Utilization of Tea Polyphenols as Color Developers in Reversible Thermochromic Dyes for Thermosensitive Color Change and Enhanced Functionality of Polyester Fabrics
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure of Biomass Thermochromic Dyes and Thermochromic Mechanism
2.2. Reversible Thermochromism of Dyed Polyester Fabric
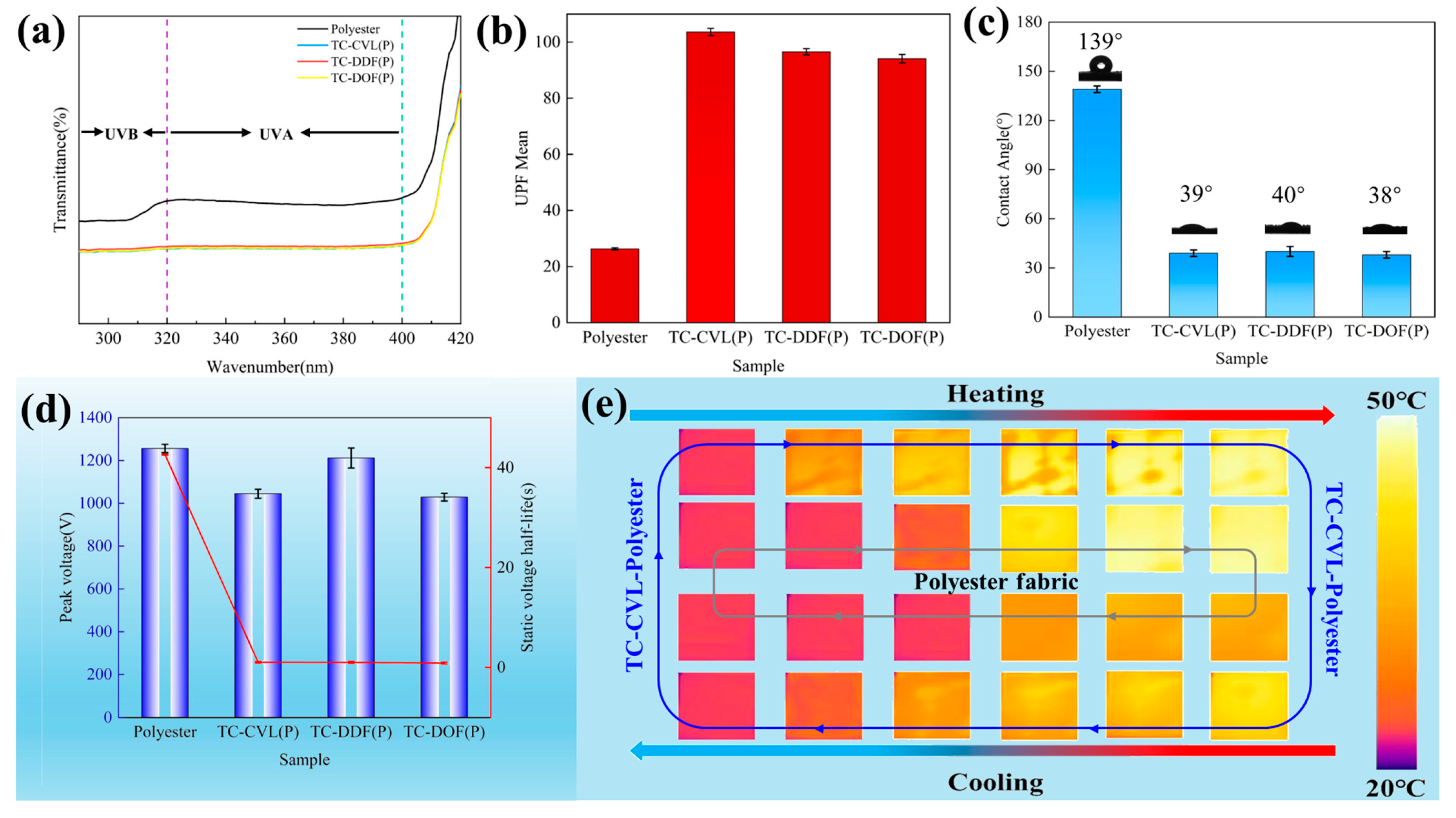
2.3. Performance of Thermochromic Polyester Fabrics
3. Materials and Methods
3.1. Materials
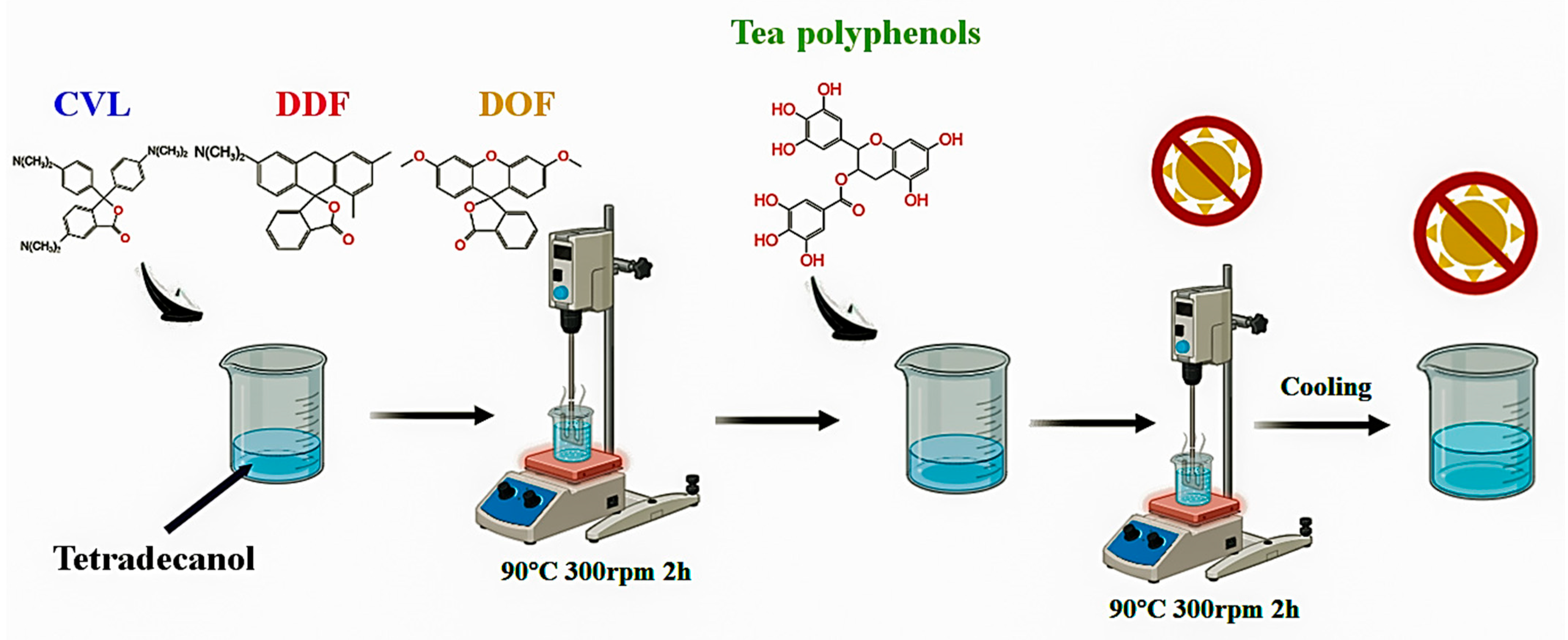
3.2. Preparation of Reversible Thermochromic Dyes
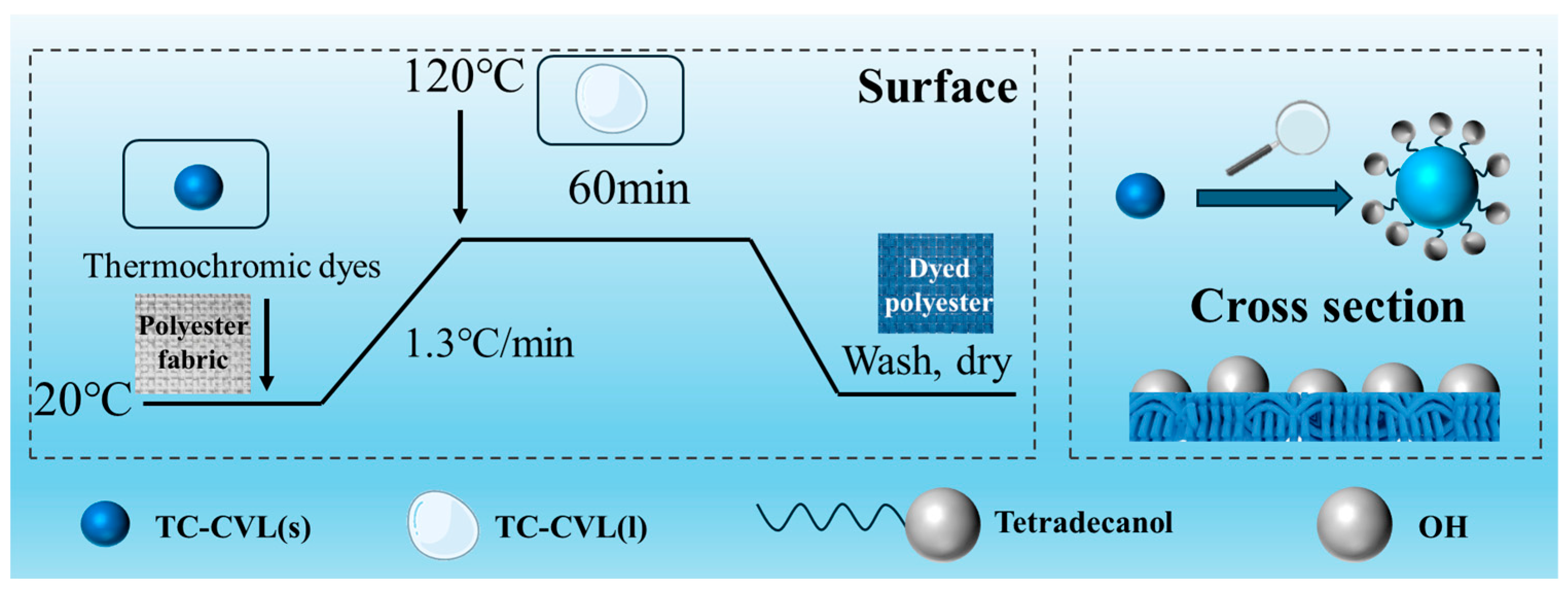
3.3. Dyeing of Polyester Fabric Using Reversible Thermochromic Dyes
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viková, M.; Pechová, M. Study of adaptive thermochromic camouflage for combat uniform. Text. Res. J. 2020, 90, 2070–2084. [Google Scholar] [CrossRef]
- Wu, S.; Sun, H.; Duan, M.; Mao, H.; Wu, Y.; Zhao, H.; Lin, B. Applications of thermochromic and electrochromic smart windows: Materials to buildings. Cell Rep. Phys. Sci. 2023, 4, 101370. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Wang, F.; Ou, J.; Li, W. Thermochromic superhydrophobic coatings for building energy conservation. Energy Build. 2021, 251, 111374. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wu, S.; Ji, Y.; Mao, Z.; Wang, D.; Xu, Z.; Wei, Q.; Feng, Q. Weavable coaxial phase change fibers concentrating thermal energy storage, photothermal conversion and thermochromic responsiveness toward smart thermoregulatory textiles. Chem. Eng. J. 2024, 483, 149281. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Li, J.; Chen, Y.; Li, B.; Zhang, L.; Fu, S. Ultrahigh-sensitivity thermochromic smart fabrics and flexible temperature sensors based on intramolecular proton-coupled electron transfer. Chem. Eng. J. 2022, 446, 136444. [Google Scholar]
