Enhanced Electrochemiluminescence of Luminol and-Dissolved Oxygen by Nanochannel-Confined Au Nanomaterials for Sensitive Immunoassay of Carcinoembryonic Antigen
Abstract
1. Introduction
2. Results and Discussion
2.1. Strategy for the Construction of Immunosensor
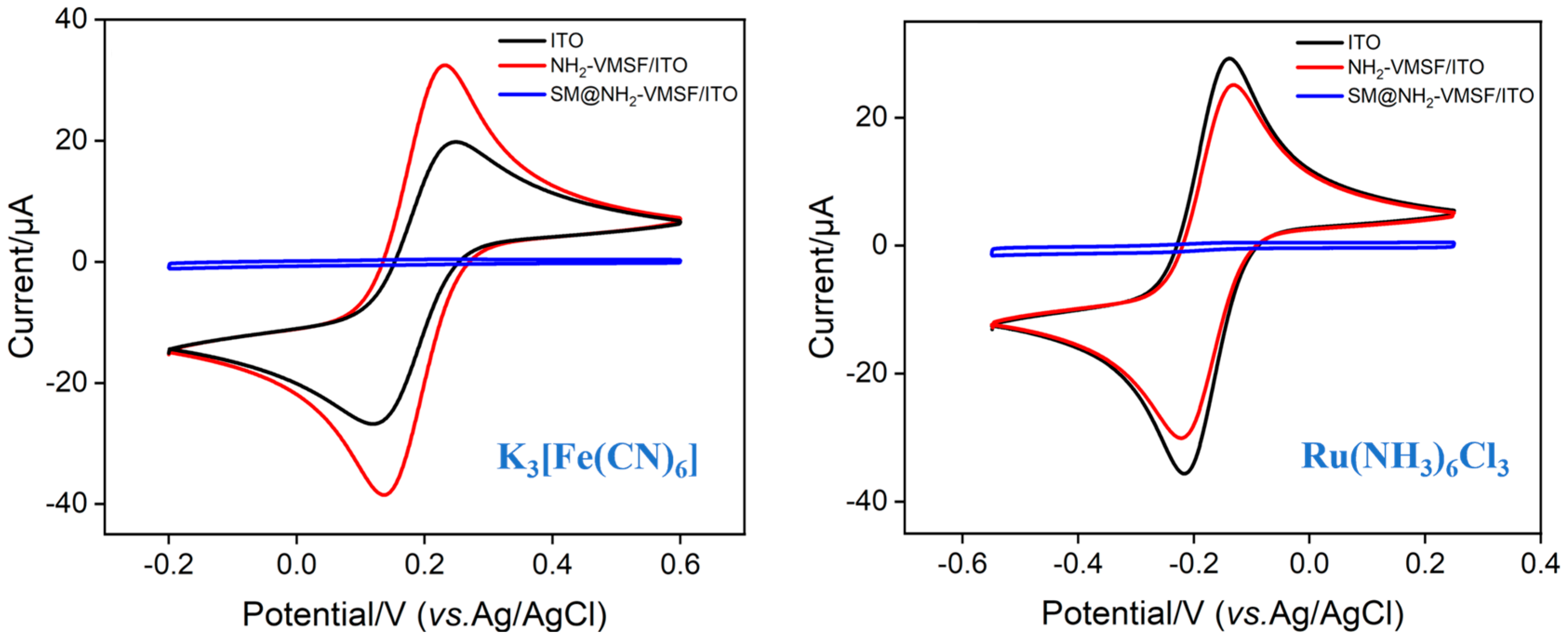
2.2. Characterization of Electrode Modified by NH2-VMSF
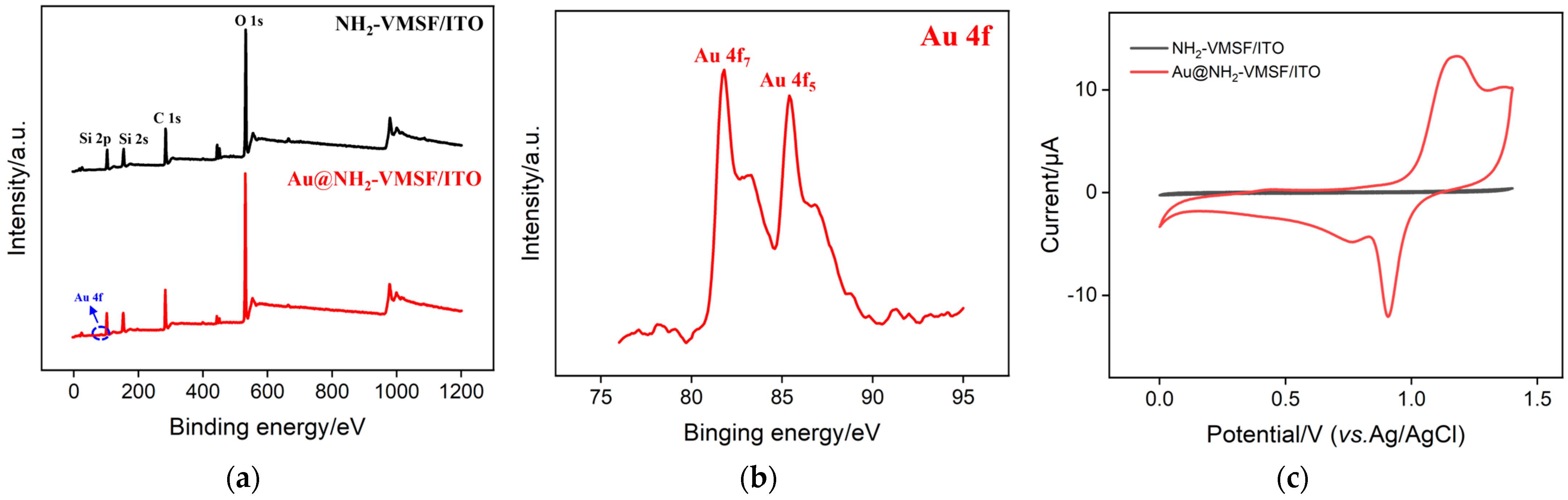
2.3. Characterization of Confined Au Nanomaterials
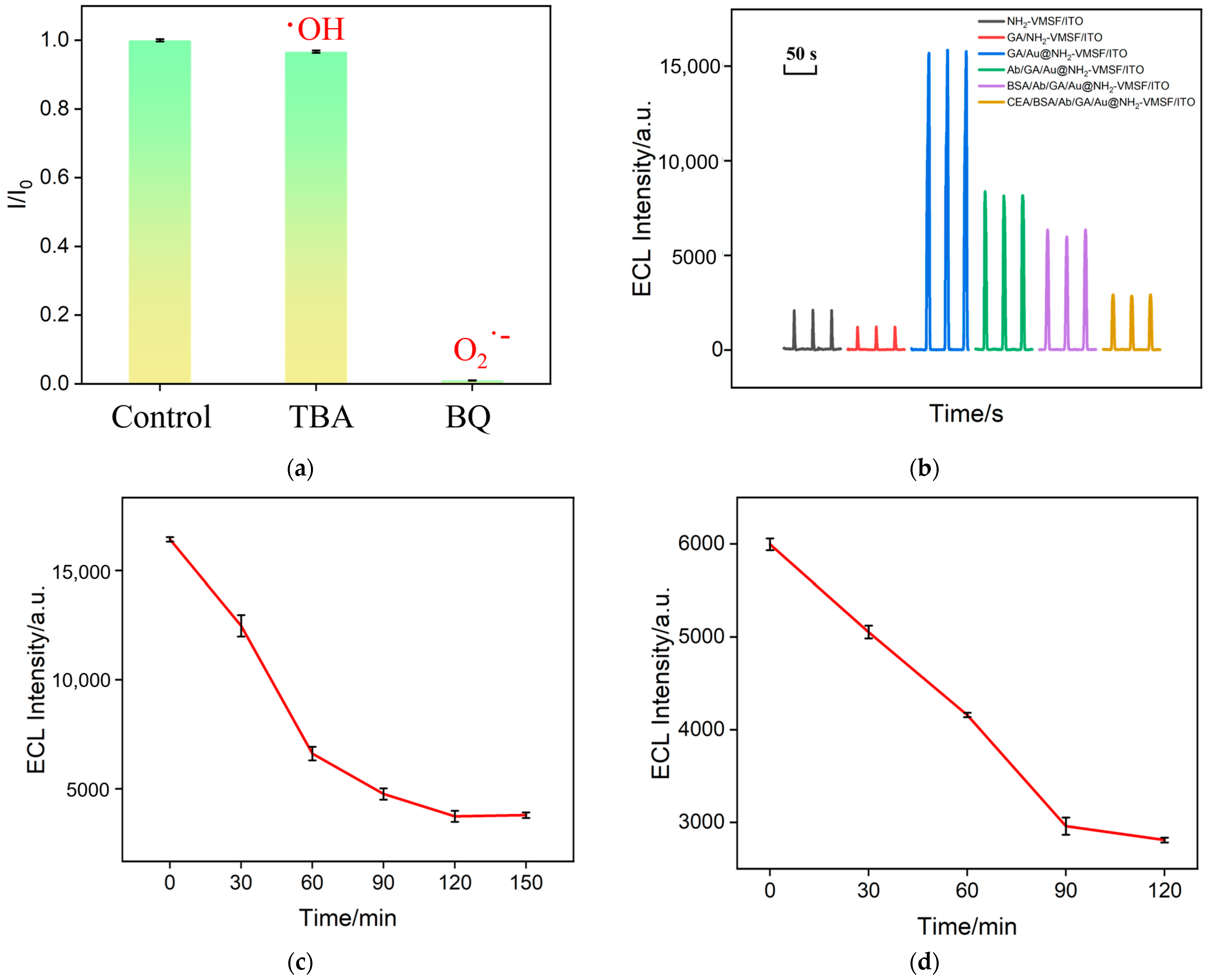
2.4. Feasibility of Immunosensor Construction
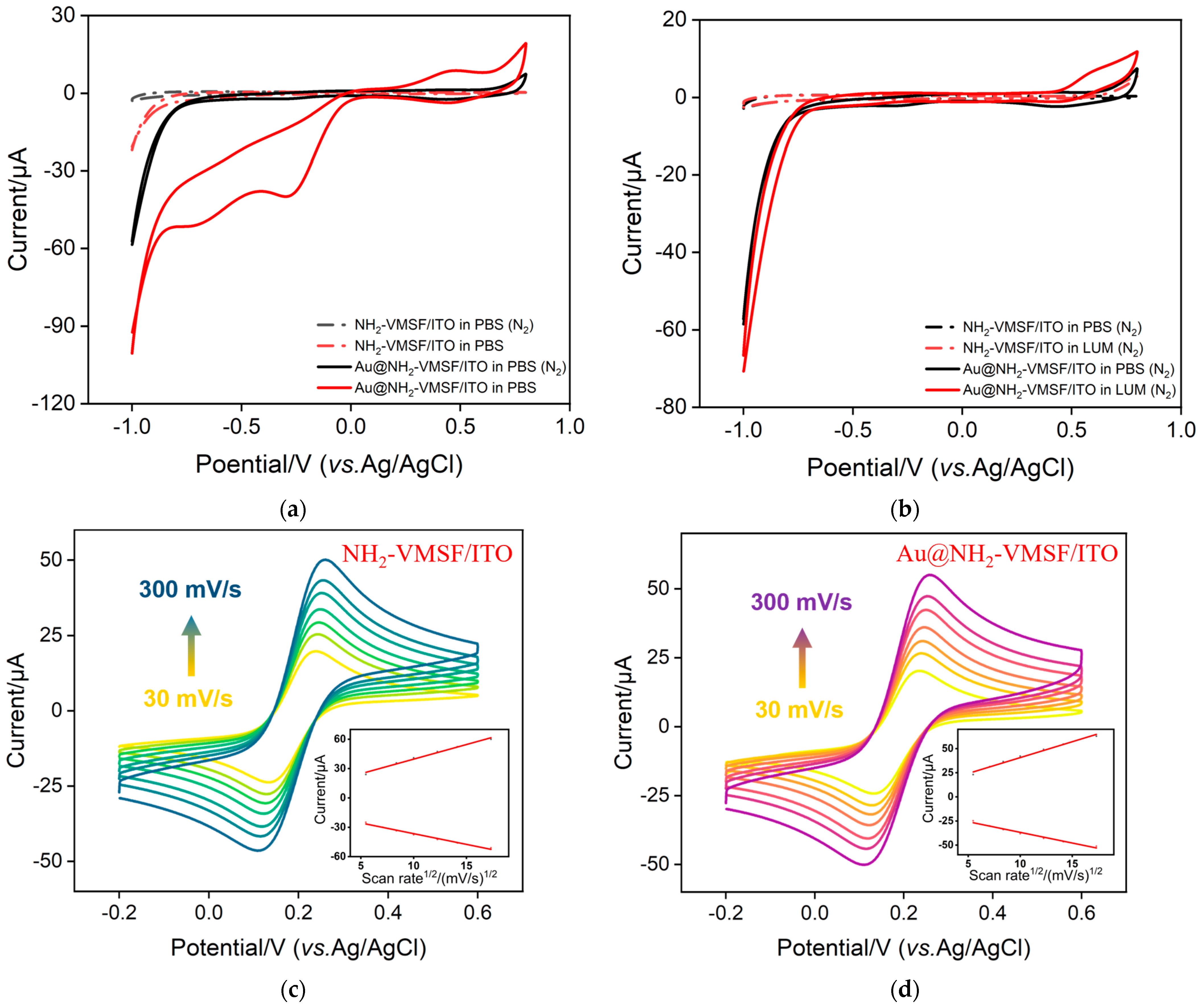
2.5. Mechanism of the Enhanced ECL by Confined Au Nanomaterials
2.6. Optimized Condition for the Fabrication of the Immunosensor
2.7. ECL Detection of CEA
2.8. Selectivity, Anti-Interference, and Stability of the Electrode
2.9. Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Measurements and Instrumentations
3.3. Fabrication of the Immunosensor
3.4. Detection of CEA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jenkins, K. Cancer and cancer care. Psycho-Oncology 2017, 26, 413. [Google Scholar] [CrossRef]
- Guerra, C.E.; Sharma, P.V.; Castillo, B.S. Multi-cancer early detection: The new frontier in cancer early detection. Annu. Rev. Med. 2024, 75, 67–81. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Z.; Zhao, C.; Shen, M.; Li, H.; Zhang, S.; Zhang, Z. Photoelectrochemical biosensing platforms for tumor marker detection. Coordin. Chem. Rev. 2022, 469, 214675. [Google Scholar] [CrossRef]
- Yin, Y.; Cao, Y.; Xu, Y.; Li, G. Colorimetric immunoassay for detection of tumor markers. Int. J. Mol. Sci. 2010, 11, 5077–5094. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, J.; Luo, F. Serum tumor markers for detection of hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Liu, J.; Qin, D. Label-Free Homogeneous electrochemical aptasensor based on size exclusion/charge-selective permeability of nanochannel arrays and 2D nanorecognitive probe for sensitive detection of alpha-fetoprotein. Molecules 2023, 28, 6935. [Google Scholar] [CrossRef]
