Acacetin, a Natural Flavone with Potential in Improving Liver Disease Based on Its Anti-Inflammation, Anti-Cancer, Anti-Infection and Other Effects
Abstract
1. Introduction
2. Overview of Liver Disease
3. Overview of Acacetin
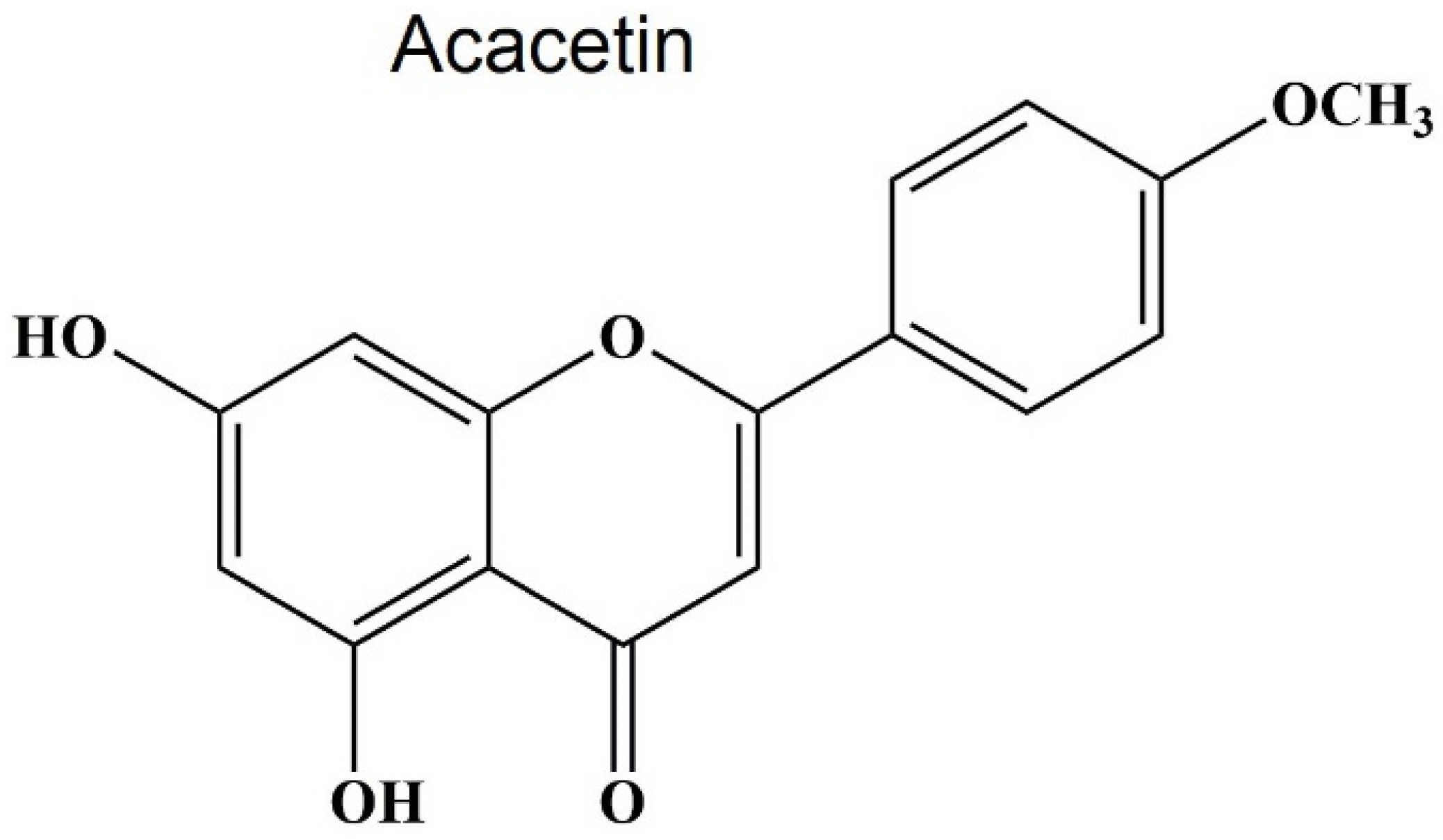
3.1. Source and Structure of Acacetin
3.2. Pharmacological Activities of Acacetin
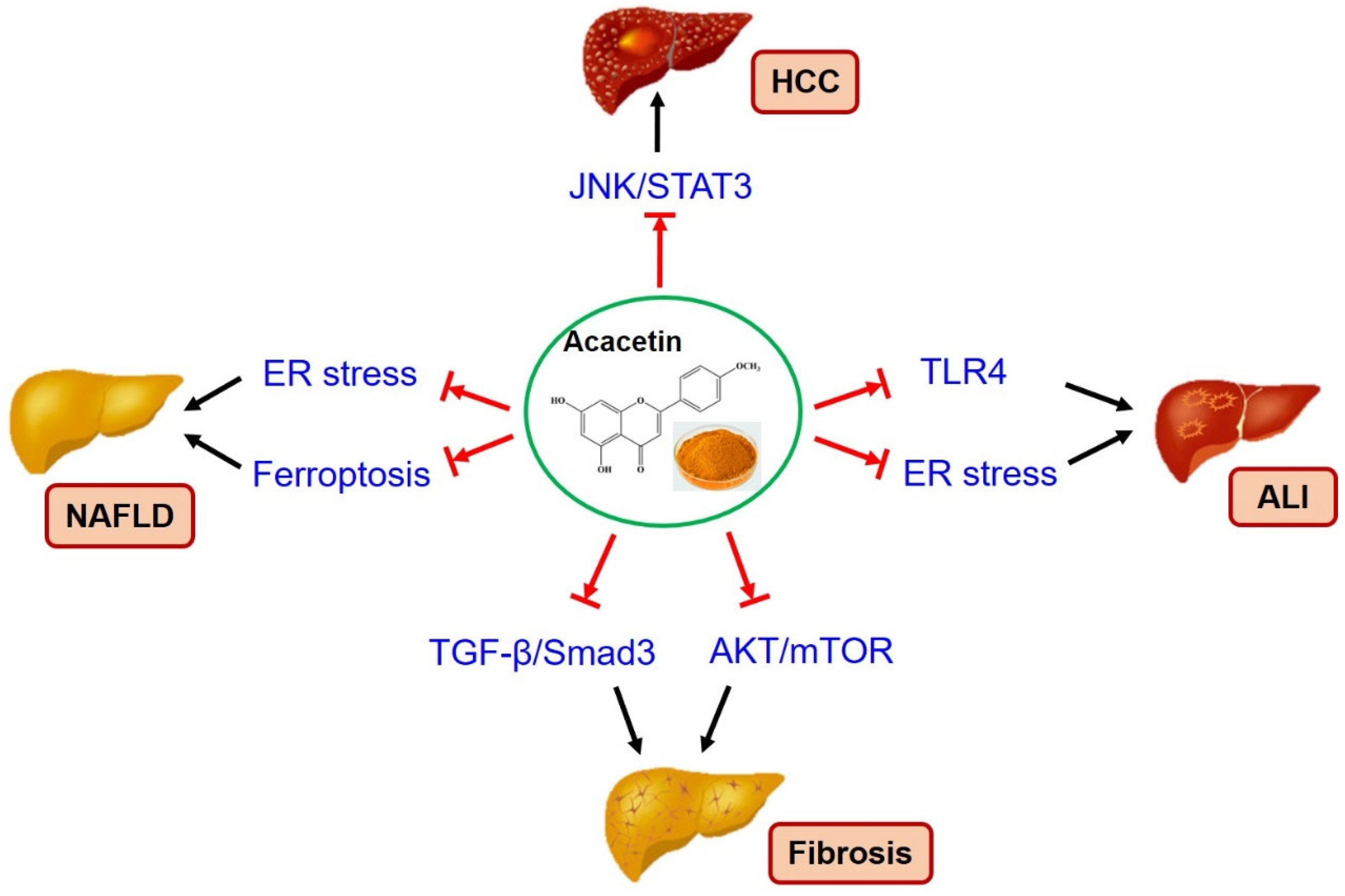
4. Therapeutic Effects of Acacetin on Liver Diseases
4.1. Anti-Hepatocellular Carcinoma
4.2. Anti-NAFLD
4.3. Anti-Fibrosis
4.4. Anti-Acute Liver Injury
5. Pharmacokinetics and Toxicity Studies
6. Discussion and Conclusions
6.1. Current Problems
6.2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griffin, C.; Agbim, U.; Ramani, A.; Shankar, N.; Kanwal, F.; Asrani, S.K. Underestimation of Cirrhosis-Related Mortality in the Medicare Eligible Population, 1999–2018. Clin. Gastroenterol. Hepatol. 2021, 21, 223–225.e3. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Luk, J.M.; Wang, X.; Liu, P.; Wong, K.; Chan, K.; Tong, Y.; Hui, C.; Lau, G.K.; Fan, S. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: From laboratory discovery to clinical evaluation. Liver Int. 2007, 27, 879–890. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef]
- Schlegel, A.; van Reeven, M.; Croome, K.; Parente, A.; Dolcet, A.; Widmer, J.; Meurisse, N.; De Carlis, R.; Hessheimer, A.; Jochmans, I.; et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J. Hepatol. 2021, 76, 371–382. [Google Scholar] [CrossRef]
- Papich, M.G.; Davis, L.E. Drugs and the liver. Vet. Clin. N. Am. Small. Anim. Pr. 1985, 15, 77–95. [Google Scholar] [CrossRef]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Cordell, G.A.; Colvard, M.D. Natural Products and Traditional Medicine: Turning on a Paradigm. J. Nat. Prod. 2012, 75, 514–525. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- Poulsen, N.B.; Lambert, M.N.T.; Jeppesen, P.B. The Effect of Plant Derived Bioactive Compounds on Inflammation: A Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2020, 64, e2000473. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.-A.; Soica, C. Main Isoflavones Found in Dietary Sources as Natural Anti-inflammatory Agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus flavanones: What is their role in cardiovascular protection? J. Agric Food Chem. 2012, 60, 8809–8822. [Google Scholar] [CrossRef]
- Martín, M.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [PubMed]
- Galle, P.R.; Dufour, J.-F.; Peck-Radosavljevic, M.; Trojan, J.; Vogel, A. Systemic Therapy of Advanced Hepatocellular Carcinoma. Futur. Oncol. 2020, 17, 1237–1251. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, S.; Esa, N.M.; Ramachandran, V.; Hamid, R.A.; Pandurangan, A.K.; Etemad, A.; Ismail, P. In vitro Antiproliferative and Apoptosis Inducing Effect of Allium Atroviolaceum Bulb Extract on Breast, Cervical, and Liver Cancer Cells. Front. Pharmacol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Sengupta, A.; Ghosh, S.; Bhattacharjee, S. Allium vegetables in cancer prevention: An overview. Asian Pac. J. Cancer Prev. 2004, 5, 237–245. [Google Scholar]
- Ng, K.T.P.; Guo, D.Y.; Cheng, Q.; Geng, W.; Ling, C.C.; Li, C.X.; Liu, X.B.; Ma, Y.Y.; Lo, C.M.; Poon, R.T.P.; et al. A Garlic Derivative, S-allylcysteine (SAC), Suppresses Proliferation and Metastasis of Hepatocellular Carcinoma. PLoS ONE 2012, 7, e31655. [Google Scholar] [CrossRef]
- Hong, H.; An, J.C.; de La Cruz, J.F.; Hwang, S.-G. Cnidium officinale Makino extract induces apoptosis through activation of caspase-3 and p53 in human liver cancer HepG2 cells. Exp. Ther. Med. 2017, 14, 3191–3197. [Google Scholar] [CrossRef][Green Version]
- Ringelhan, M.; McKeating, J.A.; Protzer, U. Viral hepatitis and liver cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160274. [Google Scholar]
- Ben Ari, Z.; Weitzman, E.; Safran, M. Oncogenic Viruses and Hepatocellular Carcinoma. Clin. Liver Dis. 2015, 19, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Paul, S. Viral hepatitis: Past, present, and future. World J. Gastroenterol. 2022, 28, 1405–1429. [Google Scholar] [CrossRef]
- Abutaleb, A.; Kottilil, S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterol. Clin. N. Am. 2020, 49, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.; Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat. Rev. Immunol. 2021, 22, 19–32. [Google Scholar] [CrossRef]
