The Dynamic Evolution Model of the Chemical and Carbon Isotopic Composition of C1–3 during the Hydrocarbon Generation Process
Abstract
1. Introduction
- (1)
- Summarize the universal evolution laws of the chemical and isotopic composition of C1–3 in the hydrocarbon generation process on the basis of fitting systematic data obtained from published papers on the pyrolysis of various hydrocarbon sources;
- (2)
- Introduce the theoretical approach and detailed steps to build the dynamic model of the chemical and isotopic evolution of C1–3 during the hydrocarbon generation process;
- (3)
- Illustrate the application of the dynamic model to the pyrolysis experiment of the Ordovician Pingliang Shale from the Ordos Basin, China, to study the chemical and isotopic evolution of C1–3 during the hydrocarbon generation process.
2. Results and Discussion
2.1. Dynamic Chemical Evolution of C1–3 during Hydrocarbon Generation Process
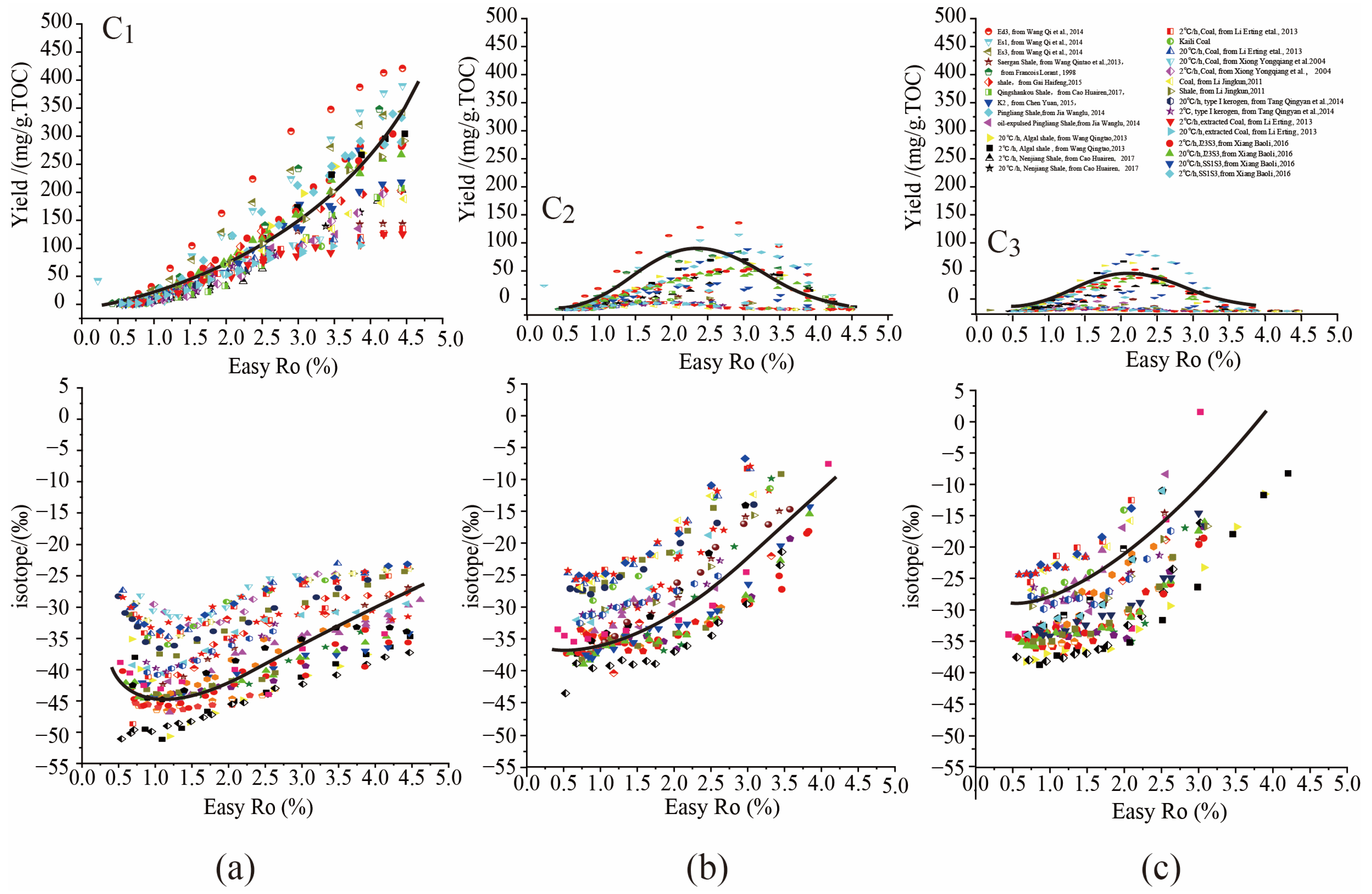
2.1.1. Dynamic Evolution of C1 Yield during Hydrocarbon Generation Process
2.1.2. Dynamic Evolution of C2–3 Yield during Hydrocarbon Generation Process
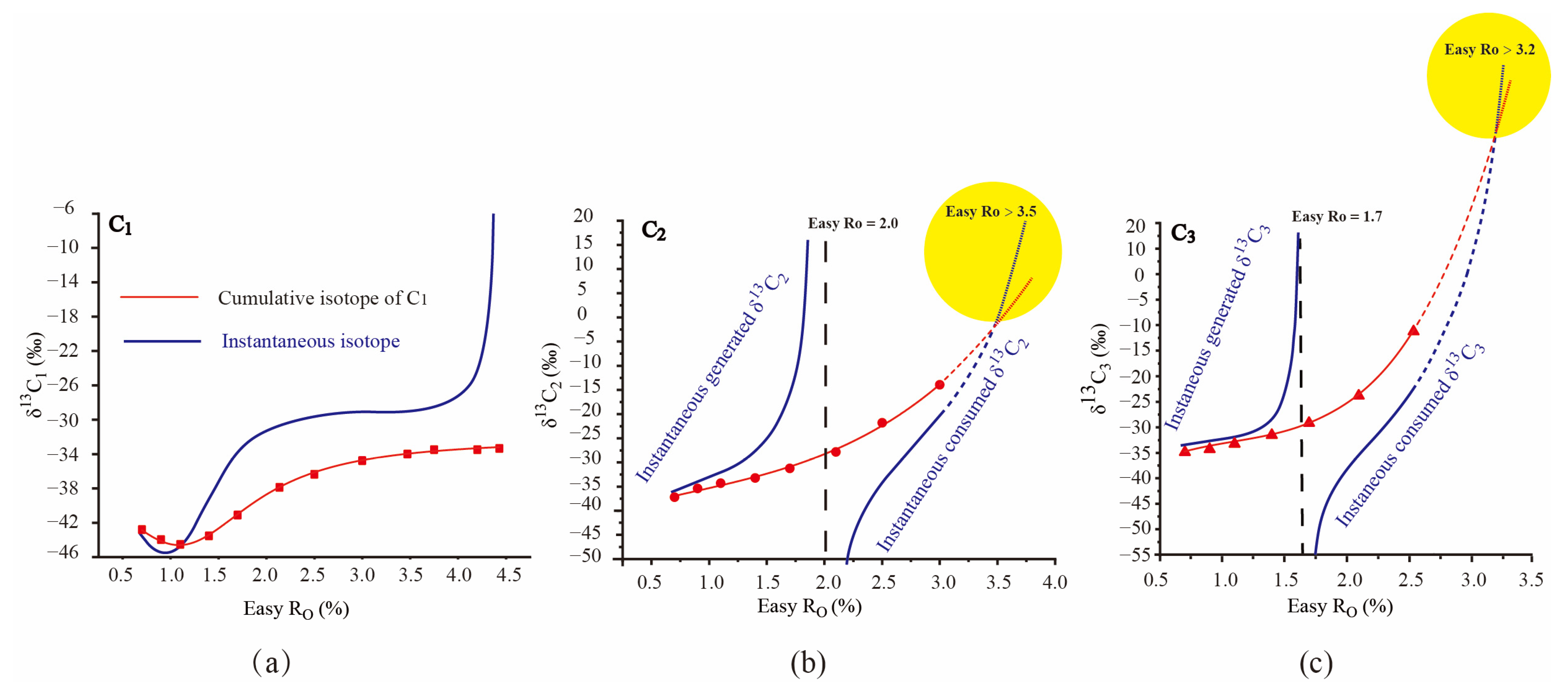
2.2. Dynamic Isotopic Evolution of δ13C1–3 during Hydrocarbon Generation Process
2.2.1. Dynamic Isotopic Evolution of δ13C1 during Hydrocarbon Generation Process
2.2.2. Dynamic Isotopic Evolution of δ13C2–3 during Hydrocarbon Generation Process
3. Theoretical Approach
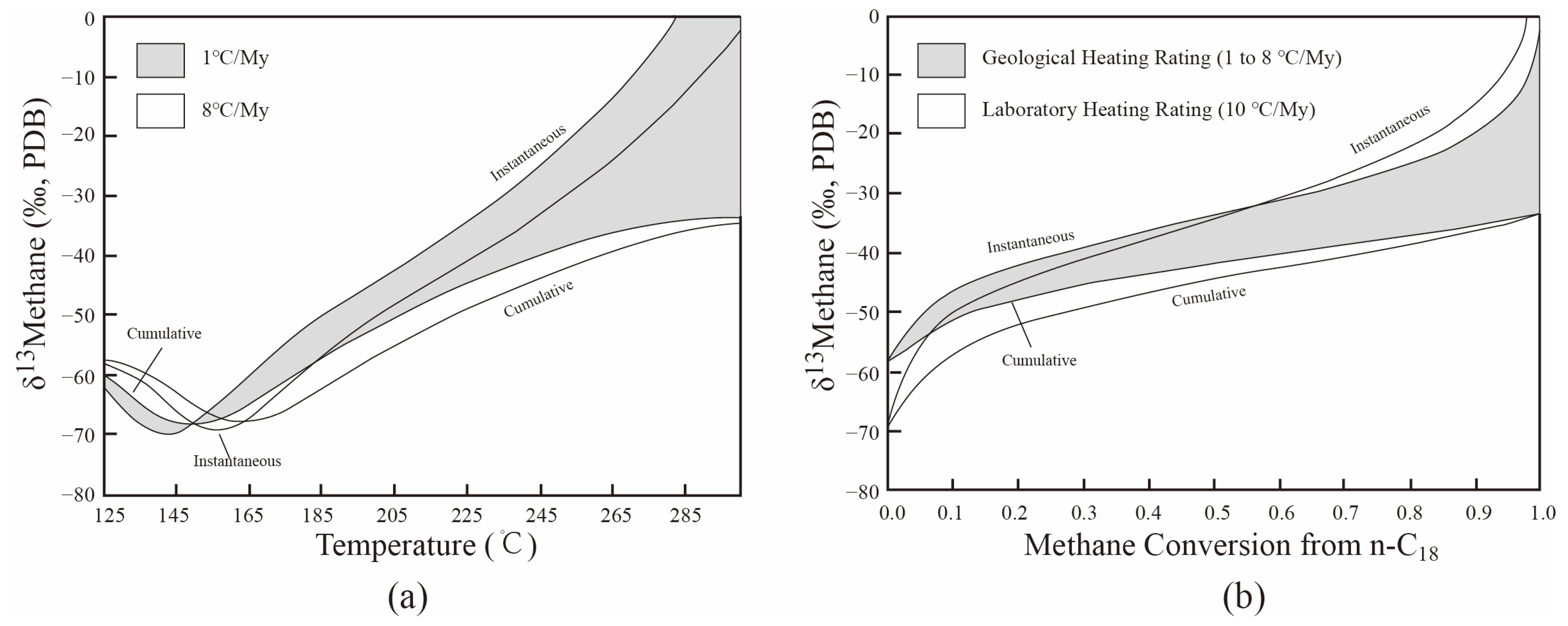
3.1. The Universal Chemical and Isotopic Evolution Laws of C1–3 in Hydrocarbon Generation Simulation Experiments
3.2. Calculation of Isotopic Composition of Mixed Natural Gas from Different Maturities (δ13Cmixed)
3.3. Dynamic Modeling of Chemical and Isotopic Variation of C1–3 during Hydrocarbon Generation Process
3.3.1. Nomenclature
- (1)
- Cumulative yield: Total yield of certain alkane gas in certain maturity interval or geological period;
- (2)
- Instantaneous yield: Yield of certain alkane gas at certain single maturity point or single point of geological time;
- (3)
- Cumulative isotope: Isotopic composition of alkane generated in certain maturity interval or geological period;
- (4)
- Instantaneous isotope: Isotopic composition of alkane generated at certain single maturity point or single point of geological time.
