Unveiling New Horizons: Advancing Technologies in Cosmeceuticals for Anti-Aging Solutions
Abstract
1. Introduction
2. Results
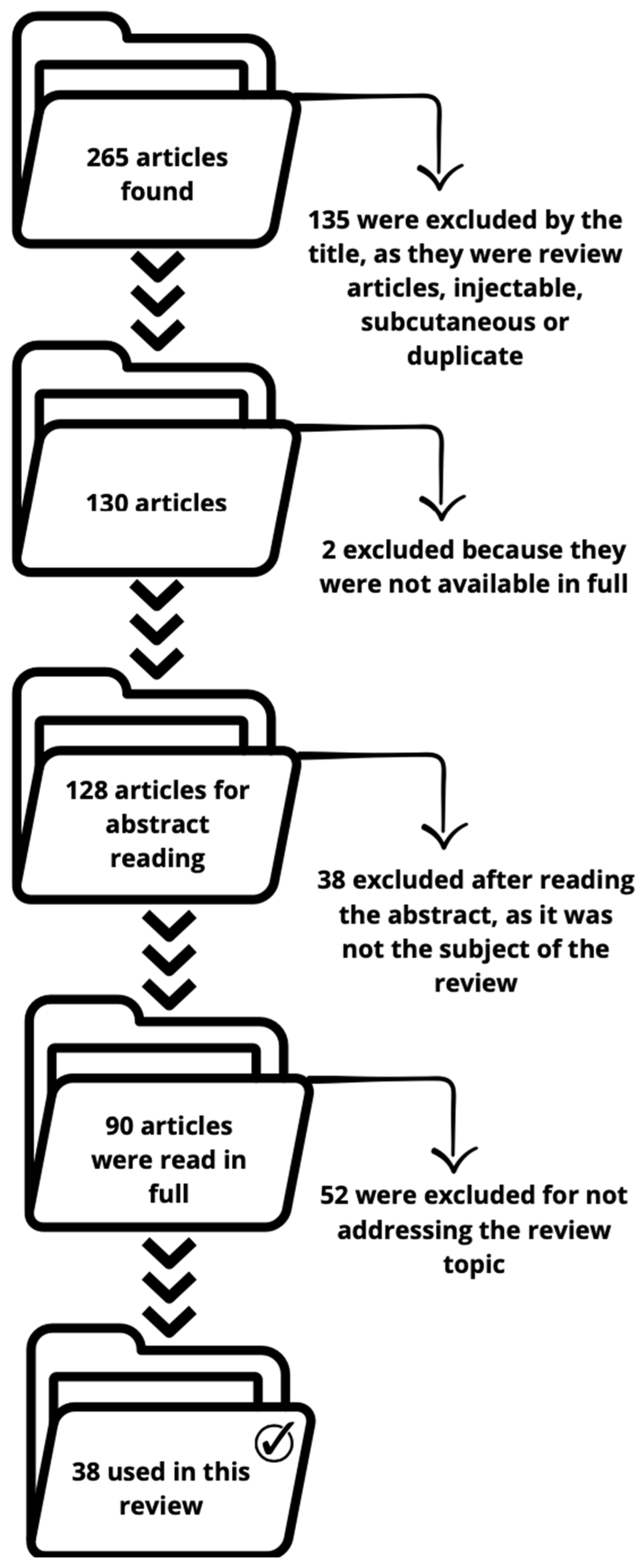
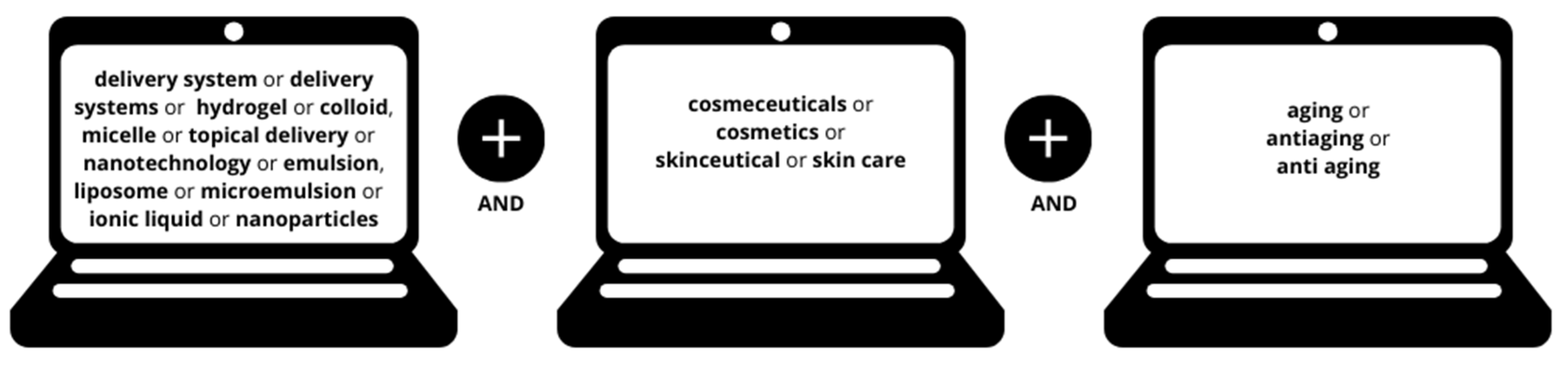
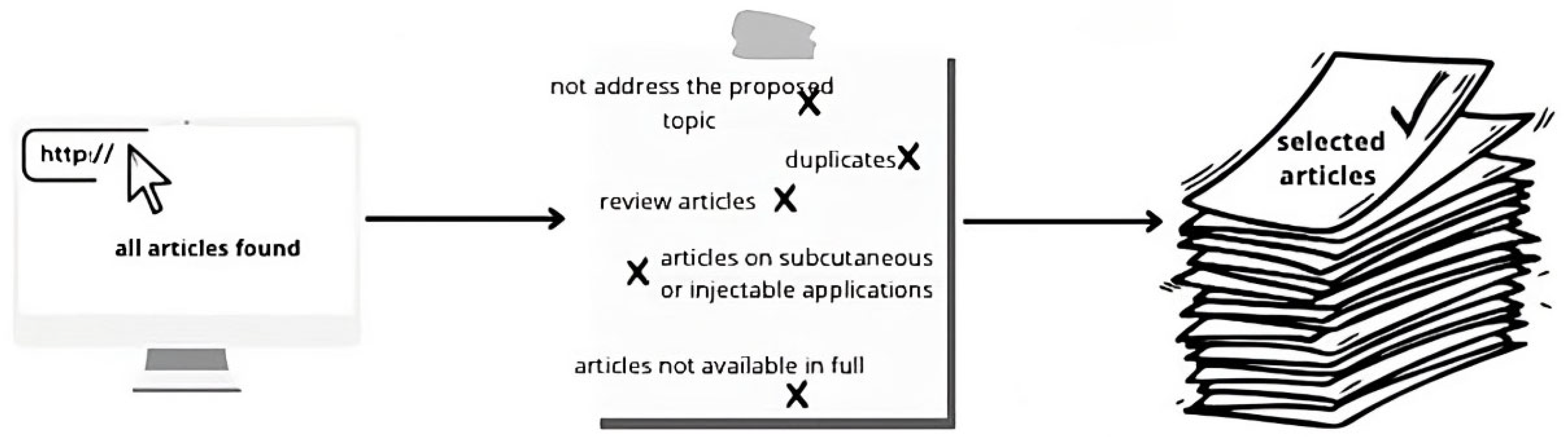
Review Methodology: Article Selection and Analysis
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bilal, M.; Iqbal, H.M.N. New Insights on Unique Features and Role of Nanostructured Materials in Cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Newburger, A.E. Cosmeceuticals: Myths and misconceptions. Clin. Dermatol. 2009, 27, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, H. A review of cosmetic skin delivery. J. Cosmet. Dermatol. 2021, 20, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Agrawal, R. Nanotechnology: A New Paradigm in Cosmeceuticals. Recent Pat. Drug Deliv. Formul. 2007, 1, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthetic Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Barnawi, G.M.; Barnawi, A.M. Truths and myths about marketed anti-aging skin products: A systematic review. Int. J. Res. Med. Sci. 2021, 9, 2445. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; Abu-Huwaij, R.; AL-Samydai, A.; Al-Halaseh, L.K.; Al-Dujaili, E.A. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. J. Cosmet. Dermatol. 2021, 20, 3818–3828. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Rathore, A.; Mahesh, G. Public perception of nanotechnology: A contrast between developed and developing countries. Technol. Soc. 2021, 67, 101751. [Google Scholar] [CrossRef]
- Oliveira, C.; Coelho, C.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Nanocarriers as Active Ingredients Enhancers in the Cosmetic Industry—The European and North America Regulation Challenges. Molecules 2022, 27, 1669. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, N.M.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 1773–2247. [Google Scholar] [CrossRef]
- Kabri, T.H.; Arab-Tehrany, E.; Belhaj, N.; Linder, M. Physico-chemical characterization of nano-emulsions in cosmetic matrix enriched on omega-3. J. Nanobiotechnol. 2011, 21, 9–41. [Google Scholar] [CrossRef]
- Sethi, M.; Rana, R.; Sambhakar, S.; Chourasia, M.K. Nanocosmeceuticals: Trends and Recent Advancements in Self Care. AAPS Pharm. Sci. Tech. 2024, 25, 51. [Google Scholar] [CrossRef] [PubMed]
- Hubbs, A.F.; Mercer, R.; Benkovic, S.A.; Harkema, J.; Sriram, K.; Schwegler-Berry, D.; Goravanahally, M.P.; Nurkiewicz, T.R.; Castranova, V.; Sargent, L.M. Nanotoxicology—A Pathologist’s Perspective. Toxicol. Pathol. 2011, 39, 301–324. [Google Scholar] [CrossRef] [PubMed]
- Shireesha, B.; Shyamala; Sathoorimanasa. Comprehensive review on cosmeceuticals. Int. J. Sci. Res. Arch. 2023, 8, 649–654. [Google Scholar] [CrossRef]
- Melo, A.; Amadeu, S.M.; Lancellotti, M.; Hollanda, M.L.; Machado, D. The role of nanomaterials in cosmetics: National and international legislative aspects. Quim. Nova 2015, 4, 38. [Google Scholar] [CrossRef]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef]
- Chaikul, P.; Khat-udomkiri, N.; Iangthanarat, K.; Manosroi, J.; Manosroi, A. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. Eur. J. Pharm. Sci. 2019, 131, 39–49. [Google Scholar] [CrossRef]
- Bellu, E.; Garroni, G.; Cruciani, S.; Balzano, F.; Serra, D.; Satta, R.; Montesu, M.A.; Fadda, A.; Mulas, M.; Sarais, G.; et al. Smart Nanofibers with Natural Extracts Prevent Senescenc Patterning in a Dynamic Cell Culture Model of Human Skin. Cells 2020, 9, 2530. [Google Scholar] [CrossRef]
- Inal, O.; Amasya, G.; Bayindir, Z.S.; Yuksel, N. Development and quality assessment of glutathione tripeptide loaded niosome containing carbopol emulgels as nanocosmeceutical formulations. Int. J. Biol. Macromol. 2023, 241, 124651. [Google Scholar] [CrossRef] [PubMed]
- Spanidi, E.; Karapetsas, A.; Voulgaridou, G.P.; Letsiou, S.; Aligiannis, N.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Mourtzinos, I.; Pappa, A.; et al. A New Controlled Release System for Propolis Polyphenols and Its Biochemical Activity for Skin Applications. Plants 2021, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Anuchapreeda, S.; Somwongin, S.; Marsup, P.; Lee, K.H.; Lin, W.C.; Lue, S.-C. Dermal Delivery Enhancement of Natural Anti-Ageing Compounds from Ocimum sanctum Linn. Extract by Nanostructured Lipid Carriers. Pharmaceutics 2020, 12, 309. [Google Scholar] [CrossRef]
- Báo, S.N.; Machado, M.; Da Silva, A.L.; Melo, A.; Cunha, S.; Sousa, S.S.; Malheiro, A.R.; Fernandes, R.; Leite, J.R.S.A.; Vasconcelos, A.G.; et al. Potential Biological Properties of Lycopene in a Self-Emulsifying Drug Delivery System. Molecules 2023, 28, 1219. [Google Scholar] [CrossRef]
- Spanidi, E.; Athanasopoulou, S.; Liakopoulou, A.; Chaidou, A.; Hatziantoniou, S.; Gardikis, K. Royal Jelly Components Encapsulation in a Controlled Release System—Skin Functionality, and Biochemical Activity for Skin Applications. Pharmaceuticals 2022, 15, 907. [Google Scholar] [CrossRef]
- Pereira, A.; Ramalho, M.J.; Silva, R.; Silva, V.; Marques-Oliveira, R.; Silva, A.C.; Pereira, M.C.; Loureiro, J.A. Vine Cane Compounds to Prevent Skin Cells Aging through Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 240. [Google Scholar] [CrossRef]