- Jeng, W.J.; Papatheodoridis, G.V.; Lok, A.S.F. Hepatitis B. Lancet 2023, 401, 1039–1052. [Google Scholar] [CrossRef]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Schall, R.A.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2012, 381, 468–475. [Google Scholar] [CrossRef]
- Liaw, Y.-F.; Sung, J.J.Y.; Chow, W.C.; Farrell, G.; Lee, C.-Z.; Yuen, H.; Tanwandee, T.; Tao, Q.-M.; Shue, K.; Keene, O.N.; et al. Lamivudine for Patients with Chronic Hepatitis B and Advanced Liver Disease. N. Engl. J. Med. 2004, 351, 1521–1531. [Google Scholar] [CrossRef]
- Rosen, H.R. Chronic hepatitis C infection. N. Engl. J. Med. 2011, 364, 2429–2438. [Google Scholar] [CrossRef]
- Potthoff, A.; Manns, M.P.; Wedemeyer, H. Treatment of HBV/HCV coinfection. Expert Opin. Pharmacother. 2010, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- González-Grande, R.; Jiménez-Pérez, M.; Arjona, C.G.; Torres, J.M. New approaches in the treatment of hepatitis C. World J. Gastroenterol. 2016, 22, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C.W.; Dusheiko, G.M.; Hellard, M.; Sonderup, M. Hepatitis C. Lancet 2019, 394, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Crouchet, E.; Wrensch, F.; Schuster, C.; Zeisel, M.B.; Baumert, T.F. Host-targeting therapies for hepatitis C virus infection: Current developments and future applications. Ther. Adv. Gastroenterol. 2018, 11, 1756284818759483. [Google Scholar] [CrossRef]
- Baumert, T.F.; Berg, T.; Lim, J.K.; Nelson, D.R. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology 2019, 156, 431–445. [Google Scholar] [CrossRef]
- Lempp, F.A.; Ni, Y.; Urban, S. Hepatitis delta virus: Insights into a peculiar pathogen and novel treatment options. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 580–589. [Google Scholar] [CrossRef]
- Koh, C.; Heller, T.; Glenn, J.S. Pathogenesis of and New Therapies for Hepatitis D. Gastroenterology 2019, 156, 461–476.e1. [Google Scholar] [CrossRef]
- Abbas, Z.; Afzal, R. Life cycle and pathogenesis of hepatitis D virus: A review. World J. Hepatol. 2013, 5, 666–675. [Google Scholar] [CrossRef]
- Aslan, A.T.; Balaban, H.Y. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J. Gastroenterol. 2020, 26, 5543–5560. [Google Scholar] [CrossRef]
- Ma, Z.; de Man, R.A.; Kamar, N.; Pan, Q. Chronic hepatitis E: Advancing research and patient care. J. Hepatol. 2022, 77, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhang, H.; Huang, W.; Harrison, T.J.; Geng, K.; Li, Z.; Wang, Y. Persistent Hepatitis E Virus Genotype 4 Infection in a Child with Acute Lymphoblastic Leukemia. Hepat. Mon. 2013, 14, e15618. [Google Scholar] [CrossRef] [PubMed]
- Todt, D.; Moeller, N.; Praditya, D.; Kinast, V.; Friesland, M.; Engelmann, M.; Verhoye, L.; Sayed, I.M.; Behrendt, P.; Thi, V.L.D.; et al. The natural compound silvestrol inhibits hepatitis E virus (HEV) replication in vitro and in vivo. Antivir. Res. 2018, 157, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Koch, D.G.; Lee, W.M. Drug-induced acute liver failure: Results of a U.S. multicenter, prospective study. Hepatology 2010, 52, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Bergquist, A.; Broomé, U.; Lindgren, S.; Wallerstedt, S.; Almer, S.; Sangfelt, P.; Danielsson, Å.; Sandberg-Gertzén, H.; Lööf, L.; et al. Acute liver failure in Sweden: Etiology and outcome. J. Intern. Med. 2007, 262, 393–401. [Google Scholar] [CrossRef]
- Reuben, A.; Tillman, H.; Fontana, R.J.; Davern, T.; McGuire, B.; Stravitz, R.T.; Durkalski, V.; Larson, A.M.; Liou, I.; Fix, O.; et al. Outcomes in Adults with Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann. Intern. Med. 2016, 164, 724–732. [Google Scholar] [CrossRef]
- Nathwani, R.A.; Kaplowitz, N. Drug hepatotoxicity. Clin. Liver Dis. 2006, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Senior, J.R. Drug Hepatotoxicity from a Regulatory Perspective. Clin. Liver Dis. 2007, 11, 507–524. [Google Scholar] [CrossRef]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef]
