The Inhibitory Effect of Agastache rugosa Essential Oil on the Dental Biofilm
Abstract
1. Introduction
2. Results
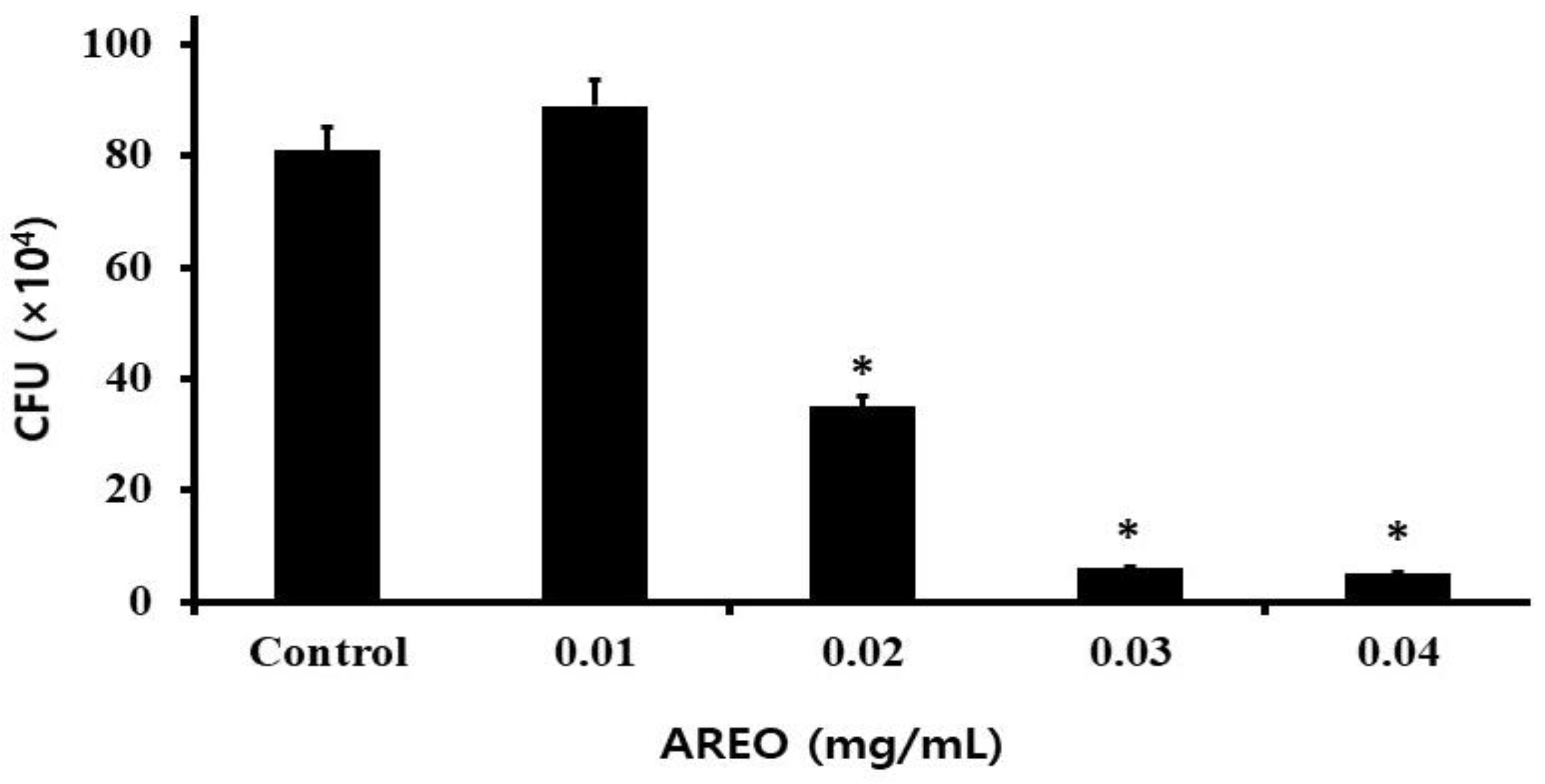
2.1. Inhibitory Effect on the Growth of S. mutans
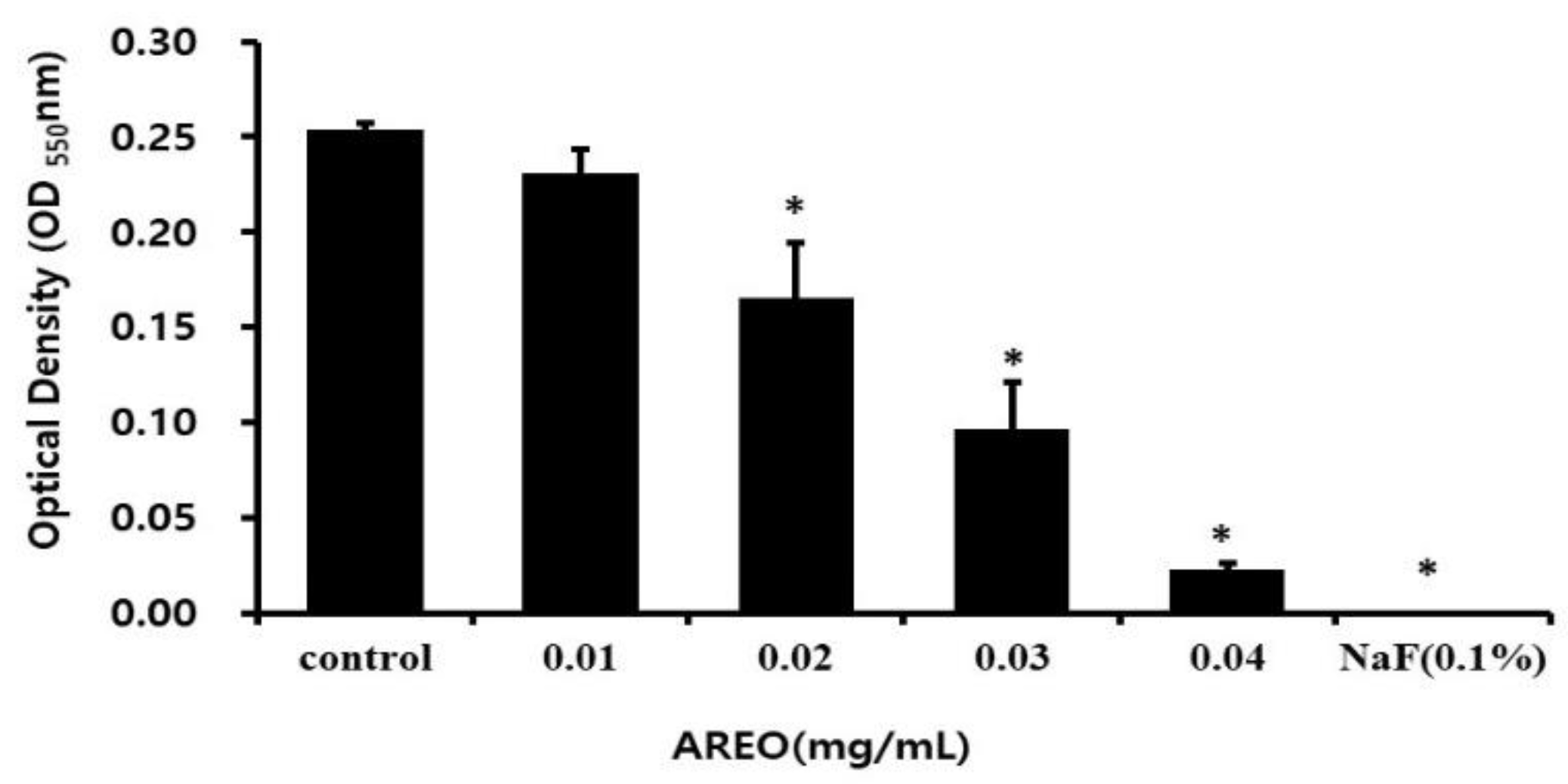
2.2. Inhibitory Effect on Adhesion to Saliva-Coated Hydroxyapatite (S-HA)
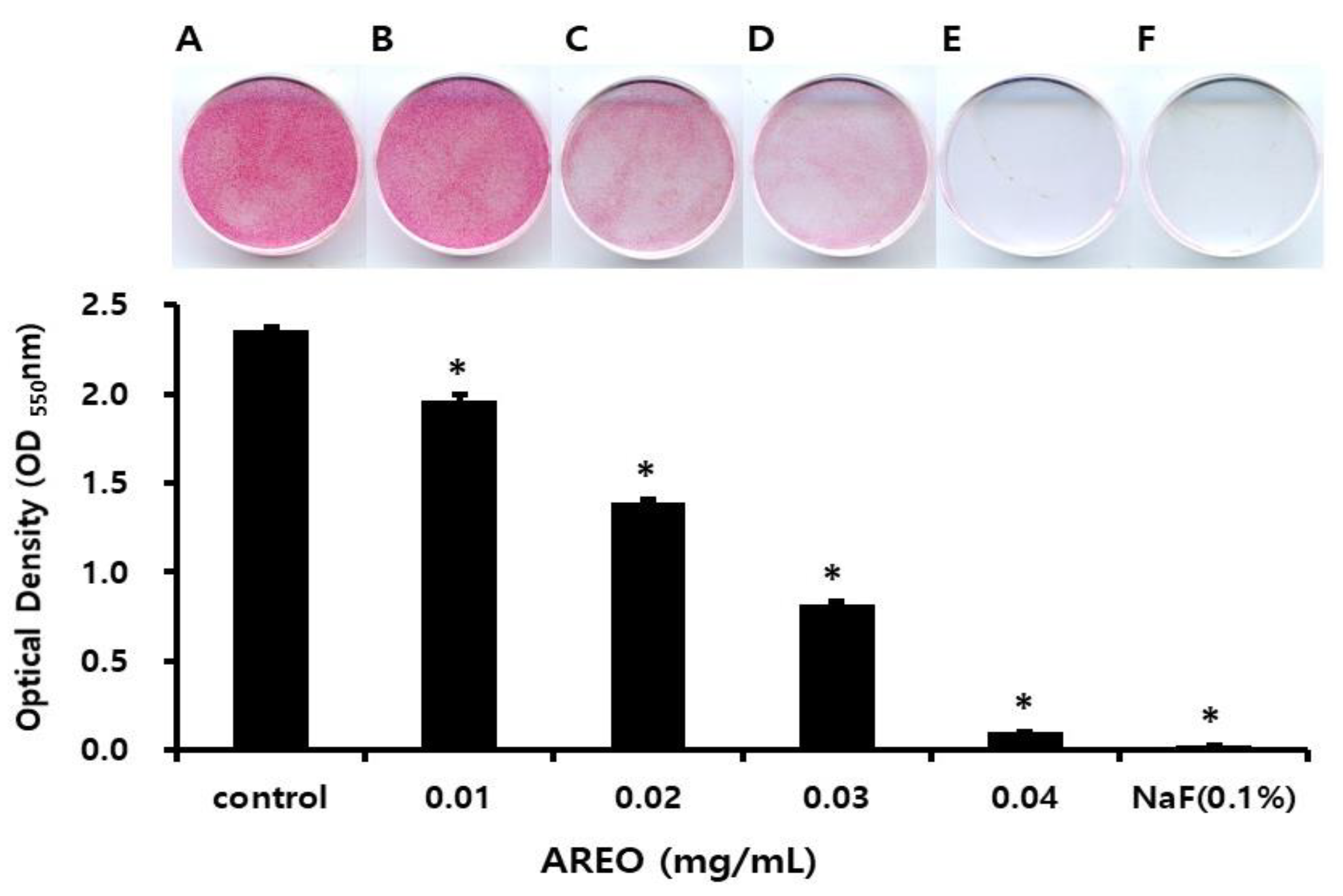
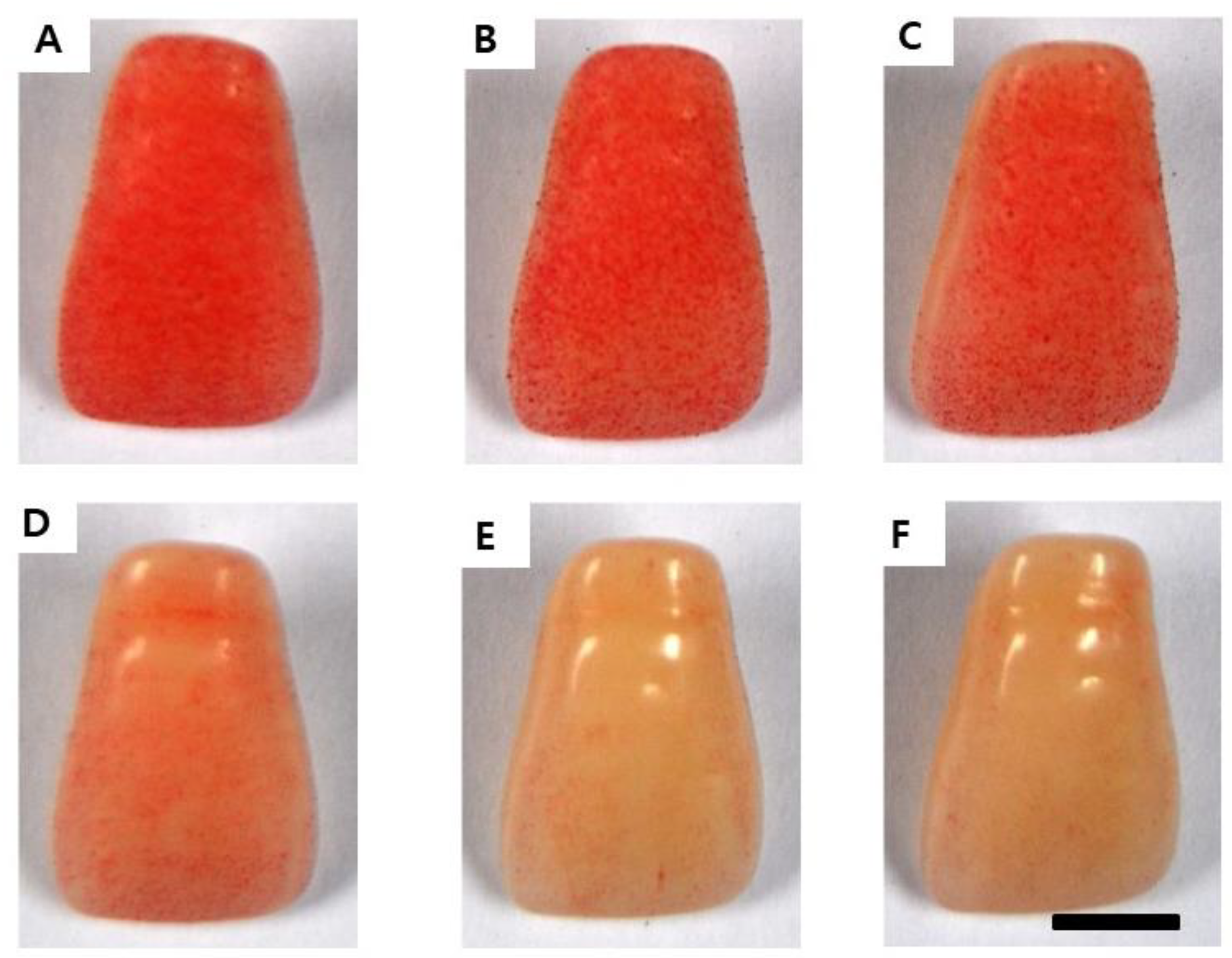
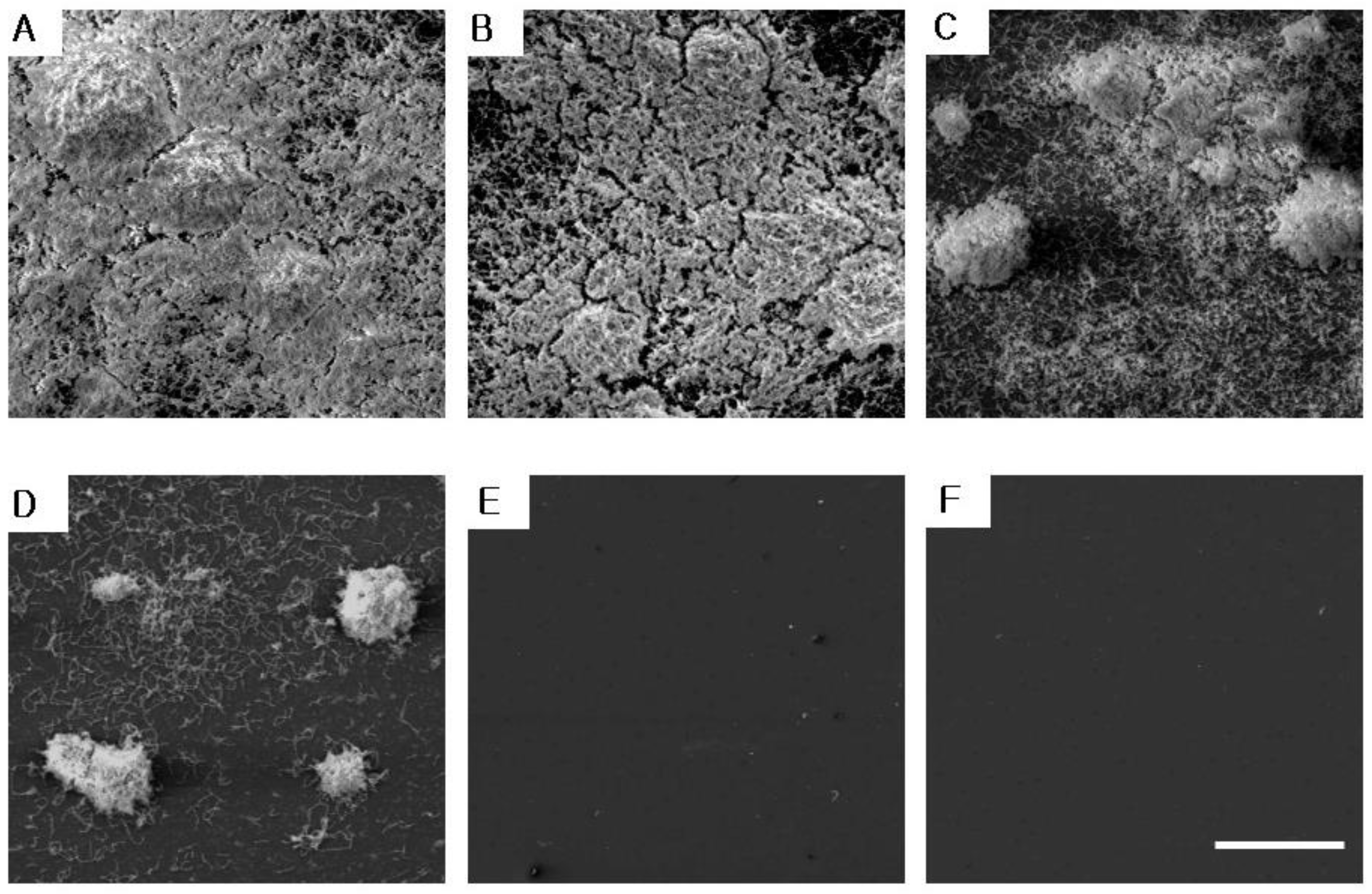
2.3. The Inhibitory Effect on S. mutans Biofilm Formation
2.4. Inhibitory Effect on the Acid Production of S. mutans
2.5. GC and GC-MS Analysis Results of AREO
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Essential Oils
4.2. Strains and Culture
4.3. Adhesion to Saliva-Coated Hydroxyapatite Beads
4.4. S. mutans Biofilm Formation
4.5. S. mutans Biofilm Formed on the Surface of the Artificial Teeth
4.6. Measurement of S. mutans Biofilm Using a Scanning Electronic Microscope
4.7. Acid Production of S. mutans
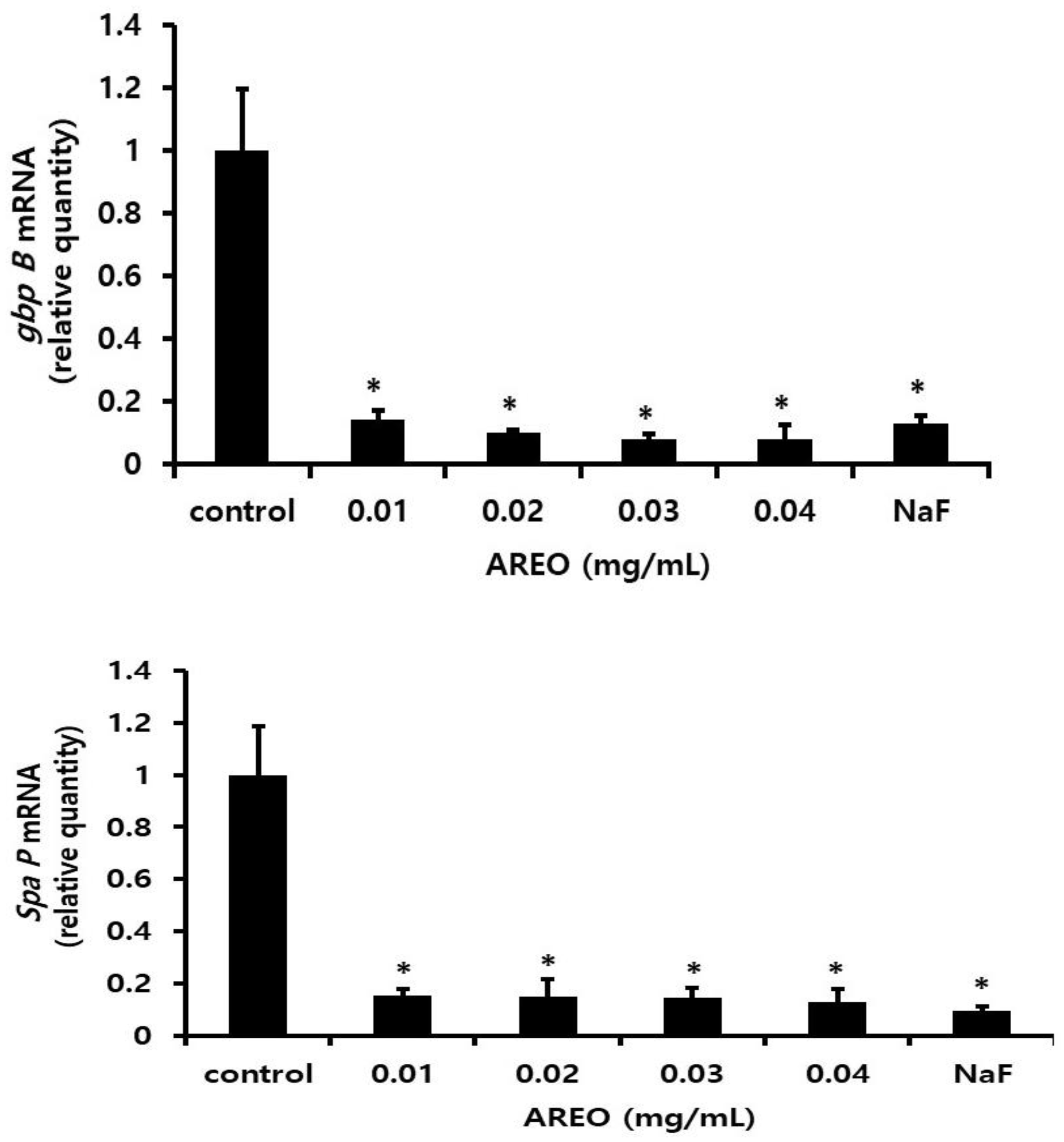
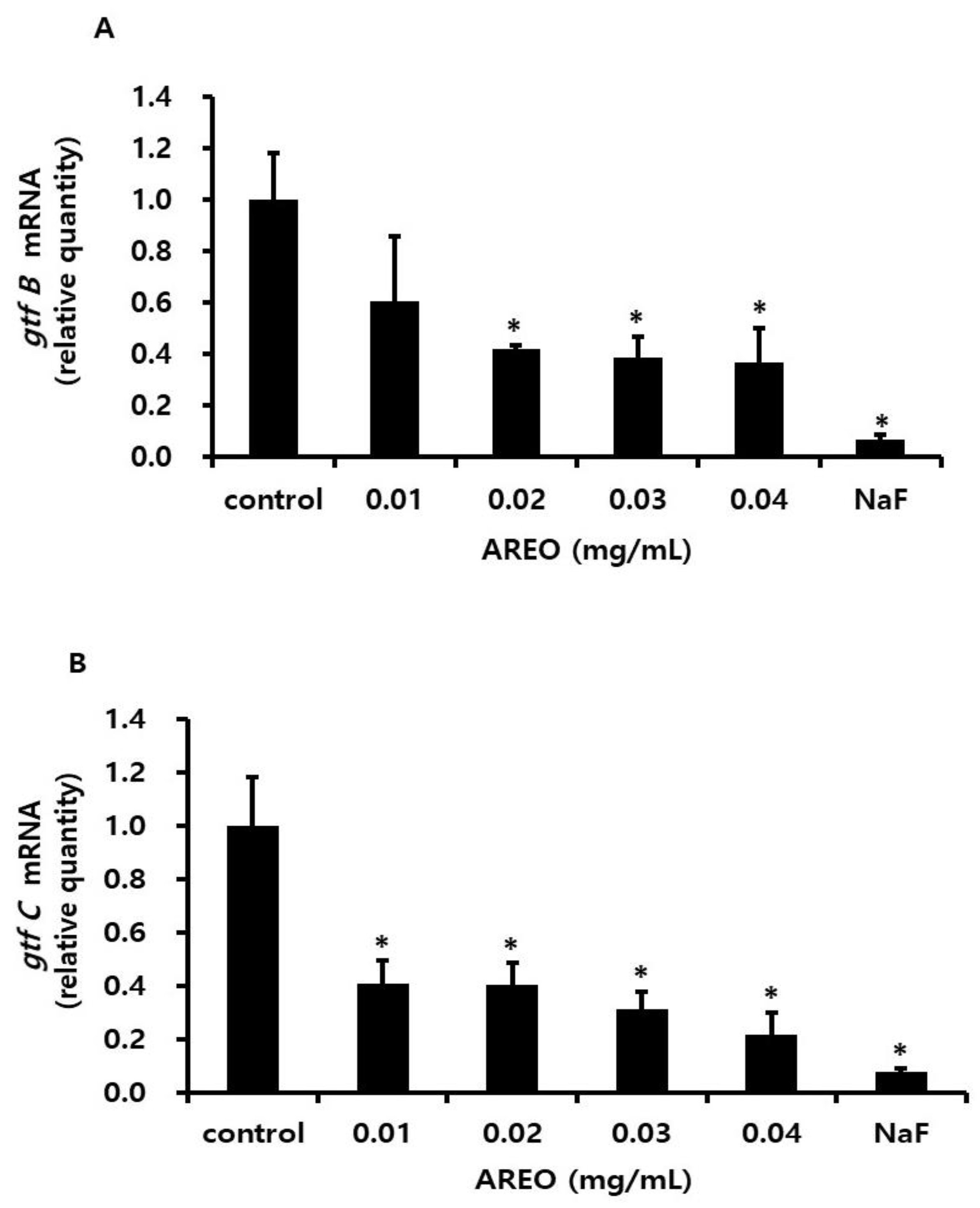
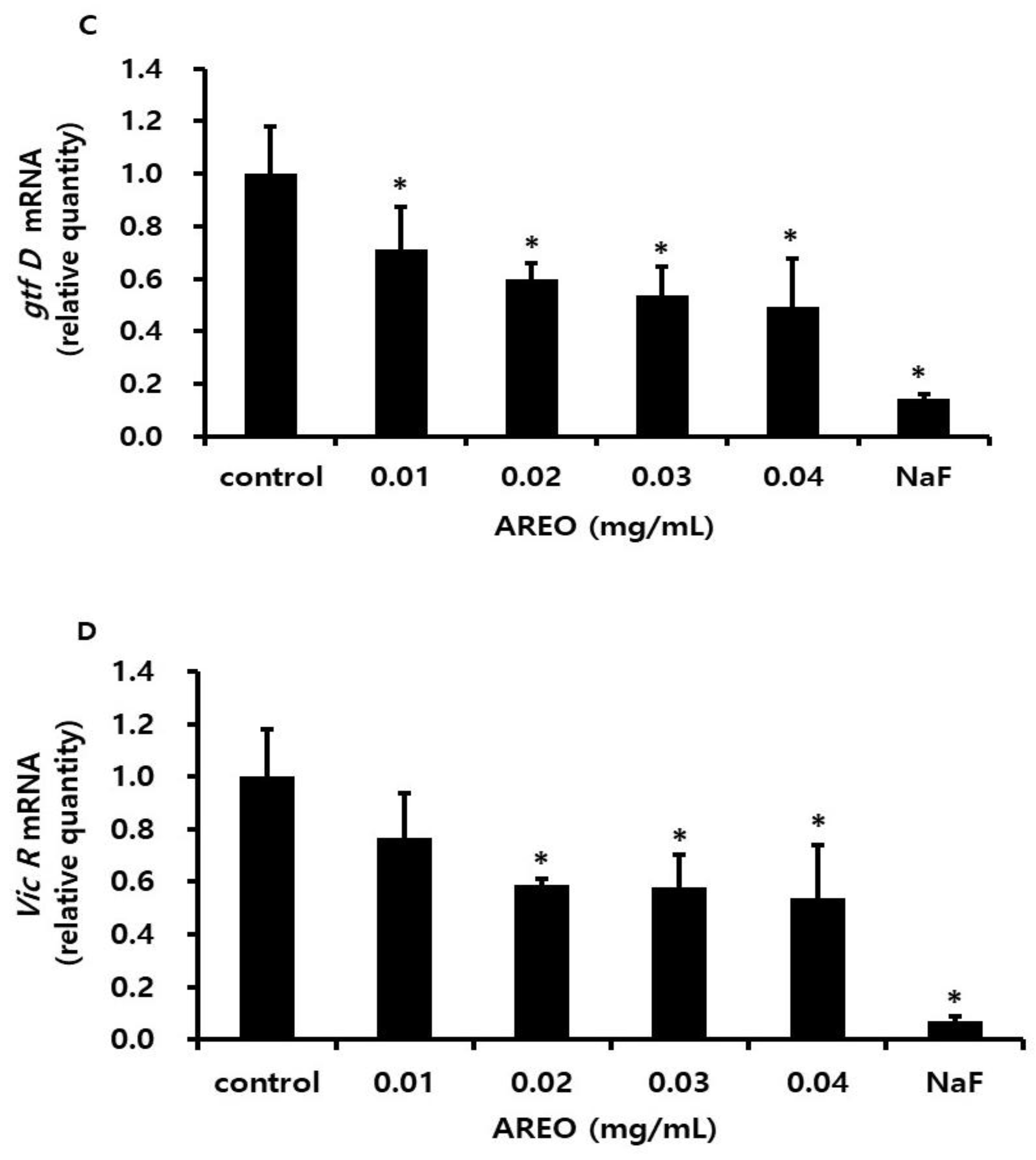
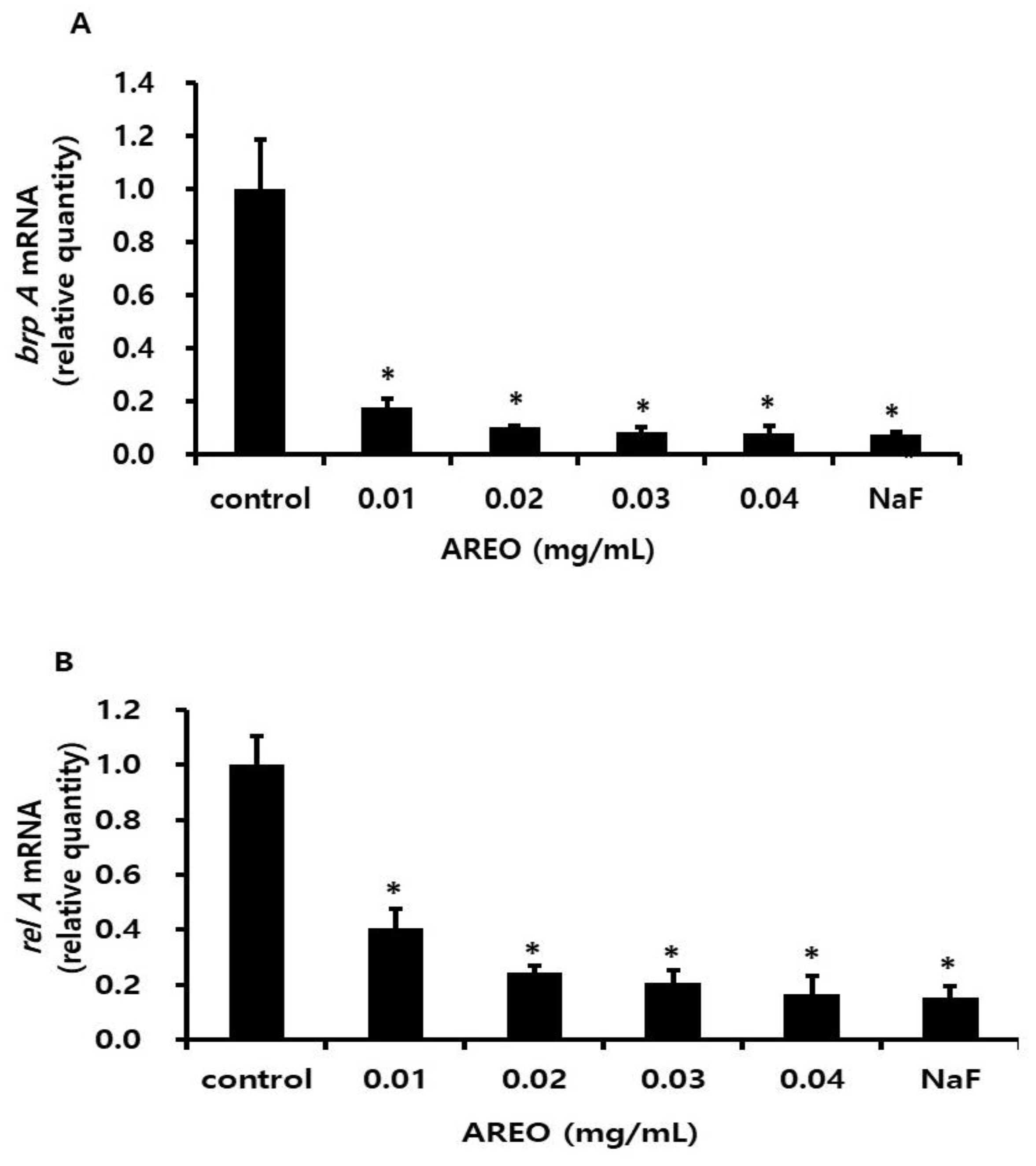
4.8. Real-Time PCR Analysis
4.9. GC and GC-MS Analyses
4.10. Statistical Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamada, S.; Torii, M. Interaction of glucosyltransferase from Streptococcus mutans with various glucans. J. Gen. Microbiol. 1980, 116, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, Q.; Chen, Z. A possible role of LIM mineralization protein 1 in tertiary dentinogenesis of dental caries treatment. Med. Hypothese 2007, 69, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Wiater, A.; Choma, A.; Szczodrak, J. Insoluble glucans synthesized by cariogenic streptococci: A structural study. J. Basic Microbiol. 1999, 39, 265–273. [Google Scholar] [CrossRef]
