Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study
Abstract
1. Introduction
2. Results
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izmestev, A.N.; Streltsov, A.A.; Kravchenko, A.N.; Gazieva, G.A. 5-Arylmethylidene-2-iminothiazolidin-4-ones in the synthesis of novel dispiro-fused oxindolepyrrolidineiminothiazolidinones. Chem. Heterocycl. Comp. 2023, 59, 309–316. [Google Scholar] [CrossRef]
- Izmestev, A.N.; Kravchenko, A.N.; Gazieva, G.A. A 1,3-dipolar cycloaddition of azomethine ylides to imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine oxindolylidene derivatives in the synthesis of novel spirooxindole derivatives. Chem. Heterocycl. Comp. 2023, 59, 594–603. [Google Scholar] [CrossRef]
- Guzyr, O.I.; Potikha, L.M.; Shishkin, S.V.; Fetyukhin, V.N.; Shermolovich, Y.G. The nitration and bromination of 2-(pentafluorosulfanyl)-1,3-benzothiazole and 2-(trifluoromethyl)-1,3-benzothiazole. Chem. Heterocycl. Comp. 2023, 59, 304–308. [Google Scholar] [CrossRef]
- Brusnakov, M.Y.; Golovchenko, O.V.; Potikha, K.M.; Brovarets, V.S. Condensed azole-based organophosphorus heterocycles. Chem. Heterocycl. Comp. 2023, 59, 217–236. [Google Scholar] [CrossRef]
- Ma, T.; Cao, G. Recent Developments in the Synthesis of Spirobenzosultams (Microreview). Chem. Heterocycl. Compd. 2023, 59, 246–248. [Google Scholar] [CrossRef]
- Los, O.V.; Sinenko, V.O.; Kobzar, O.L.; Zhirnov, V.V.; Vovk, A.I.; Brovarets, V.S. Synthesis and in Vitro Anticancer Potential of New Thiazole-Containing Derivatives of Rhodanine. Chem. Heterocycl. Compd. 2023, 59, 484–493. [Google Scholar] [CrossRef]
- Latifi, M.; Anary-Abbasinejad, M.; Mohammadi, M. A Simple Route for the Synthesis of Substituted Thiazole Derivatives by Multicomponent Reaction between Arylglyoxals, Acetylacetone or Ethyl Acetoacetate, and Thiosemicarbazones. Chem. Heterocycl. Compd. 2023, 59, 709–712. [Google Scholar] [CrossRef]
- Khodykina, E.S.; Kolodina, A.A. Recent Methods for the Synthesis of Fused Pyrazolo[3,4(4,3)-d]Thiazoles and Pyrazolo[3,4(4,3)-d][1,4]Thiazines (Microreview). Chem. Heterocycl. Compd. 2023, 59, 643–645. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of Sulphur-Heterocycles in Medicinal Chemistry: An Update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef]
- Sharma, P.K.; Amin, A.; Kumar, M. A Review: Medicinally Important Nitrogen Sulphur Containing Heterocycles. Open Med. Chem. J. 2020, 14, 49–64. [Google Scholar] [CrossRef]
- Omar, A. Review Article; Anticancer Activities of Some Fused Heterocyclic Moieties Containing Nitrogen And/Or Sulfur Heteroatoms. Al-Azhar J. Pharm. Sci. 2020, 62, 39–54. [Google Scholar] [CrossRef]
- Salah, S.; Sami, N.; Ali, S.; Khalid, T.-A.; Alnajjar, R. Natural Products as Potential Inhibitors of FLT3 for Acute Myeloid Leukemia: HTVS, Docking, and Molecular Dynamic Simulation. Sci. Radices 2023, 2, 325–346. [Google Scholar] [CrossRef]