- Pu, S.; Pan, Y.; Zhang, Q.; You, T.; Yue, T.; Zhang, Y.; Wang, M. Endoplasmic Reticulum Stress and Mitochondrial Stress in Drug-Induced Liver Injury. Molecules 2023, 28, 3160. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Clichici, S.; Olteanu, D.; Nagy, A.-L.; Oros, A.; Filip, A.; Mircea, P.A. Silymarin Inhibits the Progression of Fibrosis in the Early Stages of Liver Injury in CCl4-Treated Rats. J. Med. Food 2015, 18, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Liu, J.Y.; Wu, T.T.; Ho, P.C.; Huang, C.Y.; Shyu, J.C.; Hsieh, Y.S.; Tsai, C.C.; Liu, Y.C. Effects of silymarin on the resolution of liver fibrosis induced by carbon tetrachloride in rats. J. Viral Hepat. 2008, 15, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Silymarin in the treatment of liver diseases: What is the clinical evidence? Clin. Liver Dis. 2016, 7, 8–10. [Google Scholar] [CrossRef]
- Okigawa, M.; Hatanaka, H.; Kawano, N.; Matsunaga, I.; Tamura, Z. A new glycoside, acacetin-7-glucurono-(1 lead to 2)-glucuronide from the leaves of Clerodendron trichotomum. Tetrahedron Lett. 1970, 11, 2935–2936. [Google Scholar] [CrossRef]
- Rendiuk, T.D.; Krivut, B.A.; Glyzin, V.I. Spectrophotometric method of determining acacetin in the leaves of the thistle, Cirsium setosum (Willd.). Farmatsiia 1978, 27, 68. [Google Scholar]
- Griffiths, L.A.; Smith, G.E. Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem. J. 1972, 128, 901–911. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Terahara, N. Flavonoids in Foods: A Review. Nat. Prod. Commun. 2015, 10, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Li, G.-R.; Wang, H.-B.; Qin, G.-W.; Jin, M.-W.; Tang, Q.; Sun, H.-Y.; Du, X.-L.; Deng, X.-L.; Zhang, X.-H.; Chen, J.-B.; et al. Acacetin, a Natural Flavone, Selectively Inhibits Human Atrial Repolarization Potassium Currents and Prevents Atrial Fibrillation in Dogs. Circulation 2008, 117, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, L.; Sui, Q.; Lin, F.; Jiang, W.; Chen, J.; Lu, W.; Gao, Q. Facile synthesis of acacetin and its derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 3577–3580. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-G.; Cha, E.; Joo, J.; Hwang, J.S.; Kim, S.; Park, T.; Jeong, Y.-S.; Maeng, H.-J.; Kim, S.-B.; Yoon, I.-S. Investigation of the Factors Responsible for the Poor Oral Bioavailability of Acacetin in Rats: Physicochemical and Biopharmaceutical Aspects. Pharmaceutics 2021, 13, 175. [Google Scholar] [CrossRef]
- Yin, J.; Ma, Y.; Liang, C.; Gao, J.; Wang, H.; Zhang, L.-T. A Systematic Study of the Metabolites of Dietary Acacetin in Vivo and in Vitro Based on UHPLC-Q-TOF-MS/MS Analysis. J. Agric. Food Chem. 2019, 67, 5530–5543. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.-J.; Yang, L.; Zhou, M.; Jin, M.-W.; Xiao, G.-S.; Sun, H.-Y.; Li, G.-R. Synthesis of a highly water-soluble acacetin prodrug for treating experimental atrial fibrillation in beagle dogs. Sci. Rep. 2016, 6, 25743. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Berto, A.; Ribeiro, A.B.; Sentandreu, E.; de Souza, N.E.; Mercadante, A.Z.; Chisté, R.C.; Fernandes, E. The seed of the Amazonian fruit Couepia bracteosa exhibits higher scavenging capacity against ROS and RNS than its shell and pulp extracts. Food Funct. 2015, 6, 3081–3090. [Google Scholar] [CrossRef]
- Sun, L.-C.; Zhang, H.-B.; Gu, C.-D.; Guo, S.-D.; Li, G.; Lian, R.; Yao, Y.; Zhang, G.-Q. Protective effect of acacetin on sepsis-induced acute lung injury via its anti-inflammatory and antioxidative activity. Arch. Pharmacal Res. 2017, 41, 1199–1210. [Google Scholar] [CrossRef]
- Wu, C.; Chen, R.L.; Wang, Y.; Wu, W.Y.; Li, G. Acacetin alleviates myocardial ischaemia/reperfusion injury by inhibiting oxidative stress and apoptosis via the Nrf-2/HO-1 pathway. Pharm. Biol. 2022, 60, 553–561. [Google Scholar] [CrossRef]
- Cui, Y.-K.; Hong, Y.-X.; Wu, W.-Y.; Han, W.-M.; Wu, Y.; Wu, C.; Li, G.-R.; Wang, Y. Acacetin ameliorates cardiac hypertrophy by activating Sirt1/AMPK/PGC-1α pathway. Eur. J. Pharmacol. 2022, 920, 174858. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Zhang, H.; Du, M.; Li, T. Acacetin attenuates mice endotoxin-induced acute lung injury via augmentation of heme oxygenase-1 activity. Inflammopharmacology 2017, 26, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Mao, Y.J.; Hu, Y.; Zhao, S.S.; Wang, R.; Wu, W.Y.; Li, G.R.; Wang, Y.; Li, G. Acacetin attenuates diabetes-induced cardiomyopathy by inhibiting oxidative stress and energy metabolism via PPAR-alpha/AMPK pathway. Eur. J. Pharmacol. 2022, 92, 174916. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, L.; Wu, H.-J.; Chen, K.-H.; Lin, F.; Li, G.; Sun, H.-Y.; Xiao, G.-S.; Wang, Y.; Li, G.