Exploring Spin Distribution and Electronic Properties in FeN4-Graphene Catalysts with Edge Terminations
Abstract
1. Introduction
2. Results
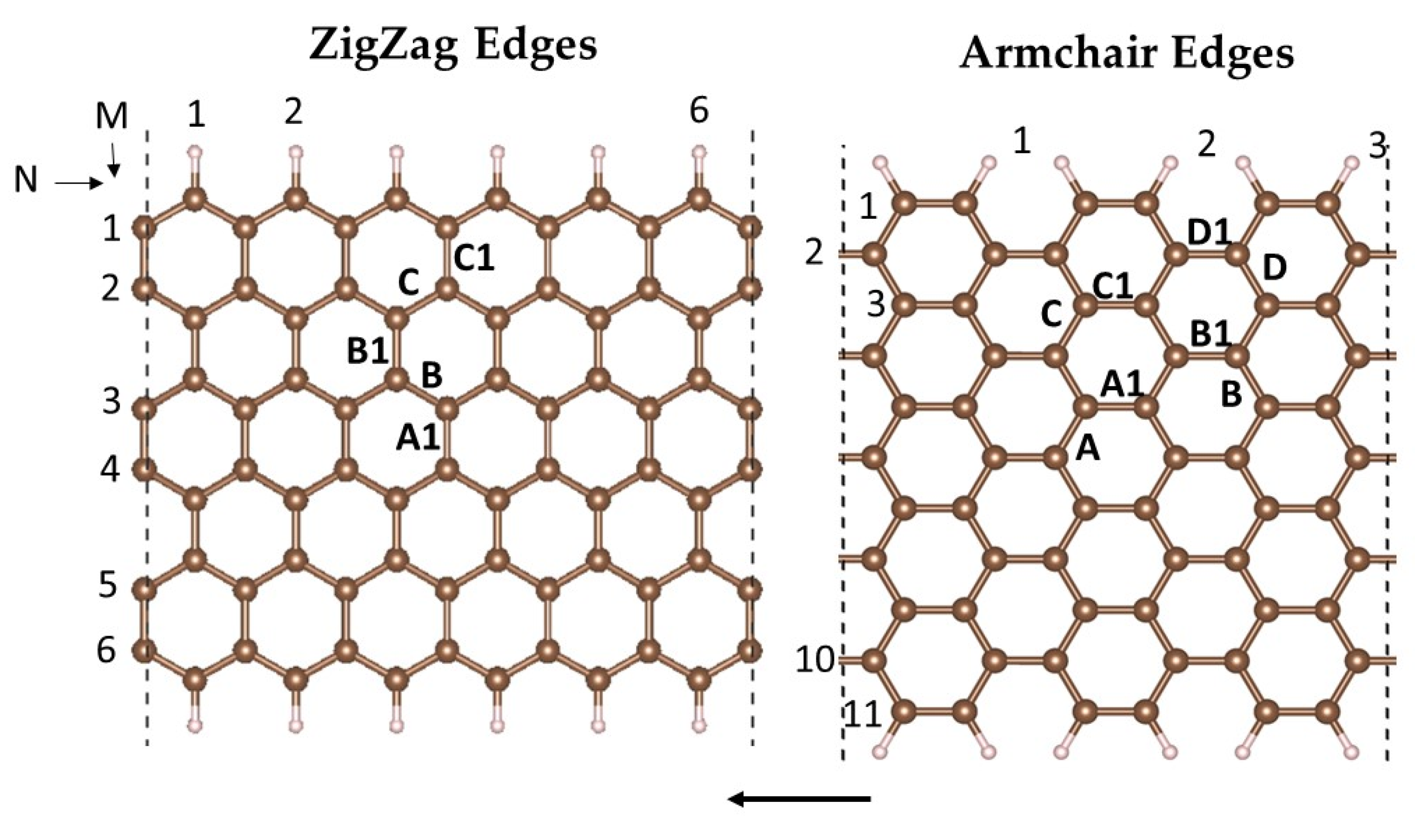
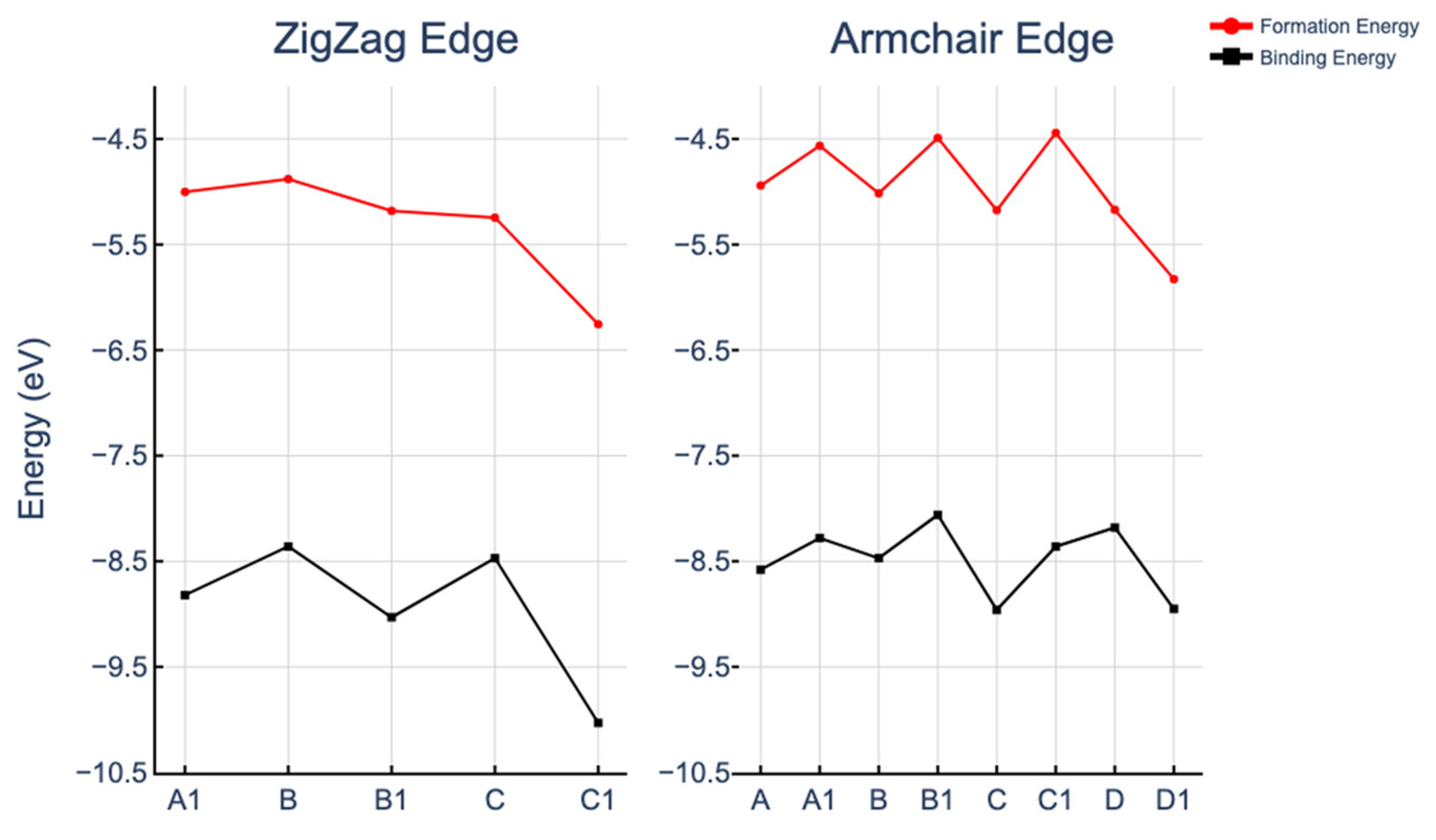
2.1. Formation and Stability of Fe(II)N4 in ZGNR and AGNR Models
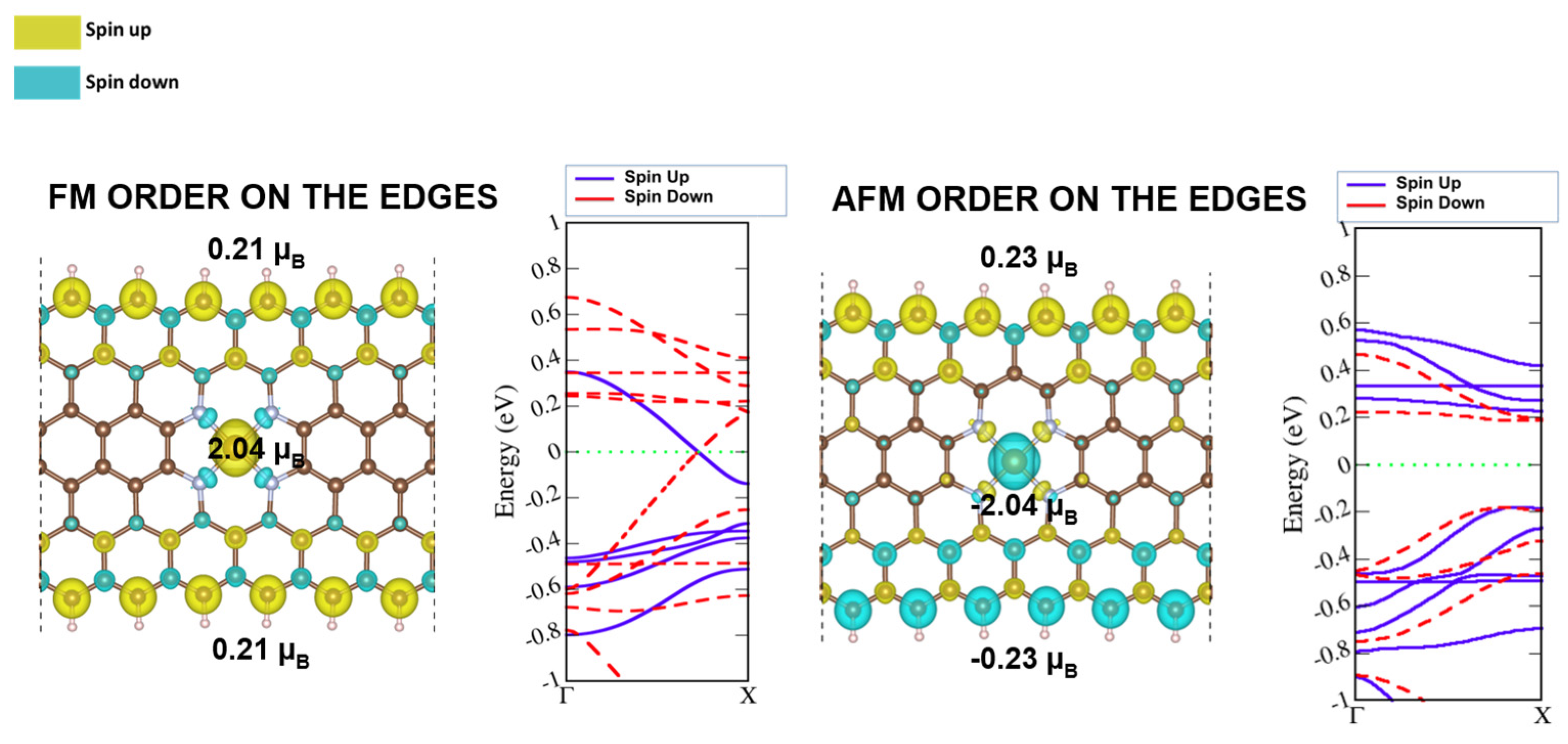
2.2. Ferromagnetic and Antiferromagnetic Spin Ordering at the ZGNR Edges
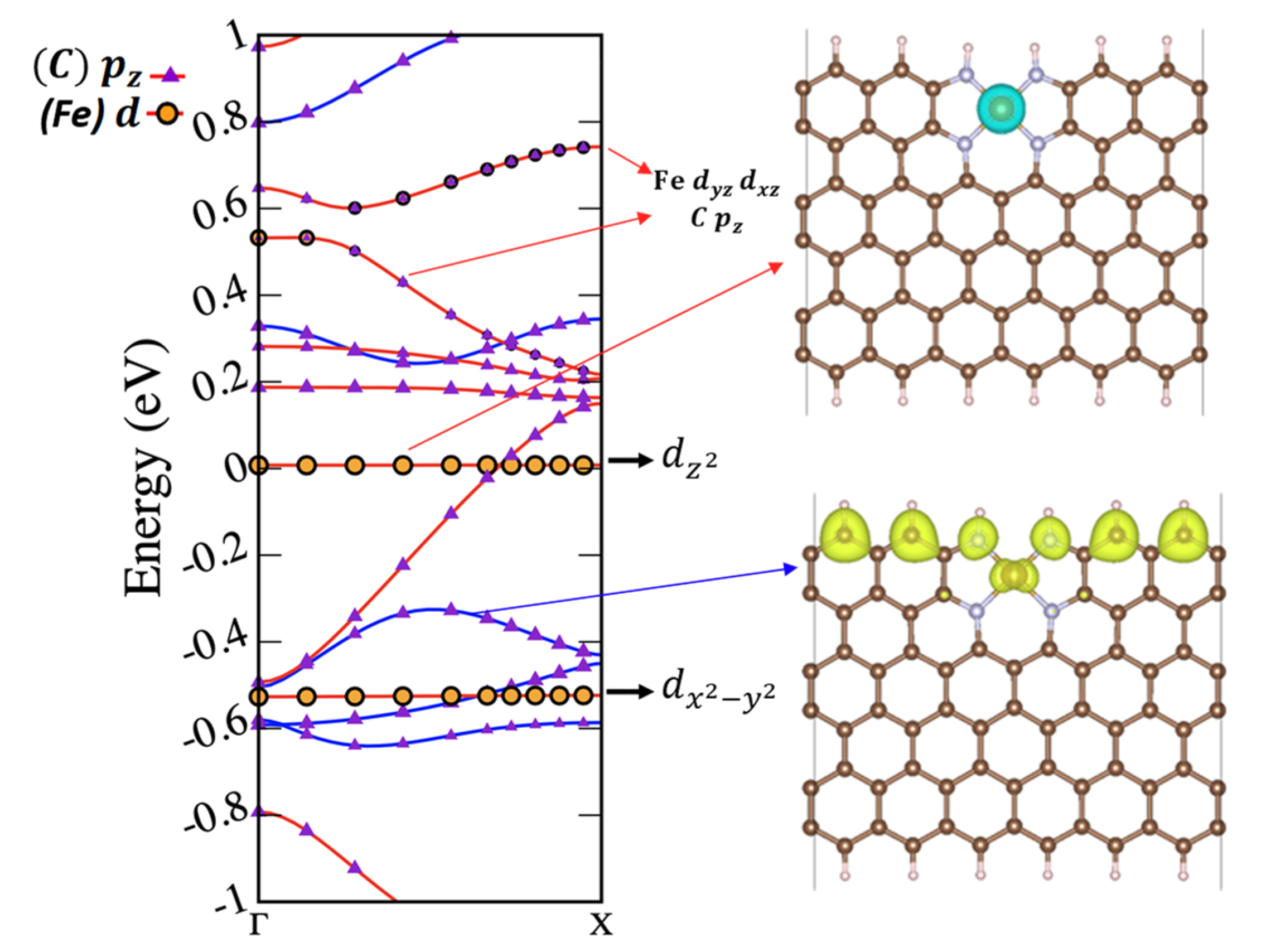
2.3. Magnetic and Electronic Properties as a Function of FeN4 Location and Edge Termination
2.4. Discussion: Implication for Catalysis
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Half-Metallic Graphene Nanoribbons. Nature 2006, 444, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Yazyev, O.V. Emergence of Magnetism in Graphene Materials and Nanostructures. Rep. Prog. Phys. 2010, 73, 056501. [Google Scholar] [CrossRef]
- Yazyev, O.V. A Guide to the Design of Electronic Properties of Graphene Nanoribbons. Acc. Chem. Res. 2013, 46, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Ejg, S.; Ayuela, A.; Sánchez-Portal, D. First-Principles Study of Substitutional Metal Impurities in Graphene: Structural, Electronic and Magnetic Properties. New J. Phys. 2010, 12, 053012. [Google Scholar] [CrossRef]
- Gao, S.; Yang, L. Edge-Insensitive Magnetism and Half Metallicity in Graphene Nanoribbons. J. Phys. Condens. Matter 2018, 30, 48LT01. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Gorjizadeh, N.; Kawazoe, Y. Multiple Spin State Analysis in Radical Carbon Edge and Oxygen Edge Graphene-like Molecules. J. Magn. Soc. Jpn. 2011, 35, 414–419. [Google Scholar] [CrossRef][Green Version]
- Ota, N.; Gorjizadeh, N.; Kawazoe, Y. Multiple Spin State Analysis of Magnetic Nano Graphene. J. Magn. Soc. Jpn. 2011, 35, 360–365. [Google Scholar] [CrossRef]