- Di Filippo, L.D.; Duarte, J.L.; Roque-Borda, C.A.; Pavan, F.R.; Meneguin, A.B.; Chorilli, M.; Melero, A.; Guillot, A.J.; Spagnol, C.M.; Correa, M.A. In Vitro Skin Co-Delivery and Antibacterial Properties of Chitosan-Based Microparticles Containing Ascorbic Acid and Nicotinamide. Life 2022, 12, 1049. [Google Scholar] [CrossRef]
- Tran, H.M.; Yang, C.Y.; Wu, T.H.; Yen, F.L. Liposomes Encapsulating Morin: Investigation of Physicochemical Properties, Dermal Absorption Improvement and Anti-Aging Activity in PM-Induced Keratinocytes. Antioxidants 2022, 11, 1183. [Google Scholar] [CrossRef]
- Mohamadi, N.; Soltanian, S.; Raeiszadeh, M.; Moeinzadeh, M.; Ohadi, M.; Sharifi, F.; Pardakhty, A.; Sharififar, F. Characteristics and in vitro anti skin aging activity and UV radiation protection of morin loaded in niosomes. J. Cosmet. Dermatol. 2022, 21, 6326–6335. [Google Scholar] [CrossRef]
- Wang, F.C.; Hudson, P.L.; Burk, K.; Marangoni, A.G. Encapsulation of cycloastragenol in phospholipid vesicles enhances transport and delivery across the skin barrier. J. Colloid Interface Sci. 2022, 608, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Tsai, Y.Y.; Chang, L.S.; Chen, Y.J. Evaluation of Gallic Acid-Coated Gold Nanoparticles as an Anti-Aging Ingredient. Pharmaceuticals 2021, 14, 1071. [Google Scholar] [CrossRef]
- Haddada, M.B.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.-L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef]
- Lewińska, A.; Domżał-Kędzia, M.; Maciejczyk, E.; Łukaszewicz, M.; Bazylińska, U. Design and Engineering of “Green” Nanoemulsions for Enhanced Topical Delivery of Bakuchiol Achieved in a Sustainable Manner: A Novel Eco-Friendly Approach to Bioretinol. Int. J. Mol. Sci. 2021, 22, 10091. [Google Scholar] [CrossRef]
- Tavakol, S.; Zare, S.; Hoveizi, E.; Tavakol, B.; Rezayat, S.M. The impact of the particle size of curcumin nanocarriers and the ethanol on beta_1-integrin overexpression in fibroblasts: A regenerative pharmaceutical approach in skin repair and anti-aging formulations. DARU J. Pharm. Sci. 2019, 27, 159–168. [Google Scholar] [CrossRef]
- Yang, H.; Yu, S.; Kim, J.; Baek, K.; Lee, Y.R.; Lee, H.S.; Choi, W.I.; Sung, D. Facile Solvent-Free Preparation of Antioxidant Idebenone-Loaded Nanoparticles for Efficient Wound Healing. Pharmaceutics 2022, 14, 521. [Google Scholar] [CrossRef]
- Ayunin, Q.; Miatmoko, A.; Soeratri, W.; Erawati, T.; Susanto, J.; Legowo, D. Improving the anti-ageing activity of coenzyme Q10 through protransfersome-loaded emulgel. Sci. Rep. 2022, 12, 906. [Google Scholar] [CrossRef]
- Kubota, Y.; Musashi, M.; Nagasawa, T.; Shimura, N.; Igarashi, R.; Yamaguchi, Y. Novel nanocapsule of α-lipoic acid reveals pigmentation improvement: α-Lipoic acid stimulates the proliferation and differentiation of keratinocyte in murine skin by topical application. Exp. Dermatol. 2019, 28, 55–63. [Google Scholar] [CrossRef]
- Baptista, S.; Baptista, F.; Freitas, F. Development of Emulsions Containing L-Ascorbic Acid and α-Tocopherol Based on the Polysaccharide FucoPol: Stability Evaluation and Rheological and Texture Assessment. Cosmetics 2023, 10, 56. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; Gaafar, P.M.E.; Khattab, M.A.; Helwah, M.K.; Noureldin, M.H.; Abbas, H. Dual-effects of caffeinated hyalurosomes as a nano-cosmeceutical gel counteracting UV-induced skin aging. Int. J. Pharm. X 2023, 5, 100170. [Google Scholar] [CrossRef]
- Amer, R.I.; Ezzat, S.M.; Aborehab, N.M.; Ragab, M.F.; Mohamed, D.; Hashad, A.; Attia, D.; Salama, M.M.; El Bishbishy, M.H. Downregulation of MMP1 expression mediates the anti-aging activity of Citrus sinensis peel extract nanoformulation in UV induced photoaging in mice. Biomed. Pharmacother. 2021, 138, 111537. [Google Scholar] [CrossRef]
- Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef]
- Liu, C.; Guo, X.; Chen, Y.; Zhao, M.; Shi, S.; Luo, Z.; Song, J.; Zhang, Z.; Yang, W.; Liu, K. Anti-photoaging effect and mechanism of flexible liposomes co-loaded with apigenin and doxycycline. Biomed. Pharmacother. 2023, 164, 114998. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J.; Xie, J.; Liu, Z.; Qiu, Z.; Teng, L. Liposomal Vitamin D3 as an Anti-aging Agent for the Skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef]
- Zewail, M.; Gaafar, P.M.E.; Youssef, N.A.H.A.; Ali, M.E.; Ragab, M.F.; Kamal, M.F.; Kamal, M.F.; Noureldin, M.H.; Abbas, H. Novel Spirulina platensis Bilosomes for Combating UVB Induced Skin Damage. Pharmaceuticals 2022, 16, 36. [Google Scholar] [CrossRef]
- Salem, M.A.; Manaa, E.G.; Osama, N.; Aborehab, N.M.; Ragab, M.F.; Haggag, Y.A.; Ibrahim, M.T.; Hamdan, D.I. Coriander (Coriandrum sativum L.) essential oil and oil-loaded nano-formulations as an anti-aging potentiality via TGFβ/SMAD pathway. Sci. Rep. 2022, 12, 6578. [Google Scholar] [CrossRef]
- Deylam, M.; Alizadeh, E.; Sarikhani, M.; Hejazy, M.; Firouzamandi, M. Zinc oxide nanoparticles promote the aging process in a size-dependent manner. J. Mater. Sci. Mater. Med. 2021, 32, 128. [Google Scholar] [CrossRef]
- Rafique, M.; Hussain, S.S.N.; Hussain, I.; Javed, I.; Nisar, N.; Riaz, R. Development of grape seed extract based formulations by using non-invasive biophysical technique and its impact on skin aging. Pak. J. Pharm. Sci. 2021, 34, 1621–1628. [Google Scholar]
- Tasneem, R.; Khan, H.M.S.; Rasool, F.; Khan, K.R.; Umair, M.; Esatbeyoglu, T.; Korma, S.A. Development of Phytocosmeceutical Microemulgel Containing Flaxseed Extract and Its In Vitro and In Vivo Characterization. Pharmaceutics 2022, 14, 1656. [Google Scholar] [CrossRef]