-R. Water-soluble acacetin prodrug confers significant cardioprotection against ischemia/reperfusion injury. Sci. Rep. 2016, 6, 36435. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Hsu, P.-C.; Wang, Y.-J. Acacetin Induces Apoptosis in Human Gastric Carcinoma Cells Accompanied by Activation of Caspase Cascades and Production of Reactive Oxygen Species. J. Agric. Food Chem. 2005, 53, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kandhari, K.; Mishra, J.P.; Agarwal, R.; Singh, R.P. Acacetin induces sustained ERK1/2 activation and RIP1-dependent necroptotic death in breast cancer cells. Toxicol. Appl. Pharmacol. 2023, 462, 116409. [Google Scholar] [CrossRef]
- Salimi, A.; Roudkenar, M.H.; Sadeghi, L.; Mohseni, A.; Seydi, E.; Pirahmadi, N.; Pourahmad, J. Selective Anticancer Activity of Acacetin Against Chronic Lymphocytic Leukemia Using Both In Vivo and In Vitro Methods: Key Role of Oxidative Stress and Cancerous Mitochondria. Nutr. Cancer 2016, 68, 1404–1416. [Google Scholar] [CrossRef]
- Wang, S.; Lin, B.; Liu, W.; Wei, G.; Li, Z.; Yu, N.; Xue, X.; Ji, G. Acacetin Induces Apoptosis in Human Osteosarcoma Cells by Modulation of ROS/JNK Activation. Drug Des. Dev. Ther. 2020, 14, 5077–5085. [Google Scholar] [CrossRef]
- Alfwuaires, M.; Elsawy, H.; Sedky, A. Acacetin Inhibits Cell Proliferation and Induces Apoptosis in Human Hepatocellular Carcinoma Cell Lines. Molecules 2022, 27, 5361. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, C.-C. Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. Biochem. Pharmacol. 2003, 67, 823–829. [Google Scholar] [CrossRef]
- Shim, H.-Y.; Park, J.-H.; Paik, H.-D.; Nah, S.-Y.; Kim, D.S.; Han, Y.S. Acacetin-induced Apoptosis of Human Breast Cancer MCF-7 Cells Involves Caspase Cascade, Mitochondria-mediated Death Signaling and SAPK/JNK1/2-c-Jun Activation. Mol. Cells 2007, 24, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Kuo, P.-L.; Liu, C.-F.; Lin, C.-C. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004, 212, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.-T.; Lin, S.-S.; Wang, C.-K.; Lee, Y.-B.; Chen, K.-S.; Fong, Y.; Shih, Y.-W. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38α MAPK signaling pathway. Mol. Cell. Biochem. 2011, 350, 135–148. [Google Scholar] [CrossRef]
- Yun, S.; Lee, Y.-J.; Choi, J.; Kim, N.D.; Han, D.C.; Kwon, B.-M. Acacetin Inhibits the Growth of STAT3-Activated DU145 Prostate Cancer Cells by Directly Binding to Signal Transducer and Activator of Transcription 3 (STAT3). Molecules 2021, 26, 6204. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, C.G.; Jung, J.Y. Acacetin (5,7-dihydroxy-4′-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-kappaB/Akt signaling in prostate cancer cells. Int. J. Mol. Med. 2014, 33, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, P.; Yim, D.; Agarwal, C.; Agarwal, R. Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: Structure-activity relationship with linarin and linarin acetate. Carcinog. 2005, 26, 845–854. [Google Scholar] [CrossRef]
- Cakmakoglu, B.; Aslan, B.; Ertugrul, B.; Iplik, E. Apoptotic effects of acacetin in human colon cancer HT-29 and HCT 116 cells. J. Cancer Res. Ther. 2021, 17, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Sharma, J.R.; Yadav, U.C.S. Induction of growth cessation by acacetin via beta-catenin pathway and apoptosis by apoptosis inducing factor activation in colorectal carcinoma cells. Mol. Biol. Rep. 2020, 47, 987–1001. [Google Scholar] [CrossRef]
- Yomogida, S.; Watanabe, K.; Kanno, S.-I.; Tomizawa, A.; Ishikawa, M. Acacetin induces apoptosis in human T cell leukemia Jurkat cells via activation of a caspase cascade. Oncol. Rep. 2011, 27, 204–209. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jun, D.Y.; Kim, K.Y.; Ha, E.J.; Woo, M.H.; Ko, J.Y.; Yun, Y.H.; Oh, I.-S.; Kim, Y.H. Pharmacologic Inhibition of Autophagy Sensitizes Human Acute Leukemia Jurkat T Cells to Acacetin-Induced Apoptosis. J. Microbiol. Biotechnol. 2017, 27, 197–205. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, J.; Lu, L.; Liu, Y.; Hu, D.; Wu, Y.; Zhao, A.; Xu, H. Acacetin exerts antitumor effects on gastric cancer by targeting EGFR. Front. Pharmacol. 