- Popov, Z.I.; Mikhaleva, N.S.; Visotin, M.A.; Kuzubov, A.A.; Entani, S.; Naramoto, H.; Sakai, S.; Sorokin, P.B.; Avramov, P.V. The Electronic Structure and Spin States of 2D Graphene/VX2 (X = S, Se) Heterostructures. Phys. Chem. Chem. Phys. 2016, 18, 33047–33052. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.; Hobza, P. Understanding the Spin-Dependent Electronic Properties of Symmetrically Far-Edge Doped Zigzag Graphene Nanoribbon from a First Principles Study. RSC Adv. 2017, 7, 46604–46614. [Google Scholar] [CrossRef]
- Wu, F.; Kan, E.; Xiang, H.; Wei, S.H.; Whangbo, M.H.; Yang, J. Magnetic States of Zigzag Graphene Nanoribbons from First Principles. Appl. Phys. Lett. 2009, 94, 223105. [Google Scholar] [CrossRef]
- Zhang, W.X.; He, C.; Li, T.; Gong, S.B. Tuning Electronic and Magnetic Properties of Zigzag Graphene Nanoribbons with a Stone-Wales Line Defect by Position and Axis Tensile Strain. RSC Adv. 2015, 5, 33407–33413. [Google Scholar] [CrossRef]
- Brede, J.; Merino-Díez, N.; Berdonces, A.; Sanz, S.; Domínguez-Celorrio, A.; Lobo-Checa, J.; Vilas-Varela, M.; Peña, D.; Frederiksen, T.; Pascual, J.I.; et al. Detecting the Spin-Polarization of Edge States in Graphene Nanoribbons. Nat. Commun. 2023, 14, 6677. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.; Švec, M.; Hapala, P.; Redondo, J.; Krejčí, O.; Lo, R.; Manna, D.; Sarmah, A.; Nachtigallová, D.; Tuček, J.; et al. Non-Covalent Control of Spin-State in Metal-Organic Complex by Positioning on N-Doped Graphene. Nat. Commun. 2018, 9, 2831. [Google Scholar] [CrossRef]
- Kinikar, A.; Xu, X.; Di Giovannantonio, M.; Gröning, O.; Eimre, K.; Pignedoli, C.A.; Müllen, K.; Narita, A.; Ruffieux, P.; Fasel, R. On-Surface Synthesis of Edge-Extended Zigzag Graphene Nanoribbons. Adv. Mater. 2023, 35, 2306311. [Google Scholar] [CrossRef]