- Aboul-Einien, M.H.; Kandil, S.M.; Abdou, E.M.; Diab, H.M.; Zaki, M.S.E. Ascorbic acid derivative-loaded modified aspasomes: Formulation, in vitro, ex vivo and clinical evaluation for melasma treatment. J. Liposome Res. 2020, 30, 54–67. [Google Scholar] [CrossRef]
- Elhabak, M.; Ibrahim, S.; Abouelatta, S.M. Topical delivery of l-ascorbic acid spanlastics for stability enhancement and treatment of UVB induced damaged skin. Drug Deliv. 2021, 28, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H.; Mohammed, H.A.; Al-Khalaf, A.M.; Khan, O.; Mostafa, M.A.H.; Al Haidari, R.A.; Taha, H.H.; Khan, R.A. Ginkgo biloba leaves extract’s cosmeceutical evaluation: A preliminary assessment on human volunteers towards achieving improved skin condition and rejuvenation. Drug Dev. Ind. Pharm. 2023, 49, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, G.; Jeon, G.; Lee, H.; Park, S.; Sohn, Y.; Park, Y.; Ryu, S. Anti-Aging and Lightening Effects of Au-Decorated Zeolite-Based Biocompatible Nanocomposites in Epidermal Delivery Systems. J. Funct. Biomater. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Lewińska, A.; Domżał-Kędzia, M.; Kierul, K.; Bochynek, M.; Pannert, D.; Nowaczyk, P.; Łukaszewicz, M. Targeted Hybrid Nanocarriers as a System Enhancing the Skin Structure. Molecules 2021, 26, 1063. [Google Scholar] [CrossRef]
- An, J.Y.; Kim, C.; Park, N.R.; Jung, H.S.; Koo, T.; Yuk, S.H.; Lee, E.H.; Cho, S.H. Clinical Anti-aging Efficacy of Propolis Polymeric Nanoparticles Prepared by a Temperature-induced Phase Transition Method. J. Cosmet. Dermatol. 2022, 21, 4060–4071. [Google Scholar] [CrossRef]
- Perugini, P.; Bleve, M.; Redondi, R.; Cortinovis, F.; Colpani, A. In vivo evaluation of the effectiveness of biocellulose facial masks as active delivery systems to skin. J. Cosmet. Dermatol. 2020, 19, 725–735. [Google Scholar] [CrossRef]
- Kashif, M.; Akhtar, N. Dermocosmetic emulgels for anti-aging effects: Evidence from chromatographic and non-invasive biophysical techniques. Pak. J. Pharm. Sci. 2019, 32, 845–852. [Google Scholar]
- Khezri, K.; Saeedi, M.; Dizaj, S.M. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Youns, M.D.; Hoheisel, J.; Efferth, T. Therapeutic and Diagnostic Applications of Nanoparticles. Curr. Drug Targets 2011, 12, 357–365. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 27, 3420204. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal Toxicol. 2016, 6, 525–2161. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Chwastowski, J.; Siudek, M.; Banach, M. Incorporation of Metallic Nanoparticles into Cosmetic Preparations and Assessment of Their Physicochemical and Utility Properties. J. Surfactants Deterg. 2018, 21, 575–591. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Dufour, E.K. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: A risk to human health? Arch. Toxicol. 2012, 86, 1063–1075. [Google Scholar] [CrossRef]
- Nery, É.M.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. A short review of alternative ingredients and technologies of inorganic UV filters. J. Cosmet. Dermatol. 2021, 20, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef]
- Singh, P.; Nanda, A. Nanotechnology in cosmetics: A boon or bane? Toxicol. Environ. Chem. 2021, 94, 1467–1479. [Google Scholar] [CrossRef]
- Mihranyan, A.; Ferraz, N.; Strømme, M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012, 57, 875–910. [Google Scholar] [CrossRef]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
| Results | Delivery System | Active Compound | Reference |
|---|---|---|---|
| The cationic CTAB niosome loaded with gallic acid demonstrated anti-skin aging activity, including a melanin suppression effect, antioxidant activity, and the inhibition of matrix metalloproteinase 2. | Niosomes | Gallic acid | [20] |
| The combination of Myrtus communis natural extract and the polycaprolactone nanofibrous scaffold (NanoPCL-M) resulted in a significant reduction in senescent cells, anti-aging effect of the pretreatment, and also prevented morphological changes associated with aging in keratinocyte populations organized in a 3D structure. | Polycaprolactone nanofibrous scaffold | Myrtus communis extract | [21] |
| The results demonstrate a successful anti-aging cosmeceutical preparation containing glutathione tripeptide loaded lipid-based niosome dispersion, suitable for topical use due to its textural and viscosity properties. | Emulgel with niosome | Glutathione tripeptide | [22] |
| The delivery system can preserve the anti-mutagenic, anti-oxidative, and anti-ageing effects of propolis polyphenols at levels similar and comparable to those of propolis methanolic extracts, making the system ideal for applications requiring non-toxic solvents and controlled release of the polyphenol content. | Liposomes | Propolis polyphenols | [23] |
| The Nanostructured lipid carriers (NLC) system containing O. sanctum extract was an attractive dermal delivery system, ensuring safety and enhancing the dermal delivery of Rosmarinic acid. This could be used in future topical anti-aging products. | Nanostructured lipid carriers, nanoemulsion, liposome, and niosome | Ocimum sanctum Linn. extract | [24] |