- Koga, T.; Asakawa, H.; Okahashi, N.; Hamada, S. Sucrose-dependent cell adherence and cariogenicity of serotype C Streptococcus mutans. J. Gen. Microbiol. 1986, 10, 2873–2883. [Google Scholar] [CrossRef]
- Loeshe, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef]
- Inoue, M.; Koga, T.; Sato, S.; Hamada, S. Synthesis adherent insoluble glucan by the concerted action of the two glucosyltranferase components of Streptococcus mutans. FEBS Lett. 1982, 143, 101–104. [Google Scholar] [CrossRef]
- Koga, T.; Hamada, S.; Murakawa, S.; Endo, A. Effect of a glucosyltransferase inhibitor on glucan synthesis and cellular adherence of Streptococcus mutans. Infect. Immun. 1982, 38, 882–886. [Google Scholar] [CrossRef]
- Wenham, D.G.; Davies, R.M.; Cole, J.A. Insoluble glucan synthesis by mutansucrase as determinant of the cariogenicity of Streptococcus mutans. J. Gen. Microbiol. 1981, 127, 407–415. [Google Scholar] [CrossRef]
- Jeng, J.H.; Hsieh, C.C.; Lan, W.H.; Chang, M.C.; Lin, S.K.; Hahn, L.J.; Kuo, M.Y. Cytotoxicity of sodium fluoride on human oral mucosal fibroblasts and its mechanisms. Cell Biol. Toxicol. 1998, 14, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Fardal, O.; Tumbull, R.S. A review of the literature on use of chlorhexidine in dentistry. J. Am. Dent. Assoc. 1986, 112, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M.Y.; Yang, D.N. The Effect of Oral Rinsing Solution on the Color Stability, Surface Microhardness and Surface Roughness Change of Composite Resin. J. Converg. Inf. Technol. 2019, 9, 159–167. [Google Scholar] [CrossRef]
- Charles, D.J.; Simon, J.E.; Widrlechner, M.P. Characterization of the essential oil of Agastache species. J. Agric. Food Chem. 1991, 39, 1946–1949. [Google Scholar] [CrossRef]
