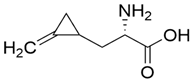 | Toronto Research Chemicals (156-56-9) | 85% |
| -(Methylenecyclopropyl)glycine (mixture of diastereomers) |  | Toronto Research Chemicals (2517-07-9) | 97% |
| (RS)-(Methylenecyclopropyl)acetic acid | 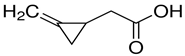 | Sigma-Aldrich (1073-00-3) | ≥95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wouters, C.P.; Klein, B.; Price, N.; Boemer, F.; Voz, M.L.; Votion, D.-M. A Zebrafish Embryo Model to Screen Potential Therapeutic Compounds in Sapindaceae Poisoning. Molecules 2024, 29, 4954. https://doi.org/10.3390/molecules29204954
Wouters CP, Klein B, Price N, Boemer F, Voz ML, Votion D-M. A Zebrafish Embryo Model to Screen Potential Therapeutic Compounds in Sapindaceae Poisoning. Molecules. 2024; 29(20):4954. https://doi.org/10.3390/molecules29204954
Chicago/Turabian StyleWouters, Clovis P., Benjamin Klein, Nicholas Price, François Boemer, Marianne L. Voz, and Dominique-Marie Votion. 2024. "A Zebrafish Embryo Model to Screen Potential Therapeutic Compounds in Sapindaceae Poisoning" Molecules 29, no. 20: 4954. https://doi.org/10.3390/molecules29204954
APA StyleWouters, C. P., Klein, B., Price, N., Boemer, F., Voz, M. L., & Votion, D.-M. (2024). A Zebrafish Embryo Model to Screen Potential Therapeutic Compounds in Sapindaceae Poisoning. Molecules, 29(20), 4954. https://doi.org/10.3390/molecules29204954