- Bundaleska, N.; Dias, A.; Bundaleski, N.; Felizardo, E.; Henriques, J.; Tsyganov, D.; Abrashev, M.; Valcheva, E.; Kissovski, J.; Ferraria, A.M.; et al. Prospects for Microwave Plasma Synthesized N-Graphene in Secondary Electron Emission Mitigation Applications. Sci. Rep. 2020, 10, 13013. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Tanaka, H.; Kubo, S.; Sato, S. Unveiling Bonding States and Roles of Edges in Nitrogen-Doped Graphene Nanoribbon by X-Ray Photoelectron Spectroscopy. Carbon 2021, 185, 342–367. [Google Scholar] [CrossRef]
- Xia, D.; Yang, X.; Xie, L.; Wei, Y.; Jiang, W.; Dou, M.; Li, X.; Li, J.; Gan, L.; Kang, F. Direct Growth of Carbon Nanotubes Doped with Single Atomic Fe–N4 Active Sites and Neighboring Graphitic Nitrogen for Efficient and Stable Oxygen Reduction Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1906174. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I. Chemical Functionalization of Graphene with Defects. Nano Lett. 2008, 8, 4374–4379. [Google Scholar] [CrossRef]
- Liao, M.S.; Scheiner, S. Electronic Structure and Bonding in Metal Porphyrins, Metal = Fe, Co, Ni, Cu, Zn. J. Chem. Phys. 2002, 117, 205–219. [Google Scholar] [CrossRef]
- Yu, G.; Lü, X.; Jiang, L.; Gao, W.; Zheng, Y. Structural, Electronic and Magnetic Properties of Transition-Metal Embedded Zigzag-Edged Graphene Nanoribbons. J. Phys. D Appl. Phys. 2013, 46, 375303. [Google Scholar] [CrossRef]
- Kattel, S. Magnetic Properties of 3d Transition Metals and Nitrogen Functionalized Armchair Graphene Nanoribbon. RSC Adv. 2013, 3, 21110–21117. [Google Scholar] [CrossRef]
- Jiang, R.; Qiao, Z.; Xu, H.; Cao, D. Novel 2D Carbon Material T-Graphene Supported 3d Transition Metal Single Atoms as Efficient Oxygen Reduction Catalysts. Nanoscale 2023, 15, 16775–16783. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhao, J.X.; Wu, H.; Cai, Q.H.; Wang, X.G.; Wang, X.Z. Chemical Functionalization of Pyridine-like and Porphyrin-like Nitrogen-Doped Carbon (CNx) Nanotubes with Transition Metal (TM) Atoms: A Theoretical Study. Theor. Chem. Acc. 2010, 127, 727–733. [Google Scholar] [CrossRef]