- Baik, J.A. Analysis, antibacterial, and insecticide effects on domestic native fragrant plants Elsholtzia ciliata and Agastache rugosa. J. People Plants Environ. 2016, 19, 79–83. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, C.K.; Cha, M.S.; Kim, J.K.; Seong, N.S.; Bang, K.H.; Bang, J.K. Antimicrobial activity of essential oils from Mentha arvensis L. var. piperascens Malivaud and Agastache rugosa O. Kuntze on Escherichia coli and Salmonella typhimurium. Korean J. Med. Crop Sci. 2002, 10, 206–211. [Google Scholar]
- Park, B.I.; Jung, Y.W.; Kim, Y.H.; Lee, S.M.; Kwon, L.S.; Kim, K.J.; An, S.Y.; Choi, N.Y.; You, Y.O. Effect of the ethanol extract of propolis on formation of Streptococcus mutans biofilm. Int. J. Oral Biol. 2016, 41, 253–262. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, J.H.; Kim, H.Y.; Kim, M.J.; Kim, S.M. Chemical composition and biological activity of essential oil of Agastache rugosa (Fisch. & C. A. Mey.) O. Kuntze. Korean J. Med. Crop Sci. 2020, 28, 95–110. [Google Scholar] [CrossRef]
- Liang, J.; Huang, X.; Ma, G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. R. Soc. Chem. Adv. 2022, 12, 29197–29213. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, M.J.; Kwon, H.D.; Park, I.H. Antimicrobial activity and components of extracts from Agastache rugosa during growth period. J. Food Sci. Nutr. 2001, 6, 10–15. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Park, S.N.; Lim, Y.K.; Kook, J.K. Antimicrobial effect of coptidis rhizome extract against mutans streptococci and periodontopathogens. Int. J. Oral Biol. 2015, 40, 79–83. [Google Scholar] [CrossRef]
- Nayak, S.S.; Ankola, A.V.; Metgud, S.C.; Bolmal, U.K. An in vitro study to determine the effect of Terminalia chebula extract and its formulation on Streptococcus mutans. J. Contemp. Dent. Pract. 2014, 15, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.K.; Jeon, J.K.; Kim, S.G.; Chang, K.W. Inhibitory effects of Schizandra chinensis extracts on the growth and adsorption to saliva coated HA beads of some oral bacteria. J. Korean Acad. Oral Health 2001, 25, 165–183. [Google Scholar]