- Xing, J.; Han, Q.; Liu, J.; Yan, Z. Electrochemical aptasensor fabricated by anchoring recognition aptamers and immobilizing redox probes on bipolar silica nanochannel array for reagentless detection of carbohydrate antigen 15-3. Front. Chem. 2023, 11, 1324469. [Google Scholar] [CrossRef]
- Xiang, W.; Lv, Q.; Shi, H.; Xie, B.; Gao, L. Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta 2020, 214, 120716. [Google Scholar] [CrossRef]
- Bao, C.; Deng, L.; Huang, F.; Yang, M.; Li, X. Signal amplification strategies in photoelectrochemical sensing of carcinoembryonic antigen. Biosens. Bioelectron. 2024, 262, 116543. [Google Scholar] [CrossRef]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, S.; Liu, J.; Xing, J. Homogeneous electrochemical aptamer sensor based on two-dimensional nanocomposite probe and nanochannel modified electrode for sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 5186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, L.; Pei, J.; Tian, Y.; Liu, J. A reagentless electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on the interface with redox probe-modified electron transfer wires and effectively immobilized antibody. Front. Chem. 2022, 10, 939736. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, T.; Chen, P.; Yan, F.; Liu, J. Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens. Actuators B Chem. 2022, 368, 132086. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Vashist, S.K. Immunosensing procedures for carcinoembryonic antigen using graphene and nanocomposites. Biosens. Bioelectron. 2017, 89, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fan, X.; Yan, F.; Wang, Y. Highly sensitive electrochemical immunosensor based on electrodeposited platinum nanostructures confined in silica nanochannels for the detection of the carcinoembryonic antigen. Front. Chem. 2023, 11, 1271556. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, J.; Han, Q.; Hu, W.; Yan, F.; Liu, J. Nanogold amplified electrochemiluminescence/electrochemistry in bipolar silica nanochannel array for ultrasensitive detection of SARS-CoV-2 pseudoviruses. Talanta 2024, 277, 126319. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef]