3.3.2. Calculations
4. Conclusions
- (1)
- Quantification of the yield and proportion of methane, ethane, and propane during the hydrocarbon generation process by the parameters of the cumulative yield of C1–3 and instantaneous yield of C1–3, providing a basis for the evaluation of natural gas resources from hydrocarbon source rock of different maturities, especially the evaluation of high-mature source rock.
- (2)
- Clarification of the cracking maturity of ethane and propane, and the proposal that there is no cracking of methane below the Easy Ro of 4.5%, which may be helpful for the natural gas exploration of deep formations.
- (3)
- Quantification of the evolution of δ13C1, δ13C2, and δ13C3 during the hydrocarbon generation process by the parameters of cumulative 13C1–3 and instantaneous 3C1–3, improving the accuracy of identifying the origin and evolutionary process of hydrocarbons by chemical and isotopic data, making it possible to study the dynamic evolution of the isotope series of C1–3 (including reversed alkane series).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoell, M. The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim. Cosmochim. Acta 1980, 44, 649–661. [Google Scholar] [CrossRef]
- Rooney, M.A.; Claypool, G.E.; Chung, H.M. Modeling thermogenic gas generation using carbon isotope ratios of natural gas hydrocarbons. Chem. Geol. 1995, 126, 219–232. [Google Scholar] [CrossRef]
- Tang, Y.; Perry, J.; Jenden, P.; Schoell, M. Mathematical modeling of stable carbon isotope ratios in natural gases. Geochim. Cosmochim. Acta 2000, 64, 2673–2687. [Google Scholar] [CrossRef]
- Galimov, E. Isotope organic geochemistry. Org. Geochem. 2006, 37, 1200–1262. [Google Scholar] [CrossRef]
- Liu, Q.; Worden, R.; Jin, Z.; Liu, W.; Li, J.; Gao, B.; Zhang, D.; Hu, A.; Yang, C. TSR versus non-TSR processes and their impact on gas geochemistry and carbon stable isotopes in Carboniferous, Permian and Lower Triassic marine carbonate gas reservoirs in the Eastern Sichuan Basin, China. Geochim. Cosmochim. Acta 2012, 100, 96–115. [Google Scholar] [CrossRef]
- Zhao, H. The Isotopic Evolution of Natural Gas During Accumulation Process—A Model of Differential Accumulation and Loss of Hydrocarbon for Isotope Reversal; Northwest Institute of Eco-Envirenment and Resources, Chinese Academy of Sciences: Lanzhou, China, 2019; (In Chinese with English Abstract). [Google Scholar]
- Zhao, H.; Liu, C.; Larson, T.E.; McGovern, G.P.; Horita, J. Bulk and position-specific isotope geochemistry of natural gases from the Late Cretaceous Eagle Ford Shale, south Texas. Mar. Pet. Geol. 2020, 122, 104659. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, H.Y.; Zhang, G.C.; Wang, Y.; Wang, X.; Qin, Y.; Zhang, D.; Liu, W. Study of the Mechanism for Identifying the Shale Gas ‘Sweet Spot’ Using the Reversed δ13C1-3 Series. Acta Geol. Sin. 2021, 95, 710–712. [Google Scholar] [CrossRef]
- Pei, L.; Liu, W.; Guo, Q.; Wang, X.; Luo, H.; Wang, Q. Genetic significance of carbon isotope curve types of methane, ethane, and propane in natural gas. Org. Geochem. 2023, 186, 104691. [Google Scholar] [CrossRef]
- Hunt, A.G.; Darrah, T.H.; Poreda, R.J. Determining the source and genetic fingerprint of natural gases using noble gas geochemistry: A northern Appalachian Basin case study. AAPG Bull. 2012, 96, 1785–1811. [Google Scholar] [CrossRef]