- Lee, K.W.; Lee, S.K.; Chang, K.W. Effects of the crude Cinnamomi cortex extract on the growth and the adherence to hydroxyapatite beads of mutans streptococci. J. Korean Acad. Oral Health 1999, 23, 25–34. [Google Scholar]
- Tsujii, T.; Kawada-Matsuo, M.; Migita, H.; Ohta, K.; Oogai, Y.; Yamasaki, Y.; Komatsuzawa, H. Antibacterial activity of phellodendron bark against Streptococcus mutans. Microbiol. Immunol. 2020, 64, 424–434. [Google Scholar] [CrossRef]
- Saeki, Y.; Kato, T.; Naito, Y.; Takazoe, I.; Okuda, K. Inhibitory effects of funoran on the adherence and colonization of mutans streptococci. Caries Res. 1996, 30, 119–125. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Fadaei Tehrani, P.; Zandi, H.; Golvardi Yazdi, R. Chemical composition and antibacterial activity of Berberis vulgaris (barberry) against bacteria associated with caries. Clin. Exp. Dent. Res. 2021, 7, 601–608. [Google Scholar] [CrossRef]
- Otake, S.; Makimura, M.; Kuroki, T.; Nishihara, Y.; Hirasawa, M. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991, 25, 438–443. [Google Scholar] [CrossRef]
- Lee, H.O.; Lee, K.H.; Park, N.K.; Jeong, S.I.; Baek, S.H.; Han, D.M. Antibacterial effects of Sophora flavescens on Streptococcus mutans. Korean J. Food Nutr. 2000, 13, 539–546. [Google Scholar]
- Nakahara, K.; Kawabata, S.; Ono, H.; Tanaka, T.; Ooshima, T.; Hamada, S. Inhibitory effect of oolong tea polyphenols on glycosyltransferases of mutans Streptococci. Appl. Environ. Microbiol. 1993, 59, 968–973. [Google Scholar] [CrossRef]
- Lee, J.H.; Yim, D.Y.; Choi, S.S. Antibacterial activity and anti-inflammatory effect of methanol extracts of Saliva miltiorrhiza against oral pathogenic bacteria. Korean J. Pharmacogn. 2021, 52, 41–48. [Google Scholar] [CrossRef]