- Jiang, J.; Xia, J.; Zang, Y.; Diao, G. Electrochemistry/photoelectrochemistry-based immunosensing and aptasensing of carcinoembryonic antigen. Sensors 2021, 21, 7742. [Google Scholar] [CrossRef]
- Gu, X.; She, Z.; Ma, T.; Tian, S.; Kraatz, H.B. Electrochemical detection of carcinoembryonic antigen. Biosens. Bioelectron. 2018, 102, 610–616. [Google Scholar] [CrossRef]
- Tan, E.; Gouvas, N.; Nicholls, R.J.; Ziprin, P.; Xynos, E.; Tekkis, P.P. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg. Oncol. 2009, 18, 15–24. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence enzyme biosensors for ultrasensitive determination of glucose using glucose dehydrogenase immobilized on vertical silica nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- Sun, J.; Sun, H.; Liang, Z. Nanomaterials in electrochemiluminescence sensors. ChemElectroChem 2017, 4, 1651–1662. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Li, L.; Zhu, J.J. Nanomaterials-based sensitive electrochemiluminescence biosensing. Nano Today 2017, 12, 98–115. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Zheng, Y.; Liu, J. Solid-phase electrochemiluminescence enzyme electrodes based on nanocage arrays for highly sensitive detection of cholesterol. Biosensors 2024, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Maraventano, G.; Ticli, G.; Cazzalini, O.; Stivala, L.A.; Ramos-Gonzalez, M.; Rodriguez, J.L.; Prosperi, E. Single cell determination of 7,8-dihydro-8-oxo-2′-deoxyguanosine by fluorescence techniques: Antibody vs. avidin labeling. Molecules 2023, 28, 4326. [Google Scholar] [CrossRef]
- Yu, H.; Raymonda, J.W.; McMahon, T.M.; Campagnari, A.A. Detection of biological threat agents by immunomagnetic microsphere-based solid phase fluorogenic- and electro-chemiluminescence. Biosens. Bioelectron. 2000, 14, 829–840. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Zhu, J.J. Recent Advances in Electrochemiluminescence Analysis. Anal. Chem. 2017, 89, 358–371. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef]