- Kashinath, K.; Snead, D.R.; Burns, J.M.; Stringham, R.W.; Gupton, B.F.; McQuade, D.T. Synthesis of an Oxathiolane Drug Substance Intermediate Guided by Constraint-Driven Innovation. Org. Process Res. Dev. 2020, 24, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.E.; Huang, M.G.; Schloman, W.W. Facile Synthesis of 1,3-Oxathiolanes from Ketones and i-Mercaptoethanol. J. Org. Chem. 1968, 33, 2133–2134. [Google Scholar] [CrossRef]
- Jones, F.N.; Andreades, S. Ethylene Thionocarbonate and 1,3-Oxathiolane-2-Thione. J. Org. Chem. 1969, 34, 3011–3014. [Google Scholar] [CrossRef]
- Belleau, B.; Brasili, L.; Chan, L.; DiMarco, M.P.; Zacharie, B.; Nguyen-Ba, N.; Jenkinson, H.J.; Coates, J.A.V.; Cameron, J.M. A Novel Class of 1,3-Oxathiolane Nucleoside Analogues Having Potent Anti-HIV Activity. Bioorg. Med. Chem. Lett. 1993, 3, 1723–1728. [Google Scholar] [CrossRef]
- Aher, U.P.; Srivastava, D.; Singh, G.P.; Jayashree, B.S. Synthetic Strategies toward 1,3-Oxathiolane Nucleoside Analogues. Beilstein J. Org. Chem. 2021, 17, 2680–2715. [Google Scholar] [CrossRef]
- Łapczuk, A. The [3 + 2] Cycloaddition Reaction as an Attractive Way for the Preparation of Nicotine Analogs (Microreview). Chem. Heterocycl. Compd. 2023, 59, 109–111. [Google Scholar] [CrossRef]
- Jasiński, R. Recent Progress in the Synthesis of Nitroisoxazoles and Their Hydrogenated Analogs via [3 + 2] Cycloaddition Reactions (Microreview). Chem. Heterocycl. Compd. 2023, 59, 730–732. [Google Scholar] [CrossRef]
- Dresler, E. The Participation of Oleic Acid and Its Esters in [3 + 2] Cycloaddition Reactions: A Mini-Review. Sci. Radices 2024, 3, 53–61. [Google Scholar] [CrossRef]
- Sadowski, M.; Mudyna, A.; Knap, K.; Demchuk, O.M.; Łapczuk, A. Synthesis of (Z)-N-Aryl-C-(Pyrid-3-Yl)-Nitrones. Sci. Radices 2023, 2, 319–324. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3 + 2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Jasiński, R. In the Searching for Zwitterionic Intermediates on Reaction Paths of [3 + 2] Cycloaddition Reactions between 2,2,4,4-Tetramethyl-3-Thiocyclobutanone S-Methylide and Polymerizable Olefins. RSC Adv. 2015, 5, 101045–101048. [Google Scholar] [CrossRef]
- Linden, A.; Mlostoń, G.; Grzelak, P.; Heimgartner, H. Chemo- and Regioselective [3 + 2]-Cycloadditions of Thiocarbonyl Ylides: Crystal Structures of Trans -8-Benzoyl-1,1,3,3-Tetramethyl-7-Trifluoromethyl-5-Thiaspiro[3.4]Octan-2-One and Trans-3-Benzoyl-2,2-Diphenyl-4-(Trifluoromethyl)Tetrahydrothiophene. Acta Crystallogr. Sect. E Crystallogr. Commun. 2018, 74, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.K.; Obijalska, E.; Mlostoń, G.; Heimgartner, H. Generation and Reactions of Thiocarbonyl S-(2,2,2-Trifluoroethanides). Synthesis of Trifluoromethylated 1,3-Dithiolanes, Thiiranes and Alkenes. J. Fluor. Chem. 2017, 200, 102–108. [Google Scholar] [CrossRef]
- Mlostoń, G.; Hamera-Fałdyga, R.; Linden, A.; Heimgartner, H. Synthesis of Ferrocenyl-Substituted 1,3-Dithiolanes via [3 + 2]-Cycloadditions of Ferrocenyl Hetaryl Thioketones with Thiocarbonyl S-Methanides. Beilstein J. Org. Chem. 2016, 12, 1421–1427. [Google Scholar] [CrossRef]