- Ban, S.H.; Kim, J.E.; Pandit, S.; Jeon, J.G. Influences of Dryopteris crassirhizoma extract on the viability, growth and virulence properties of Streptococcus mutans. Molecules 2012, 17, 9231–9244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Antibacterial and anti-inflammatory effects of Platycodon grandiflorum extracts. J. Digit. Converg. 2014, 12, 359–366. [Google Scholar] [CrossRef]
- Cho, Y.H.; Chiang, M.H. Essential oil composition and antibacterial activity of Artemisia capillaris, Artemisia argyi, and Artemisia princeps. J. Korean Soc. Int. Agric. 2001, 13, 313–320. [Google Scholar]
- Doh, E.S.; Yoo, J.H. Antibacterial activity of bio-fermented Galla Rhios extract. Korea J. Herbol. 2014, 29, 21–27. [Google Scholar] [CrossRef]
- Shin, A.R.; Ohk, S.H.; Choi, C.H.; Hong, S.J. Identification and partial purification of antibacterial compounds against Streptococcus mutans from Galla Rhois. J. Korean Acad. Oral Health 2016, 40, 3–8. [Google Scholar] [CrossRef][Green Version]
- Kim, E.H.; Kang, S.Y.; Park, B.I.; Kim, Y.H.; Lee, Y.R.; Hoe, J.H.; Choi, N.Y.; Ra, J.Y.; An, S.Y.; You, Y.O. Chamaecyparis obtusa suppresses virulence genes in Streptococcus mutans. Evid. Based Complement. Altern. Med. 2016, 2396404. [Google Scholar] [CrossRef]
- Carvalho Êni, S.; Ayres, V.F.S.; Oliveira, M.R.; Corrêa, G.M.; Takeara, R.; Guimarães, A.C.; Santiago, M.B.; Oliveira, T.A.S.; Martins, C.H.G.; Crotti, A.E.M.; et al. Anticariogenic activity of three essential oils from Brazilian Piperaceae. Pharmaceuticals 2022, 15, 972. [Google Scholar] [CrossRef]
- Park, B.I.; You, Y.O.; Mo, J.S.; An, S.Y.; Choi, N.Y.; Kim, K.J. Anti-cariogenic properties of ɑ-pinene, a monoterpene in plant essential oil. Int. J. Oral Biol. 2017, 42, 25–31. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.G.; Baik, B.J.; Yang, Y.M.; Lee, K.Y.; Lee, H.Y.; Kim, M.A. Antimicrobial effect of essential oils on oral bacteria. J. Korean Acad. Pediatr. Dent. 2009, 36, 1–11. [Google Scholar]