2023, 14, 1121643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Z.; Dong, J.; Zhou, W.; Zhang, Z.; Que, Z.; Zhu, X.; Xu, Y.; Cao, N.; Zhao, A. Acacetin inhibits invasion, migration and TGF-beta1-induced EMT of gastric cancer cells through the PI3K/Akt/Snail pathway. BMC Complement. Med. Ther. 2022, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Ge, M.; Cui, J.; Dong, X.; Shao, Y. Acacetin attenuates the pancreatic and hepatorenal dysfunction in type 2 diabetic rats induced by high-fat diet combined with streptozotocin. J. Nat. Med. 2023, 77, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Wu, S.J.; Shen, S.C.; Chen, L.C.; Chen, Y.L.; Huang, W.C. Acacetin Protects against Non-Alcoholic Fatty Liver Disease by Regulating Lipid Accumulation and Inflammation in Mice. Int. J. Mol. Sci. 2022, 23, 4687. [Google Scholar] [CrossRef]
- Ren, J.; Yue, B.; Wang, H.; Zhang, B.; Luo, X.; Yu, Z.; Zhang, J.; Ren, Y.; Mani, S.; Wang, Z.; et al. Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 2020, 11, 577237. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.-G.; Yu, S.-Y.; Li, C.-E.; Kang, S.-M. Protective effect of acacetin in human periodontal ligament cells via regulation of autophagy and inflammation. Pharmazie 2020, 75, 436–439. [Google Scholar] [CrossRef]
- Ha, S.K.; Moon, E.; Lee, P.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. Acacetin Attenuates Neuroinflammation via Regulation the Response to LPS Stimuli In Vitro and In Vivo. Neurochem. Res. 2012, 37, 1560–1567. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Wang, Y.-J.; Ho, C.-T. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem. Pharmacol. 2006, 72, 1293–1303. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Z.; Pang, Z.; Zhou, T.; Gu, Y. Acacetin Alleviates Inflammation and Matrix Degradation in Nucleus Pulposus Cells and Ameliorates Inter-vertebral Disc Degeneration in vivo. Drug Des. Devel. Ther. 2020, 14, 4801–4813. [Google Scholar] [CrossRef]
- Wei, Y.; Jing, J.; Peng, Z.; Liu, X.; Wang, X. Acacetin ameliorates insulin resistance in obesity mice through regulating Treg/Th17 balance via MiR-23b-3p/NEU1 Axis. BMC Endocr. Disord. 2021, 21, 57. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, J.; Shi, S.; Wang, H.-Q.; Niu, X.-S.; Zhao, Z.-F.; Wu, W.-D.; Zhang, X.-L.; Ma, Z.; Zhang, Y.-J.; et al. Acacetin protects against cerebral ischemia-reperfusion injury via the NLRP3 signaling pathway. Neural Regen. Res. 2019, 14, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, H.; Peng, J.; Qing, D.; Zhang, X.; Guo, D.; Meng, P.; Luo, Z.; Wang, X.; Peng, Q. Acacetin protects against depression-associated dry eye disease by regulating ubiquitination of NLRP3 through gp78 signal. Front. Pharmacol. 2022, 13, 984475. [Google Scholar] [CrossRef]
- Bu, J.; Zhang, Y.; Mahan, Y.; Shi, S.; Wu, X.; Zhang, X.; Wang, Z.; Zhou, L. Acacetin improves cognitive function of APP/PS1 Alzheimer’s disease model mice via the NLRP3 inflammasome signaling pathway. Transl. Neurosci. 2022, 13, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Q.; Chen, K.; Shi, Q.; Kilkuskie, R.E.; Cheng, Y.-C.; Lee, K.-H. Anti-AIDS Agents, 10. Acacetin-7-O-β-D-galactopyranoside, an Anti-HIV Principle from Chrysanthemum Morifolium and a Structure-Activity Correlation with Some Related Flavonoids. J. Nat. Prod. 1994, 57, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV Activation in Latently Infected Cells by Flavonoid Compounds. AIDS Res. Hum. Retroviruses 1996, 12, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Bao, J.; Yang, J.; Wu, C. Potential mechanism of action of Jing Fang Bai Du San in the treatment of COVID-19 using docking and network pharmacology. Int. J. Med. Sci. 2022, 19, 213–224. [Google Scholar] [CrossRef]
- Parvez, M.K.; Alhowiriny, T.A.; Al-Dosari, M.S.; Amina, M.; Rehman, M.T.; Al-Yousef, H.M.; Alanzi, A.R.; Alajmi, M.F. Inhibition of hepatitis B virus activities by Rhazya stricta-derived acacetin and acetyl-beta-carboline. Exp. Ther. Med. 2023, 26, 327. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, W.; Jia, H.; Zheng, L.; Xing, J.; Liu, A.; Du, G. Discovery of Multitarget-Directed Ligands Against Influenza A Virus from Compound Yizhihao Through a Predictive System for Compound-Protein Interactions. Front. Cell. Infect. Microbiol. 2020, 10, 16. [Google Scholar] [CrossRef]
- Cai, C.; Xu, L.; Fang, J.; Dai, Z.; Wu, Q.; Liu, X.; Wang, Q.; Fang, J.; Liu, A.L.; Du, G.H. In Silico Prediction and Bioactivity Evaluation of Chemical Ingredients Against Influenza A Virus from Isatis Tinctoria L. Front. Pharmacol. 2021, 12, 755396. [Google Scholar] [CrossRef]