- Chan, K.T.; Neaton, J.B.; Cohen, M.L. First-Principles Study of Metal Adatom Adsorption on Graphene. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 235430. [Google Scholar] [CrossRef]
- Longo, R.C.; Carrete, J.; Ferrer, J.; Gallego, L.J. Structural, Magnetic, and Electronic Properties of Nin and Fen Nanostructures (N = 1–4) Adsorbed on Zigzag Graphene Nanoribbons. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 115418. [Google Scholar] [CrossRef]
- Zheng, S.; Yan, X.; Yang, Y.; Xu, J. Identifying Structure-Property Relationships through SMILES Syntax Analysis with Self-Attention Mechanism. J. Chem. Inf. Model. 2019, 59, 914–923. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Shen, P.; Chen, Z. Spin Gapless Semiconductor-Metal-Half-Metal Properties in Nitrogen-Doped Zigzag Graphene Nanoribbons. ACS Nano 2009, 3, 1952–1958. [Google Scholar] [CrossRef]
- Holby, E.F.; Taylor, C.D. Control of Graphene Nanoribbon Vacancies by Fe and N Dopants: Implications for Catalysis. Appl. Phys. Lett. 2012, 101, 064102. [Google Scholar] [CrossRef]
- Luo, F.; Roy, A.; Silvioli, L.; Cullen, D.A.; Zitolo, A.; Sougrati, M.T.; Oguz, I.C.; Mineva, T.; Teschner, D.; Wagner, S.; et al. Author Correction: P-Block Single-Metal-Site Tin/Nitrogen-Doped Carbon Fuel Cell Cathode Catalyst for Oxygen Reduction Reaction (Nature Materials, (2020), 19, 11, (1215-1223), 10.1038/S41563-020-0717-5). Nat. Mater. 2023, 22, 146. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Chen, Z.; Faust Akl, D.; Mitchell, S.; Pérez-Ramírez, J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120, 11703–11809. [Google Scholar] [CrossRef]