- Tilley, B.; McLellan, S.; Hiebert, S.; Quartero, B.; Veilleux, B.; Muehlenbachs, K. Gas isotope reversals in fractured gas reservoirs of the western Canadian Foothills: Mature shale gases in disguise. AAPG Bull. 2011, 95, 1399–1422. [Google Scholar] [CrossRef]
- Dai, J.; Zou, C.; Dong, D.; Ni, Y.; Wu, W.; Gong, D.; Wang, Y.; Huang, S.; Huang, J.; Fang, C.; et al. Geochemical characteristics of marine and terrestrial shale gas in China. Mar. Pet. Geol. 2016, 76, 444–463. [Google Scholar] [CrossRef]
- Shen, B.-J.; He, Z.-L.; Tao, C.; Shen, J.-C.; Hu, Z.-Q.; Li, Z.-M.; Cao, Y.-H.; Chen, W. A mathematical diffusion model of carbon isotopic reversals inside ultra-tight Longmaxi shale matrixes. Pet. Sci. 2022, 19, 2014–2026. [Google Scholar] [CrossRef]
- Liu, C.; Liu, P.; McGovern, G.P.; Horita, J. Molecular and intramolecular isotope geochemistry of natural gases from the Woodford Shale, Arkoma Basin, Oklahoma. Geochim. Cosmochim. Acta 2019, 255, 188–204. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, L.; Li, Y.; Huntington, K.W.; Jin, T.; Schauer, A.J.; Wang, C. Late Mesozoic elevation history of the north Taihang Mountains, China: Constraints from clumped isotope geochemistry. GSA Bull. 2022, 135, 1794–1806. [Google Scholar] [CrossRef]
- Xia, X.; Tang, Y. Isotope fractionation of methane during natural gas flow with coupled diffusion and adsorption/desorption. Geochim. Cosmochim. Acta 2012, 77, 489–503. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, X.F.; Wang, X.Z.; Shi, B.; Luo, X.; Zhang, L.; Lei, W.; Jiang, C.; Meng, Q. Carbon isotopic fractionation by desorption of shale gases. Mar. Pet. Geol. 2015, 60, 79–86. [Google Scholar] [CrossRef]
- Stolper, D.A.; Lawson, M.; Davis, C.L.; Ferreira, A.A.; Neto, E.V.S.; Ellis, G.S.; Lewan, M.D.; Martini, A.M.; Tang, Y.; Schoell, M.; et al. Formation temperatures of thermogenic and biogenic methane. Science 2014, 344, 1500–1503. [Google Scholar] [CrossRef]
- Gilbert, A.; Yamada, K.; Suda, K.; Ueno, Y.; Yoshida, N. Measurement of position-specific 13C isotopic composition of propane at the nanomole level. Geochim. Cosmochim. Acta 2016, 177, 205–216. [Google Scholar] [CrossRef]
- Piasecki, A.; Sessions, A.; Lawson, M.; Ferreira, A.; Neto, E.S.; Eiler, J.M. Analysis of the site-specific carbon isotope composition of propane by gas source isotope ratiomass spectrometer. Geochem. Cosmochim. Acta 2016, 188, 58–72. [Google Scholar] [CrossRef]
- Stahl, W.J.; Carey, B.B., Jr. Source rock identification by isotope analyses of natural gases from fields in the Val Varde Delaware Basins, West Texas. Chem. Geol. 1975, 16, 257–267. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Hue, A. Genetic and post-genetic molecular and isotopic fractionation in natural gas. Chem. Geol. 1995, 126, 281–290. [Google Scholar] [CrossRef]
- Liu, W.H.; Xu, Y.C. A two stage model of carbon isotopic fractionation in coal gas. Geochimica 1999, 28, 359–366. [Google Scholar]
- Clayton, C. Carbon isotope fractionation during natural gas generation from kerogen. Mar. Pet. Geol. 1991, 8, 232–240. [Google Scholar] [CrossRef]
- Berner, U.; Faber, E.; Stahl, W. Mathematical simulation of the carbon isotopic fractionation between huminitic coals and related methane. Chem. Geol. Isot. Geosci. 1992, 94, 315–319. [Google Scholar] [CrossRef]