- Ma, N.; Xu, S.; Wu, W.; Liu, J. Electrochemiluminescence aptasensor with dual signal amplification by silica nanochannel-based confinement effect on nanocatalyst and efficient emitter enrichment for highly sensitive detection of C-reactive protein. Molecules 2023, 28, 7664. [Google Scholar] [CrossRef]
- Zhou, X.; Gu, X.; Zhang, S.; Zou, Y.; Yan, F. Magnetic graphene oxide and vertically-ordered mesoporous silica film for universal and sensitive homogeneous electrochemiluminescence aptasensor platform. Microchem. J. 2024, 200, 110315. [Google Scholar] [CrossRef]
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef] [PubMed]
- Giussani, A.; Farahani, P.; Martinez-Munoz, D.; Lundberg, M.; Lindh, R.; Roca-Sanjuan, D. Molecular basis of the chemiluminescence mechanism of luminol. Chemistry 2019, 25, 5202–5213. [Google Scholar] [CrossRef]
- Liang, W.; Wang, M.; Ma, C.; Wang, J.; Zhao, C.; Hong, C. NiCo-LDH hollow nanocage oxygen evolution reaction promotes luminol electrochemiluminescence. Small 2024, 20, e2306473. [Google Scholar] [CrossRef]
- Gu, W.; Wang, H.; Jiao, L.; Wu, Y.; Chen, Y.; Hu, L.; Gong, J.; Du, D.; Zhu, C. Single-atom iron boosts electrochemiluminescence. Angew. Chem. Int. Ed. 2020, 59, 3534–3538. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, C.; Zhang, F.; Li, W.; Hong, C.; Bao, F. Co3O4/NiCo2O4 heterojunction as oxygen evolution reaction catalyst for efficient luminol anode electrochemiluminescence. J. Colloid Interface Sci. 2024, 659, 728–738. [Google Scholar] [CrossRef]
- An, J.; Zhang, C.; Yan, F.; Ma, P. Nanochannel-confined platinum nanostructure for enhancement of luminol-dissolved oxygen electrochemiluminescence coupled with gated aptasensor for sensitive detection of carcinoembryonic antigen. Microchem. J. 2024, 206, 111413. [Google Scholar] [CrossRef]