| The self-emulsifying system containing Lycopene prevents DNA degradation, exhibits antioxidant activity, and inhibits enzymes (tyrosinase and elastase) involved in the skin aging process, without causing cytotoxicity in HaCaT cells. | Self-emulsifying | Lycopene | [25] |
| The study addressed the stability issues of royal jelly, maintaining its stability for 6 months. Furthermore, the double encapsulation of royal jelly in cyclodextrin/liposomes allows a time-controlled release of 10-HDA, potentially valuable for dermal applications. The novel delivery system aims not only to eliminate the stability challenges of the sensitive royal jelly component, 10-HDA but also to achieve controlled release, particularly of 10-HDA, enhancing bioactivity in cosmeceutical applications. | Liposomes | Royal jelly (10-hydroxy-2-decenoic acid) | [26] |
| The combination of EGCG-loaded solid lipid nanoparticles (SLNs) with resveratrol-loaded SLNs proved to have the highest protection against induced oxidative stress. Encapsulating EGCG, resveratrol and myricetin in SLNs seems to be a suitable strategy for the delivery of these antioxidants to the skin, improving their bioavailability | Solid lipid nanoparticles | Epigallocatechin Gallate (EGCG), resveratrol and myricetin | [27] |
| Microparticles containing AA and NIC showed antibacterial activity, suggesting they may be a useful additive in cosmetic products. Together, these results describe a technology based on a natural polymer for skin cosmetic products, facilitating the topical release of antioxidant drugs. | Encapsulated microparticles (Chitosan) | Ascorbic acid (AA) and nicotinamide (NIC) | [28] |
| Both morin and liposomal morin effectively protected keratinocytes against ROS generation induced by PM exposure. They further provided anti-aging and anti-skin pollution properties by reducing MMP-1 expression via downregulation of the MAPK signaling pathway. | Liposomes | Morin | [29] |
| Morin-loaded niosome had potent anti-aging (antioxidative and anti-stain) activities, including reduction in melanin formation due to tyrosinase inhibitory activity and intracellular ROS scavenging activity. The product demonstrated effective UVR protection (SPF > 30). | Niosomes | Morin | [30] |
| The results suggest that CA could permeate the skin barrier, both when encapsulated in transetosomes and when included in cosmetic creams containing CA-loaded transetosomes. These findings provide opportunities for research and development in other applications of the encapsulation of CA, a telomerase activator, in topical products such as cosmetics, as anti-aging active ingredients, or even in topical pharmaceuticals and medicines. | Liposomes, ethosomes, and transethosomes | Cycloastragenol (CA) | [31] |
| The GA–AuNPs inhibited oxidative stress, MMP-1 upregulation, and the release of type I collagen by high glucose. Thes study suggests that GA–AuNPs are valuable as an active ingredient in anti-aging products, particularly for glycation-induced skin aging. | Metallic nanoparticles | Gallic acid | [32] |
| Hubertia ambavilla extract contains bioactive constituents used for synthesizing gold nanoparticles with interesting properties, particularly for cosmetic applications. GAuNP are non-toxic to fibroblast and dermal cells, efficiently scavenging free radicals. They also protect against UV-A induced damage to fibroblasts and dermal cells. | Metallic nanoparticles | Hubertia ambavilla | [33] |
| The improved permeability and antiaging potential of the bakuchiol-encapsulated rich extract were observed, indicating that the obtained ecological nanoemulsions are competitive with commercial retinol formulations. | Nanoemulsions | Bakuchiol (bioretinol) | [34] |
| A curcumin nanoformulation, sized at 77 nm and containing 3% ethanol, proved more effective in increasing β1-integrin gene over-expression, preventing apoptosis in fibroblast cells, and decreasing Bax and NFκB gene expression compared to a particle size of 50 nm. Such a formulation may be considered a valuable candidate in anti-aging and wound-healing formulations. | Nano-carriers | Curcumin | [35] |
| IB@NPs showed excellent biocompatibility, inhibited oxidative damage to mouse NIH-3T3 fibroblasts, and reduced intracellular ROS generation, as indicated by an in vitro DPPH antioxidant assay. Therefore, as carriers with excellent stability, IB@NPs hold potential applications in cosmetics and pharmaceuticals. | Nanoemulsions | Idebenone (IB) | [36] |
| Results | Delivery Sistem | Active Compound | Reference |
|---|---|---|---|
| Protransf-CoQ10 emulgel increased collagen density and the number of fibroblast cells in UV radiation-induced skin-aged mice, indicating its potential for repairing the skin aging process. CoQ10 emulgels, including Protransf-CoQ10 emulgel, significantly increased the number of fibroblasts compared to the control group, indicating increased collagen production. | Emulgel | Coenzyme Q10 | [37] |
| Topical application of Nanolipoic acid (nLA) effectively improved UV-induced pigmentation and epidermal thickening, demonstrating the efficacy of nanoencapsulation in enhancing α-lipoic acid’s therapeutic potential. | Micelles | α-lipoic acid | [38] |
| FucoPol-based cream showed positive physical–chemical, rheological, stability, antioxidant, and bioactivity properties throughout 60 days. The potential of FucoPol for use as a standalone anti-aging cream or for enhancing UV-blocking capacity of other formulations has been established. | Micelles | α-lipoic acid | [39] |