- Kosylo, N.; Hotynchan, A.; Skrypska, O.; Horak, Y.; Obushak, M. Synthesis and Prediction of Toxicological and Pharmacological Properties of Schiff Bases Containing Arylfuran and Pyrazole Moiety. Sci. Radices 2024, 3, 63–72. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums: The Lore and the Science; RSC Publ.: Cambridge, UK, 2010; ISBN 978-0-85404-190-9. [Google Scholar]
- He, Q.; Kubec, R.; Jadhav, A.P.; Musah, R.A. First Insights into the Mode of Action of a “Lachrymatory Factor Synthase”—Implications for the Mechanism of Lachrymator Formation in Petiveria Alliacea, Allium Cepa and Nectaroscordum Species. Phytochemistry 2011, 72, 1939–1946. [Google Scholar] [CrossRef]
- Castro, V.; Carpena, M.; Fraga-Corral, M.; Lopez-Soria, A.; Garcia-Perez, P.; Barral-Martinez, M.; Perez-Gregorio, R.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Sulfur-Containing Compounds from Plants. In Natural Secondary Metabolites; Carocho, M., Heleno, S.A., Barros, L., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 363–402. ISBN 978-3-031-18586-1. [Google Scholar]
- Loredana, L.; Giuseppina, A.; Filomena, N.; Florinda, F.; Marisa, D.M.; Donatella, A. Biochemical, Antioxidant Properties and Antimicrobial Activity of Different Onion Varieties in the Mediterranean Area. J. Food Meas. Charact. 2019, 13, 1232–1241. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. A Mechanistic Study of the Participation of Azomethine Ylides and Carbonyl Ylides in [3+2] Cycloaddition Reactions. Tetrahedron 2015, 71, 1050–1057. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Molecular Electron Density Theory Study of the Reactivity of Azomethine Imine in [3 + 2] Cycloaddition Reactions. Molecules 2017, 22, 750. [Google Scholar] [CrossRef] [PubMed]
- Kras, J.; Sadowski, M.; Zawadzińska, K.; Nagatsky, R.; Woliński, P.; Kula, K.; Łapczuk, A. Thermal [3 + 2] Cycloaddition Reactions as Most Universal Way for the Effective Preparation of Five-Membered Nitrogen Containing Heterocycles. Sci. Radices 2023, 2, 247–267. [Google Scholar] [CrossRef]
- Cailleuxa, P.; Pieta, J.C.; Benhaouaaib, H.; Carrie, R. Cycloaddition Des Methylazide Et Phenylazide Au p-nitrostyrene Et Au Nitroprene Homologue. Bull. Soc. Chim. Belg. 1996, 105, 45–51. [Google Scholar] [CrossRef]
- Risaliti, A.; Forchiassin, M.; Valentin, E. Vinylamines—VIII: The reaction of cyclohexanone enamines with 1- and 2-nitropropene. Tetrahedron 1968, 24, 1889–1898. [Google Scholar] [CrossRef]
- Wilkendorf, R.; Trénel, M. Zur Kenntnis Aliphatischer Nitro-alkohole (II). Ber. Dtsch. Chem. Ges. A/B 1924, 57, 306–309. [Google Scholar] [CrossRef]
- Jasiński, R. A Stepwise, Zwitterionic Mechanism for the 1,3-Dipolar Cycloaddition between (Z)-C-4-Methoxyphenyl-N-Phenylnitrone and Gem-Chloronitroethene Catalysed by 1-Butyl-3-Methylimidazolium Ionic Liquid Cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Gold, M.H.; Hamel, E.E.; Klager, K. Preparation and Characterization of 2,2-Dinitroethanol1. J. Org. Chem. 1957, 22, 1665–1667. [Google Scholar] [CrossRef]