- Li, Y.; Peng, W.; You, X. Determination of dopamine by exploiting the catalytic effect of hemoglobin–stabilized gold nanoclusters on the luminol–NaIO4 chemiluminescence system. Microchim. Acta 2017, 184, 3539–3545. [Google Scholar] [CrossRef]
- Huang, X.; Deng, X.; Qi, W.; Wu, D. Highly sensitive luminol electrochemiluminescence immunosensor based on platinum-gold alloy hybrid functionalized zinc oxide nanocomposites for catalytic amplification. Sens. Actuators B Chem. 2018, 273, 466–472. [Google Scholar] [CrossRef]
- Xu, S.L.; Cui, H. Luminol chemiluminescence catalysed by colloidal platinum nanoparticles. Luminescence 2007, 22, 77–87. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.; Dong, X.; Hao, N.; Chen, S.; You, T.; Mao, H.; Wang, K. Copper(I) oxide nanospheres decorated with graphene quantum dots display improved electrocatalytic activity for enhanced luminol electrochemiluminescence. Microchim. Acta 2016, 183, 1591–1599. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, X.; Zhou, Z.; Hua, R.; Zhang, Y.; Liu, Q.; Qian, J.; Li, H.; Wang, K. AgBr nanoparticles/3D nitrogen-doped graphene hydrogel for fabricating all-solid-state luminol-electrochemiluminescence Escherichia coli aptasensors. Biosens. Bioelectron. 2017, 97, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A. Electroinduced surfactant self-assembly driven to vertical growth of oriented mesoporous films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Tananaiko, O.; Walcarius, A. Composite silica-based films as platforms for electrochemical sensors. Chem. Rec. 2024, 24, e202300194. [Google Scholar] [CrossRef]
- Fan, X.; Wu, J.; Zhang, T.; Liu, J. Electrochemical/electrochemiluminescence sensors based on vertically-ordered mesoporous silica films for biomedical analytical applications. ChemBioChem 2024, e202400320. [Google Scholar] [CrossRef]