| Caffeinated hyalurosomes were successfully developed as an anti-aging nanoplatform for caffeine delivery. They exhibited prolonged in vitro drug release and superior in vivo anti-aging efficacy compared to caffeine solution or caffeine gel. | Hialurosomes | Caffeine | [40] |
| Citrus sinensis L. peel standardized extract (CSPE) exhibits potent anti-aging activity via downregulation of MMP1 mRNA expression, anti-inflammatory, and antioxidant effects. The visible appearance of UV-induced photoaging in mice significantly improved after the topical application of CSPE-NLC cream for 5 weeks, with increased levels of collagen and SOD observed in the CSPE- NLC group. | Lipid nanoparticles | Citrus sinensis peel extract | [41] |
| Different PPEE-SLNs cream formulations (2% and 5%) were assessed for possible anti-wrinkle activity against UV-induced photoaging in a mouse model using a wrinkle scoring method. They demonstrated a highly significant protective effect against UV, as evidenced by tissue biomarkers (SOD) and histopathological studies. The current study demonstrates that Prunus persica leaf by-products provide an interesting, valuable resource for natural cosmetic ingredients. | Lipid nanoparticles | Prunus persica (L.) leaf extract | [42] |
| In cellular experiments, A/D-FLip inhibited oxidative stress damage, reduced inflammatory factors and decreased the activation of MMPs in human immortalized keratinocytes (HaCaT) cells. In animal experiments, A/D-FLip inhibited skin damage and reduced skin collagen loss by decreasing the activation of MMPs, thus inhibiting skin photoaging in mice. A/D-FLip has good anti-photoaging effects and it has the potential to become an effective skincare product. | Flexible liposomes | Apigenin and Doxycycline | [43] |
| The results indicated that liposomes could significantly improve the stability of vitamin D3. Vitamin D3 liposomes repaired the surface morphology of skin in the photoaging model and promoted the production of new collagen fibers. Vitamin D3 liposomes, as a potential skincare agent, significantly improved skin appearance and repair damage in the histology of photoaging. | Liposomes | Vitamin D3 | [44] |
| Spirulina Bilosomes (SPR-BS), a novel anti-aging nanoplatform for SPR delivery, exhibits enhanced in vitro drug release and superior in vivo anti-aging efficacy compared to free SPR | Bilosomes | Spirulina | [45] |
| Coriander oil possesses anti-wrinkle activity. Coriander oil cream and CEOLNs formulations attenuated in vivo UV-induced skin photoaging, manifested by significantly decreased MDA, COX-2, PGE-2, MMP-1, JNK, and AP-1 levels. These pharmaceutical dosage forms significantly increased skin collagen content compared to the UV-injured group. | Lipid nanoparticle/Nanoemulgel | Coriandrum sativum L. essential oil | [46] |
| The results of the β-galactosidase test showed the promotion of the aging process in the cells treated with the smaller size of ZnO NPs. Both sizes of the NP were found to upregulate the aging-related genes NF-kB and p53 and downregulate the anti-aging gene Nanog. The smaller size of ZnO NPs can enhance the aging process in the cells. | Metallic nanoparticles | Zinc oxide (ZnO) | [47] |
| Results | Delivery System | Active Compound | Reference |
|---|---|---|---|
| Both formulations showed anti-aging potential (anti-wrinkle action, promotion of skin hydration). The emulgel showed greater antioxidant potential. | Emulsion W/O Emulgel | Grape seed extract | [48] |
| The active formulation recovered the skin from any UV-related imperfections in terms of erythema, melanin, sebum levels, moisture, and elasticity. | Micro emulgel | Flaxseed extract | [49] |
| It demonstrated effectiveness in treating melasma, surpassing conventional chemical peeling treatment, without causing side effects. | Aspasomes | Ascorbic acid derivative | [50] |
| The formulation provided clinical improvement of UV-induced damaged skin and ultrastructure, suggesting that spanlastic nanovesicles had a synergistic effect on collagen synthesis and melanin formation in the skin. | Spanlastic vesicles | L-Ascorbic acid | [51] |
| The formulation demonstrated efficacy at the topical level, offering benefits when applied daily during the two-week trial duration. The formulation also provided visually observable anti-wrinkle effects on the skin, with visible improvements in the skin’s shape and texture. | Micro emulsion | Ginkgo biloba extract | [52] |
| The adenosine delivery system exhibited enhanced wrinkle improvement of 203% compared to 0.04 wt% of the pure adenosine system. The niacinamide- and sulforaphane-loaded Au-decorated zeolite nanocomposites decreased the skin surface melanin content by 123% and 222%, respectively, compared to 2 and 0.01 wt% of pure niacinamide and sulforaphane systems, respectively. | Metallic nanoparticles | Niacinamide, sulforaphane, and adenosine | [53] |
| The cream tested on volunteers increased skin hydration, elasticity and had a softening effect. Regarding wrinkles, the cream reduced both their area and depth. | Levan Nanoparticles and Nanoemulsion | Levan from microbial fermentation with Bacillus subtilis natto KB1 | [54] |