- Munaf Kharbuli, A.; Lyngdoh, R.H.D. Ozonolysis of Methyl, Amino and Nitro Substituted Ethenes: A Semi-Empirical Molecular Orbital Study. J. Mol. Struct. THEOCHEM 2008, 860, 150–160. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gostyński, B. Nitrosubstituted Analogs of Isoxazolines and Isoxazolidines: A Surprising Estimation of Their Biological Activity via Molecular Docking. Sci. Radices 2023, 2, 25–46. [Google Scholar] [CrossRef]
- Sadowski, M.; Kula, K. Nitro-Functionalized Analogues of 1,3-Butadiene: An Overview of Characteristic, Synthesis, Chemical Transformations and Biological Activity. Curr. Chem. Lett. 2024, 13, 15–30. [Google Scholar] [CrossRef]
- Sadowski, M.; Synkiewicz-Musialska, B.; Kula, K. (1E,3E)-1,4-Dinitro-1,3-Butadiene—Synthesis, Spectral Characteristics and Computational Study Based on MEDT, ADME and PASS Simulation. Molecules 2024, 29, 542. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3 + 2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 49–69. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Kula, K. Application of β-Phosphorylated Nitroethenes in [3 + 2] Cycloaddition Reactions Involving Benzonitrile N-Oxide in the Light of a DFT Computational Study. Organics 2021, 2, 26–37. [Google Scholar] [CrossRef]
- Kula, K.; Sadowski, M. Regio- and Stereoselectivity of [3 + 2] Cycloaddition Reactions between (Z)-1-(Anthracen-9-Yl)-N-Methyl Nitrone and Analogs of Trans-β-Nitrostyrene on the Basis of MEDT Computational Study. Chem. Heterocycl. Compd. 2023, 59, 138–144. [Google Scholar] [CrossRef]
- Huisgen, R.; Mlostoń, G.; Giera, H.; Langhals, E.; Polborn, K.; Sustmann, R. Aliphatic Thiocarbonyl Ylides and Thiobenzophenone: Experimental Study of Regiochemistry and Methylene Transfer in Cycloadditions. Eur. J. Org. Chem. 2005, 2005, 1519–1531. [Google Scholar] [CrossRef]
- Domingo, L. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Fox, Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Domingo, L.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational Tools for the Electron Localization Function Topological Analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Thiéry, M.-M.; Rérat, C. Calculation of Crystal and Molecular Structures of Carbon Disulfide CS2. J. Chem. Phys. 2005, 122, 044503. [Google Scholar] [CrossRef]
- Rothenberg, S.; Schaefer, H.F. Theoretical Study of SO2 Molecular Properties. J. Chem. Phys. 1970, 53, 3014–3019. [Google Scholar] [CrossRef]
- Silvi, B. The Synaptic Order: A Key Concept to Understand Multicenter Bonding. J. Mol. Struct. 2002, 614, 3–10. [Google Scholar] [CrossRef]
- Jasiński, R.; Jasińska, E.; Dresler, E. A DFT Computational Study of the Molecular Mechanism of [3 + 2] Cycloaddition Reactions between Nitroethene and Benzonitrile N-Oxides. J. Mol. Model. 2017, 23, 13. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Regioselectivity and the Molecular Mechanism of [3 + 2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: A DFT Computational Study. Molecules 2022, 27, 8441. [Google Scholar] [CrossRef] [PubMed]