- Cheng, Z.; Lei, L.; Zhao, B.; Zhu, Y.; Yu, T.; Yang, W.; Li, Y. High performance reversible thermochromic composite films with wide thermochromic range and multiple colors based on micro/nanoencapsulated phase change materials for temperature indicators. Compos. Sci. Technol. 2023, 240, 110091. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.M.; Abdeldayem, S.A.; Elshafai, N. Simultaneous Thermochromic pigment printing and Se-NP multifunctional finishing of cotton fabrics for smart childrenswear. Cloth. Text. Res. J. 2020, 38, 182–195. [Google Scholar] [CrossRef]
- Liu, J.; Tan, J.; Liu, H.; Yin, Y.; Wang, C. Multicolor-tunable biomass thermochromic dyes utilizing tea polyphenols color developer for temperature-controlled linen fabric. Ind. Crops Prod. 2023, 204, 117254. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Q.; Liu, L.; Ma, R. Thermochromic conductive fibers with modifiable solar absorption for personal thermal management and temperature visualization. ACS Nano 2023, 17, 20299–20307. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Ye, C.; Pei, Y.; Ling, S. Thermochromic silks for temperature management and dynamic textile displays. Nano-Micro Lett. 2021, 13, 72. [Google Scholar] [CrossRef]
- Wang, X.; Dong, L.; Li, J.; Wang, S.; Yao, B.; Chen, Y.; Yang, H. Reversible thermochromic properties of bisphenol A aromatic phenolic resin’s composite natural rubber film. Opt. Mater. 2024, 147, 114698. [Google Scholar] [CrossRef]
- Geng, X.; Li, W.; Wang, Y.; Lu, J.; Wang, J.; Wang, N.; Li, J.; Zhang, X. Reversible thermochromic microencapsulated phase change materials for thermal energy storage application in thermal protective clothing. Appl. Energy 2018, 217, 281–294. [Google Scholar] [CrossRef]
- Simonelli, A.; Guadagni, R.; De Franciscis, P.; Colacurci, N.; Pieri, M.; Basilicata, P.; Pedata, P.; Lamberti, M.; Sannolo, N.; Miraglia, N. Environmental and occupational exposure to bisphenol A and endometriosis: Urinary and peritoneal fluid concentration levels. Int. Arch. Occup. Environ. Health 2016, 90, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Schönfelder, G.; Schneider, M.R. Endocrine Disruptors: Adverse Health Effects Mediated by EGFR? Trends Endocrinol. Metab. 