- Lorant, F.; Prinzhofer, A.; Behar, F.; Huc, A.-Y. Carbon isotopic and molecular constraints on the formation and the expulsion of thermogenic hydrocarbon gases. Chem. Geol. 1998, 147, 249–264. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Y.; Ellis, G.S.; Wang, Y.; Kralert, P.G.; Gillaizeau, B.; Ma, Q.; Hwang, R. A kinetic model for thermally induced hydrogen and carbon isotope fractionation of individual n-alkanes in crude oil. Geochim. Cosmochim. Acta 2005, 69, 4505–4520. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, F.; Wang, Z.; Zhao, C.; Wang, H.; Liu, J.; Lu, J.; Geng, A.; Liu, D. Thermal cracking history by laboratory kinetic simulation of Paleozoic oil in eastern Tarim Basin, NW China, implications for the occurrence of residual oil reservoirs. Org. Geochem. 2006, 37, 1803–1815. [Google Scholar] [CrossRef]
- Tian, H.; Xiao, X.; Wilkins, R.; Li, X.; Gan, H. Gas sources of the YN2 gas pool in the Tarim Basin—Evidence from gas generation and methane carbon isotope fractionation kinetics of source rocks and crude oils. Mar. Pet. Geol. 2007, 24, 29–41. [Google Scholar] [CrossRef]
- Pan, C.; Jiang, L.; Liu, J.; Zhang, S.; Zhu, G. The effects of calcite and montmorillonite on oil cracking in confined pyrolysis experiments. Org. Geochem. 2010, 41, 611–626. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Q.; Liu, J.; Peng, P.; Li, B.; Lu, J. The effect of oil expulsion or retention on further thermal degradation of kerogen at the high maturity stage: A pyrolysis study of type II kerogen from Pingliang shale, China. Org. Geochem. 2014, 71, 17–29. [Google Scholar] [CrossRef]
- Xiang, B.; Li, E.; Gao, X.; Wang, M.; Wang, Y.; Xu, H.; Huang, P.; Yu, S.; Liu, J.; Zou, Y.; et al. Petroleum generation kinetics for Permian lacustrine source rocks in the Junggar Basin, NW China. Org. Geochem. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Shuai, Y.; Zou, Y.; Liu, J. Carbon Isotope Modeling of Coal-derived Methane and Ethane from the Upper Paleozoic of the Ordos Basin, China. Geol. Rev. 2005, 51, 665–671, (In Chinese with English Abstract). [Google Scholar]
- Shuai, Y.; Zou, Y.; Peng, P. Kinetic modeling of stable carbon isotope ratios of ethane from coal in confined system and its significance in geological application. Geochimica 2006, 35, 151–156, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.; Tang, Y. Kinetics of early methane generation from Green River shale. Chin. Sci. Bull. 1998, 43, 1908–1912. [Google Scholar] [CrossRef]
- Sweeney, J.J.; Burnham, A.K. Evaluation of a Simple Model of Vitrinite Reflectance Based on Chemical Kinetics. AAPG Bull. 1990, 74, 1559–1570. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Wang, Z.Y.; Zhang, S.C.; Wang, H.; Zhao, C.; Hu, G. Successive generation of natural gas from organic materials and its significance in future exploration. Pet. Explor. Dev. 2005, 32, 1–7, (In Chinese with English Abstract). [Google Scholar]
- Li, J.; Ma, W.; Wang, Y.; Wang, D.; Xie, Z.; Li, Z.; Ma, C. Modeling of the whole hydrocarbon-generating process of sapropelic source rock. Pet. Explor. Dev. 2018, 45, 461–471. [Google Scholar] [CrossRef]
- Ni, L.J.; Zhang, L.G.; Ni, J.F.; Yuan, W.K. Structural kinetic model of pyrolysis process of paraffins and its simulation. J. Chem. Ind. Eng. 1995, 46, 562–570, (In Chinese with English Abstract). [Google Scholar]
- ZHANG, H.M.; ZHANG, H.W.; GU, P.P.; Zhao, L. Molecular simulation of propane pyrolysis reaction. Acta Pet. Sin. (Pet. Process. Sect.) 2012, 28, 146–150, (In Chinese with English Abstract). [Google Scholar]