| The developed polymeric nanoparticles were effective in alleviating wrinkles and can find applications in pharmaceutical formulations utilizing propolis for its antiseptic, anti-inflammatory, antimycotic, antifungal, antibacterial, antiulcer, anticancer, and immunomodulatory properties. | Polymeric nanoparticles | Propolis extract | [55] |
| A decrease in skin roughness and wrinkle breadth, along with an improvement in dermal homogeneity and firmness, were observed after two months of treatment with “anti-aging” masks. A significant improvement in skin firmness and elasticity was observed after one month of treatment with “lifting” masks. Furthermore, a one-month treatment with “cell renewal” masks promoted the production of new skin cells through a mild exfoliating action. | Bacterial cellulose | Anti-aging mask * Lifting mask * Purifying and regenerative mask * | [56] |
| The surface evaluation of living skin index values significantly reduced for the emulgels loaded with kaki fruit extract, indicating a reduction in skin wrinkles, roughness and scaliness. | Emulgels | Kaki fruit extract | [57] |
| Types of Systems | Carrier Types | Reference |
|---|---|---|
| Polymeric-based nanosystems | Polycaprolactone nanofibrous scaffold | [21] |
| Polymeric nanoparticles | [55] | |
| Lipid-based nanosystems | Emulgel with niosome | [22] |
| Liposomes | [23,24,26,29,31,43,44] | |
| Nanostructured lipid carriers | [24] | |
| Nanoemulsion | [24,34,36] | |
| Niosome | [20,24,30] | |
| Solid lipid nanoparticles | [27] | |
| Ethosomes | [31] | |
| Aspasomes | [50] | |
| Transethosomes | [30] | |
| Nano-carriers | [35] | |
| Hialurosomes | [40] | |
| Lipid nanoparticles | [41] | |
| Bilosomes | [45] | |
| Spanlastic vesicles | [51] | |
| Metal-based nanosystems | Metallic nanoparticles | [32,33,47,53] |
| Additional systems | Self-emulsifying | [25] |
| Encapsulated microparticles | [28] | |
| Emulgel | [37,57] | |
| Micelles | [38,39] | |
| Nanoemulgel | [46] | |
| Emulsion W/O | [48] | |
| Micro emulgel | [49,52] | |
| Levan Nanoparticles | [54] | |
| Bacterial cellulose | [56] |
| Article | Study Limitation | Stability | Delivery System Ingredients |
|---|---|---|---|
| [20] | Although the study reports the anti-aging activity on the skin, it does not delve into the mechanisms by which gallic acid exerts its effects when administered through niosomes. | 3 months | Polyoxyethylene 2 cetyl ether, cholesterol, cetyltrimethylammonium bromide |
| [21] | The experiments focus on short-term exposure to UV stress and the immediate effects of pre-treatment with nanoPcl-M. Long-term effects and the potential for cumulative damage over time are not addressed. | 7 days | Polycaprolactone nanofibrous scaffold (PCL), chloroform, ethanol |
| [22] | The study does not include long-term efficacy data regarding the anti-aging effects of the formulations. The article acknowledges financial support from a cosmetics company. | Not provided | Glycerin dibehenate, cetyl alcohol, cetearyl alcohol, squalene, L-α-phosphatidyl choline from soybean, dimethyl amino ethyl (DMAE), isopropyl palmitate, Tween® 80, Span® 60, cholesterol, 2-phenoxy ethanol, ascorbyl palmitate, disodium EDTA, citric acid, α-tocopherol |
| [23] | The experiments were conducted over relatively short incubation periods (e.g., 48 to 72 h). The long-term effects of CRPP on cell viability and gene expression were not evaluated. | 8 weeks | Cyclodextrins, propanediol, lecithin, tocopherol, sunflower seed oil, Hydrolite® 5 green (pentylene glycol), disodium EDTA |
| [24] | The study uses a single marker of rosmarinic acid (RA) for the quantitative determination of the extract. Although the study reports zeta potential values and formulation stability, it does not explore the long-term stability of these systems under various environmental conditions. | 8 days | Cetyl palmitate, tea seed oil, Tween® 20 (polysorbate 20), Plantacare 2000® (Decyl glucoside), distilled water (DI water), glycerin, propylene glycol, cholesterol |
| [25] | The study measured only a limited number of cytokines (TNF-α, IL-6, and IL-8) to assess pro-inflammatory activity. | 10 months at 5 °C | Fucan-coated acetylated cashew gum nanoparticles, glutaraldehyde, dimethyl sulfoxide (DMSO), lipopolysaccharides (LPS) |
| [26] | One of the main limitations highlighted is the requirement for royal jelly to be stored at low temperatures and in the absence of light to maintain its stability. | 6 months at 6 °C | 10-hydroxy-2-decenoic Acid (10-HDA), cyclodextrins, phenolic compounds |
| [27] | The study does not mention the sample size or the number of independent experiments conducted. | 30 days | Precirol® 5 ATO (glyceryl distearate), Gelucire® 39/01, cetyl palmitate, Suppocire DM pellets, Compritol® HD5 ATO, Gelucire® 50/13 (stearoyl polyoxyl-32 glycerides), Suppocire NA15 Pellets, Gelucire® 43/01 (hard fat compounds), Apifil® (PEG-8 beeswax), Dynasan® 114 (glyceryl tristearate), Softisan® 100 (hydrogenated coco-glycerides), Softisan® 154 (hydrogenated palm oil), Witepsol® E76 (hard fat compounds), glyceryl monostearate stearic acid, Pluronic® F-127 (a surfactant), pure anhydrous ethanol, HEPES hemisodium salt, 2,2-diphenyl-1-picrylhydrazyl, DPPH0 uranyl acetate |
| [28] | The study encountered difficulties in quantifying ascorbic acid (AA) during ex vivo permeation experiments, as the microparticles released AA gradually, resulting in amounts that fell below the detection limits of the analytical method. | Not provided | Acetic acid, trifluoroacetic acid, anhydrous monobasic sodium phosphate, anhydrous bibasic sodium phosphate |