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial Constituents of Artemisia Afra Jacq. ex Willd. against Periodontal Pathogens. Evid. Based Complement. Altern. Med. 2012, 2012, 252758. [Google Scholar] [CrossRef]
- Li, S.; Xu, X.; Wei, L.; Wang, L.; Lv, Q. Acacetin Alleviates Listeria monocytogenes Virulence Both In Vitro and In Vivo via the Inhibition of Listeriolysin O. Foodborne Pathog. Dis. 2022, 19, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, Y.; Xu, L.; Li, S.; Shen, X.; Li, L.; Deng, X.; Zhou, Y. Acacetin attenuates Streptococcus suis virulence by simultaneously targeting suilysin and inflammation. Microb. Pathog. 2022, 162, 105354. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lim, D.J.; Yang, H.J.; Choi, E.K.; Shin, M.H.; Ahn, K.S.; Jung, S.H.; Um, J.Y.; Jung, H.J.; Lee, J.H.; et al. The multi-targeted effects of Chrysanthemum herb extract against Escherichia coli O157:H7. Phytother. Res. 2013, 27, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Komape, N.P.M.; Aderogba, M.; Bagla, V.P.; Masoko, P.; Eloff, J.N. Anti-bacterial and anti-oxidant activities of leaf extracts of Combretum vendae (Combretecacea) and the isolation of an anti-bacterial compound. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Dong, X.; Zhong, X.; Cai, H.; Wang, D.; Wang, L. Acacetin Protects Mice from Staphylococcus aureus Bloodstream Infection by Inhibiting the Activity of Sortase A. Molecules 2016, 21, 1285. [Google Scholar] [CrossRef]
- Li, S.; Lv, Q.; Sun, X.; Tang, T.; Deng, X.; Yin, Y.; Li, L. Acacetin inhibits Streptococcus pneumoniae virulence by targeting pneumolysin. J. Pharm. Pharmacol. 2020, 72, 1092–1100. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Frankowski, R.; Kobierecki, M.; Wittczak, A.; Różycka-Kosmalska, M.; Pietras, T.; Sipowicz, K.; Kosmalski, M. Type 2 Diabetes Mellitus, Non-Alcoholic Fatty Liver Disease, and Metabolic Repercussions: The Vicious Cycle and Its Interplay with Inflammation. Int. J. Mol. Sci. 2023, 24, 9677. [Google Scholar] [CrossRef]
- Wang, N.; Gao, Q.; Shi, J.; Yulan, C.; Ji, W.; Sheng, X.; Zhang, R. Acacetin antagonized lipotoxicity in pancreatic beta-cells via ameliorating oxidative stress and endoplasmic reticulum stress. Mol. Biol. Rep. 2022, 49, 8727–8740. [Google Scholar] [CrossRef]
- Juárez-Reyes, K.; Brindis, F.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Bye, R.; Linares, E.; Mata, R. Hypoglycemic, antihyperglycemic, and antioxidant effects of the edible plant Anoda cristata. J. Ethnopharmacol. 2015, 161, 36–45. [Google Scholar] [CrossRef]
- Kwon, E.-B.; Kang, M.-J.; Ryu, H.W.; Lee, S.; Lee, J.-W.; Lee, M.K.; Lee, H.-S.; Lee, S.U.; Oh, S.-R.; Kim, M.-O. Acacetin enhances glucose uptake through insulin-independent GLUT4 translocation in L6 myotubes. Phytomedicine 2020, 68, 153178. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M.; Chen, X.C.; Li, G.R.; Wang, Y. Acacetin Protects Against High Glucose-Induced Endothelial Cells Injury by Preserving Mitochondrial Function via Activating Sirt1/Sirt3/AMPK Signals. Front. Pharmacol. 2020, 11, 607796. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiang, J.; Huang, G.; Kang, L.; Yang, G.; Wu, H.; Jiang, K.; Liang, Z.; Yang, S. Inhibition of Podocytes DPP4 Activity Is a Potential Mechanism of Lobeliae Chinensis Herba in Treating Diabetic Kidney Disease. Front. Pharmacol. 2021, 12, 779652. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Li, J.; Wu, Z.-S.; Zhang, C.-L.; Meng, X.-L. STAT3 activation in monocytes accelerates liver cancer progression. BMC Cancer 2011, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Karin, M. NF-kappaB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef]
- Yang, C.; Cai, W.-C.; Dong, Z.-T.; Guo, J.-W.; Zhao, Y.-J.; Sui, C.-J.; Yang, J.-M. lncARSR promotes liver cancer stem cells expansion via STAT3 pathway. Gene 2018, 687, 73–81. [Google Scholar] [CrossRef]