2018, 29, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Yu, Q.; Zhang, Y.; Li, G.; Huang, Y. Improving the thermal performance of novel low-temperature phase change materials through the configuration of 1-dodecanol-tetradecane nanofluids/expanded graphite composites. J. Mol. Liq. 2021, 322, 114948. [Google Scholar] [CrossRef]
- Hou, M.; Kong, X.; Li, H.; Yang, H.; Chen, W. Experimental study on the thermal performance of composite phase change ventilated roof. J. Energy Storage 2021, 33, 102060. [Google Scholar] [CrossRef]
- Hsieh, S.; Omu, A.; Orehounig, K. Comparison of solar thermal systems with storage: From building to neighbourhood scale. Energy Build. 2017, 152, 359–372. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Yang, R.; Ke, J.; Di, X.; Liu, F.; Zhang, W.; Wang, C. Composite phase change materials with good reversible thermochromic ability in delignified wood substrate for thermal energy storage. Appl. Energy 2018, 212, 455–464. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, W.; Feng, F.; Wang, Z.; Wang, X.; Zhang, Y. Polydopamine-assisted load of palygorskite on polyester fabric for moisture absorption and perspiration. Appl. Clay Sci. 2022, 230, 106720. [Google Scholar] [CrossRef]
- Fan, L.; Tan, Y.; Amesimeku, J.; Yin, Y.; Wang, C. A novel functional disperse dye doped with graphene oxide for improving antistatic properties of polyester fabric using one-bath dyeing method. Text. Res. J. 2019, 90, 655–665. [Google Scholar] [CrossRef]
- Panák, O.; Držková, M.; Kaplanová, M. Insight into the evaluation of colour changes of leuco dye based thermochromic systems as a function of temperature. Dye. Pigment. 2015, 120, 279–287. [Google Scholar] [CrossRef]
- Panák, O.; Držková, M.; Kaplanová, M.; Novak, U.; Klanjšek Gunde, M. The relation between colour and structural changes in thermochromic systems comprising crystal violet lactone, bisphenol A, and tetradecanol. Dye. Pigment. 2017, 136, 382–389. [Google Scholar] [CrossRef]