| [29] | The study does not explore the long-term effects nor the efficacy of liposomal morin in other applications. | Not provided | Lecinolws-50 (Lysolecithin), Tween®-80, Pluronic F-68 |
| [30] | The characterization of niosomes focused on size, zeta potential, morphology, and encapsulation efficiency. Other important factors, such as the release kinetics in different biological environments and the potential for skin irritation or allergic reactions, were not extensively addressed. | 3 months at −4 °C | Sorbitan monostearate (Span® 40), polyoxyethylenesorbitan monopalmitate (Tween® 40), cholesterol |
| [31] | Although the study suggests that cycloastragenol (CA) may penetrate the skin barrier when encapsulated in phospholipid vesicles, direct measurement of this penetration remains unconfirmed. The study does not provide long-term data on the efficacy and safety of CA when used in topical applications. | Not provided | Phospholipon® 20H, Sunlipon® 90, Neobee® M-5 oil, xanthan gum, potassium sorbate, glyceryl monostearate (GMS), Alphadim 90 SB, glyceryl monostearate (GMS), Alphadim 90 SB |
| [32] | The study does not address the long-term effects of GA–AuNPs on skin health and aging. | Not provided | Span® 40 (sorbitan monostearate), Tween® 40 (polyoxyethylenesorbitan monopalmitate), cholesterol, isopropyl alcohol, chloroform and acetone, sodium borate buffer solution (pH 9.1) |
| [33] | The study does not include long-term exposure assessments, which are crucial for understanding the chronic effects and potential cumulative toxicity of AuNP over time. | 25 days | Chloroauric acid, sodium citrate, citrate |
| [34] | The article does not provide comprehensive data on the long-term stability of nanoemulsions under various environmental conditions (e.g., light exposure, humidity). | 30 days | Coco-betaine, surfactin, carbon dioxide |
| [35] | The study evaluates the immediate effects on cell viability and gene expression but does not address the long-term impacts of these nanocarriers on skin repair and aging processes. | Not provided | Mineral, sesame, and soybean oils, ethanol, nanocarrier base |
| [36] | The effects of prolonged storage or exposure to different environmental conditions on the stability of IB@NPs were not evaluated. | 28 days | Idebenone (IB), polyethylene glycol-40 hydrogenated castor oil (PEG-40 HCO), deionized water (DIW) |
| Article | Study Limitation | Stability | Delivery System Ingredients |
|---|---|---|---|
| [37] | Data on the drug release profile and its dermal penetration are missing. The ages of the mice used in the anti-aging activity and skin irritation tests were not specified. | 28 days | Coenzyme Q10 (CoQ10) L-α-Phosphatidylcholine oleic acid, Tween® 80, Span® 80, Carbopol 940, triethanolamine (TEA) |
| [38] | The study used 5-week-old mice to assess epidermal proliferation and 8-week-old male guinea pigs to investigate pharmacological effects. However, the number of animals used in the experiment was not reported. Alpha-lipoic acid was applied as an antioxidant supplement for anti-aging purposes, but the study did not mention the aging factor of the animals. In addition, the study did not report any stability testing. | Not conducted | Alpha-lipoic acid (α-LA), non-ionic surfactant (BS-20), magnesium chloride (MgCl2), sodium bicarbonate (NaCO), sodium hydroxide solution (NaOH) HEPES |
| [39] | The article highlights the physical instability of emulsions with L-ascorbic acid, despite no perceptible degradation of the ingredient. | 45 days | FucoPol L-ascorbic acid (vitamin C), α-tocopherol (vitamin E), olive oil (Olea europaea), sweet almond oil (Prunus amygdalus dulcis), cetyl alcohol, glycerin methylparaben triethanolamine (TEA) |
| [40] | The experiment was conducted on the shaved dorsal skin of adult male Wistar rats, aged between 6 and 8 weeks. | 3 months | Caffeine (CAF), phospholipids (Phospholipon® 90G), hyaluronic acid (hyaluronan), Carbopol 940 |
| [41] | The study does not present more robust stability tests, such as long-term tests under different temperature and humidity conditions, and it also does not address the potential sensitivity of human skin to the formulation or allergic reactions. | Not provided | Sweet orange peel extract (citrus sinensis), lipid nanoparticles (LNPs), cocoa butter, olive oil, Tween®-80, phosphatidylcholine (Lecithin) |
| [42] | The mice used in the tests were young—only 4 weeks old. | 2 months | Peach leaf extract (PPEE), solid lipid nanoparticles (SLNs): glyceryl monostearate, Tween® 80. Cream base ingredients: Span® 80, Cetearate-20, liquid paraffin, cetostearyl alcohol, beeswax biocrol WS2, propylene glycol, purified water |
| [43] | The age of the mice was not provided. | 28 days | Apigenin (98% purity), doxycycline (98% purity), lecithin, cholesterol, sodium cholate, Tween® 80, vitamin E, phosphate buffer saline (PBS) |
| [44] | The age of the rats was not provided. | 9 days | Egg phosphatidylcholine (Egg PC), phosphate buffer saline (PBS), phospholipids, cholesterol, vitamin D3, ethanol |
| [45] | The experiment was conducted on the shaved dorsal skin of adult male Wistar rats, aged between 6 and 8 weeks. | 3 months | Sodium deoxycholate (SDC), phospholipids, phosphate, buffer saline (PBS), spirulina (SPR), lipoid S100 (l-α-PC), cholesterol |