- Zhu, X.; Song, G.; Zhang, S.; Chen, J.; Hu, X.; Zhu, H.; Jia, X.; Li, Z.; Song, W.; Chen, J.; et al. Asialoglycoprotein Receptor 1 Functions as a Tumor Suppressor in Liver Cancer via Inhibition of STAT3. Cancer Res. 2022, 82, 3987–4000. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, C.; Cheng, H.; Wu, Y.L.; Liu, J.; Chen, Z.; Huang, J.G.; Ericksen, R.E.; Chen, L.; Zhang, H.; et al. Targeting to the non-genomic activity of retinoic acid receptor-gamma by acacetin in hepatocellular carcinoma. Sci. Rep. 2017, 7, 348. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, C.; Zhao, S.; Wang, M.; Shang, L.; Zhou, J.; Ma, Y. The role of tumor-associated macrophages in hepatocellular carcinoma progression: A narrative review. Cancer Med. 2023, 12, 22109–22129. [Google Scholar] [CrossRef]
- Choi, H.-J.; Chung, T.-W.; Ha, K.-T. Luteolin inhibits recruitment of monocytes and migration of Lewis lung carcinoma cells by suppressing chemokine (C–C motif) ligand 2 expression in tumor-associated macrophage. Biochem. Biophys. Res. Commun. 2016, 470, 101–106. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Gao, S.; Lee, J.K.M.; Chan, Y.Y.; Wong, E.C.W.; Zheng, T.; Li, X.X.; Shaw, P.C.; Simmonds, M.S.; Lau, C.B.S. A Natural Flavone Tricin from Grains Can Alleviate Tumor Growth and Lung Metastasis in Colorectal Tumor Mice. Molecules 2020, 25, 3730. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis in NAFLD/NASH: From pathophysiology towards diagnostic and therapeutic strategies. Mol. Aspects Med. 2024, 95, 101231. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Wu, S.J.; Chen, L.C.; Yeh, K.W.; Chen, C.Y.; Huang, W.C. Acacetin from Traditionally Used Saussurea Involucrata Kar. et Kir. Suppressed Adipogenesis in 3T3-L1 Adipocytes and Attenuated Lipid Accumulation in Obese Mice. Front. Pharmacol. 2017, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, H.; Miao, J.; Sheng, Q.; Xu, J.; Gao, Z.; Zhang, X.; Song, Y.; Chen, K. The natural flavone acacetin protects against high-fat diet-induced lipid accumulation in the liver via the endo-plasmic reticulum stress/ferroptosis pathway. Biochem. Biophys. Res. Commun. 2023, 640, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Q.; Xiong, X.; Yin, T.; Chen, S.; Yuan, W.; Zeng, G.; Huang, Q. Acacetin alleviates energy metabolism disorder through promoting white fat browning mediated by AC-cAMP pathway. J. Physiol. Biochem. 2023, 79, 529–541. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Li, Z.Y.; Lv, S.; Qiao, J.; Wang, S.Q.; Ji, F.; Li, D.; Yan, J.; Wei, Y.; Wu, L.; Gao, C.; et al. Acacetin Alleviates Cardiac Fibrosis via TGF-beta1/Smad and AKT/mTOR Signal Pathways in Spontaneous Hypertensive Rats. Gerontology 2023, 69, 1076–1094. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Gao, L.; Zheng, X.; Feng, W. Acacetin improves endothelial dysfunction and aortic fibrosis in insulin-resistant SHR rats by estrogen receptors. Mol. Biol. Rep. 2020, 47, 6899–6918. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, R.; Cai, F.-F.; Zhou, W.-J.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Sun, M.-Y.; Su, S.-B. Development of a novel anti-liver fibrosis formula with luteolin, licochalcone A, aloe-emodin and acacetin by network pharmacology and transcriptomics analysis. Pharm. Biol. 2021, 59, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Xu, W.; Wang, S.; Hu, Z.; Zhang, Q.; Deng, X.; Wang, J.; Zhang, J.; Guo, C. Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGF-beta/Smad signalling pathways. Liver Int. 2015, 35, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Cao, P.; Yin, Z.; Wang, X.; Chen, Y.; Yu, M.; Xu, B.; Liao, C.; Duan, Y.; Zhang, S.; et al. Apigenin protects mice against 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced cholestasis. Food Funct. 2021, 12, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yu, Q.; Dai, W.; Wu, L.; Feng, J.; Zheng, Y.; Li, Y.; Guo, C. Apigenin Alleviates Liver Fibrosis by Inhibiting Hepatic Stellate Cell Activation and Autophagy via TGF-beta1/Smad3 and p38/PPARalpha Pathways. PPAR Res. 2021, 2021, 6651839. [Google Scholar] [CrossRef] [PubMed]
- Tujios, S.; Stravitz, R.T.; Lee, W.M. Management of Acute Liver Failure: Update 2022. Semin. Liver Dis. 2022, 42, 362–378. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef]
- Chen, T.; Li, R.; Chen, P. Gut Microbiota and Chemical-Induced Acute Liver Injury. Front. Physiol. 2021, 12, 688780. [Google Scholar] [CrossRef]
- Bernal, W.; Wendon, J. Acute liver failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef]
- Morris, S.M.; Chauhan, A. The role of platelet mediated thromboinflammation in acute liver injury. Front. Immunol. 2022, 13, 1037645. [Google Scholar] [CrossRef]