- Kula, K.; Zawadzińska, K. Local Nucleophile-Electrophile Interactions in [3+2] Cycloaddition Reactions between Benzonitrile N-Oxide and Selected Conjugated Nitroalkenes in the Light of MEDT Computational Study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. How Does the Global Electron Density Transfer Diminish Activation Energies in Polar Cycloaddition Reactions? A Molecular Electron Density Theory Study. Tetrahedron 2017, 73, 1718–1724. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Molecular Mechanism of Thermal and LA-Catalysed Diels–Alder Reactions between Cyclopentadiene and Isopropyl 3-Nitroprop-2-Enate. Molecules 2023, 28, 5289. [Google Scholar] [CrossRef]
- Wen, C.; Dechsupa, N.; Yu, Z.; Zhang, X.; Liang, S.; Lei, X.; Xu, T.; Gao, X.; Hu, Q.; Innuan, P.; et al. Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile. Molecules 2023, 28, 4856. [Google Scholar] [CrossRef]
- Aitouna, A.O.; Barhoumi, A.; Zeroual, A. A Mechanism Study and an Investigation of the Reason for the Stereoselectivity in the [4+2] Cycloaddition Reaction between Cyclopentadiene and Gem-Substituted Ethylene Electrophiles. Sci. Radices 2023, 2, 217–228. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Energetic Aspects and Molecular Mechanism of 3-Nitro-Substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study. Molecules 2024, 29, 3042. [Google Scholar] [CrossRef]
- Dresler, E.; Woliński, P.; Wróblewska, A.; Jasiński, R. On the Question of Zwitterionic Intermediates in the [3 + 2] Cycloaddition Reactions between Aryl Azides and Ethyl Propiolate. Molecules 2023, 28, 8152. [Google Scholar] [CrossRef] [PubMed]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Kras, J.; Zawadzińska, K.; Demchuk, O.M.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-Ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials 2022, 15, 7584. [Google Scholar] [CrossRef]
- Mondal, A.; Mohammad-Salim, H.A.; Acharjee, N. Unveiling Substituent Effects in [3 + 2] Cycloaddition Reactions of Benzonitrile N-Oxide and Benzylideneanilines from the Molecular Electron Density Theory Perspective. Sci. Radices 2023, 2, 75–92. [Google Scholar] [CrossRef]
- Doming, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Based on the Flux of the Electron Density. Sci. Radices 2023, 2, 1–24. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kacka-Zych, A.; Kula, K. Recent progress in the field of cycloaddition reactions involving conjugated nitroalkenes. Curr. Chem. Lett. 2019, 8, 13–38. [Google Scholar] [CrossRef]
- Siadati, S.A. Beyond the Alternatives That Switch the Mechanism of the 1,3-Dipolar CyCloadditions from Concerted to Stepwise or Vice Versa: A Literature Review. Prog. React. Kinet. Mech. 2016, 41, 331–344. [Google Scholar] [CrossRef]