| [46] | The mechanisms underlying the anti-aging properties of coriander oil have not yet been fully characterized. Furthermore, the stability tests are not clearly described in the methodology. Eight-week-old female mice were used in the tests, where photoaging was induced by UV radiation, and erythema formation was assessed 24 h after exposure. | Not provided | Coriander essential oil-loaded solid lipid nanoparticle (COEOLNs): coriander essential oil, cocoa butter, olive oil or sesame oil and lecithin; nanoemulgel with CEONLIC: xanthan gum, deionized water, Tween® 80, triethanolamine. |
| [47] | The study highlights a size-dependent toxicity of nanoparticles (NPs); however, it does not delve deeper into the underlying mechanisms of this phenomenon. Furthermore, the study does not report on the stability of the system. | Not provided | Zinc oxide (ZnO), phosphate buffer saline (PBS) |
| Article | Study Limitation | Stability | Delivery System Ingredients |
|---|---|---|---|
| [48] | The tested formulation included potentially harmful ingredients, such as parabens. The study involved 40 individuals, all classified as healthy. Furthermore, the lack of demographic data, such as the participants’ ages, is a relevant limitation. | 90 days | Emulsion W/O: propylene, paraben, paraffinoil, Abil-EM, methylparaben, olive oil Emulgel: Emulsion ingredients + Carbapol 940, triethanolamine |
| [49] | The study included 14 healthy Asian women, with the participants’ age range varying only between 25 and 40 years. Another limitation to consider is that this is a monocentric and single-blind study. | 90 days at 25 °C | Tween® 80, Span® 80, ethanol, isopropyl myristate |
| [50] | The study included only 20 Egyptian women, with an age range of 25 to 60 years. Additionally, it is a monocentric, single-blind study. A positive aspect is that the participants had melasma, rather than being only healthy individuals. | 3 months | Ascorbyl palmitate, lecithin, cholesterol, magnesium ascorbyl phosphate, phosphate buffered saline |
| [51] | Although the study was evaluated both in vitro and in vivo, the research involving humans was quite limited, as it included only 6 healthy female volunteers, all with normal skin condition. | 6 months | Span® (40, 60, or 80), tween® (60 or 80) or tocopherol polyethylene glycol 1000 succinate, ethanol |
| [52] | The formulation contains parabens. The study included only 12 participants of both sexes, conducted at a single center in Egypt. | 1 month, both 4 and 25 °C | Stearic acid, potassium hydroxide, methylparaben, glycerol |
| [53] | The study presents significant limitations, such as the lack of an exact number of participants, indicating only that there were more than 20 women. Additionally, no information was provided about the sex and age range, mentioning only that all were over 19 years old. The conclusion is quite general. | Not provided | Calcined zeolite 13X, gold chloride hydrate, oleic acid |
| [54] | The study had a small sample size, with only 10 women aged between 42 and 54 years. | 90 days | Nanoemulsion: sodium surfactin powder, 2-(2 ethoxyethoxy) ethanol, ascorbyl tetraisopalmitate |
| [55] | In this study, no significant limitations were identified. It is a double-blind study with an adequate number of volunteers, covering an appropriate age range. | 6 months at 25 ± 2 °C, 3 months at 40 ± 2 °C | PN7-PEG 400; Poloxamer 188; Poloxamer 407 |
| [56] | The study included about 20 to 25 volunteers for each type of application. Although the sample size is relatively small, there was careful consideration of the age range of the participants: the anti-aging mask was tested on individuals aged between 42 and 64 years, the lifting mask on people aged between 38 and 57 years, and the purifying and regenerative mask on participants aged between 25 and 40 years. | Not provided | Bacterial Cellulose |
| [57] | The sample consisted of 13 healthy men of Asian origin, aged between 25 and 37 years. The study used a single-blind method. Additionally, the formulation included parabens, a potentially harmful ingredient. | Not provided | Span® 80, Tween® 80, methylparaben, water, Carbopol-940 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.L.M.; Nieri, V.; Moreli, F.d.C.; Constantino, E.; de Souza, J.; Oshima-Franco, Y.; Grotto, D. Unveiling New Horizons: Advancing Technologies in Cosmeceuticals for Anti-Aging Solutions. Molecules 2024, 29, 4890. https://doi.org/10.3390/molecules29204890
Alves PLM, Nieri V, Moreli FdC, Constantino E, de Souza J, Oshima-Franco Y, Grotto D. Unveiling New Horizons: Advancing Technologies in Cosmeceuticals for Anti-Aging Solutions. Molecules. 2024; 29(20):4890. https://doi.org/10.3390/molecules29204890
Chicago/Turabian StyleAlves, Patrícia Lius Melo, Vitor Nieri, Fernanda de Campos Moreli, Ederson Constantino, Jocimar de Souza, Yoko Oshima-Franco, and Denise Grotto. 2024. "Unveiling New Horizons: Advancing Technologies in Cosmeceuticals for Anti-Aging Solutions" Molecules 29, no. 20: 4890. https://doi.org/10.3390/molecules29204890
APA StyleAlves, P. L. M., Nieri, V., Moreli, F. d. C., Constantino, E., de Souza, J., Oshima-Franco, Y., & Grotto, D. (2024). Unveiling New Horizons: Advancing Technologies in Cosmeceuticals for Anti-Aging Solutions. Molecules, 29(20), 4890. https://doi.org/10.3390/molecules29204890