- Jeong, S.I.; Kim, B.S.; Keum, K.S.; Lee, K.H.; Kang, S.H.; Park, B.I.; Lee, Y.R.; You, Y.O. Kaurenoic acid from Aralia continentalis inhibits biofilm formation of Streptococcus mutans. Evid.-Based Complement. Altern. Med. 2013, 160592. [Google Scholar] [CrossRef]
- Hay, D.I.; Gibbons, R.J.; Spinell, D.M. Characteristics ofsome high molecular weight constituents with bacterial aggregating activity from whole saliva and dentalplaque. Caries. Res. 1971, 5, 111–123. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Hilary, H.; James, P.O. Carriage of both the fnbA and fnbB genes and growth at 37 °C promote FnBP mediated biofilm development in meticillin-resistant Staphylococcus aureus clinical isolates. J. Med. Microbiol. 2009, 58, 399–402. [Google Scholar] [CrossRef]
- Kwang, H.L.; Kim, B.S.; Keum, K.S.; Yu, H.H.; Kim, Y.H.; Chang, B.S.; Ra, J.Y.; Moon, H.D.; Seo, B.R.; Choi, N.Y.; et al. Essential oil of Curcuma longa inhibits Streptococcus mutans biofilm formation. J. Food Sci. 2011, 76, H226–H230. [Google Scholar] [CrossRef]
- Ooshimaa, T.; Osakaa, Y.; Sasakia, H.; Osawab, K.; Yasudab, H.; Matsumuraa, M.; Sobuea, S.; Matsumotoa, M. Caries inhibitory activity of cacao bean husk extract in in-vitro and animal experiments. Arch. Oral Biol. 2000, 45, 639–645. [Google Scholar] [CrossRef]
- VanDenDool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
| Concentration (mg/mL) | pH (Before Incubation) | pH (After Incubation) |
|---|---|---|
| Control | 7.38 ± 0.05 | 5.41 ± 0.05 |
| 0.01 | 7.38 ± 0.05 | 5.48 ± 0.10 |
| 0.02 | 7.38 ± 0.05 | 5.74 ± 0.00 * |
| 0.03 | 7.38 ± 0.05 | 6.54 ± 0.11 * |
| 0.04 | 7.38 ± 0.00 | 7.19 ± 0.05 * |
| NaF (0.1%) | 7.38 ± 0.00 | 7.37 ± 0.00 * |
| Compound | Retention Index | Peak Area (%) | |