- Cho, H.-I.; Park, J.-H.; Choi, H.-S.; Kwak, J.H.; Lee, D.-U.; Lee, S.K.; Lee, S.-M. Protective Mechanisms of Acacetin against d-Galactosamine and Lipopolysaccharide-Induced Fulminant Hepatic Failure in Mice. J. Nat. Prod. 2014, 77, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Yao, S.; Sun, H.; Jiang, Z.; Gao, Z.; Xu, J.; Chen, K. Protective Effect of Water-Soluble Acacetin Prodrug on APAP-Induced Acute Liver Injury Is Associated with Upregulation of PPARgamma and Alleviation of ER Stress. Int. J. Mol. Sci. 2023, 24, 11320. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Akhshi, N.; Raissi, F.; Shiravi, A.; Alvani, A.; Vaezi, G. Acacetin Alleviates Hepatitis Following Renal Ischemia-Reperfusion in Male Balb/C Mice by Antioxidants Regulation and Inflammatory Markers Suppression. J. Investig. Surg. 2021, 34, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-H.; Li, X.; Chen, D.-Y.; Zhang, N.; Wang, Y.; Shan, Y.; Hu, Y.; Xu, R.-A.; Jin, J.; Ge, R.-S. Determination of acacetin in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study. J. Chromatogr. B 2015, 986, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.-K.; Rashid, M.; Lee, G.; Kim, D.-Y.; Ryu, H.W.; Oh, S.-R.; Park, J.; Lee, H.; Hong, J.; Jung, B.H. Metabolites identification for major active components of Agastache rugosa in rat by UPLC-Orbitap-MS: Comparison of the difference between metabolism as a single component and as a component in a multi-component extract. J. Pharm. Biomed. Anal. 2022, 220, 114976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.Q.; Zhang, L.C.; Wang, K.X.; Zhang, L.T.; Li, D.Q. The development and validation of a sensitive HPLC-MS/MS method for the quantitative and pharmacokinetic study of the seven components of Buddleja lindleyana Fort. RSC Adv. 2021, 11, 26016–26028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tu, Y.; Zhou, Q.; Hua, A.; Geng, P.; Chen, F.; Han, A.; Liu, J.; Dai, D.; Wang, S.; et al. Evaluation of acacetin inhibition potential against cytochrome P450 in vitro and in vivo. Chem. Interact. 2020, 329, 109147. [Google Scholar] [CrossRef]
- Shokri, V.; Jalili, C.; Raissi, F.; Akhshi, N.; Ghanbari, A. Evaluating the effects of acacetin versus a low dose of cisplatin drug on male reproductive system and kidney in mice: With emphasis on inflammation process. Andrologia 2019, 52, e13444. [Google Scholar] [CrossRef]
- Tacke, F.; Weiskirchen, R. Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: Mechanisms, treatment and prevention. Ann. Transl. Med. 2021, 9, 729. [Google Scholar] [CrossRef]
| Samples | Source | References | |
|---|---|---|---|
| Flavonols | Quercetin Kaempferol Fisetin | Fresh capers Anethum graveolens Red onion Fennel | [13] |
| Flavones | Apigenin Acacetin Luteolin | Chamaemelum nobile Tanacetum vulgare Origanum vulgare Petroselinumcrispum | [14] |
| Isoflavones | Daidzein Genistein Glycitein | Soybeans Natto | [15] |
| Anthocyanidins | Cyaniding Delphinidin Malvidin | Apples Berries Stone fruits Grapes | [16] |
| Flavanones | Naringenin Hesperetin Eriodictyol | Orange Tangerine Lemon Grapefruit | [17] |
| Flavanols | Epigallocatechin gallate Epicatechin Theflavin-3,3′-digallate | Cocoa Tea Berries Apricots | [18] |
| Chalcones | Lonchocarpin Cardamonin licochalcones | Lonchocarpussericeus Campomanesia adamantium Glycyrrhiza urakensis | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Gao, Z. Acacetin, a Natural Flavone with Potential in Improving Liver Disease Based on Its Anti-Inflammation, Anti-Cancer, Anti-Infection and Other Effects. Molecules 2024, 29, 4872. https://doi.org/10.3390/molecules29204872
Chen K, Gao Z. Acacetin, a Natural Flavone with Potential in Improving Liver Disease Based on Its Anti-Inflammation, Anti-Cancer, Anti-Infection and Other Effects. Molecules. 2024; 29(20):4872. https://doi.org/10.3390/molecules29204872
Chicago/Turabian StyleChen, Kuihao, and Zhe Gao. 2024. "Acacetin, a Natural Flavone with Potential in Improving Liver Disease Based on Its Anti-Inflammation, Anti-Cancer, Anti-Infection and Other Effects" Molecules 29, no. 20: 4872. https://doi.org/10.3390/molecules29204872
APA StyleChen, K., & Gao, Z. (2024). Acacetin, a Natural Flavone with Potential in Improving Liver Disease Based on Its Anti-Inflammation, Anti-Cancer, Anti-Infection and Other Effects. Molecules, 29(20), 4872. https://doi.org/10.3390/molecules29204872