- Kattel, S.; Atanassov, P.; Kiefer, B. A Density Functional Theory Study of Oxygen Reduction Reaction on Non-PGM Fe-Nx-C Electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 13800–13806. [Google Scholar] [CrossRef]
- Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M.T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of Catalytic Sites for Oxygen Reduction in Iron- and Nitrogen-Doped Graphene Materials. Nat. Mater. 2015, 14, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Li, J.; Jia, Q.; Stamatin, S.; Harrington, G.F.; Lyth, S.M.; Krtil, P.; Mukerjee, S.; et al. Identification of Catalytic Sites in Cobalt-Nitrogen-Carbon Materials for the Oxygen Reduction Reaction. Nat. Commun. 2017, 8, 957. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.P.; Varela, A.S.; Sinev, I.; Bon, V.; Roldan Cuenya, B.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding Activity and Selectivity of Metal-Nitrogen-Doped Carbon Catalysts for Electrochemical Reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, Y.; Yang, W.; Liu, N.; Chen, X. The Relationship between the Local Environment, N-Type, Spin State and Catalytic Functionality of Carbon-Hosted FeII/III-N4 for the Conversion of CO2 to CO. Phys. Chem. Chem. Phys. 2023, 25, 18889–18902. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Li, Q.K.; Cheng, J.; Liu, L.; Yan, Q.; Wu, Y.; Zhang, X.H.; Wang, Z.Y.; Qiu, Q.; Luo, Y. Conversion of Dinitrogen to Ammonia by FeN3-Embedded Graphene. J. Am. Chem. Soc. 2016, 138, 8706–8709. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Wang, G. A Density Functional Theory Study of Oxygen Reduction Reaction on Me-N 4 (Me = Fe, Co, or Ni) Clusters between Graphitic Pores. J. Mater. Chem. A Mater. 2013, 1, 10790–10797. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Feng, Y.; Allen, C.S.