- Liu, W.; Wang, J.; Tenger; Qin, J.; Zheng, L. Stable carbon isotopes of gaseous alkanes as genetic indicators inferred from laboratory pyrolysis experiments of various marine hydrocarbon source materials from southern China. Sci. China Earth Sci. 2012, 55, 966–974. [Google Scholar] [CrossRef]
- Tian, H.; Xiao, X.; Wilkins, R.W.; Tang, Y. An experimental comparison of gas generation from three oil fractions: Implications for the chemical and stable carbon isotopic signatures of oil cracking gas. Org. Geochem. 2012, 46, 96–112. [Google Scholar] [CrossRef]
- Li, E.; Pan, C.; Yu, S.; Jin, X.; Liu, J. Hydrocarbon generation from coal, extracted coal and bitumen rich coal in confined pyrolysis experiments. Org. Geochem. 2013, 64, 58–75. [Google Scholar] [CrossRef]
- Cao, H.R. The Paleo-Environment of Source Rock Formation and Geological Evaluation of Shale Oil in the Songliao Basin; Guangzhou Institute of Geochemistry, Chinese Academy of Sciences: Guangzhou, China, 2017; (In Chinese with English Abstract). [Google Scholar]
- Wang, Q.; Zou, H.; Hao, F.; Zhu, Y.; Zhou, X.; Wang, Y.; Tian, J.; Liu, J. Modeling hydrocarbon generation from the Paleogene source rocks in Liaodong Bay, Bohai Sea: A study on gas potential of oil-prone source rocks. Org. Geochem. 2014, 76, 204–219. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Geng, A.S.; Liu, J.Z. Kinetic modeling of carbon isotope fractionation of coal-derived methane. Geochimica 2004, 33, 545–550, (In Chinese with English Abstract). [Google Scholar]
- Xiong, Y.Q.; Geng, A.S.; Liu, J.Z.; Wang, Y.P.; Liu, D.H.; Jia, R.F.; Shen, J.G. Kinetic simulating experiment combined with GC-IRMS analysis: Application to identification of effective source rock. Geochimica 2002, 31, 21–25, (In Chinese with English Abstract). [Google Scholar]
- Hill, R.J.; Tang, Y.; Kaplan, I.R. Insights into oil cracking based on laboratory experiments. Org. Geochem. 2003, 34, 1651–1672. [Google Scholar] [CrossRef]
- Gong, S.; Peng, P.A.; Lu, Y.H.; Xiao, Z.X.; Jia, W.L.; Wang, Z.Q.; Yu, C.L.; Liu, D.H.; Lu, J.L.; Liu, J.Z. The second heating experiment of biodegraded asphalt sand. Chin. Sci. Bull. 2004, 49, 39–47. (In Chinese) [Google Scholar]
- Yin, Q.; Song, Z.G.; Liu, J.Z. Influences of sulfur on composition of oil cracked gas and carbon isotopes. Oil Gas Geol. 2010, 31, 309–314, (In Chinese with English Abstract). [Google Scholar]
- Li, J.K.; Fang, W.; Zeng, H.S.; Liu, W.; Zou, Y.R.; Liu, J.Z. Possible origins for inverse stable carbon isotopes of gaseous alkanes from the Xujiaweizi fault depression. Acta Pet. Sin. 2011, 32, 54–61, (In Chinese with English Abstract). [Google Scholar]
- Tang, Q.Y.; Zhang, M.J.; Yu, M.; Zhang, T.W.; Liu, J.Z.; Zhang, M.C. Pyrolysis constraints on the generation mechanism of shale gas. J. China Coal Soc. 2013, 38, 742–747, (In Chinese with English Abstract). [Google Scholar]
- Tang, Q.Y.; Zhang, M.J.; Zhang, T.W.; Liu, J.J.; Yu, M. Kinetic pyrolysis simulation of hydrocarbon generation in shale system: A case study on Pearl River Mouth Basin, China. Geochimica 2014, 43, 518–528, (In Chinese with English Abstract). [Google Scholar]
- Wang, Q.T.; Lu, H.; Gao, L.H.; Xiong, P.; Shen, C.C.; Liu, J.Z.; Peng, P.A. Geochemical characterization of thermogenic gas during the simulation experiments of the mature Salgan Shale. J. China Coal Soc. 2013, 38, 754–759. [Google Scholar]