- Yan, F.; Chen, J.; Jin, Q.; Zhou, H.; Sailjoi, A.; Liu, J.; Tang, W. Fast one-step fabrication of a vertically-ordered mesoporous silica-nanochannel film on graphene for direct and sensitive detection of doxorubicin in human whole blood. J. Mater. Chem. C 2020, 8, 7113–7119. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Ru, H.; Yan, F.; Liu, J. Silica nanochannels as nanoreactors for the confined synthesis of ag nps to boost electrochemical stripping chemiluminescence of the luminol-O2 system for the sensitive aptasensor. Anal. Chem. 2024, 96, 10264–10273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Dong, G.; Zhu, S.; Yan, F.; Liu, J. Direct and sensitive electrochemical detection of bisphenol A in complex environmental samples using a simple and convenient nanochannel-modified electrode. Front. Chem. 2022, 10, 900282. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, T.; Wang, M.; Yan, F.; Liu, J. Disposable electrochemical sensors for highly sensitive detection of chlorpromazine in human whole blood based on the silica nanochannel array modified screen-printed carbon electrode. Molecules 2022, 27, 8200. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Luo, T.; Jin, Q.; Zhou, H.; Sailjoi, A.; Dong, G.; Liu, J.; Tang, W. Tailoring molecular permeability of vertically-ordered mesoporous silica-nanochannel films on graphene for selectively enhanced determination of dihydroxybenzene isomers in environmental water samples. J. Hazard. Mater. 2021, 410, 124636. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, M.; Jin, Q.; Zhou, H.; Xie, L.; Tang, H.; Liu, J. Vertically-ordered mesoporous silica films on graphene for anti-fouling electrochemical detection of tert-butylhydroquinone in cosmetics and edible oils. J. Electroanal. Chem. 2021, 881, 114969. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Yan, F.; Liu, J. Vertical silica nanochannels and o-phenanthroline chelator for the detection of trace Fe(II). ACS Appl. Nano Mater. 2024, 7, 7743–7752. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef]