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H.; et al. General Synthesis and Definitive Structural Identification of MN4C4 Single-Atom Catalysts with Tunable Electrocatalytic Activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Ma, J.; Wang, L.; Deng, Y.; Zhang, W.; Wu, T.; Song, Y. Mass production of high-performance single atomic FeNC electrocatalysts via sequenced ultrasonic atomization and pyrolysis process. Sci. China Mater. 2021, 64, 631–641. [Google Scholar] [CrossRef]
- Saputro, A.G.; Kasai, H. Oxygen Reduction Reaction on Neighboring Fe-N4 and Quaternary-N Sites of Pyrolized Fe/N/C Catalyst. Phys. Chem. Chem. Phys. 2015, 17, 3059–3071. [Google Scholar] [CrossRef]
- Sougrati, M.T.; Goellner, V.; Schuppert, A.K.; Stievano, L.; Jaouen, F. Probing Active Sites in Iron-Based Catalysts for Oxygen Electro-Reduction: A Temperature-Dependent 57Fe Mössbauer Spectroscopy Study. Catal. Today 2016, 262, 110–120. [Google Scholar] [CrossRef]
- Mineva, T.; Matanovic, I.; Atanassov, P.; Sougrati, M.T.; Stievano, L.; Clémancey, M.; Kochem, A.; Latour, J.M.; Jaouen, F. Understanding Active Sites in Pyrolyzed Fe-N-C Catalysts for Fuel Cell Cathodes by Bridging Density Functional Theory Calculations and 57Fe Mössbauer Spectroscopy. ACS Catal. 2019, 9, 9359–9371. [Google Scholar] [CrossRef]
- Li, J.; Sougrati, M.T.; Zitolo, A.; Ablett, J.M.; Oğuz, I.C.; Mineva, T.; Matanovic, I.; Atanassov, P.; Huang, Y.; Zenyuk, I.; et al. Identification of Durable and Non-Durable FeNx Sites in Fe–N–C Materials for Proton Exchange Membrane Fuel Cells. Nat. Catal. 2021, 4, 10–19. [Google Scholar] [CrossRef]
- Szakacs, C.E.; Lefèvre, M.; Kramm, U.I.; Dodelet, J.P.; Vidal, F. A Density Functional Theory Study of Catalytic Sites for Oxygen Reduction in Fe/N/C Catalysts Used in H2/O2 Fuel Cells. Phys. Chem. Chem. Phys. 2014, 16, 13654–13661. [Google Scholar] [CrossRef]