|---|---|---|---|
| Calculated 1) | Literature 2) | ||
| 2-Ethyl furan | 712 | 713 | t |
| n-Hexanal | 799 | 800 | t |
| trans-2-Hexenal | 862 | 862 | 0.06 |
| cis-3-Hexen-1-ol | 867 | 858 | t |
| α-Pinene | 930 | 939 | t |
| Camphene | 950 | 953 | 0.08 |
| Benzaldehyde | 958 | 961 | 0.12 |
| Sabinene | 967 | 971 3) | t |
| 1-Octen-3-ol | 985 | 978 | 0.45 |
| 3-Octanone | 987 | 986 | 0.08 |
| Myrcene | 991 | 991 | 0.05 |
| Limonene | 1031 | 1031 | 2.29 |
| Benzyl alcohol | 1037 | 1032 | 0.06 |
| Phenylacetaldehyde | 1039 | 1043 | 0.07 |
| Linalool | 1102 | 1098 | t |
| 1-Octen-3-yl acetate | 1121 | 1119 | 0.24 |
| trans-Isopulegone | 1181 | 1175 | 0.13 |
| Estragole | 1219 | 1196 | 88.69 |
| Chavicol | 1287 | 1265 3) | 1.05 |
| Isopiperitenone | 1292 | 1282 3) | 0.08 |
| trans-Anethole | 1300 | 1285 | t |
| p-Vinylguaiacol | 1323 | 1324 | t |
| Eugenol | 1366 | 1356 | 0.22 |
| Unidentified | 1379 | - | 0.24 |
| β-Bourbonene | 1383 | 1384 | 0.05 |
| cis-Jasmone | 1395 | 1394 | 0.05 |
| Methyl eugenol | 1404 | 1401 | 0.06 |
| β-Caryophyllene | 1424 | 1418 | 2.56 |
| α-Humulene | 1447 | 1454 | 0.12 |
| Germacrene D | 1479 | 1480 | 0.80 |
| Bicyclogermacrene | 1493 | 1494 | 0.35 |
| trans-Methyl isoeugenol | 1505 | 1495 3) | 0.07 |
| trans,trans-α-Farnesene | 1508 | 1508 | t |
| δ-Cadinene | 1521 | 1524 | 0.08 |
| Spathulenol | 1570 | 1576 | 0.07 |
| Caryophyllene oxide | 1573 | 1581 | 0.06 |
| τ-Cadinol | 1627 | 1625 | t |
| τ-Muurolol | 1635 | 1641 | 0.05 |
| α-Cadinol | 1648 | 1653 | 0.07 |
| 6,10,14-Trimethylpentadecan-2-one | 1842 | 1843 | t |
| Benzyl salicylate | 1883 | 1876 3) | t |
| Oleic acid | 2113 | 2115 3) | 0.27 |
| Total | 98.58 | ||
| Genes * | Genes Description | Primer Sequences (5′-3′) | |
|---|---|---|---|
| 16S rRNA | Normalizing internal standards | Forward | CCTACGGGAGGCAGCAGTAG |
| Reverse | CAACAGAGCTTTACGATCCGAAA | ||