- Siadati, S.A.; Kula, K.; Babanezhad, E. The Possibility of a Two-Step Oxidation of the Surface of C20 Fullerene by a Single Molecule of Nitric (V) Acid. Chem. Rev. Lett. 2019, 2, 2–6. [Google Scholar]
- Pandey, S.K.; Yadava, U.; Sharma, M.L.; Upadhyay, A.; Gupt, M.P.; Dwivedi, A.R.; Khatoon, A. Synthesis, Molecular Structure Investigation, Biological Evaluation and Docking Studies of Novel Spirothiazolidinones. Results Chem. 2023, 5, 100726. [Google Scholar] [CrossRef]
- Nuti, E.; Cantelmo, A.R.; Gallo, C.; Bruno, A.; Bassani, B.; Camodeca, C.; Tuccinardi, T.; Vera, L.; Orlandini, E.; Nencetti, S.; et al. N-O -Isopropyl Sulfonamido-Based Hydroxamates as Matrix Metalloproteinase Inhibitors: Hit Selection and in Vivo Antiangiogenic Activity. J. Med. Chem. 2015, 58, 7224–7240. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Abualhasan, M.; Qaoud, M.T.; Joudeh, Y.; Jaber, Z.; Sawalmeh, M.; Zarour, A.; Mousa, A.; Arar, M. Molecular Docking Studies and Biological Evaluation of Isoxazole-Carboxamide Derivatives as COX Inhibitors and Antimicrobial Agents. 3 Biotech 2022, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, M. A density functional theory study on the [3 + 2]cycloaddition of N-(p-methylphenacyl)benzothiazolium ylideand 1-nitro-2-(p-methoxyphenyl) ethene: The formation of twodiastereomeric adducts via two different mechanisms. Theor. Chem. Acc. 2019, 138, 87. [Google Scholar] [CrossRef]
- Sobhi, C.; Nacereddine, A.K.; Djerourou, A.; Ríos-Gutiérrez, M.; Domingo, L.R. A DFT study of the mechanism and selectivities of the [3 + 2] cycloaddition reaction between 3-(benzylideneamino)oxindole and trans-β-nitrostyrene. J. Phys. Org. Chem. 2017, 30, e3637. [Google Scholar] [CrossRef]
- Aitouna, O.; Barhoumi, A.; El Abdallaoui, E.A.; Mazoir, N.; Belghiti, M.E.; Syed, A.; Bahkali, A.H.; Verma, M.; Zeroual, A. Explaining the selectivities and the mechanism of [3+2] cycloloaddition reaction between isoalantolactone and diazocyclopropane. J. Mol. Model. 2023, 29, 280. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Domingo, L.R. A New C–C Bond Formation Model Based on the Quantum Chemical Topology of Electron Density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative Characterization of the Global Electrophilicity Power of Common Diene/Dienophile Pairs in Diels–Alder Reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R. 1999–2024, a Quarter Century of the Parr’s Electrophilicity ω Index. Sci. Radices 2024, 3, 157–186. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the Local Reactivity in Polar Organic Reactions through Electrophilic and Nucleophilic Parr Functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major Enhancements for Small-Molecule Docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.C.; Kollman, P.A. An Approach to Computing Electrostatic Charges for Molecules. J. Comput. Chem. 1984, 5, 129–145. [Google Scholar] [CrossRef]
- Crystallography: Protein Data Bank. Nat. New Biol. 1971, 233, 223. [CrossRef]


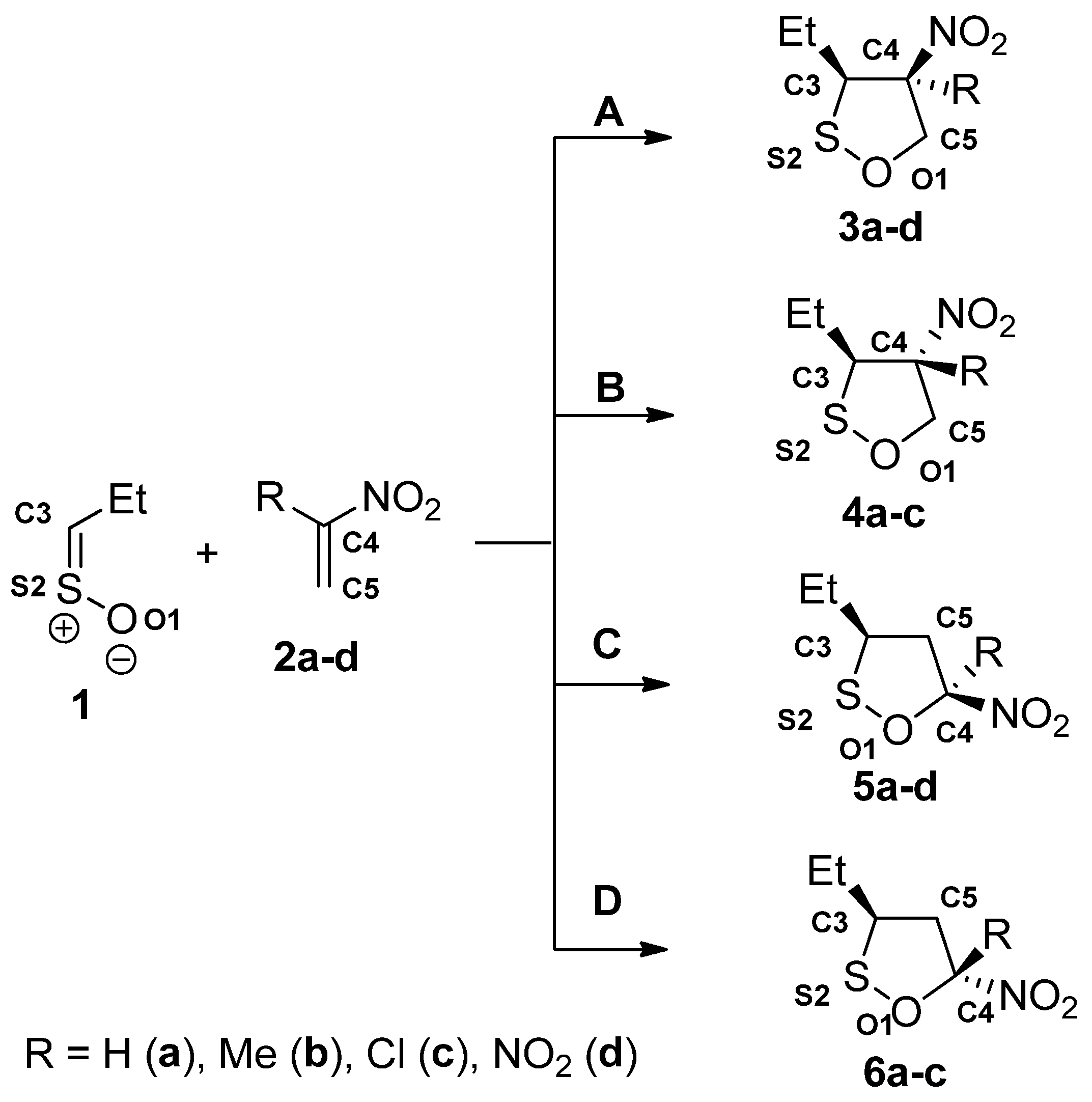

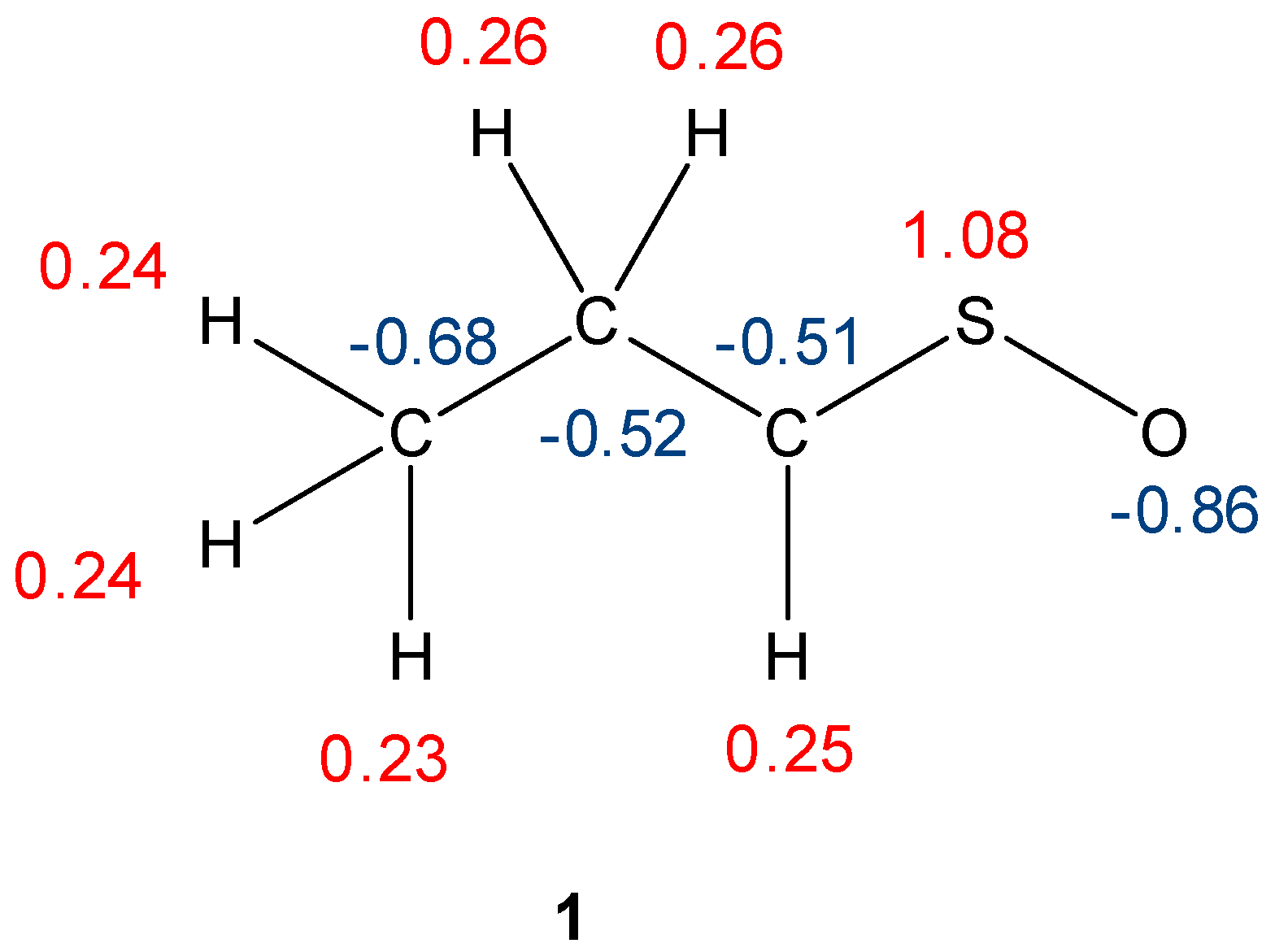