- Kramm, U.I.; Abs-Wurmbach, I.; Herrmann-Geppert, I.; Radnik, J.; Fiechter, S.; Bogdanoff, P. Influence of the Electron-Density of FeN[Sub 4]-Centers Towards the Catalytic Activity of Pyrolyzed FeTMPPCl-Based ORR-Electrocatalysts. J. Electrochem. Soc. 2011, 158, B69. [Google Scholar] [CrossRef]
- Wagner, S.; Auerbach, H.; Tait, C.E.; Martinaiou, I.; Kumar, S.C.N.; Kübel, C.; Sergeev, I.; Wille, H.C.; Behrends, J.; Wolny, J.A.; et al. Elucidating the Structural Composition of an Fe–N–C Catalyst by Nuclear- and Electron-Resonance Techniques. Angew. Chem. Int. Ed. 2019, 58, 10486–10492. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Liu, L.; Yan, Q.; Li, Q.K.; Wang, Y.; Deng, M.; Qiu, Q. Strong Current Polarization and Perfect Negative Differential Resistance in Few-FeN4-Embedded Zigzag Graphene Nanoribbons. Phys. Chem. Chem. Phys. 2017, 19, 2674–2678. [Google Scholar] [CrossRef]
- Wu, L.; Cao, X.; Hu, W.; Ji, Y.; Zhu, Z.Z.; Li, X.F. Improving the Oxygen Reduction Reaction Activity of FeN4-Graphene via Tuning Electronic Characteristics. ACS Appl. Energy Mater. 2019, 2, 6634–6641. [Google Scholar] [CrossRef]
- Cervantes-Sodi, F.; Csányi, G.; Piscanec, S.; Ferrari, A.C. Edge-Functionalized and Substitutionally Doped Graphene Nanoribbons: Electronic and Spin Properties. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 165427. [Google Scholar] [CrossRef]
- Holby, E.F.; Wu, G.; Zelenay, P.; Taylor, C.D. Structure of Fe − N. J. Phys. Chem. C 2014, 118, 14388–14393. [Google Scholar]
- Yu, S.S.; Zheng, W.T.; Wen, Q.B.; Jiang, Q. First Principle Calculations of the Electronic Properties of Nitrogen-Doped Carbon Nanoribbons with Zigzag Edges. Carbon 2008, 46, 537–543. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Sun, L.; Krasheninnikov, A. V Gold-Embedded Zigzag Graphene Nanoribbons as Spin Gapless Semiconductors. Phys. Rev. B 2012, 86, 195418. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Theoretical Surface Science and Catalysis—Calculations and Concepts. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2000; Volume 45, pp. 71–129. ISBN 0360-0564. [Google Scholar]