- Zhu, C.F.; Wu, A.B. Studies on the synthesis and thermochromic properties of crystal violet lactone and its reversible thermochromic complexes. Thermochim. Acta 2005, 425, 7–12. [Google Scholar] [CrossRef]
- Zhou, W.M.; Yang, Q.; Tao, S.X.; Zhou, S.Y.; Zhu, J.; Li, R.M.; Xu, L.H.; Pan, H.; Zhang, H.J.; Zhao, H.; et al. Fabrication and reversible thermotropic characterization of polyurethane/dye microcapsules composite films for thermal management. Colloids Surf. A Physicochem. Eng. Aspect. 2024, 703, 135226. [Google Scholar] [CrossRef]
- Pei, L.; Li, H.; Zhang, H.; Wang, Z.; Wang, J. Migration and chemical characterization of cyclic oligomers from polyester fiber in waterless dyeing system. Fibers Polym. 2022, 23, 2648–2656. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, X.; Zhang, H.; Zhuang, X.; Yan, X. Facile dip-coating process towards multifunctional nonwovens: Robust noise reduction, abrasion resistance and antistatic electricity. Text. Res. J. 2017, 88, 2568–2578. [Google Scholar] [CrossRef]
- Elabid, A.E.A.; Zhang, J.; Shi, J.; Guo, Y.; Ding, K.; Zhang, J. Improving the low temperature dyeability of polyethylene terephthalate fabric with dispersive dyes by atmospheric pressure plasma discharge. Appl. Surf. Sci. 2016, 375, 26–34. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, S.S.; Liang, N.-Y. Fabrication and characterization of antistatic fiber with segmented pie structure. Text. Res. J. 2015, 86, 1828–1836. [Google Scholar] [CrossRef]
- kaplan, Ö.; gökşen Tosun, N.; Gökçe, İ.; Alkan, C. Diacid esters of 1-dodecanol as new alternatives to solid-liquid phase change materials for solar heat storage systems. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 608–622. [Google Scholar] [CrossRef]
- Gambichler, T.; Laperre, J.; Hoffmann, K. The European standard for sun-protective clothing: EN 13758. J. Eur. Acad. Dermatol. Venereol. JEADV 2006, 20, 125–130. [Google Scholar] [CrossRef]
- GBT127031-2021; Textiles—Test Methods for Electrostatic Propensity—Part 1 Test Method Using Corona Charging. China National Standardization Administration: Beijing, China, 2021.