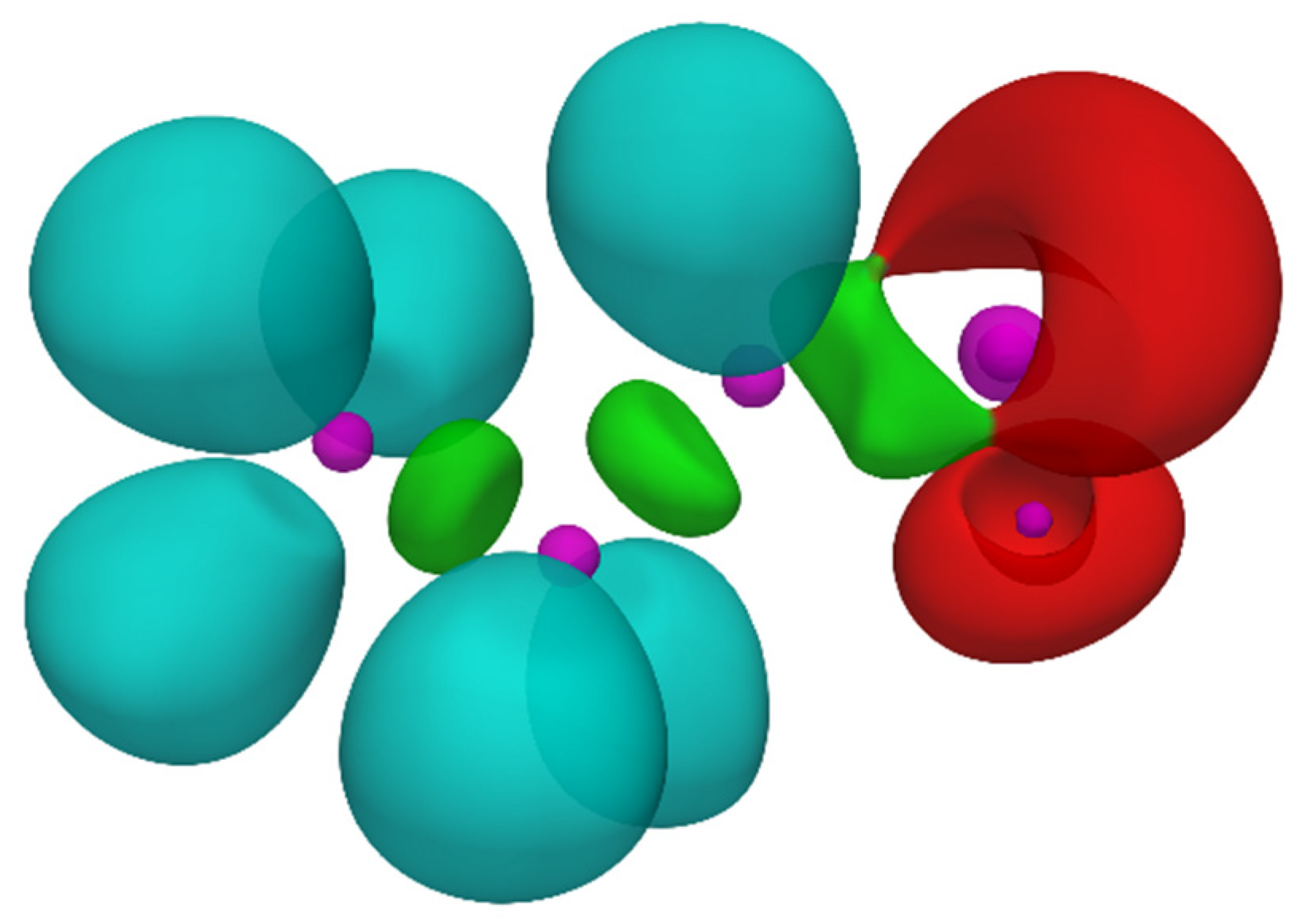
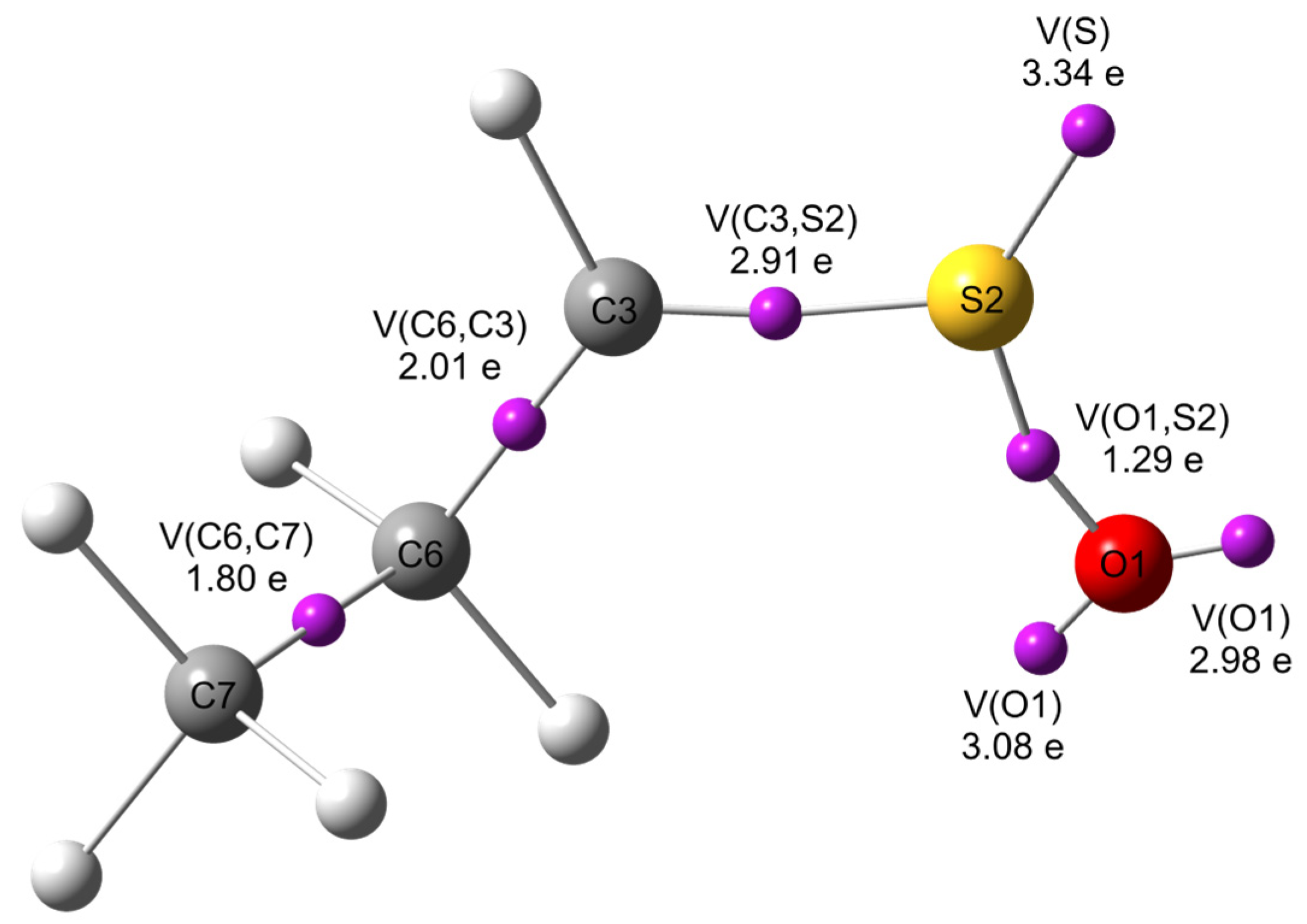
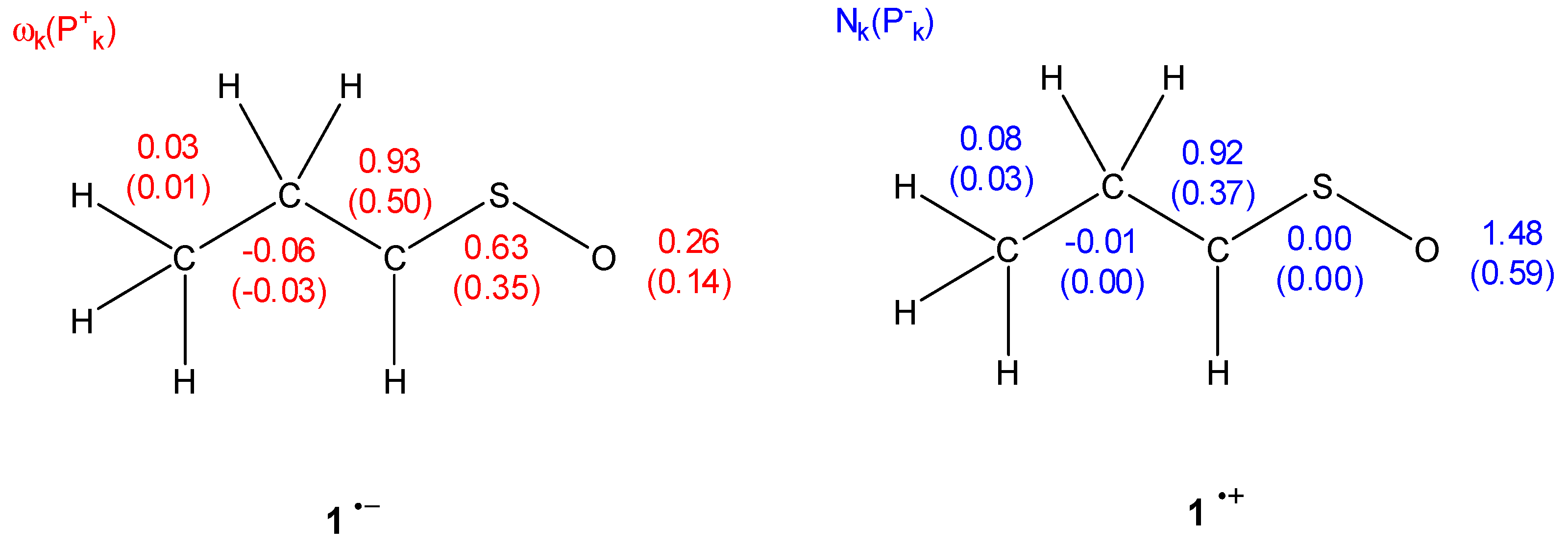

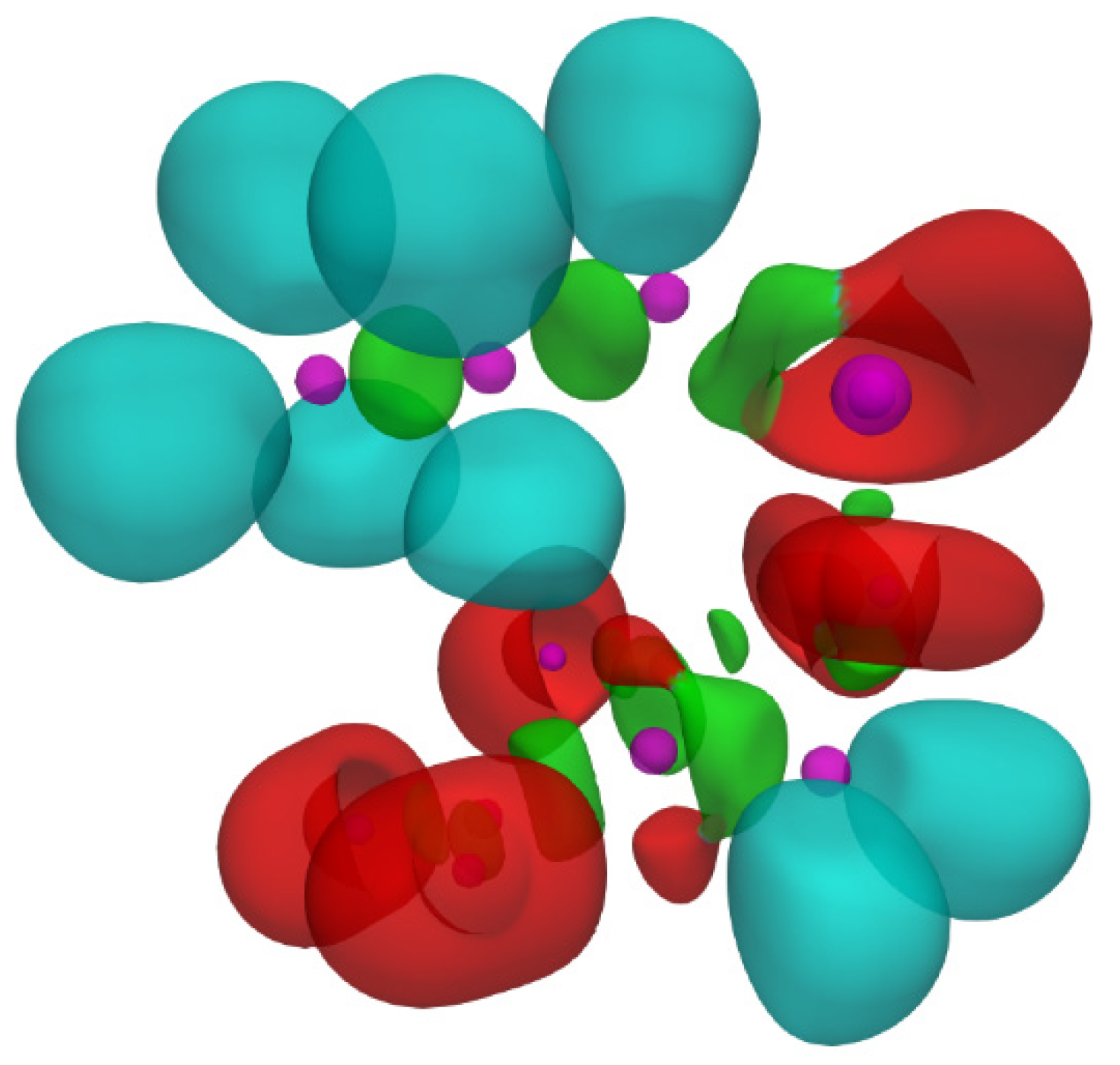

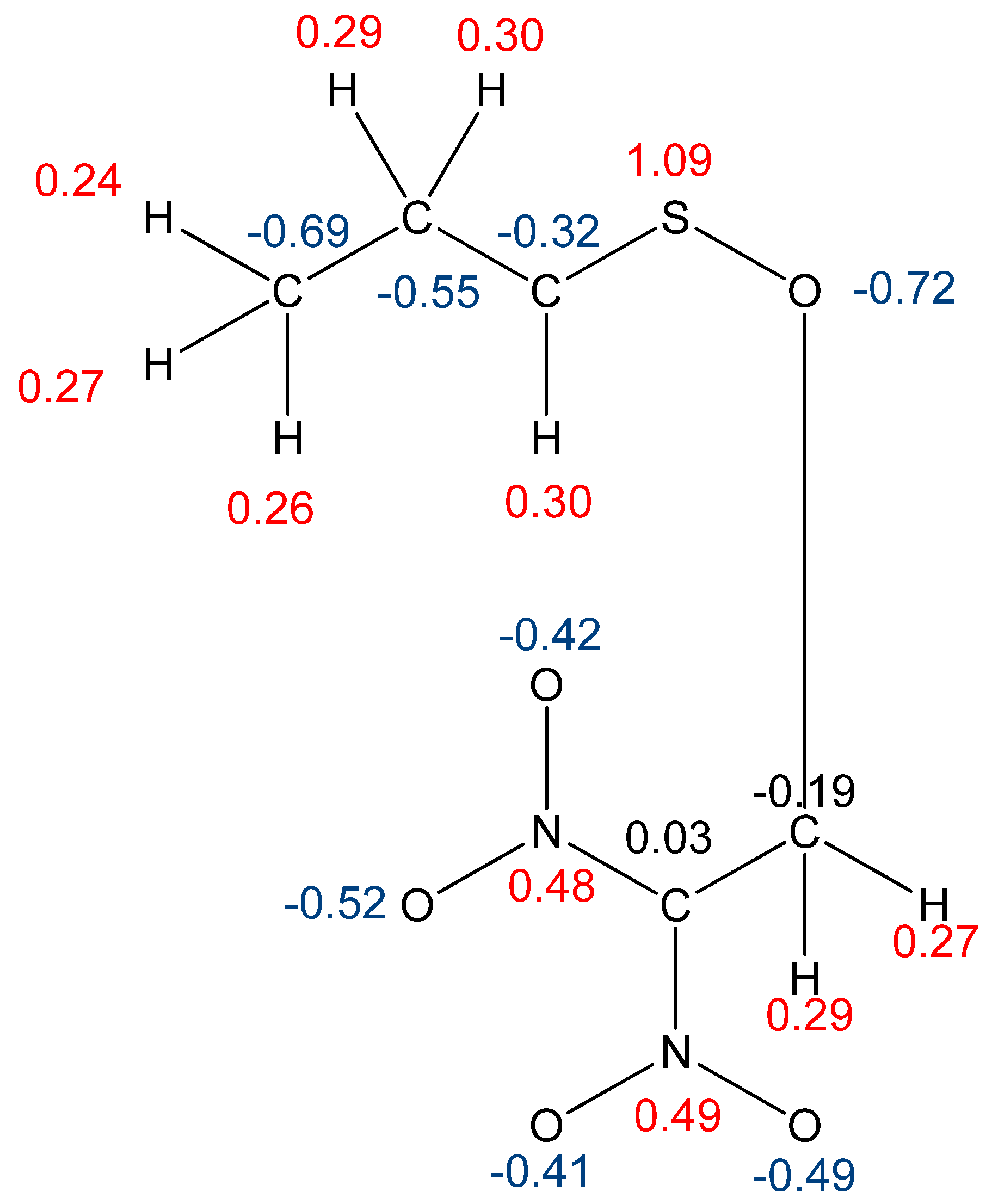
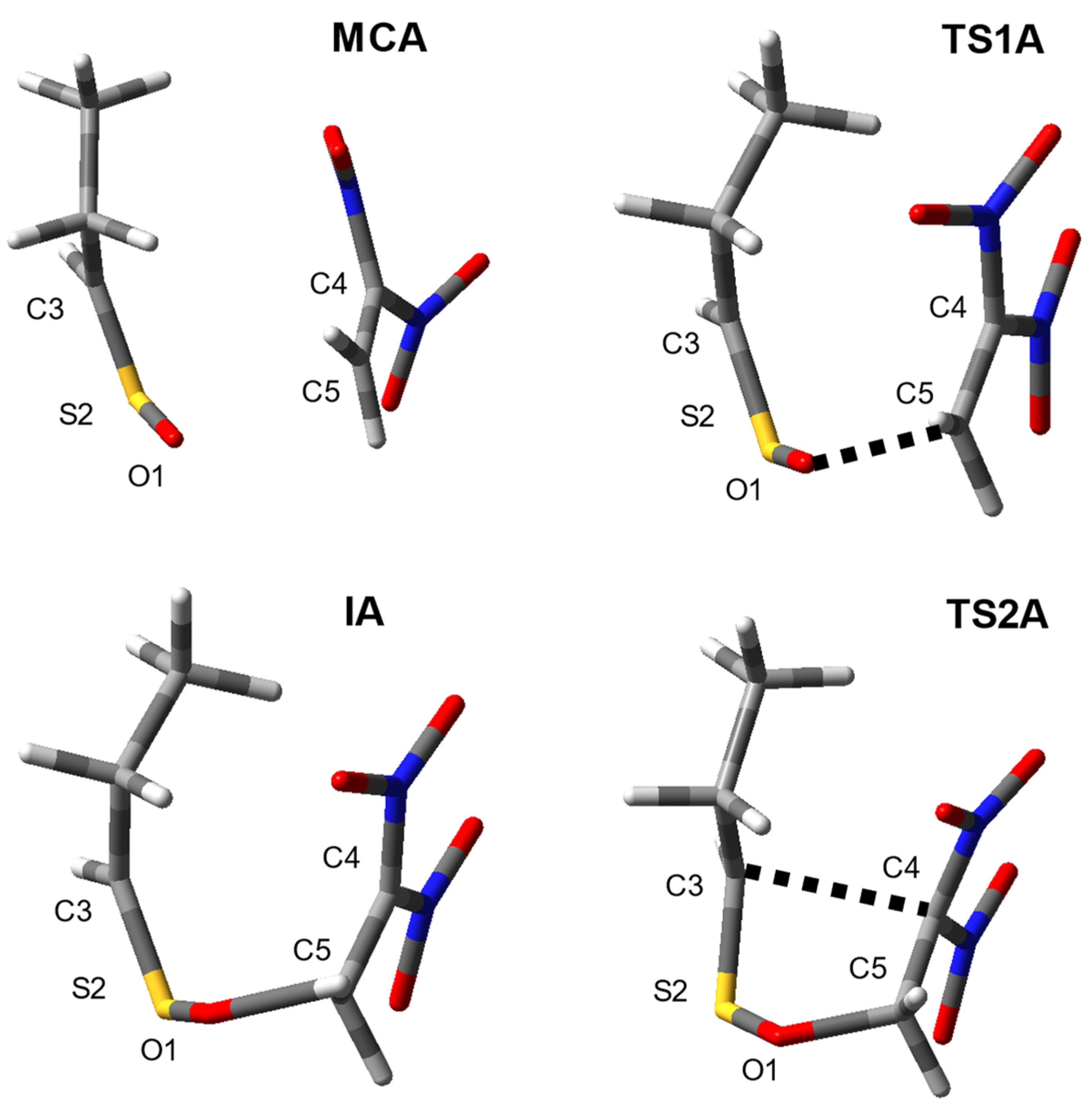

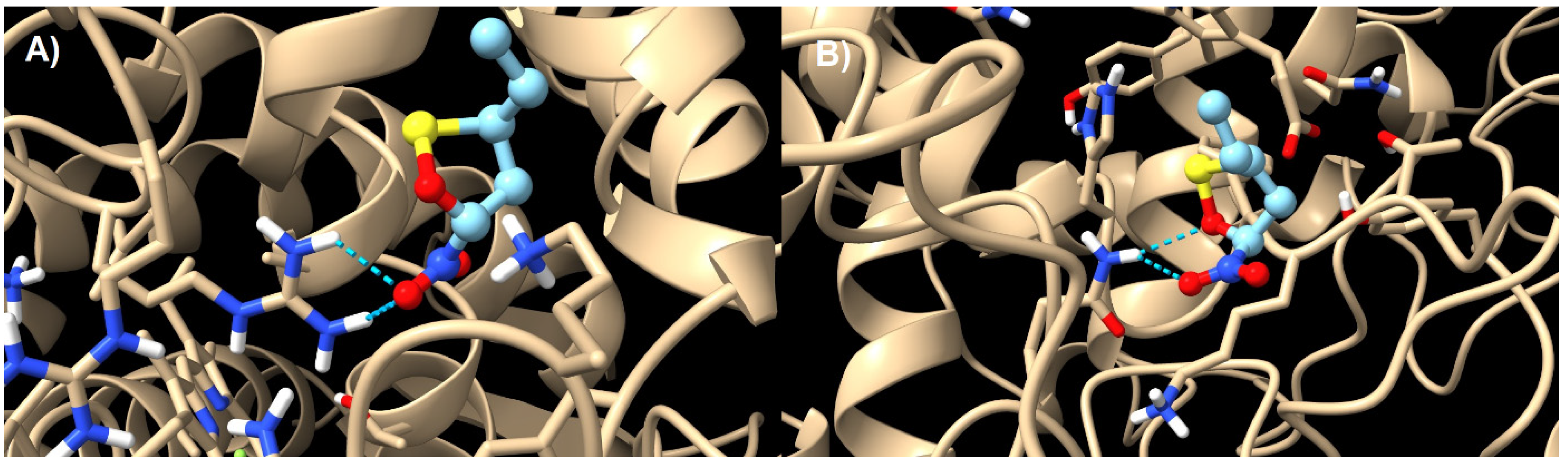
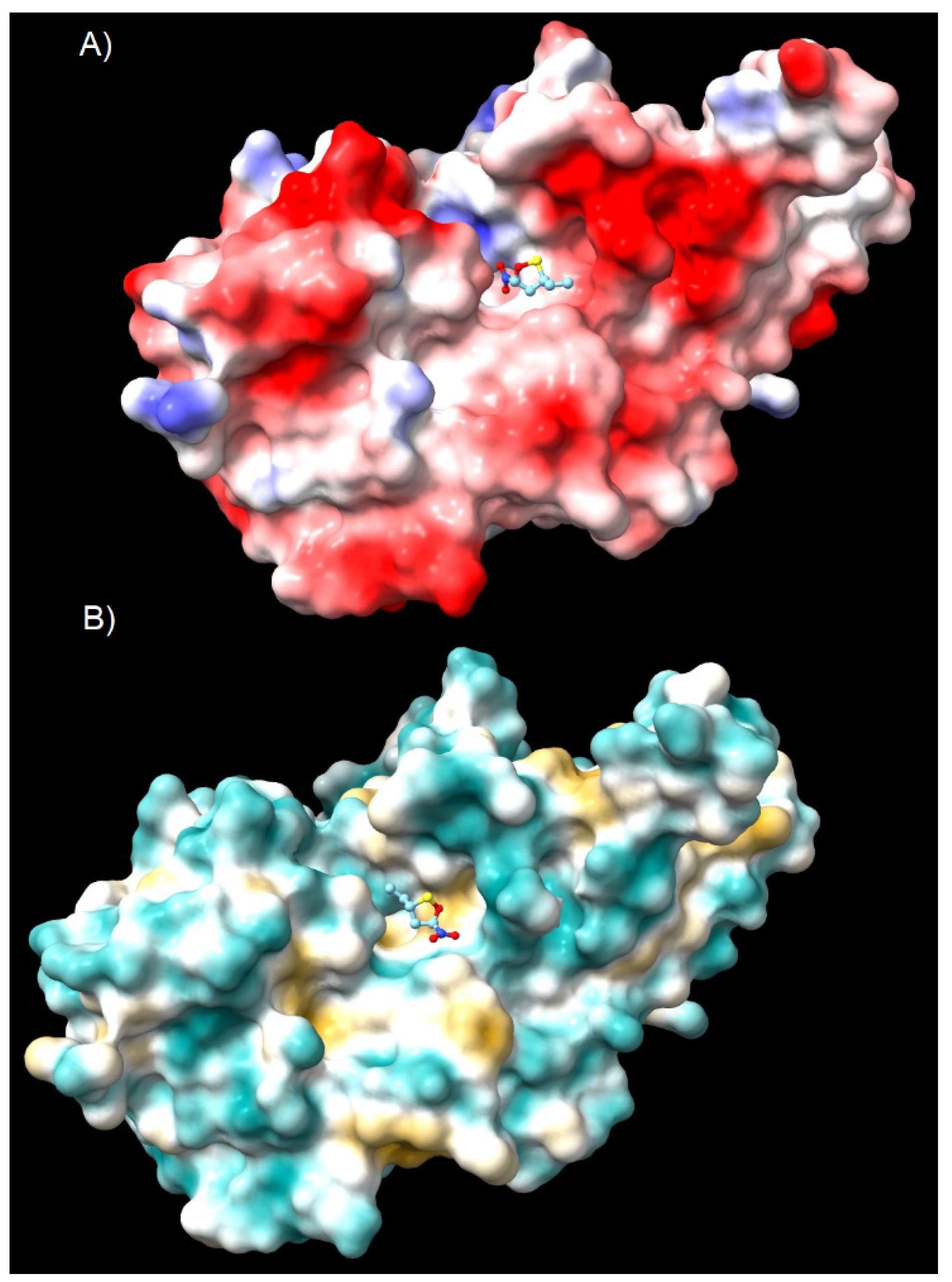
| Reaction | Transition | ∆H | ∆S | ∆G |
|---|---|---|---|---|
| 1 + 2a | 1 + 2a→MCA | −4.1 | −32.7 | 5.6 |
| 1 + 2a→TSA | 18.9 | −49.2 | 33.6 | |
| 1 + 2a→3a | −32.4 | −50.3 | −17.4 | |
| 1 + 2a→MCB | −4.3 | −38.3 | 7.1 | |
| 1 + 2a→TSB | 16.0 | −47.7 | 30.2 | |
| 1 + 2a→4a | −35.6 | −48.7 | −21.1 | |
| 1 + 2a→MCC | −3.1 | −32.5 | 6.6 | |
| 1 + 2a→TSC | 19.4 | −47.6 | 33.6 | |
| 1 + 2a→5a | −39.4 | −50.1 | −24.4 | |
| 1 + 2a→MCD | −3.6 | −32.6 | 6.2 | |
| 1 + 2a→TSD | 17.1 | −48.4 | 31.6 | |
| 1 + 2a→6a | −38.6 | −48.6 | −24.1 | |
| 1 + 2b | 1 + 2b→MCA | −4.3 | −33.2 | 5.6 |
| 1 + 2b→TSA | 20.9 | −49.1 | 35.5 | |
| 1 + 2b→3b | −31.8 | −53.5 | −15.8 | |
| 1 + 2b→MCB | −4.1 | −35.7 | 6.6 | |
| 1 + 2b→TSB | 17.4 | −50.3 | 32.4 | |
| 1 + 2b→4b | −33.6 | −54.1 | −17.5 | |
| 1 + 2b→MCC | −4.1 | −28.4 | 4.3 | |
| 1 + 2b→TC | 18.7 | −49.3 | 33.4 | |
| 1 + 2b→5b | −40.5 | −51.2 | −25.2 | |
| 1 + 2b→MCD | −4.2 | −35.2 | 6.3 | |
| 1 + 2b→TSD | 15.3 | −48.8 | 29.8 | |
| 1 + 2b→6b | −39.6 | −51.0 | −24.4 | |
| 1 + 2c | 1 + 2c→MCA | −4.7 | −33.2 | 5.2 |
| 1 + 2c→TSA | 16.4 | −51.1 | 31.6 | |
| 1 + 2c→3c | −32.9 | −53.1 | −17.1 | |
| 1 + 2c→MCB | −4.6 | −32.3 | 5.0 | |
| 1 + 2c→TSB | 15.1 | −51.6 | 30.5 | |
| 1 + 2c→4c | −34.7 | −53.0 | −18.9 | |
| 1 + 2c→MCC | −3.8 | −33.7 | 6.3 | |
| 1 + 2c→TC | 17.8 | −48.6 | 32.3 | |
| 1 + 2c→5c | −41.1 | −50.6 | −26.1 | |
| 1 + 2c→MCD | −3.8 | −33.9 | 6.4 | |
| 1 + 2c→TSD | 14.7 | −48.9 | 29.3 | |
| 1 + 2c→6c | −40.5 | −49.9 | −25.7 | |
| 1 + 2d | 1 + 2d→MCA | −5.8 | −37.7 | 5.4 |
| 1 + 2d→TS1A | 0.9 | −49.6 | 15.7 | |
| 1 + 2d→IA | −0.1 | −49.7 | 14.7 | |
| 1 + 2d→TS2A | 5.6 | −55.4 | 22.1 | |
| 1 + 2d→3d | −36.6 | −55.2 | −20.1 | |
| 1 + 2d→MC | −4.3 | −39.2 | 7.4 | |
| 1 + 2d→TSC | 12.1 | −51.5 | 27.5 | |
| 1 + 2d→5d | −44.4 | −53.2 | −28.6 |
| Reaction | Path | Structure | Interatomic Distance [Å] | GEDT [e] | ||||
|---|---|---|---|---|---|---|---|---|
| O1-S2 | S2-C3 | C3-C4(5) | C4-C5 | C5(4)-O1 | ||||
| 1 + 2a | A | MCA | 1.498 | 1.616 | 4.532 | 1.322 | 2.867 | 0.00 |
| TSA | 1.578 | 1.654 | 2.704 | 1.419 | 1.688 | 0.45 | ||
| 3a | 1.690 | 1.827 | 1.555 | 1.545 | 1.419 | |||
| B | MCB | 1.499 | 1.616 | 3.645 | 1.322 | 2.952 | 0.00 | |
| TSB | 1.567 | 1.649 | 2.552 | 1.405 | 1.766 | 0.33 | ||
| 4a | 1.699 | 1.844 | 1.539 | 1.538 | 1.407 | |||
| C | MCC | 1.497 | 1.616 | 4.334 | 1.320 | 2.884 | 0.00 | |
| TSC | 1.527 | 1.685 | 2.070 | 1.377 | 2.303 | 0.20 | ||
| 5a | 1.728 | 1.837 | 1.536 | 1.521 | 1.364 | |||
| D | MCD | 1.498 | 1.615 | 4.550 | 1.319 | 2.959 | 0.00 | |
| TSD | 1.511 | 1.683 | 2.014 | 1.381 | 2.494 | 0.24 | ||
| 6a | 1.715 | 1.822 | 1.540 | 1.544 | 1.372 | |||