- Groome, C.; Ngo, H.; Li, J.; Wang, C.S.; Wu, R.; Ragan, R. Influence of Magnetic Moment on Single Atom Catalytic Activation Energy Barriers. Catal. Lett. 2022, 152, 1347–1357. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Erratum: Generalized Gradient Approximation Made Simple (Physical Review Letters (1996) 77 (3865)). Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Tang, S.; Case, D.A. Vibrational Averaging of Chemical Shift Anisotropies in Model Peptides. J. Biomol. NMR 2007, 38, 255–266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
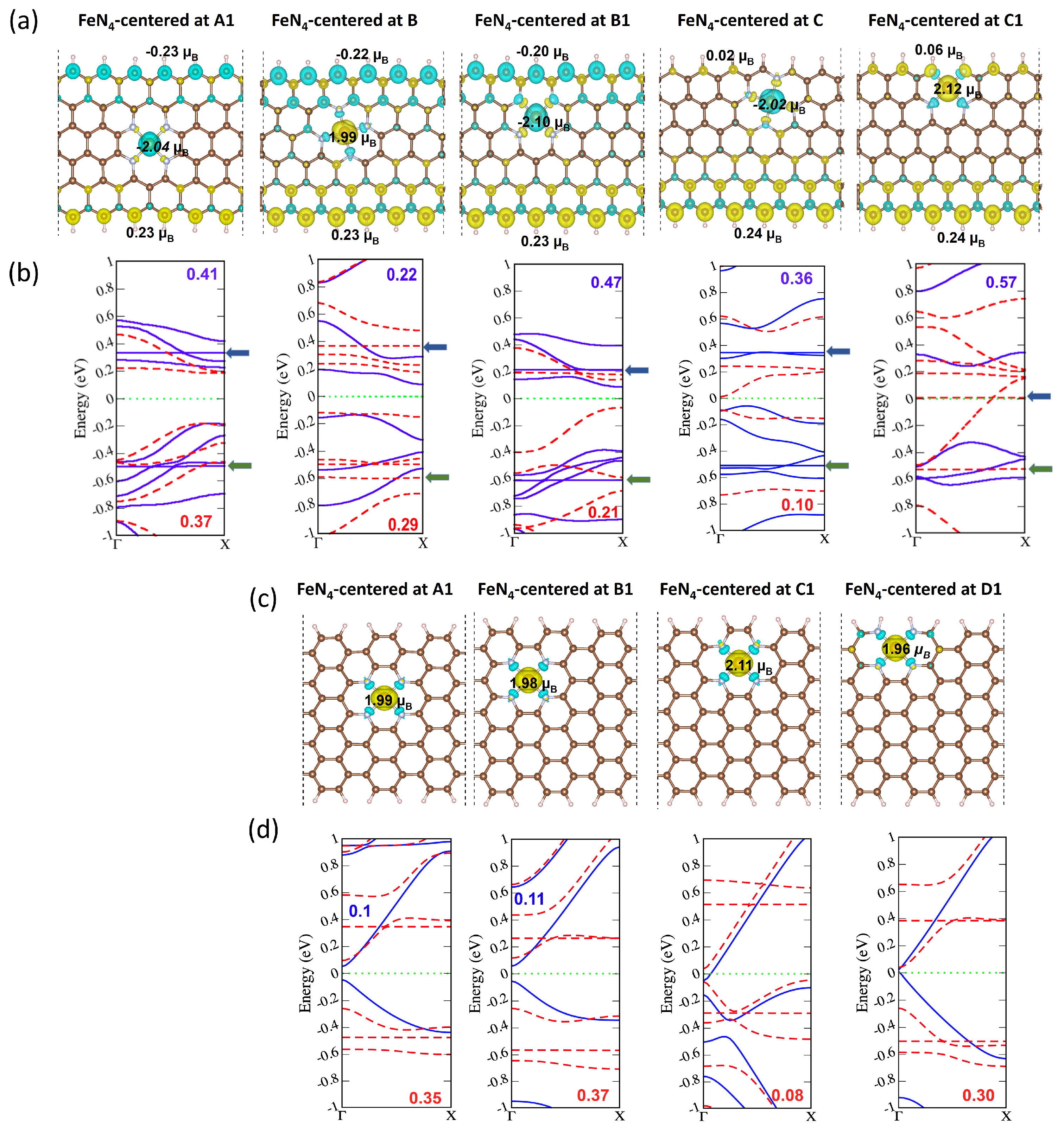
| FeN4 Position | A1 | B | B1 | C | C1 |
|---|---|---|---|---|---|
| μTotal | −2.00 | 2.02 | −1.97 | −0.09 | 4.00 |
| μFe | −2.04 | 1.99 | −2.10 | −2.02 | 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oguz, I.C.; Jaouen, F.; Mineva, T. Exploring Spin Distribution and Electronic Properties in FeN4-Graphene Catalysts with Edge Terminations. Molecules 2024, 29, 479. https://doi.org/10.3390/molecules29020479
Oguz IC, Jaouen F, Mineva T. Exploring Spin Distribution and Electronic Properties in FeN4-Graphene Catalysts with Edge Terminations. Molecules. 2024; 29(2):479. https://doi.org/10.3390/molecules29020479
Chicago/Turabian StyleOguz, Ismail Can, Frederic Jaouen, and Tzonka Mineva. 2024. "Exploring Spin Distribution and Electronic Properties in FeN4-Graphene Catalysts with Edge Terminations" Molecules 29, no. 2: 479. https://doi.org/10.3390/molecules29020479
APA StyleOguz, I. C., Jaouen, F., & Mineva, T. (2024). Exploring Spin Distribution and Electronic Properties in FeN4-Graphene Catalysts with Edge Terminations. Molecules, 29(2), 479. https://doi.org/10.3390/molecules29020479