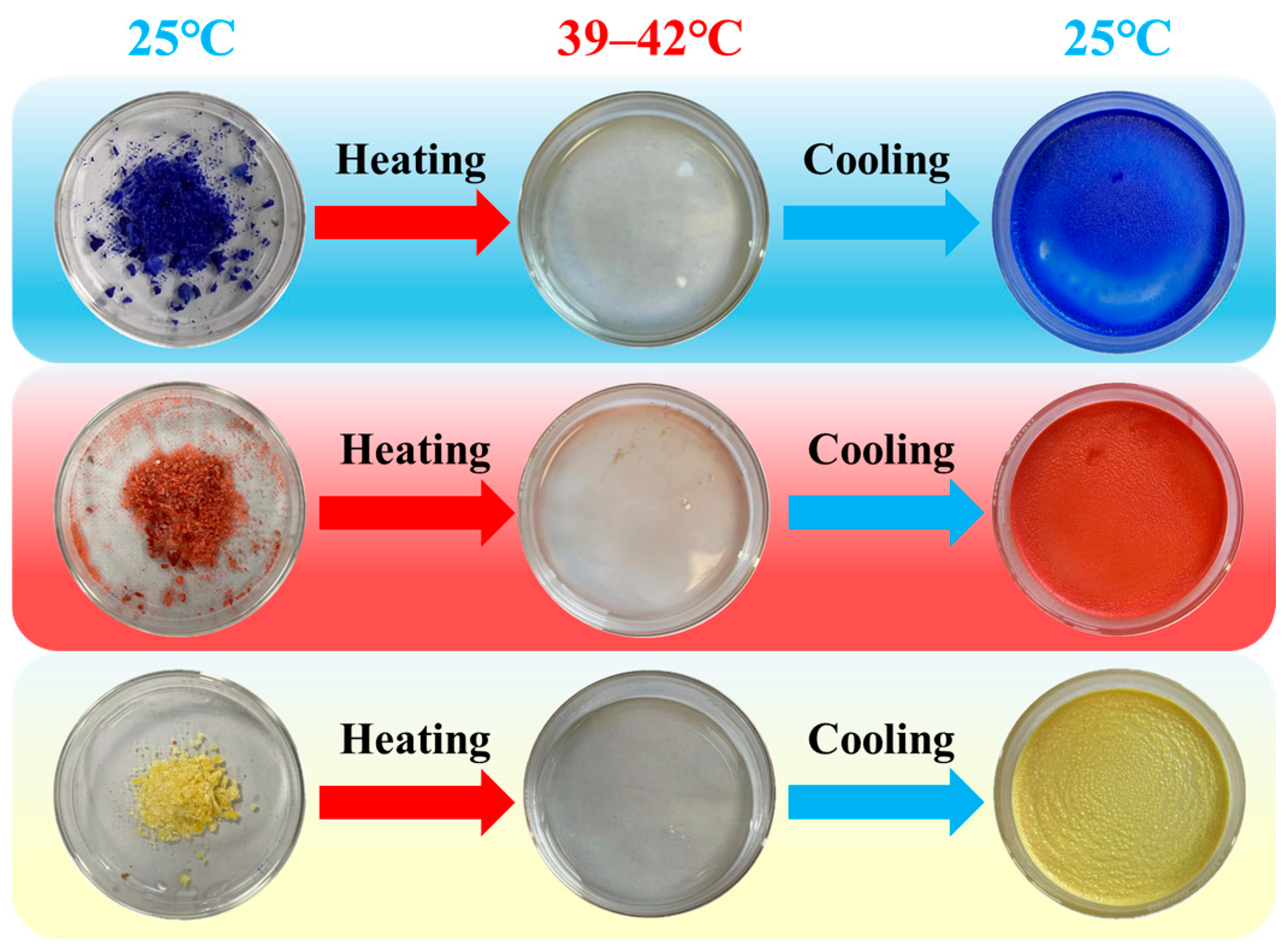
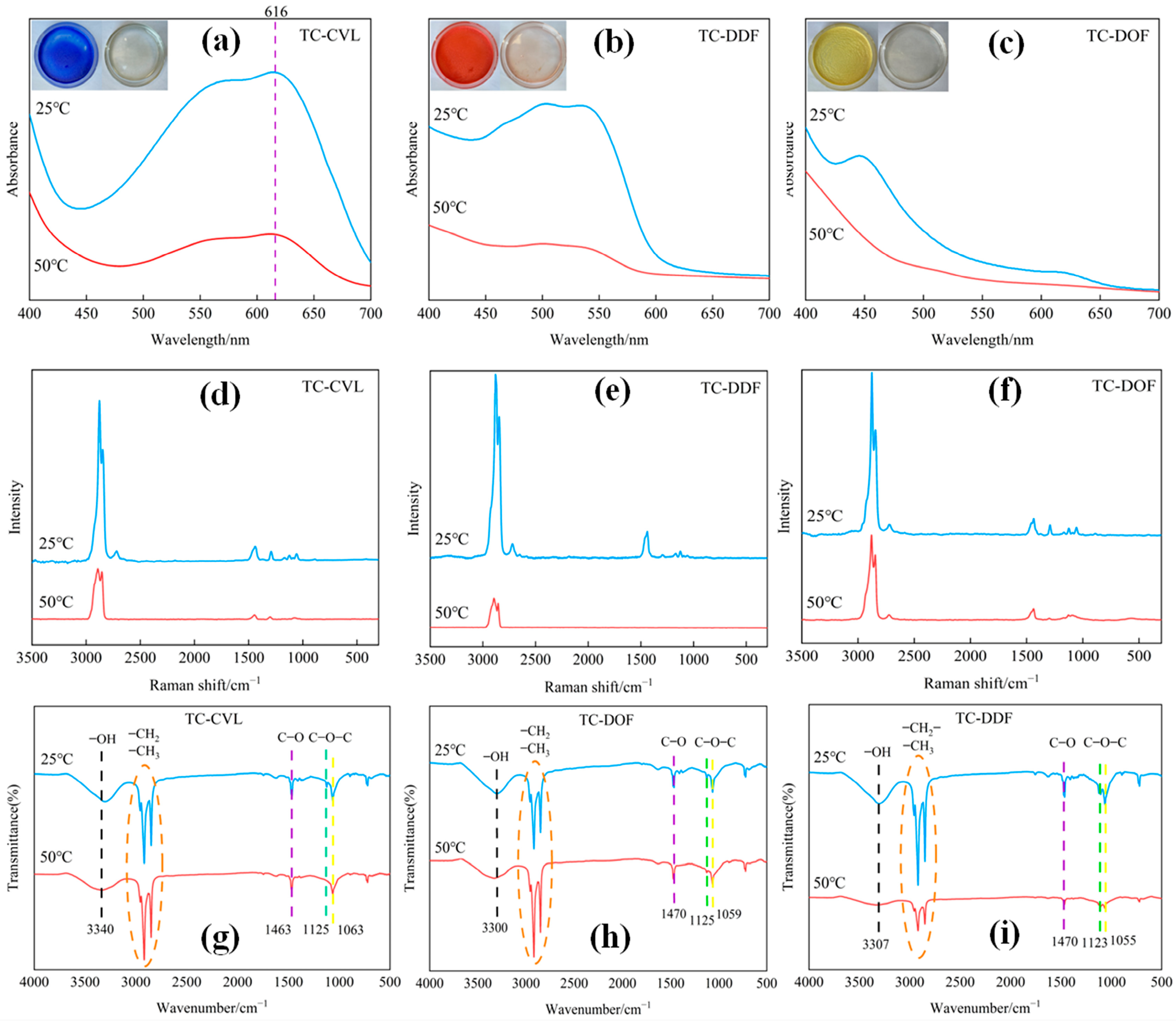
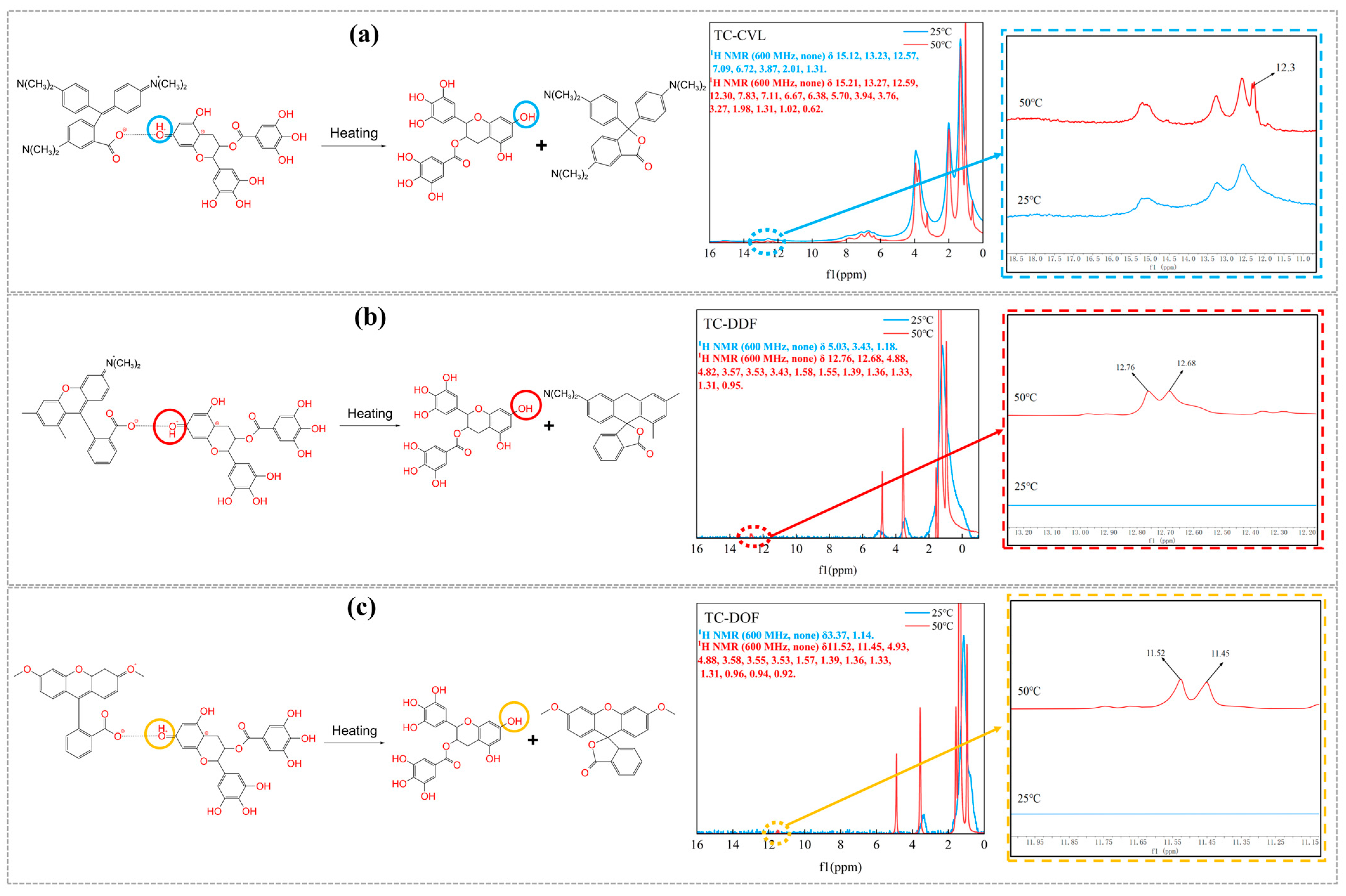
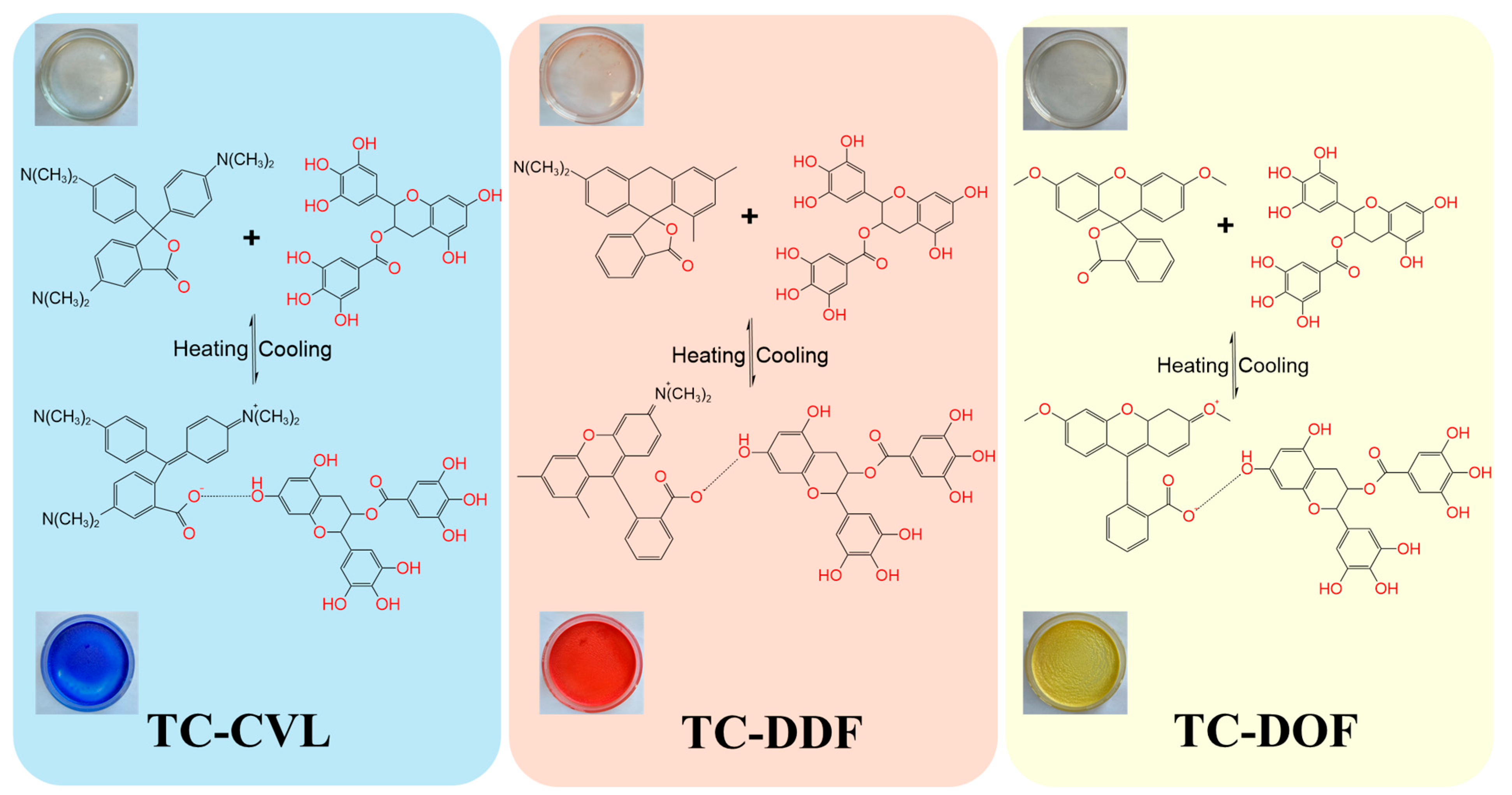


| Samples | Leuco Dyes | Chromogenic Agents | Solvent |
|---|---|---|---|
| TC-CVL | Crystal violet lactone | Tea polyphenols | Tetradecanol |
| TC-DDF | 6′-(Diethylamino)-1′,3′-dimethyl-fluoran | Tea polyphenols | Tetradecanol |
| TC-DOF | 3′,6′-Dimethoxyfluoran | Tea polyphenols | Tetradecanol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Yang, Q.; Tao, S.; Cui, J.; Zhu, J.; Zhou, S.; Li, R.; Su, J.; Zhang, N.; Xu, L.; et al. Utilization of Tea Polyphenols as Color Developers in Reversible Thermochromic Dyes for Thermosensitive Color Change and Enhanced Functionality of Polyester Fabrics. Molecules 2024, 29, 4944. https://doi.org/10.3390/molecules29204944
Zhou W, Yang Q, Tao S, Cui J, Zhu J, Zhou S, Li R, Su J, Zhang N, Xu L, et al. Utilization of Tea Polyphenols as Color Developers in Reversible Thermochromic Dyes for Thermosensitive Color Change and Enhanced Functionality of Polyester Fabrics. Molecules. 2024; 29(20):4944. https://doi.org/10.3390/molecules29204944
Chicago/Turabian StyleZhou, Weimian, Qun Yang, Sixuan Tao, Jin Cui, Jie Zhu, Siyu Zhou, Ruimiao Li, Juan Su, Ning Zhang, Lihui Xu, and et al. 2024. "Utilization of Tea Polyphenols as Color Developers in Reversible Thermochromic Dyes for Thermosensitive Color Change and Enhanced Functionality of Polyester Fabrics" Molecules 29, no. 20: 4944. https://doi.org/10.3390/molecules29204944
APA StyleZhou, W., Yang, Q., Tao, S., Cui, J., Zhu, J., Zhou, S., Li, R., Su, J., Zhang, N., Xu, L., Pan, H., & Wang, J. (2024). Utilization of Tea Polyphenols as Color Developers in Reversible Thermochromic Dyes for Thermosensitive Color Change and Enhanced Functionality of Polyester Fabrics. Molecules, 29(20), 4944. https://doi.org/10.3390/molecules29204944