- Ma, N.; Luo, X.; Wu, W.; Liu, J. Fabrication of a disposable electrochemical immunosensor based on nanochannel array modified electrodes and gated electrochemical signals for sensitive determination of c-reactive protein. Nanomaterials 2022, 12, 3981. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, X.; Yan, F.; Mou, Y. Electrostatic nanocage-confined probe for electrochemical detection of CA19-9 in human serum. ACS Omega 2023, 8, 48491–48498. [Google Scholar] [CrossRef]
- Xing, J.; Wang, H.; Yan, F. Carbon nitride nanosheets as an adhesive layer for stable growth of vertically-ordered mesoporous silica film on a glassy carbon electrode and their application for CA15-3 immunosensor. Molecules 2024, 29, 4334. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]





| Sample | Added (ng mL−1) | Found (ng mL−1) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| fetal bovine serum a | 0.100 | 0.0958 ± 0.001 | 95.8 | 1.0 |
| 1.00 | 1.12 ± 0.03 | 112 | 2.7 | |
| 10.0 | 9.77 ± 0.12 | 97.7 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yu, R.; Xi, F. Enhanced Electrochemiluminescence of Luminol and-Dissolved Oxygen by Nanochannel-Confined Au Nanomaterials for Sensitive Immunoassay of Carcinoembryonic Antigen. Molecules 2024, 29, 4880. https://doi.org/10.3390/molecules29204880
Li W, Yu R, Xi F. Enhanced Electrochemiluminescence of Luminol and-Dissolved Oxygen by Nanochannel-Confined Au Nanomaterials for Sensitive Immunoassay of Carcinoembryonic Antigen. Molecules. 2024; 29(20):4880. https://doi.org/10.3390/molecules29204880
Chicago/Turabian StyleLi, Weibin, Ruliang Yu, and Fengna Xi. 2024. "Enhanced Electrochemiluminescence of Luminol and-Dissolved Oxygen by Nanochannel-Confined Au Nanomaterials for Sensitive Immunoassay of Carcinoembryonic Antigen" Molecules 29, no. 20: 4880. https://doi.org/10.3390/molecules29204880
APA StyleLi, W., Yu, R., & Xi, F. (2024). Enhanced Electrochemiluminescence of Luminol and-Dissolved Oxygen by Nanochannel-Confined Au Nanomaterials for Sensitive Immunoassay of Carcinoembryonic Antigen. Molecules, 29(20), 4880. https://doi.org/10.3390/molecules29204880