- Gao, L.; Schimmelmann, A.; Tang, Y.; Mastalerz, M. Isotope rollover in shale gas observed in laboratory pyrolysis experiments: Insight to the role of water in thermogenesis of mature gas. Org. Geochem. 2014, 68, 95–106. [Google Scholar] [CrossRef]
- Chen, Y. Mechanisms and Evaluation of Shale Gas Generation from Organic-Rich Marine Shales; Guangzhou Institute of Geochemistry, Chinese Academy of Sciences: Guangzhou, China, 2015; (In Chinese with English Abstract). [Google Scholar]
- Gai, H.; Xiao, X.; Cheng, P.; Tian, H.; Fu, J. Gas generation of shale organic matter with different contents of residual oil based on a pyrolysis experiment. Org. Geochem. 2014, 78, 69–78. [Google Scholar] [CrossRef]
- Shao, D.; Ellis, G.S.; Li, Y.; Zhang, T. Experimental investigation of the role of rock fabric in gas generation and expulsion during thermal maturation: Anhydrous closed-system pyrolysis of a bitumen-rich Eagle Ford Shale. Org. Geochem. 2018, 119, 22–35. [Google Scholar] [CrossRef]
- Xia, X.Y.; Li, C.Y.; Zhao, L. Influence of mixture on isotope indices in gas source discrimination. Pet. Explor. Dev. 1998, 25, 89–93, (In Chinese with English Abstract). [Google Scholar]
- Qin, Y.; Zhang, Y.S.; Zhu, Y.M.; Fan, B.H.; Jiang, B.; Li, T.Z. Lagging and reaction kinetic mechanism of hydrocarbonregeneration from organic matters in coals. Earth Sci. J. China Univ. Geosci. 2000, 25, 278–282. [Google Scholar]
- Jin, Q.; Wang, X.H.; Hu, X.Q.; Wang, L.; Wang, J.; Song, G.Q. Kinetics of primary and secondary generation of coal-derived gases and its applications to genesis of natural gases found in Gubei area, Zhanhua Depression. Geochimica 2008, 37, 239–244, (In Chinese with English Abstract). [Google Scholar]

| Proportion of End Member A (%) | Proportion of End Member B (%) | δ13Cmixed according to Definition Formula (‰) | δ13Cmixed according to Simplified Formula (‰) |
|---|---|---|---|
| 0.01 | 99.99 | −49.996 | −49.996 |
| 0.1 | 99.9 | −49.960 | −49.960 |
| 1 | 99 | −49.600 | −49.600 |
| 5 | 95 | −48.001 | −48.000 |
| 10 | 90 | −46.002 | −46.000 |
| 20 | 80 | −42.003 | −42.000 |
| 30 | 70 | −38.004 | −38.000 |
| 40 | 60 | −34.004 | −34.000 |
| 50 | 50 | −30.004 | −30.000 |
| 60 | 40 | −26.004 | −26.000 |
| 70 | 30 | −22.004 | −22.000 |
| 80 | 20 | −18.003 | −18.000 |
| 90 | 10 | −14.002 | −14.000 |
| 95 | 5 | −12.001 | −12.000 |
| 99 | 1 | −10.400 | −10.400 |
| 99.9 | 0.1 | −10.040 | −10.040 |
| 99.99 | 0.01 | −10.004 | −10.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Li, Y.; Liu, W.; Zhang, G.; Wang, Y. The Dynamic Evolution Model of the Chemical and Carbon Isotopic Composition of C1–3 during the Hydrocarbon Generation Process. Molecules 2024, 29, 476. https://doi.org/10.3390/molecules29020476
Zhao H, Li Y, Liu W, Zhang G, Wang Y. The Dynamic Evolution Model of the Chemical and Carbon Isotopic Composition of C1–3 during the Hydrocarbon Generation Process. Molecules. 2024; 29(2):476. https://doi.org/10.3390/molecules29020476
Chicago/Turabian StyleZhao, Heng, Yanjie Li, Wenhui Liu, Guchun Zhang, and Yanjun Wang. 2024. "The Dynamic Evolution Model of the Chemical and Carbon Isotopic Composition of C1–3 during the Hydrocarbon Generation Process" Molecules 29, no. 2: 476. https://doi.org/10.3390/molecules29020476
APA StyleZhao, H., Li, Y., Liu, W., Zhang, G., & Wang, Y. (2024). The Dynamic Evolution Model of the Chemical and Carbon Isotopic Composition of C1–3 during the Hydrocarbon Generation Process. Molecules, 29(2), 476. https://doi.org/10.3390/molecules29020476