| 1 + 2b | A | MCA | 1.498 | 1.616 | 3.588 | 1.326 | 3.144 | 0.00 |
| TSA | 1.576 | 1.654 | 2.711 | 1.421 | 1.707 | 0.45 | ||
| 3b | 1.694 | 1.848 | 1.552 | 1.544 | 1.402 | |||
| B | MCB | 1.499 | 1.615 | 3.747 | 1.325 | 3.108 | 0.00 | |
| TSB | 1.578 | 1.637 | 2.767 | 1.431 | 1.655 | 0.38 | ||
| 4b | 1.706 | 1.845 | 1.548 | 1.545 | 1.407 | |||
| C | MCC | 1.497 | 1.616 | 4.250 | 1.325 | 2.993 | 0.00 | |
| TSC | 1.526 | 1.683 | 2.091 | 1.381 | 2.351 | 0.17 | ||
| 5b | 1.720 | 1.840 | 1.535 | 1.525 | 1.372 | |||
| D | MCD | 1.497 | 1.616 | 4.001 | 1.324 | 3.012 | 0.00 | |
| TSD | 1.512 | 1.683 | 2.036 | 1.383 | 2.545 | 0.20 | ||
| 6b | 1.708 | 1.823 | 1.541 | 1.546 | 1.380 | |||
| 1 + 2c | A | MCA | 1.500 | 1.616 | 3.811 | 1.323 | 2.863 | 0.00 |
| TSA | 1.594 | 1.650 | 2.760 | 1.441 | 1.587 | 0.53 | ||
| 3c | 1.699 | 1.850 | 1.547 | 1.546 | 1.398 | |||
| B | MCB | 1.499 | 1.616 | 3.885 | 1.322 | 2.865 | 0.00 | |
| TSB | 1.601 | 1.642 | 2.719 | 1.457 | 1.645 | 0.53 | ||
| 4c | 1.706 | 1.847 | 1.541 | 1.542 | 1.401 | |||
| C | MCC | 1.497 | 1.617 | 4.185 | 1.321 | 2.945 | 0.00 | |
| TSC | 1.518 | 1.685 | 2.041 | 1.381 | 2.413 | 0.20 | ||
| 5c | 1.735 | 1.831 | 1.532 | 1.521 | 1.348 | |||
| D | MCD | 1.496 | 1.616 | 4.061 | 1.321 | 2.949 | 0.00 | |
| TSD | 1.507 | 1.681 | 2.038 | 1.380 | 2.575 | 0.24 | ||
| 6c | 1.726 | 1.823 | 1.532 | 1.537 | 1.354 | |||
| 1 + 2d | A | MCA | 1.503 | 1.617 | 3.950 | 1.321 | 2.631 | 0.00 |
| TS1A | 1.550 | 1.620 | 3.490 | 1.391 | 1.813 | 0.54 | ||
| IA | 1.594 | 1.617 | 3.439 | 1.455 | 1.524 | 0.73 | ||
| TS2A | 1.614 | 1.662 | 2.523 | 1.474 | 1.510 | 0.61 | ||
| 3d | 1.692 | 1.830 | 1.544 | 1.554 | 1.409 | |||
| C | MC | 1.499 | 1.616 | 3.887 | 1.318 | 2.775 | 0.00 | |
| TS | 1.493 | 1.677 | 2.033 | 1.383 | 2.641 | 0.36 | ||
| 5d | 1.731 | 1.823 | 1.536 | 1.527 | 1.348 | |||
| CYP51 1EA1 | MMP-9 4XCT | COX-1 3KK6 | COX-2 5KIR | |
|---|---|---|---|---|
| ΔG (kcal/mol) | ||||
| 3a | −3.79 | −5.48 | −4.70 | −4.43 |
| 4a | −3.35 | −4.95 | −4.30 | −4.05 |
| 5a | −3.60 | −5.13 | −4.55 | −4.56 |
| 6a | −3.49 | −4.84 | −4.33 | −4.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowski, M.; Dresler, E.; Zawadzińska, K.; Wróblewska, A.; Jasiński, R. Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study. Molecules 2024, 29, 4892. https://doi.org/10.3390/molecules29204892
Sadowski M, Dresler E, Zawadzińska K, Wróblewska A, Jasiński R. Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study. Molecules. 2024; 29(20):4892. https://doi.org/10.3390/molecules29204892
Chicago/Turabian StyleSadowski, Mikołaj, Ewa Dresler, Karolina Zawadzińska, Aneta Wróblewska, and Radomir Jasiński. 2024. "Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study" Molecules 29, no. 20: 4892. https://doi.org/10.3390/molecules29204892
APA StyleSadowski, M., Dresler, E., Zawadzińska, K., Wróblewska, A., & Jasiński, R. (2024). Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study. Molecules, 29(20), 4892. https://doi.org/10.3390/molecules29204892