| gtfB | Glucosyltransferase B (gtfB) | Forward | AGCAATGCAGCCAATCTACAAAT |
| Reverse | ACGAACTTTGCCGTTATTGTCA | ||
| gtfC | Glucosyltransferase SI (gtfC) | Forward | GGTTTAACGTCAAAATTAGCTGTATTAGC |
| Reverse | CTCAACCAACCGCCACTGTT | ||
| gtfD | Glucosyltransferase-I (gtfD) | Forward | ACAGCAGACAGCAGCCAAGA |
| Reverse | ACTGGGTTTGCTGCGTTTG | ||
| brpA | Biofilm-regulation protein | Forward | GGAGGAGCTGCATCAGGATTC |
| Reverse | AACTCCAGCACATCCAGCAAG | ||
| gbpB | Glucan-binding protein | Forward | ATGGCGGTTATGGACACGTT |
| Reverse | TTTGGCCACCTTGAACACCT | ||
| relA | Guanosine tetra (penta)-phosphate synthetase | Forward | ACAAAAAGGGTATCGTCCGTACAT |
| Reverse | AATCACGCTTGGTATTGCTAATTG | ||
| spaP | Cell surface antigen | Forward | GACTTTGGTAATGGTTATGCATCAA |
| Reverse | TTTGTATCAGCCGGATCAAGTG | ||
| vicR | Two-component system regulatory | Forward | TGACACGATTACAGCCTTTGATG |
| Reverse | CGTCTAGTTCTGGTAACATTAAGTCCAATA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.S.; Park, B.-I.; Kim, Y.-H.; Kang, J.; You, Y.-O. The Inhibitory Effect of Agastache rugosa Essential Oil on the Dental Biofilm. Molecules 2024, 29, 4907. https://doi.org/10.3390/molecules29204907
Kim ES, Park B-I, Kim Y-H, Kang J, You Y-O. The Inhibitory Effect of Agastache rugosa Essential Oil on the Dental Biofilm. Molecules. 2024; 29(20):4907. https://doi.org/10.3390/molecules29204907
Chicago/Turabian StyleKim, Eun Sook, Bog-Im Park, Young-Hoi Kim, Jooyi Kang, and Yong-Ouk You. 2024. "The Inhibitory Effect of Agastache rugosa Essential Oil on the Dental Biofilm" Molecules 29, no. 20: 4907. https://doi.org/10.3390/molecules29204907
APA StyleKim, E. S., Park, B.-I., Kim, Y.-H., Kang, J., & You, Y.-O. (2024). The Inhibitory Effect of Agastache rugosa Essential Oil on the Dental Biofilm. Molecules, 29(20), 4907. https://doi.org/10.3390/molecules29204907







